Abstract
Background:
Kidney iron deposition (KID) is caused by iron overload that is observed in kidney diseases and anemia. The protective effects of deferoxamine (DF) and silymarin (SM) were studied against iron overload-induced KID in rat model.
Methods:
Rats received iron dextran (200 mg/kg) for a period of 4 weeks every other day, but at the beginning of week 3, they also were subjected to a 2-week (every other day) treatment with vehicle (group 2, positive control), SM (200 mg/kg; group 3), DF (50 mg/kg; group 4), SM (400 mg/kg; group 5), and combination of SM and DF (200 and 50 mg/kg, respectively; group 6). Group 1, as the negative control, received saline alone during the study. The levels of serum creatinine (Cr), blood urea nitrogen (BUN), iron, ferritin, and nitrite were determined, and the kidney was removed for histopathological investigations.
Results:
Before treatment, the serum levels of iron and ferritin in all iron dextran receiver groups were significantly higher than those of the negative control group (P < 0.05). However, the serum levels of BUN, Cr, and nitrite were not different between the groups. No statistical differences were detected in kidney weight and the serum levels of BUN, Cr, iron, ferritin, and nitrite after 2 weeks of treatment with SM, DF, or combination of both. The SM and DF treatments reduced the intensity of the KID, but only in the SM (200 mg/kg) group, a significant reduction in KID was observed (P < 0.05).
Conclusion:
It seems that SM is a nephroprotectant agent against KID in acute iron overload animal models.
Keywords: Deferoxamine, iron overload, kidney iron deposition, silymarin
INTRODUCTION
Iron overload disturbs many physiological functions and provides iron deposition in different organs including kidney. Kidney iron deposition (KID) has been observed in patients with kidney diseases,[1] sickle cell disease,[2] and aplastic anemia.[3,4] KID has been also developed in experimental animals for different research purposes.[5–8] Under some conditions, the KID may not disturb the renal function,[7] but iron overload potentially could enhance the formation of hydroxyl radical, which promote organ damaging including tubular damage.[8,9]
It has been reported that nitric oxide protected the rats’ kidney from iron-induced nephrotoxicity,[10,11] and sesame oil is nephroprotectant against iron-induced injury in mice.[12]
Clinically, thalassemic patients usually struggle with iron overload and deferoxamine (DF) as an iron chelator is the most common drug to prevent increasing of the iron level in plasma or specific organs tissues in patients[13–15] and experimental animals.[16,17]
Silymarin (SM), as an antioxidant agent, is derived from the herb milk thistle (Silybum marianum) and potentially has the ability to chelate iron.[18–21] The antioxidant effect of SM in the renal ischemia/reperfusion injury[22,23] and renal toxicity[24,25] has been investigated before.
Iron overload in particular diseases such as thalassemia is a continuous process. The patients are usually treated by DF, but due to the administration procedure, it is a subject of complaints from the patients and their relatives. To simulate this process, we designed an iron overload model, which consisted of two phases. In phase 1, iron overload was induced by administration of iron dextran and in phase 2, iron overload was continued and the protective role of SM, DF, or combination of both against KID was studied.
METHODS
Animals
Thirty-six adult male Wistar rats (Animal Centre, Ahvaz University of Medical Sciences, Ahvaz, Iran) with the mean weight of 201 ± 4 g were used. The rats were individually housed at a temperature of 23-25°C. Rats had free access to water and chow. The experimental procedures were in advance approved by the Isfahan University of Medical Sciences Ethics Committee.
Experimental protocol
The animals were randomly divided into six experimental groups as follows:
Group 1 (n = 6, negative control group): The animal received vehicle (0.5 mL of saline) every other day during the 4 weeks of study.
Group 2 (n = 6, positive control group): The animals received iron dextran (Vifor Inc., Switzerland) 200 mg/kg every other day during the 4 weeks of study. They also received vehicle during weeks 3 and 4. The dose of iron dextran for overloading was selected based on other studies.[7,10,16]
Groups 3 to 6: The animals in these groups had regimen the same as group 2, except that they received SM (200 mg/kg, orally by feeding tube, group 3), DF (50 mg/kg, i.p., group 4), SM (400 mg/kg, orally, group 5), and combination of SM and DF (200 mg/kg, orally and 50 mg/kg, i.p., group 6) instead of vehicle. The dose of SM and DF was selected based on other studies.[16]
Blood samples were obtained 2 weeks after overloading (before treatment) and 4 weeks after overloading (end of the experiment; after treatment). The serum samples were separated to measure the parameters. Then, the rats were anesthetized and sacrificed. The kidneys were removed and weighted immediately, and were prepared for histopathological procedures.
Measurements
The levels of serum creatinine (Cr), blood urea nitrogen (BUN), and iron were determined using quantitative diagnostic kits (Pars Azmoon, Iran). The serum level of nitrite [stable nitric oxide (NO) metabolite] was measured using a colorimetric ELISA kit (Promega Corporation, USA) that involves the Griess reaction. The serum level of ferritin was measured using enzyme immunoassay ELISA kit for rat (Immunology Consultants Laboratory Inc., USA).
Histopathological procedures
The removed kidneys were fixed in 10% neutral formalin solution and embedded in paraffin for staining to examine iron deposition in the kidney. The tissue was also subjected to hematoxylin and eosin staining to examine the tissue damage. The KID was evaluated by two independent pathologists who were totally blind to the study. On the basis of the intensity of KID and tissue damage, the kidney tissue damage score (KTDS) was graded from 1 to 5, while score 0 was assigned to normal kidney tissue without damage and iron deposition. This scoring was modified by our pathologists based on study by Senturk et al.[22]
Statistical analysis
Data are expressed as mean ± standard error of the mean. The after treatment serum levels of BUN, Cr, iron, nitrite, and ferritin were compared between the groups using univariate analysis of variance (ANOVA) and before treatment parameters as covariate. The one-way ANOVA was applied to compare the KTDS between the groups. The statistical P ≤ 0.05 was considered as significant.
RESULTS
Before treatment (baseline data)
Effect of iron overload on serum levels of BUN, Cr, iron, ferritin, and nitrite
The serum levels of iron and ferritin were increased in all iron dextran receiver groups and the levels were significantly different from the values obtained for the negative control group (P < 0.05). However, no statistically significant differences in serum levels of BUN, Cr, and nitrite were observed between the groups [Table 1]. These data supported that iron overload have no effect on BUN and Cr as kidney function biomarkers.
Table 1.
Serum levels of BUN, Cr, iron, ferritin, and nitrite in six groups of experiment 2 weeks after iron overload (before treatment). Iron dextran receiver groups were significantly different from the negative control group in this respect (P < 0.05)

After treatment
The effect of SM and DF accompanied with iron overload on serum levels of BUN, Cr, iron, ferritin, and nitrite
The data indicated that SM200, DF + SM200, and SM400 had no significant effects on serum levels of BUN, Cr, iron, ferritin, and nitrite [Figure 1]. With regard to the serum levels of BUN and Cr, it seems that there was no renal dysfunction, but based on histological investigations there was iron deposition in the kidney tissue of groups 2 to 6 [Figure 2].
Figure 1.

The serum levels of BUN, Cr, iron, ferritin, and nitrite after 2 weeks of treatment with SM (200 mg/kg; group 3), DF (50 mg/kg; group 4), SM (400 mg/kg; group 5), and combination of SM and DF (200 and 50 mg/kg, respectively; group 6) compared with the positive control group (group 2). No significant differences were detected among treatment and control groups. The group 1 is not shown in the figure because the treated groups (groups 3 to 6) were compared with group 2 (positive control group) only
Figure 2.

The kidney weight per 100 g of body weight and kidney pathology score (KTDS), which corresponds to KID after 2 weeks of treatment with SM (200 mg/kg; group 3), DF (50 mg/kg; group 4), SM (400 mg/kg; group 5), and combination of SM and DF (200 and 50 mg/kg, respectively; group 6) compared with the positive control group (group 2). The star (*) indicates significant difference from the positive control group (group 2) (P< 0.05). The group 1 is not shown in the figure because the treated groups (groups 3 to 6) were compared with group 2 (positive control group) only
The kidneys from all animals in the negative control group (group 1) were free of iron deposition with 0 score, and significant differences were observed between this negative control group and others groups (P < 0.05). The lower intensity of KID and damage in group 3, which was treated by SM (200 mg/kg) revealed the lower KTDS, and group 3 was significantly different from the positive control group in this regard (P < 0.05). However, no statistical difference in KTDS was detected between the positive control group on the one hand and groups 4-6 on the other hand [Figure 3].
Figure 3.
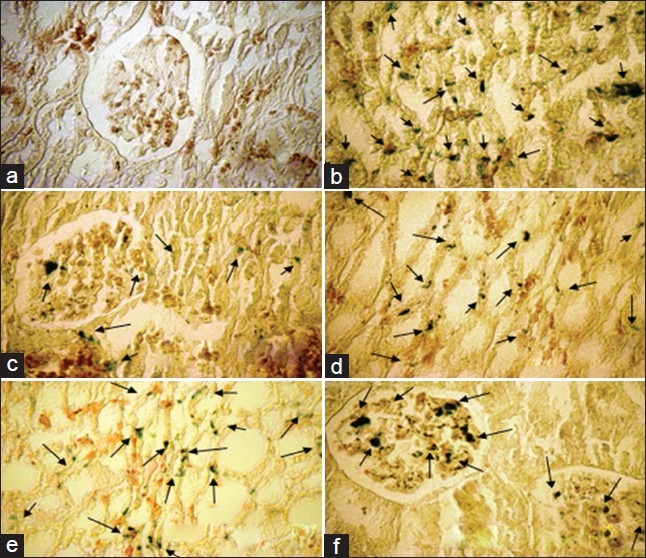
The sample images of kidney tissue stained with Persian blue to examine iron deposition in the kidney of six experimental groups; a= Vehicle, b= Fe, c= SM200+Fe: d: DF50+Fe, e= SM400+Fe, f=SM200+DF50+Fe. ‘a’ received vehicle during the study, and the groups 2 to 6 received iron dextran for a period of 4 weeks but during weeks 3 and 4, they were treated by vehicle (b; positive control group), SM (200 mg/kg; c), DF (50 mg/kg; d), SM (400 mg/kg; e), and combination of SM and DF (200 and 50 mg/kg, respectively; f), respectively. The arrows show Fe deposition in the tissue. Lower Fe deposition was observed in group 3 (SM200)
DISCUSSION
The main objective of this study was to investigate the protective role of SM and DF against deposition of iron in an iron overload rat model. Our finding indicated that SM at the dose of 200 mg/kg reduced the KID. Iron is required for metabolic processes in the kidney, but iron overload enhances hydroxyl radical formation, resulting tubular damage[8,9] and may disturb physiological function of the cellular environment of the kidney.[26] Clinically, thalassemic patients are the best example of iron overload, and usually they use DF as an iron chelator. However, renal dysfunction is one of the side effects of iron overload in these patients. SM as an antioxidant agent is also an iron chelator.[18–20]
In the study, iron overload was induced by iron dextran, which is a safe agent,[27,28] but due to formation of oxidative stress, it may damage the organs.[29] Serum levels of both iron and ferritin increased significantly after 2 weeks of iron overload [Table 1]. This expected finding supported the process of overloading. Iron deposition was detected in the kidney tissue via pathological investigations. However, based on the serum levels of BUN and Cr, no renal dysfunction occurred. This may be attributed to the lower potency of iron dextran in enhancing the oxidative stress and location of iron deposition in the kidney that does not disturb the regular renal function.[7] There is also little evidence available that accumulated iron via polynuclear iron complexes participate in free radical reactions to disturb the functions of various organ.[30] However, a dose of 600 mg/kg iron dextran for 24 h increased the serum level of urea and Cr.[10]
High iron stores may disturb endothelial function in patients,[31,32] and iron chelators may improve the endothelial function.[33,34] In this study, we measured NO metabolite (nitrite) as a marker of endothelial function; however, no change in nitrite level was observed. It is reported that chronic iron overload enhances inducible NO synthase expression in some organ;[35] however, our model was an acute iron overloading model, and it seems for such acute model iron-induced endothelial dysfunction may not achieve.
The data obtained in our study indicated that SM, DF, or combination of both did not demonstrate an iron chelation effect in reducing the iron serum level. The reduction of the serum level of iron by SM in a different iron overload model was reported before.[16] In this model, SM (200 mg/kg) was administrated accompanied with iron dextran for 2 weeks, but the usage dose of iron dextran was half of our usage dose. SM also used as iron chelator,[18,20] protective agent against gentamicin-induced nephrotoxicity,[36] or treatment of liver diseases.[37] It seems that our model and the high usage dose of iron dextran (200 mg/kg) was suitable to obtain a KID animal model, but possibly the dose of iron dextran was high enough to saturate the iron and ferritin capacities, so SM, DF, or combination of both were not enough potentiate to reduce the saturated level of iron and ferritin in animal serum. On the contrary, the histopathological data revealed that SM (200 mg/kg) alone reduced the KID significantly. This finding supports the antioxidant effects of SM similar to other experimental models.[22–25] DF also has antioxidant effect in rat kidneys,[38] and it reduced the KID in our study nonsignificantly. Future studies are needed to compare the antioxidant potency of SM and DF. In our iron overload model, coadministration of SM and DF did not protect the kidney tissue from iron deposition, possibly due to pharmacokinetic agents’ interactions, and finally our findings demonstrated unexpected results that KID obtained from the treated animals with 200 mg/kg of SM was less than KID obtained from treated animals with 400 mg/kg of SM. The reason may relate to the dose of antioxidants. The effect of some flavonoids antioxidant such as galangin in fructose-fed-induced renal damage was dose-dependent, and the lower dose provide a better protection effect for renal damage,[39] so possibly the SM as a flavonoids at the dose of 200 mg/kg was more effective than the higher dose.
CONCLUSION
SM is an effective antioxidant to reduce the KID in an iron overload model. Our finding demonstrated that SM with the dose of 200 mg/kg potentially was more protective than DF against KID. This observation need to be verified in serial experimental studies with different intensity of iron overload.
ACKNOWLEDGMENT
This research was supported by Isfahan University of Medical Sciences (grant # 290201). The authors thank Dr. Ali-Reza Rezaei for his assistance.
Footnotes
Source of Support: Isfahan University of Medical Sciences (grant # 290201)
Conflict of Interest: None declared
REFERENCES
- 1.Wang H, Nishiya K, Ito H, Hosokawa T, Hashimoto K, Moriki T. Iron deposition in renal biopsy specimens from patients with kidney diseases. Am J Kidney Dis. 2001;38:1038–44. doi: 10.1053/ajkd.2001.28593. [DOI] [PubMed] [Google Scholar]
- 2.Schein A, Enriquez C, Coates TD, Wood JC. Magnetic resonance detection of kidney iron deposition in sickle cell disease: A marker of chronic hemolysis. J Magn Reson Imaging. 2008;28:698–704. doi: 10.1002/jmri.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhusudhan KS, Oberoi R. Renal iron deposition in aplastic anemia: Magnetic resonance imaging appearance. Indian J Nephrol. 2011;21:134–5. doi: 10.4103/0971-4065.82145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rakow-Penner R, Glader B, Yu H, Vasanawala S. Adrenal and renal corticomedullary junction iron deposition in red cell aplasia. Pediatr Radiol. 2010;40:1955–7. doi: 10.1007/s00247-010-1824-2. [DOI] [PubMed] [Google Scholar]
- 5.Ceyssens B, Pauwels M, Meulemans B, Verbeelen D, Van den Branden C. Increased oxidative stress in the mouse adriamycin model of glomerulosclerosis is accompanied by deposition of ferric iron and altered GGT activity in renal cortex. Ren Fail. 2004;26:21–7. doi: 10.1081/jdi-120028539. [DOI] [PubMed] [Google Scholar]
- 6.Ishizaka N, Aizawa T, Yamazaki I, Usui S, Mori I, Kurokawa K, et al. Abnormal iron deposition in renal cells in the rat with chronic angiotensin II administration. Lab Invest. 2002;82:87–96. doi: 10.1038/labinvest.3780398. [DOI] [PubMed] [Google Scholar]
- 7.Ishizaka N, Saito K, Noiri E, Sata M, Mori I, Ohno M, et al. Iron dextran causes renal iron deposition but not renal dysfunction in angiotensin II-treated and untreated rats. Nephron Physiol. 2004;98:107–13. doi: 10.1159/000081559. [DOI] [PubMed] [Google Scholar]
- 8.Kondo A, Deguchi J, Okada S. Intranuclear iron deposition in hepatocytes and renal tubular cells in mice treated with ferric nitrilotriacetate. Virchows Arch. 1998;433:543–8. doi: 10.1007/s004280050287. [DOI] [PubMed] [Google Scholar]
- 9.Sponsel HT, Alfrey AC, Hammond WS, Durr JA, Ray C, Anderson RJ. Effect of iron on renal tubular epithelial cells. Kidney Int. 1996;50:436–44. doi: 10.1038/ki.1996.334. [DOI] [PubMed] [Google Scholar]
- 10.Kadkhodaee M, Gol A. The role of nitric oxide in iron-induced rat renal injury. Hum Exp Toxicol. 2004;23:533–6. doi: 10.1191/0960327104ht485oa. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Sharma S, Chopra K. Reversal of iron-induced nephrotoxicity in rats by molsidomine: A nitric oxide donor. Food Chem Toxicol. 2008;46:537–43. doi: 10.1016/j.fct.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Li YH, Chien SP, Chu PY, Liu MY. Prophylactic and therapeutic effects of a subcutaneous injection of sesame oil against iron-induced acute renal injury in mice. JPEN J Parenter Enteral Nutr. 2012;36:344–8. doi: 10.1177/0148607111415530. [DOI] [PubMed] [Google Scholar]
- 13.Franchini M, Gandini G, Veneri D, Aprili G. Safety and efficacy of subcutaneous bolus injection of deferoxamine in adult patients with iron overload: An update. Blood. 2004;103:747–8. doi: 10.1182/blood-2003-10-3373. [DOI] [PubMed] [Google Scholar]
- 14.Franchini M, Gandini G, de Gironcoli M, Vassanelli A, Borgna-Pignatti C, Aprili G. Safety and efficacy of subcutaneous bolus injection of deferoxamine in adult patients with iron overload. Blood. 2000;95:2776–9. [PubMed] [Google Scholar]
- 15.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 16.Najafzadeh H, Jalali MR, Morovvati H, Taravati F. Comparison of the prophylactic effect of silymarin and deferoxamine on iron overload-induced hepatotoxicity in rat. J Med Toxicol. 2010;6:22–6. doi: 10.1007/s13181-010-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obejero-Paz CA, Yang T, Dong WQ, Levy MN, Brittenham GM, Kuryshev YA, et al. Deferoxamine promotes survival and prevents electrocardiographic abnormalities in the gerbil model of iron-overload cardiomyopathy. J Lab Clin Med. 2003;141:121–30. doi: 10.1067/mlc.2003.18. [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson C, Bomford A, Geissler CA. The iron-chelating potential of silybin in patients with hereditary haemochromatosis. Eur J Clin Nutr. 2010;64:1239–41. doi: 10.1038/ejcn.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gharagozloo M, Khoshdel Z, Amirghofran Z. The effect of an iron (III) chelator, silybin, on the proliferation and cell cycle of Jurkat cells: A comparison with desferrioxamine. Eur J Pharmacol. 2008;589:1–7. doi: 10.1016/j.ejphar.2008.03.059. [DOI] [PubMed] [Google Scholar]
- 20.Borsari M, Gabbi C, Ghelfi F, Grandi R, Saladini M, Severi S, et al. Silybin, a new iron-chelating agent. J Inorg Biochem. 2001;85:123–9. doi: 10.1016/s0162-0134(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 21.Pietrangelo A, Borella F, Casalgrandi G, Montosi G, Ceccarelli D, Gallesi D, et al. Antioxidant activity of silybin in vivo during long-term iron overload in rats. Gastroenterology. 1995;109:1941–9. doi: 10.1016/0016-5085(95)90762-9. [DOI] [PubMed] [Google Scholar]
- 22.Senturk H, Kabay S, Bayramoglu G, Ozden H, Yaylak F, Yucel M, et al. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008;26:401–7. doi: 10.1007/s00345-008-0256-1. [DOI] [PubMed] [Google Scholar]
- 23.Turgut F, Bayrak O, Catal F, Bayrak R, Atmaca AF, Koc A, et al. Antioxidant and protective effects of silymarin on ischemia and reperfusion injury in the kidney tissues of rats. Int Urol Nephrol. 2008;40:453–60. doi: 10.1007/s11255-008-9365-4. [DOI] [PubMed] [Google Scholar]
- 24.Ninsontia C, Pongjit K, Chaotham C, Chanvorachote P. Silymarin selectively protects human renal cells from cisplatin-induced cell death. Pharm Biol. 2011;49:1082–90. doi: 10.3109/13880209.2011.568506. [DOI] [PubMed] [Google Scholar]
- 25.Kaur G, Athar M, Alam MS. Dietary supplementation of silymarin protects against chemically induced nephrotoxicity, inflammation and renal tumor promotion response. Invest New Drugs. 2011;28:703–13. doi: 10.1007/s10637-009-9289-6. [DOI] [PubMed] [Google Scholar]
- 26.Ponka P. Cellular iron metabolism. Kidney Int Suppl. 1999;69:S2–11. doi: 10.1046/j.1523-1755.1999.055suppl.69002.x. [DOI] [PubMed] [Google Scholar]
- 27.Dahdah K, Patrie JT, Bolton WK. Intravenous iron dextran treatment in predialysis patients with chronic renal failure. Am J Kidney Dis. 2000;36:775–82. doi: 10.1053/ajkd.2000.17663. [DOI] [PubMed] [Google Scholar]
- 28.Hood SA, O’Brien M, Higgins R. The safety of intravenous iron dextran (Dexferrum) during hemodialysis in patients with end stage renal disease. Nephrol Nurs J. 2000;27:41–2. [PubMed] [Google Scholar]
- 29.Zhou XJ, Laszik Z, Wang XQ, Silva FG, Vaziri ND. Association of renal injury with increased oxygen free radical activity and altered nitric oxide metabolism in chronic experimental hemosiderosis. Lab Invest. 2000;80:1905–14. doi: 10.1038/labinvest.3780200. [DOI] [PubMed] [Google Scholar]
- 30.Legssyer R, Geisser P, McArdle H, Crichton RR, Ward RJ. Comparison of injectable iron complexes in their ability to iron load tissues and to induce oxidative stress. Biometals. 2003;16:425–33. doi: 10.1023/a:1022547819506. [DOI] [PubMed] [Google Scholar]
- 31.Mascitelli L, Pezzetta F. High iron stores and impaired endothelial function in prediabetic subjects. Am J Cardiol. 2006;97:1550. doi: 10.1016/j.amjcard.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Gaenzer H, Marschang P, Sturm W, Neumayr G, Vogel W, Patsch J, et al. Association between increased iron stores and impaired endothelial function in patients with hereditary hemochromatosis. J Am Coll Cardiol. 2002;40:2189–94. doi: 10.1016/s0735-1097(02)02611-6. [DOI] [PubMed] [Google Scholar]
- 33.Mascitelli L, Pezzetta F, Sullivan JL. Iron chelation by green tea flavonoids and improved endothelial function in chronic smokers. Circ J. 2006;70:1523. doi: 10.1253/circj.70.1523. [DOI] [PubMed] [Google Scholar]
- 34.Duffy SJ, Biegelsen ES, Holbrook M, Russell JD, Gokce N, Keaney JF, Jr, et al. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation. 2001;103:2799–804. doi: 10.1161/01.cir.103.23.2799. [DOI] [PubMed] [Google Scholar]
- 35.Cornejo P, Varela P, Videla LA, Fernandez V. Chronic iron overload enhances inducible nitric oxide synthase expression in rat liver. Nitric Oxide. 2005;13:54–61. doi: 10.1016/j.niox.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Varzi HN, Esmailzadeh S, Morovvati H, Avizeh R, Shahriari A, Givi ME. Effect of silymarin and vitamin E on gentamicin-induced nephrotoxicity in dogs. J Vet Pharmacol Ther. 2007;30:477–81. doi: 10.1111/j.1365-2885.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 37.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139–43. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 38.Kadikoylu G, Bolaman Z, Demir S, Balkaya M, Akalin N, Enli Y. The effects of desferrioxamine on cisplatin-induced lipid peroxidation and the activities of antioxidant enzymes in rat kidneys. Hum Exp Toxicol. 2004;23:29–34. doi: 10.1191/0960327104ht413oa. [DOI] [PubMed] [Google Scholar]
- 39.Sivakumar AS, Viswanathan P, Anuradha CV. Dose-dependent effect of galangin on fructose-mediated insulin resistance and oxidative events in rat kidney. Redox Rep. 2010;15:224–32. doi: 10.1179/135100010X12826446921545. [DOI] [PMC free article] [PubMed] [Google Scholar]


