Abstract
Abnormal auditory adaptation is a standard clinical tool for diagnosing auditory nerve disorders due to acoustic neuromas. In the present study we investigated auditory adaptation in auditory neuropathy owing to disordered function of inner hair cell ribbon synapses (temperature-sensitive auditory neuropathy) or auditory nerve fibres. Subjects were tested when afebrile for (i) psychophysical loudness adaptation to comfortably-loud sustained tones; and (ii) physiological adaptation of auditory brainstem responses to clicks as a function of their position in brief 20-click stimulus trains (#1, 2, 3 … 20). Results were compared with normal hearing listeners and other forms of hearing impairment. Subjects with ribbon synapse disorder had abnormally increased magnitude of loudness adaptation to both low (250 Hz) and high (8000 Hz) frequency tones. Subjects with auditory nerve disorders had normal loudness adaptation to low frequency tones; all but one had abnormal adaptation to high frequency tones. Adaptation was both more rapid and of greater magnitude in ribbon synapse than in auditory nerve disorders. Auditory brainstem response measures of adaptation in ribbon synapse disorder showed Wave V to the first click in the train to be abnormal both in latency and amplitude, and these abnormalities increased in magnitude or Wave V was absent to subsequent clicks. In contrast, auditory brainstem responses in four of the five subjects with neural disorders were absent to every click in the train. The fifth subject had normal latency and abnormally reduced amplitude of Wave V to the first click and abnormal or absent responses to subsequent clicks. Thus, dysfunction of both synaptic transmission and auditory neural function can be associated with abnormal loudness adaptation and the magnitude of the adaptation is significantly greater with ribbon synapse than neural disorders.
Keywords: auditory brainstem response, loudness adaptation, auditory neuropathy, temperature-sensitive deafness
Introduction
Adaptation to a sensory stimulus, while diminishing the perception of that signal, is accompanied by enhancement of the processing of other stimuli. For example, in the visual system, adaptation to light is accompanied by improved ability to detect changes in brightness compared with the resting state (Craik, 1938). In the somatosensory system, exposure to a vibrating cutaneous stimulus enhances the detection of changes in frequency of vibration compared with the resting state (Tommerdahl et al., 2005). For the auditory system, adaptation to a steady tone enhances the perceived loudness of changes of intensity compared with the resting state (Bekesy, 1929). These perceptual changes accompanying adaptation have been attributed in varying proportion to functional alterations of receptors (Dowling, 1963), afferent synapses (Smith and Brachman, 1982), afferent nerves (Sato, 1972), and central sensory pathways (Goble and Hollins, 1993; Tommerdahl et al., 2005).
Clinical studies of loudness adaptation in the auditory system over the past 50 years showed loudness adaptation to be both more rapid and significantly greater in patients with acoustic neuromas (Carhart, 1957; Johnson, 1979) compared with patients with cochlear sensory hearing disorders (Owens, 1964). The finding led to the concept that abnormal loudness adaptation was a sign of retrocochlear neural disorders. In contrast, physiological studies in experimental animals suggested that auditory adaptation is primarily governed by changes of cochlear inner hair cell ribbon synapses (Eggermont, 1973) and not of auditory nerves. In the present study, we attempt to address the relative contribution of inner hair cell synaptic and neural disorders to loudness adaptation by studying patients with auditory neuropathy (Starr et al., 1996), a hearing disorder affecting temporal auditory processes (Starr et al., 1991). The clinical features of auditory neuropathy in adults include impaired speech comprehension out of proportion to impairment of audibility, abnormal or absent auditory brainstem responses (ABRs) reflecting activity of auditory nerve and auditory brainstem pathways, and preserved cochlear outer hair cell function measures including cochlear microphonics and otoacoustic emissions (Starr et al., 1996; Zeng et al., 1999; Rance, 2005). These features accompany disordered function of either auditory nerves (Kovach et al., 1999; Starr et al., 2003; Shaia et al., 2005) or inner hair cell ribbon synapses (e.g. mutations in OTOF, Rodríguez-Ballesteros et al., 2008; Reisinger et al., 2011). The benefits of cochlear implantation for improving speech comprehension differ depending on the site and severity of dysfunction; electrical stimulation provides a clear benefit to patients with OTOF ribbon synapse disorders (Rouillon et al., 2006), whereas benefits for neural forms of auditory neuropathy vary (Berlin et al., 2010).
We had previously noted abnormal loudness adaptation in auditory neuropathy (Dimitrijevic et al., 2011) while investigating the ability of subjects with auditory neuropathy to detect brief changes of intensity or frequency in a continuous tone. Subjects with temperature-sensitive auditory neuropathy (Starr et al., 1998) due to OTOF mutation affecting ribbon synapse function (Varga et al., 2006; Marlin et al., 2010) reported that the steady background tone ‘disappeared’, whereas the changes in intensity and frequency were correctly identified and perceived as ‘brief tones’. Subjects with auditory neuropathy due to neural disorders did not report any ‘disappearance’ of the steady background tone.
Here we evaluated psychophysical and physiological measures of auditory adaptation in subjects with auditory neuropathy: four with ribbon synapse disorder, and six with neural disorders. We employed two measures of adaptation: (i) changes of subjective ‘loudness’ in response to 3-min continuous tones; and (ii) changes of ABR latency and amplitude to rapidly repeated click stimuli (Don et al., 1977). We hypothesized that abnormal psychoacoustic measures of loudness adaptation would be accompanied by abnormal measures of adaptation of auditory nerve and brainstem activities and that these measures would distinguish between auditory neuropathy due to ribbon synapse disorders and auditory neuropathy due to neural disorders.
Materials and methods
Subjects
Ten subjects with auditory neuropathy participated in the study. We retained the auditory neuropathy subject number system used in our previous publications (Zeng et al., 2005; Michalewski et al., 2009; Dimitrijevic et al., 2011). Table 1 contains data about site of disorder (synaptic or neural), demographics (age, gender), audibility (pure tone average), ABRs (Wave V latency), gap detection threshold, gene mutation (if known), and special clinical features. Cochlear microphonics were normal in all. Otoacoustic emissions were present in 7 of 10 subjects in keeping with our experience that the incidence of normal otoacoustic emissions is ∼70% in auditory neuropathy (Starr et al., 2001; Rance, 2005).
Table 1.
Features of subjects with auditory neuropathy
| Subject | Site of auditory neuropathy | Age (years) | Gender | Ear tested | OAEs | ABR Wave V latency | PTA | Gap (ms) | Gene | Special features | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Low) (dBHL) | (High) (dBHL) | ||||||||||
| AN30 | Synapse | 24 | M | Left | Yes | 7.1 | 8 | 2 | 5.8 | OTOF | Temperature sensitive |
| Right | Yes | 6.9 | 12 | 7 | |||||||
| AN31 | Synapse | 9 | M | Left | Yes | Absent | 20 | 8 | 15 | OTOF | Temperature sensitive |
| Right | Yes | 7 | 23 | 17 | 15 | ||||||
| AN32 | Synapse | 18 | F | Left | Yes | 7.5 | 28 | 40 | 12 | OTOF | Temperature sensitive |
| AN33 | Synapse | 13 | M | Left | Yes | 7.6 | 17 | 10 | 11 | OTOF | Temperature sensitive |
| AN2 | Nerve | 65 | F | Left | Yes | Absent | 77 | DNT | 15 | MPZ | CMT neuropathy |
| Right | Yes | 85 | 52 | 9 | |||||||
| AN3 | Nerve | 30 | F | Right | No | Absent | 83 | 90 | 130 | ? | Neuropathy |
| AN13 | Nerve | 37 | F | Left | Yes | Absent | 52 | 27 | 8 | ? | Peripheral neuropathy |
| AN34 | Nerve | 15 | F | Left | No | Absent | 28 | 35 | DNT | FX | FRDA neuropathy |
| Right | No | Absent | 27 | 27 | |||||||
| AN36 | Nerve | 56 | F | Left | Yes | 6.2 | 62 | 18 | 10 | ? | Neuropathy |
| Right | Yes | Absent | 47 | 30 | 10 | ||||||
| AN40 | Nerve | 14 | M | Right | Yes | Absent | 50 | 35 | 11 | ? | Optic neuropathy |
| All AN subjects | 10 | 9–65 | 6F 4M | Both | 7/10 | 7.1 | 42 | 35 | 23.9 | ||
AN = auditory neuropathy; CMT = Charcot–Marie–Tooth; dBHL = decibels hearing level; DNT = did not test; FRDA = Friedreich’s ataxia; Nerve = neural auditory neuropathy; OAE = otoacoustic emission; PTA (high) = pure tone threshold average at 4.0, 6.0, 8.0 kHz; PTA (low) = pure tone threshold average at 0.5, 1.0, 2.0 kHz; Synapse = ribbon synapse disorder; ? = unknown.
Four subjects with auditory neuropathy (Subjects AN30, AN31, AN32 and AN33) have compound heterozygous mutations of OTOF with ‘deafness’ expressed when body temperature is elevated (Starr et al., 1998; Varga et al., 2006; Marlin et al., 2010; Wang et al., 2010). When body temperature is normal, both audiometric thresholds and speech perception in quiet are normal or mildly affected. Mutations of OTOF affect neurotransmitter release from inner hair cell ribbon synapses (Roux et al., 2006; Pangrsic et al., 2010).
Six subjects have clinical evidence of involvement of other cranial and/or peripheral nerves; two have mutations [MPZ (Subject AN2), FX (Subject AN34)] associated with axonal loss and demyelination of VIII nerve (Spoendlin, 1974; Starr et al., 2003). Subject AN40 has a mitochondrial disorder of unknown aetiology. The other three subjects (Subjects AN3, AN13 and AN36) have clinical evidence of involvement of other cranial (optic, vestibular) and/or peripheral nerves of unknown aetiology.
Eight subjects with normal audibility (ages 22–35 years, male/female: 3/5) served as control subjects in the psychophysical loudness adaptation experiment. Seven different subjects with normal audibility (ages 18–26 years, male/female: 2/5) served as control subjects for the physiological ABR adaptation experiment.
Two additional subjects with hearing impairments served as control subjects for low frequency hearing loss that is commonly found in auditory neuropathy (Zeng et al., 1999) and was present in five of our subjects with auditory neuropathy. The first, a 21-year-old male, had a moderate familial low frequency hearing loss. There was no evidence of involvement of cranial or peripheral nerves. Clinical neurological examination, nerve conduction studies, and ABRs were normal.
The second, a 62-year-old female, had a small (2.5 mm) acoustic neuroma treated by gamma knife radiation. She subsequently developed low frequency hearing loss. ABRs were absent. The ear with the auditory neuroma also served as a control for proximal site of auditory nerve involvement that can be associated with abnormal loudness adaptation, which has historically been used as a diagnostic test for tumours of the auditory nerve (Carhart, 1957).
Before testing, all subjects signed informed consent forms following University of California guidelines for testing human subjects.
Psychoacoustics
Adaptation was studied by evaluating the change-over time in the subjective loudness of a constant-intensity continuous tone (Scharf, 1983). Subjects were seated in a comfortable chair inside a well-illuminated sound attenuating acoustic chamber for testing. Subjects were tested when afebrile. Two of the subjects with auditory neuropathy (Subjects 3 and 31) were not available to test for loudness adaptation. Two tones were used as stimuli: (i) a low frequency tone of 250 Hz; and (ii) a high frequency tone of 8000 Hz. There were three reasons for using tones of different frequencies: first, individuals with normal hearing experience more loudness adaptation to high frequency tones than to low frequency tones (Hellman et al., 1997; Tang et al., 2006); second, in other psychophysical tasks (e.g. frequency discrimination), subjects with auditory neuropathy are significantly more impaired to low frequency tones than high frequency tones (Zeng et al., 2005); and third, our previous work using cortical evoked potentials to low and high frequency tones showed that low frequency responses were delayed in subjects with ribbon synapse disorders but were of normal latency to high frequencies (Dimitrijevic et al., 2011).
Pure tones lasting 185 s were generated digitally at a sampling rate of 44.1 kHz and then presented monaurally at a ‘comfortable’ intensity (50–70 dB sound pressure level for normal control subjects and 60–110 dB sound pressure level for subjects with auditory neuropathy and special control subjects) through circumaural headphones (Sennheiser HDA200). Each ear was tested separately at both tone frequencies for a total of four stimulus combinations. The first stimulus presentation was always the 250 Hz tone presented to the ‘better’ ear. The other three combinations of ear and frequency were presented in random order. For each combination, subjects estimated the loudness of the tone by entering a value between zero and 10 using a keyboard. A value of zero indicated that the sound was inaudible and a value of 10 indicated that the sound was intolerably loud. Subjects could enter any number between zero and 10, including decimal or fractional numbers, as a loudness estimate (Scharf, 1983). Loudness judgements were made immediately following tone onset and every 30 s thereafter. A visual cue on a video monitor signalled the subject when to enter an estimate. Five-minute rest breaks were provided after each stimulus condition. In order to adjust for variability due to different initial loudness judgements, loudness estimates were normalized using Equation 1:
| (1) |
where Lt is how loud the subject estimated the test tone to be at time t and L0 is the initial loudness judgement (Tang et al., 2006). A per cent adaptation of −100% indicated that the test tone became inaudible, 0% indicated no change in loudness, and a positive per cent indicated that the subject perceived the sound to be louder than at onset. For each frequency condition, the normalized loudness judgements were averaged between the two ears to obtain a single adaptation profile for each subject at each frequency. Initial raw loudness judgements differed between ears by no more than one point on the (0–10) scale in all but one subject (Subject AN40).
The time course and magnitude of adaptation are described by Equation 2:
| (2) |
(Tang et al., 2006). The parameter s corresponds to the magnitude of adaptation and the parameter τ corresponds to the rate of adaptation. y(t) represents the amount of loudness adaptation at a given time t, s represents the amount of adaptation to an infinitely long tone, and τ represents the amount of time necessary to reach 63% of the maximum adaptation (the ‘time constant’). The parameter s was constrained to the limits of audibility (s ≥ −100%) and the parameter τ964; was constrained to the time course of loudness judgments (0 ≤ τ 964; ≤ 180 s). ANOVA procedures were used to evaluate the fitted values for the normal control subjects, neural disorder and ribbon synapse disorder groups. A two-factor repeated measures ANOVA included group (three) and frequency (two). Post hoc tests of the means were carried out with the Tukey procedure. Fisher’s exact test was used to compare the number of subjects with ribbon synapse disorder and neural disorders whose fitted magnitude of adaptation (s) was within the normal range. Significance levels were set at P ≤ 0.05 or better.
Electrophysiology
ABRs were tested bilaterally in four subjects (Subjects AN30, 31, 34 and 36) and from the ‘better’ ear in six subjects (Subjects AN2, 3, 13, 32, 33 and 40). All subjects were tested when afebrile. Digitization of scalp potentials was at 20 kHz sampling frequency and included a 2 ms prestimulus period and a 10 ms post-stimulus period. Amplitude and latency of Wave V were defined from Cz-inion montage (Pratt et al., 2004). One of the authors (S.B.) identified and measured Wave V amplitude from baseline to Wave V peak. Another author (A.S.) reviewed and agreed with the Wave V measures. Wave V was considered present when both evaluators defined the component to be present.
Two separate measures of auditory neural function were investigated. The first was a ‘clinical’ measure of ABRs averaging responses to 4000 clicks presented at a rate of 23.3/s. The second was an ‘adaptation’ measure of ABRs to trains of clicks. The stimulus trains were presented at a slow rate of 1.9/s that reflected the time between the last click in the stimulus train and the first click in the next stimulus train. Thus the ABR to the first click in the train provided a measure of auditory nerve function in an ‘unadapted’ state. ABRs to subsequent clicks in the train presented at rapid rates (e.g. 76.9/s) provided measures of auditory nerve function during adaptation. Stimulus trains have also been used to quantify activity-dependent changes in neural function that accompany peripheral neuropathies (Park et al., 2011).
Grand auditory brainstem response average to 4000 clicks: clinical measure
ABRs were averaged both to 2000 condensation and to 2000 rarefaction clicks, presented at 90 dB sound pressure level and at 23.3/s rate. The two ABRs were summed to attenuate cochlear microphonics that are out of phase and enhance neural responses that are in phase (Starr et al., 1991). The summed average was band pass filtered (100–2000 Hz; Butterworth) for making measures of latency and amplitude of Wave V.
Auditory brainstem responses to 20 individual clicks in a brief click train: adaptation measure
ABRs were recorded to a 20-click stimulus train presented every 553 ms (1.9 trains/s). A total of 4000 trains were presented: 2000 with condensation clicks and 2000 with rarefaction clicks. Successive click trains alternated between condensation and rarefaction. Brain activity was averaged separately to each click in the train (e.g. #1, 2, … , 20), and measures of Wave V latency and amplitude were made for each click within the train. Clicks in the trains were presented at a rate of 76.9/s for all subjects with auditory neuropathy except in the first two subjects tested (Subjects AN32, 33), who were tested at a rate of 111/s. In our normal control subjects, ABR Wave V latencies did not significantly differ at these two stimulus rates. Subject AN13 was not tested for adaptation of ABRs.
Non-linear regression procedures with bootstrapping procedures were used to evaluate changes of Wave V latencies and amplitudes during the click train. A growth-curve model [at / (b + t); Michaelis and Menten, 1913] (Equation 3) was selected to define adaptation effects in the period over the first 10 clicks of the train where rapid adaptation was most apparent. For reference purposes, goodness-of-fit was estimated with pseudo r-squared values and was interpreted in a manner similar to r-squared in linear regression. Curve differences among subject groups were evaluated using inequality randomization tests. Test differences were accepted as significant for levels of P ≤ 0.05. Monte Carlo iterative methods were used to generate approximate probability levels. Statistical software (NCSS) was used to perform the computations and test comparisons.
Results
Psychophysics
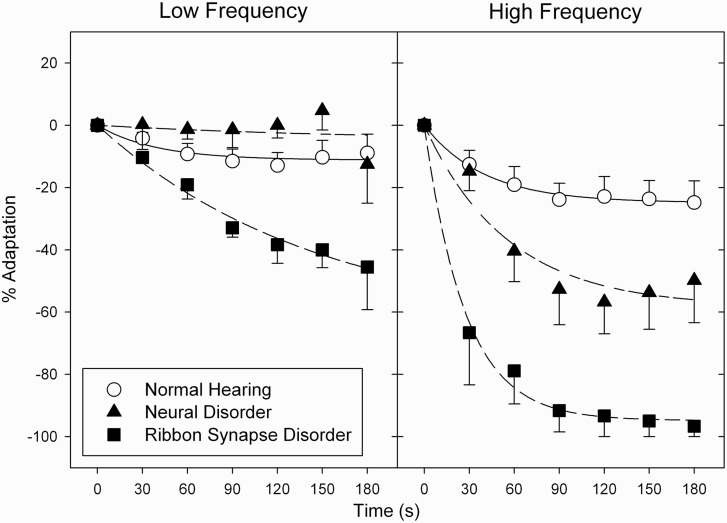
The major psychophysical results were that (i) subjects with auditory neuropathy perceived continuous tones to decrease in loudness significantly more than did normal listeners; (ii) the magnitude of adaptation was significantly greater in subjects with both normal hearing and auditory neuropathy to high than to low frequencies; and (iii) within the auditory neuropathy group, adaptation was greater in ribbon synapse disorder than in neural disorders for both low and high frequency tones (Fig. 1).
Figure 1.
Average loudness adaptation in subjects with ribbon synapse disorder (filled squares), neural disorder (filled triangles), and normal hearing (open circles). Error bars are ± SEM. Adaptation is greater for high than low frequencies and for both, the magnitude of adaptation is greater in ribbon synapse than neural disorders.
There was a significant difference in the magnitude of adaptation (s) between those with normal hearing and the two auditory neuropathy groups showing a main effect of group [ribbon synapse disorder versus neural disorder versus normal hearing; F(2,27) = 31.585, P < 0.001] and tone frequency [250 Hz versus 8000 Hz; F(1,28) = 11.749, P = 0.005]; there was no significant interaction between group and frequency [F(2,27) = 1.469, P = 0.269]. Post hoc tests indicated significant differences between ribbon synapse disorder and normal hearing groups (P < 0.001) and between the two auditory neuropathy groups (P < 0.001), but not between the subjects with neural disorder auditory neuropathy and normal hearing control subjects (P = 0.495). No significant main or interaction effects were found for the rate of adaptation (τ).
Individual adaptation profiles are displayed for ribbon synapse and neural disorders (Fig. 2) relative to the upper and lower limits of adaptation in normal hearing control subjects. Adaptation was abnormal to the low frequency tone in two of the three subjects with ribbon synapse disorder but in none of the subjects with neural disorders. In contrast, adaptation to the high frequency tone was abnormal in all but one of the subjects with auditory neuropathy (Subject AN13). The subject with acoustic neuroma had complete (−100%) loudness adaptation to the high frequency tone and profound adaptation (−74%) to the low frequency tone. The magnitude of adaptation was within normal limits in the subject with sensorineural hearing loss (downward-pointing open triangles).
Table 2.
Features of control subjects
| Control | Age (years) | Gender | Ear tested | OAEs | ABR Wave V Latency | PTA (low) (dBHL) | PTA (high) (dBHL) | Gap (ms) | Special features |
|---|---|---|---|---|---|---|---|---|---|
| Normal | 18–26 | 5F 2M | Left | Yes | 5.7 | 5 | 5 | 4.8 | |
| SNHL | 21 | M | Right | No | 5.8 | 47 | 13 | Did not test | Low frequency hearing loss |
| Neuroma | 62 | F | Right | No | Absent | 50 | 57 | 115 | Acoustic neuroma |
Neuroma = acoustic neuroma control; OAE = otoacoustic emissions; SNHL = low-frequency sensorineural hearing loss control; PTA (high) = pure tone threshold average at 4.0, 6.0, 8.0 kHz; PTA (low) = pure tone threshold average at 0.5, 1.0, 2.0 kHz.
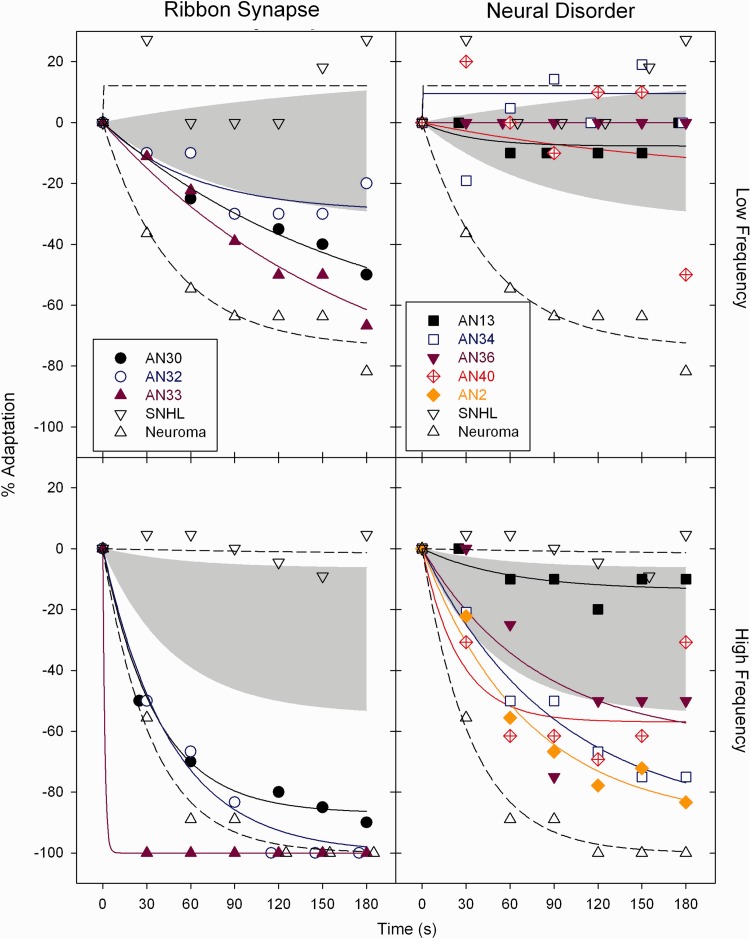
Figure 2.
Individual adaptation profiles for subjects with auditory neuropathy with ribbon synapse disorder (left) and neural disorder (right) for 250 Hz (top) and 8000 Hz (bottom) tones. The grey shaded area is the range of adaptation found in control subjects with normal hearing. Adaptation profiles are also shown for a subject with (i) sensorineural hearing loss (SNHL, downward-pointing open triangles); and (ii) another subject with acoustic neuroma (upward-pointing open triangles). The magnitude of adaptation in ribbon synapse disorders is similar to that found in the control subject with acoustic neuroma.
Table 3 contains measures of the magnitude (s) and rate (τ) of adaptation for both individual subjects and the group means of subjects with auditory neuropathy with ribbon synapse disorder and neural disorders, and the upper and lower limits found in normal control subjects. R-squared values are listed in Table 3 as a measure of goodness-of-fit of Equation 2. The best-fitting exponential function accounted for >95% of the variation in the group means for subjects with ribbon synapse disorder at both low and high frequencies; it accounted for 93% of the variation in the group means for subjects with neural disorders at high frequencies but <10% for the average data at low frequencies. The fitted magnitude of adaptation to the 250 Hz tone was beyond the normal range in two of three subjects with ribbon synapse disorder but not in any of the four subjects with neural disorders. The fitted magnitude of adaptation to the 8000 Hz tone was abnormal in all three subjects with ribbon synapse disorder and in four of five subjects with neural disorders. The difference in the incidence of abnormal adaptation between ribbon synapse and neural disorders was significant for low frequency (P = 0.029) but not high frequency (P = 0.196) tones.
Table 3.
Adaptation in individual ribbon synapse and neural disorders
| Group | Subject | Low frequency |
High frequency |
||||
|---|---|---|---|---|---|---|---|
| s (%) | τ (s) | r2 | s (%) | τ (s) | r2 | ||
| Ribbon synapse | AN30 | −74.2 | 175.4 | 0.98 | −87 | 36.36 | 0.99 |
| AN32 | −32.6 | 91.74 | 0.62 | −100 | 46.08 | 0.99 | |
| AN33 | −97.2 | 180 | 0.97 | −100 | 1.31 | 1 | |
| Average | −65.1 | 147.1 | 0.98 | −94.8 | 27.32 | 0.99 | |
| Neural disorder | AN13 | −7.69 | 32.57 | 0.34 | −14 | 63.69 | 0.6 |
| AN34 | 9.53 | 0 | 0.01 | −89.4 | 91.74 | 0.98 | |
| AN36 | 0 | N/A | 1 | −63.5 | 77.52 | 0.68 | |
| AN40 | −18.1 | 180 | 0.11 | −57 | 25.13 | 0.72 | |
| AN2 | N/A | N/A | N/A | −89.7 | 71.43 | 0.97 | |
| Average | −5.03 | 180 | 0.09 | −58.4 | 55.87 | 0.93 | |
| Normal hearing limits | Upper | 16.43 | 180 | 0.69 | −6.51 | 55.25 | 0.42 |
| Lower | −32.8 | 83.33 | 0.97 | −54.4 | 48.08 | 1 | |
| Average | −11.17 | 39.06 | 0.87 | −24.85 | 40.16 | 0.99 | |
| Special controls | SNHL | 12.1 | 0.01 | 0.12 | −2.1 | 180 | 0.03 |
| Neuroma | −74 | 47.1 | 0.96 | −100 | 33.61 | 0.99 | |
s is the magnitude of adaptation to an infinitely long tone; τ is the time to achieve 63% of maximal adaptation (rate of adaptation).
SNHL = low-frequency sensorineural hearing loss.
Bold magnitudes are outside the range of normal hearing.
In summary, loudness adaptation was abnormal in subjects with ribbon synapse disorders regardless of the stimulus frequency, whereas in subjects with neural disorders, loudness adaptation was abnormal to high but not low frequencies.
Electrophysiology
Grand auditory brainstem response average to 4000 clicks: clinical measure
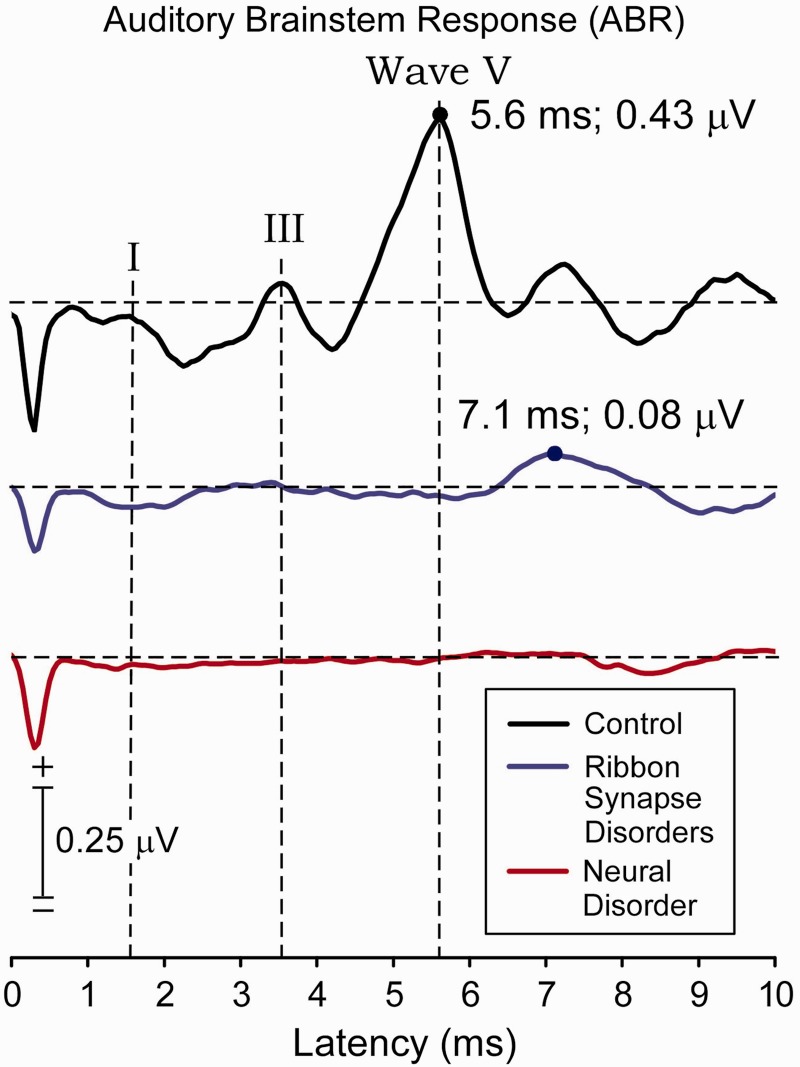
ABR Waves I, III and V were present in the control subjects (Fig. 3). Only Wave V, delayed in latency and reduced in amplitude, was present in ribbon synapse disorders. Waves I, III and V were absent in neural disorders but a late negative shift that peaked in control subjects at ∼8 ms was also found in both the neural group (∼8.5 ms) and the ribbon synapse disorder group (∼9 ms). ABRs from individual ears of subjects within each group showed Wave V to be present to stimulation of all tested ears of control subjects, five of six ears in ribbon synapse disorder, and only one of eight ears in neural disorders. Wave V was of significantly longer latency in subjects with ribbon synapse disorder compared with control subjects (P < 0.001). The difference in Wave V amplitude between subjects with ribbon synapse disorder and control subjects approached significance (P = 0.09). There was a significant difference in the incidence of detecting ABR Wave V between the ribbon synapse and neural auditory neuropathy groups (P = 0.016; Fisher’s exact test).
Figure 3.
Grand average of ABRs to 4000 clicks (‘clinical’ measure) presented at 90 dB sound pressure level for control subjects (black trace), ribbon synapse disorder (blue trace), and neural disorder (red trace). Waves I, III and V are identified in the control group. The peak measure of component V, when present, is indicated by a filled circle. Wave V is not identified in the neural disorders group. A late negative wave occurring between 8 and 10 ms latency was identified in all three groups. The negative deflection at onset of the waveforms is an artefact of the stimulus voltage applied to the transducer.
Auditory brainstem responses to 20 individual clicks in a brief click train: adaptation measure
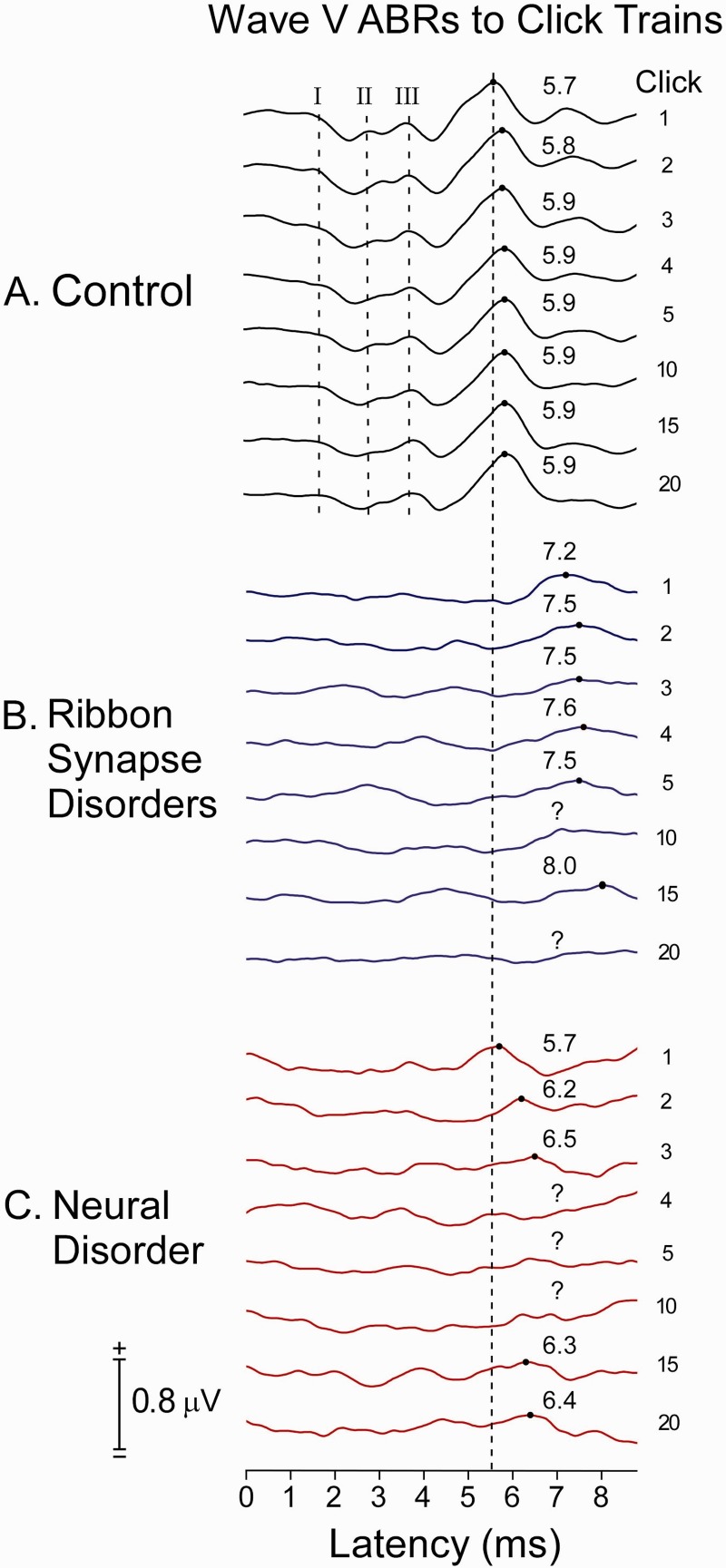
ABRs to clicks as a function of position in the train (#1, 2, 3, 4, 5, 10, 15, 20) in control subjects with normal audibility and subjects with auditory neuropathy are shown in Fig. 4. In control subjects, ABR Waves I, III and V were present to every click of the train. Wave V latency to the first click was 5.6 ms and was delayed on average 0.2 ms by the 20th click. In subjects with ribbon synapse disorder, ABR Wave V to the first click was delayed to 7.2 ms and was absent (Fig. 4B, click #10) or further delayed (Fig. 4B, click #15) to later clicks. In the five subjects with neural disorders, ABRs were absent to every click in the train in all but one (Subject AN36). In Subject AN36, Wave V was of normal latency (5.7 ms) to the first click in both ears, and then was absent or abnormally delayed to subsequent clicks.
Figure 4.
Grand averaged ABRs to clicks as a function of their position (#1, 2, 3…20 indicated on the right side of the displays) in a click train with interstimulus interval of 13 ms between clicks (76.9/s rate) and 553 ms (1.9/s rate) between each train. The averages are from (A) seven normal control subjects (seven ears), (B) four subjects with ribbon synapse disorder (six ears), and (C) both ears of the single subject with auditory nerve disorder with a Wave V to any of the clicks (Subject AN36).
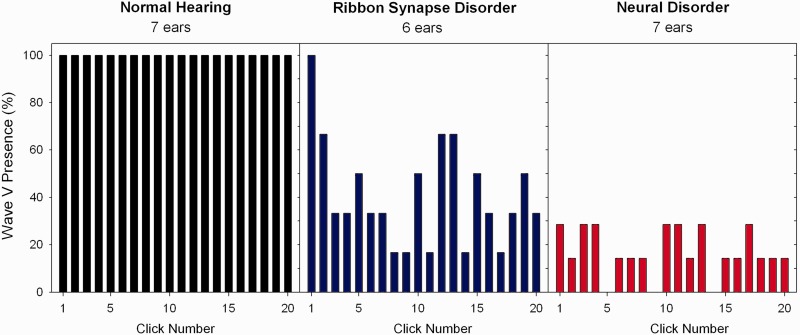
The incidence of detecting ABR Wave V as a function of click position is shown in Fig. 5. In normal control subjects Wave V was present to every click in the train. In subjects with ribbon synapse disorder Wave V was present 100% to the first click and then decreased to ∼40% for the remainder of the train. In the only subject with neural disorder (Subject AN36) with a Wave V to any click in the train, Wave V was present in both ears to the first click and identified to ∼75% of subsequent clicks in the right ear (average latency: 6.4 ms) and 30% of clicks in the left ear (average latency: 6.9 ms). The subject with low frequency sensorineural hearing loss exhibited ABRs to every click that were of normal latency. No ABR Wave V was found to any click in the subject with an acoustic neuroma.
Figure 5.
Incidence of ABR Wave V detection to individual clicks as a function of the position of the click in the click train in subjects with normal hearing (left), ribbon synapse disorder (middle), and neural disorder (right).
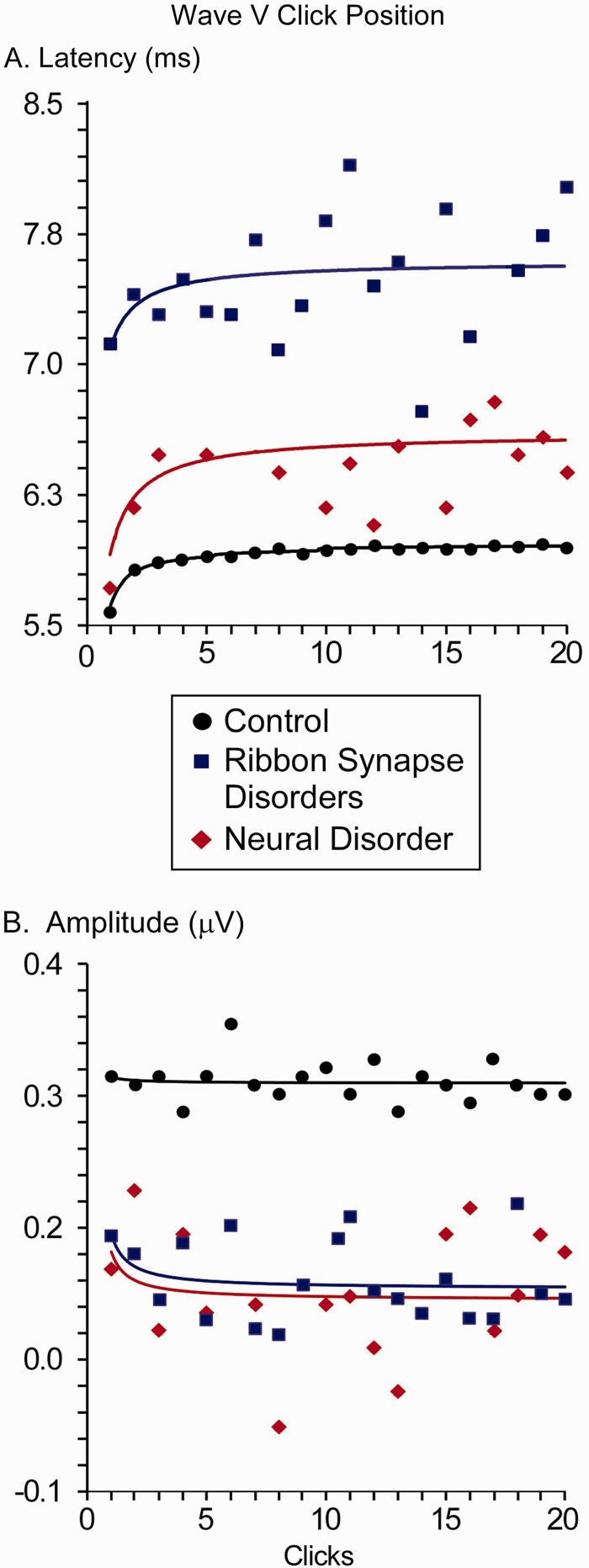
For latency of Wave V (Fig. 6A), the growth model was well-fitted for control subjects (r2 = 0.89) in contrast with the poorly-fitted responses of both ribbon synapse disorder (r2 = 0.12) and the one subject with neural disorder with a Wave V to any click (r2 = 0.24). Latencies of Wave V for all clicks in ribbon synapse auditory neuropathies were significantly delayed compared with control subjects (P < 0.005), while the latency for the one subject with neural disorder was within the normal range for the first click but abnormally delayed for subsequent clicks. Comparing the difference between this subject with single neural auditory neuropathy with the normal group approached significance (P = 0.08). Between the two auditory neuropathy groups, the latencies of the ribbon synapse group were prolonged compared with the neural disorder (P = 0.006).
Figure 6.
Michaelis-Menten growth curves for Wave V (A) latency and (B) amplitude in control subjects (seven ears), ribbon synapse disorder (six ears), and neural disorder (one ear). Growth curves were fit to the first 10 clicks of the train and extrapolated to the last 10 clicks.
For amplitude of Wave V (Fig. 6B), the growth model poorly fitted the data for all subject groups (control subjects: r2 = 0.004; ribbon synapse disorder: r2 = 0.09; neural disorder: r2 = 0.04). As suggested by Fig. 6, control subjects had significantly larger amplitudes than the either the ribbon synapse (P < 0.023) or neural disorder (P = 0.037) groups. Amplitude differences between the two auditory neuropathy groups (P = 0.8) were not indicated.
Table 4 compares individual ABR Wave V latency when the ABR was collected as (i) a ‘clinical’ average of 4000 clicks presented at 23.3/s; and (ii) the average to the first click in the stimulus train presented at a slow rate (1.9/s). All seven control subjects had longer latency of Wave V to the clinical ABR than the first click in the train and their mean values differed significantly (t = 6.00, P = 0.001, paired t-test).
Table 4.
ABR Wave V latency to clicks presented at 23.3/s (clinical response) and the first click in the train presented every 1.9/s (unadapted response)
| Group | Subject | Ear tested | Clinical ABR V latency (ms) | Click 1 ABR V latency (ms) |
|---|---|---|---|---|
| Ribbon synapse | AN30 | Left | 7.1 | 7.5 |
| Right | 6.9 | 7 | ||
| AN31 | Left | Absent | 7 | |
| Right | 7 | 6.8 | ||
| AN32 | Left | 7.5 | 7 | |
| AN33 | Left | 7.6 | 7.7 | |
| Average | Both | 7.22 ± 0.31 | 7.17 ± 0.35 | |
| Neural disorder | AN2 | Left | Absent | Absent |
| AN3 | Right | Absent | Absent | |
| AN13 | Left | Absent | Not tested | |
| AN34 | Left | Absent | Absent | |
| Right | Absent | Absent | ||
| AN36 | Left | 6.2 | 5.7 | |
| Right | Absent | 5.7 | ||
| AN40 | Left | Absent | Absent | |
| Normal hearing | 1 | Left | 6.05 | 5.95 |
| 2 | Left | 5.7 | 5.6 | |
| 3 | Left | 5.65 | 5.55 | |
| 4 | Left | 5.6 | 5.45 | |
| 5 | Left | 6.05 | 5.8 | |
| 6 | Left | 5.8 | 5.7 | |
| 7 | Left | 5.85 | 5.75 | |
| Average | Left | 5.81 ± 0.18 | 5.69 ± 0.17 | |
| Special control subjects | SNHL | Right | 5.8 | 5.7 |
| Neuroma | Right | Absent | Absent |
SNHL = low-frequency sensorineural hearing loss.
Average values are given as mean ± standard deviation.
‘Clinical ABR V latency’ is the latency of the grand averaged ABR Wave V to clicks presented at rate of 23.3 clicks/s.
‘Click 1 ABR V latency’ is the latency of the grand averaged ABR Wave V to the first click in trains of clicks presented at 1.9 trains/s.
Clicks within each train were presented at a rapid rate of 76.9 s (except for Subjects AN32 and AN33, who were tested at 111/s).
In ribbon synapse disorder, half of the ears tested (3/6) showed a longer latency ABR Wave V to the clinical ABR than to the first click in the train. The group mean ABR Wave V latency in subjects with ribbon synapse disorder was not significantly longer to the clinical ABR than the first click in the train (t = 0.131, P = 0.902, paired t-test).
In the sole subject with neural disorder (Subject AN36) with preserved ABRs, Wave V to stimulation of either ear was of normal latency to the first click of the stimulus train but absent in one ear and abnormally delayed in the other ear in the clinical ABR.
Analysis of relationship between adaptation of auditory brainstem response and loudness
ANOVA procedures were used to evaluate the effect of ABR Wave V adaptation on the fitted values for loudness adaptation. A two-factor repeated measures ANOVA included ABR Wave V presence (two) and tone frequency (two). There were seven subjects with auditory neuropathy (three with ribbon synapse disorder, four with neural disorder) who participated in both the psychophysical and electrophysiological adaptation experiments. No correlation analyses were performed between loudness adaptation and ABR adaptation as the number of subjects with auditory neuropathy with ABR Wave V was <5 and considered too small to test for significance.
There were significant differences in the magnitude of adaptation (s) showing a main effect of tone frequency [250 Hz versus 8000 Hz; F(1,12) = 7.883, P = 0.038] but not of ABR Wave V presence [presence versus absence; F(1,12) = 4.653, P = 0.083], most likely reflecting that only one subject with neural disorder showed an ABR to any of the clicks in the train. There were no significant main effects of tone frequency [F(1,12) = 2.461, P = 0.192] or Wave V presence [F(1,12) = 1.150, P = 0.344] on the fitted rate of adaptation (τ). No significant interactions between tone frequency and ABR Wave V presence were indicated for either parameter.
Discussion
There are two major new findings in this study of loudness adaptation in auditory neuropathy. First, adaptation was abnormally increased in both ribbon synapse and neural forms of auditory neuropathy. Second, measures of ABRs to individual clicks in brief stimulus trains provided evidence for suggesting mechanisms of abnormal adaptation. We will discuss these findings in relation to (i) adaptation in normal hearing; (ii) synaptic and neural mechanisms of abnormal adaptation in auditory neuropathy; (iii) consequences of abnormal adaptation for hearing; and (iv) clinical relevance of abnormal adaptation for diagnosis and treatment of auditory neuropathy.
Adaptation in normal hearing
In subjects with normal hearing, loudness adaptation is greater to high than low frequency tones (Hellman et al., 1997; Tang et al., 2006). Experimental animal studies of adaptation have shown that discharge rates of individual auditory nerve fibres decrease exponentially over time scales ranging from milliseconds (Smith, 1977) to minutes (Kiang, 1965; Javel, 1996). However, these functions do not significantly differ as a function of the best frequency of the fibres (Javel, 1996). In contrast, the population size of auditory nerve fibres activated by low (1 kHz) and high (5 kHz) frequency tones differs with approximately three times more fibres activated to low than high tones (Kim et al., 1990; Kim and Parham, 1991). The data suggest that the magnitude of loudness adaptation in normal hearing may be related, in part, to the population size of auditory nerve fibres activated. Moreover, the increased adaptation found in auditory neuropathy may be related to the reduced number of nerve fibres identified in temporal bones (Starr et al., 2003). Central auditory structures also participate in adaptation as documented by loudness reduction of ‘sounds’ experienced during direct electrical stimulation of inferior colliculus (Lim et al., 2008). Experimental animal PET studies suggest that auditory cortex is the major central site of adaptation based on the reduction of metabolic activity during continuous acoustic stimulation whereas subcortical structures maintained metabolic activity at high levels (Jang et al., 2012).
Synaptic and neural mechanisms of adaptation
The abnormalities of loudness adaptation identified in subjects with auditory neuropathy are similar to those described in patients with acoustic neuromas (Johnson, 1979). In both auditory neuropathy and acoustic neuromas loudness adaptation can develop rapidly and be complete within 60 s. Mechanisms of impaired hearing accompanying acoustic neuromas are thought to be secondary to pressure resulting in (i) conduction blocks in nerve fibres adjacent to the tumour; and/or (ii) ischaemia of the cochlea (Matsunaga and Kanzaki, 2000) affecting both nerve fibres and hair cells (Roosli et al., 2012). The presence of conduction block is supported by the rapid recovery of both hearing and ABRs in some neuroma patients after removal of the tumour (Fukaya et al., 1993). The one patient with acoustic neuroma that we tested had complete adaptation of loudness similar to that defined in our subjects with ribbon synapse disorder. The absence of both Wave I generated by distal auditory nerve and Wave V generated by brainstem auditory pathways suggests that conduction blocks were unlikely to account for the complete loudness adaptation. We suggest that pressure from the tumour may have impaired blood supply to both nerve terminals and inner hair cells leading to complete adaptation.
The control subject with sensorineural low frequency hearing loss had normal loudness adaptation and normal ABR Wave V latency both to clinical measures and to the first click in the stimulus train. These results are consistent with previous reports of normal adaptation in sensorineural hearing loss (Owens, 1964).
Our results in subjects with auditory neuropathy suggest that there are at least three separate mechanisms that may contribute to abnormal loudness adaptation: (i) conduction block in abnormal nerves; (ii) loss of auditory ganglion cells; and (iii) dys-synchronous activation of auditory nerve fibres.
Conduction block was identified in a neural form of auditory neuropathy (Subject AN36) using ABRs to click trains that showed the presence of normal latency Wave V to the initial click of the train but delayed or absent ABRs to subsequent clicks. Thus, there was normal transmission of auditory nerve activity to brainstem structures only at stimulus onset.
Auditory ganglion cell loss was considered likely in four neural disorder subjects who did not have ABRs to any of the clicks in the train. Two of these subjects have mutations, FRDA (in Subject AN34) and MPZ (in Subject AN2), that have been shown to be associated with marked loss of auditory ganglion cells in temporal bones (Spoendlin, 1974; Starr et al., 2003).
Dys-synchronous activation of auditory nerve fibres may be likely in subjects with temperature-sensitive ribbon synapse disorder due to mutation of OTOF (Varga et al., 2006; Marlin et al., 2010). ABRs were abnormal (delayed latency, reduced amplitude) to the first click in the stimulus train and then were further delayed or absent to subsequent clicks. The ABR findings reflect impaired neurotransmitter release to the initial click that is further impaired to subsequent clicks in the train. Moreover the magnitude of the ribbon synapse disorder likely varies among ribbon synapses to affect dys-synchrony of activation of nerve fibres. There was no evidence of conduction block since ABRs were abnormal to all clicks. Furthermore, auditory nerve fibres in ribbon synapse disorders appear normal and can respond to rapid rates of electrical stimulation (Santarelli et al., 2008).
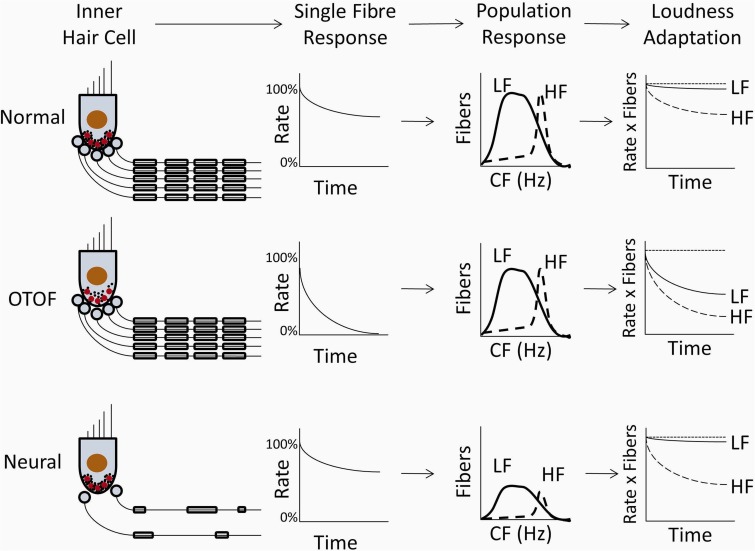
Figure 7 summarizes the relative contributions of synaptic and neural activities to loudness adaptation in subjects with normal hearing, temperature-sensitive auditory neuropathy due to ribbon synapse disorder, and auditory neuropathy due to neural disorders. In normal hearing (Fig. 7), the firing rate of single nerve fibres adapts exponentially from a high onset rate to an asymptotic final rate. The adaptation of single fibres is modulated by the size of the neural population, which is larger for low than high tonal frequencies, resulting in greater adaptation to high than low frequency tones.
Figure 7.
Model of synaptic and neural functions affecting loudness adaptation in normal hearing (top) and their presumed changes in ribbon synapse disorder (OTOF, middle) and neural disorder (bottom). The first column depicts inner hair cells, their ribbon synapses, and their corresponding nerve fibres. In normal hearing, synaptic vesicles (black dots) are attached to ribbon synapses (red circles) along with the auditory nerve fibres (black lines) activated by those synapses. In ribbon synapse disorder, production and transport of synaptic vesicles is abnormal leading to decreases in the number of attached vesicles (black dots), while in neural disorders, both the number of nerve fibres and their myelination (rounded rectangles surrounding black lines) are abnormally reduced. The second column depicts normalized firing rate of a typical single auditory nerve fibre in response to a continuous stimulus; firing rate decreases exponentially from 100% (onset rate) to an asymptotic final rate that is abnormally reduced to both low and high frequency tones only in ribbon synapse disorder. The third column depicts the population of auditory nerve fibres activated by low frequency (solid line) and high frequency (dashed line) tones showing a reduced number of fibres only in neural disorder. The final column depicts subjective loudness adaptation to those tones compared with no adaptation (dotted line). Loudness adaptation in normal hearing and ribbon synapse disorder is greater to high than low frequency tones, whereas in neural disorders adaptation is normal to low frequency tones despite the abnormally reduced number of activated nerve fibres. HF = high frequency; LF = low frequency; CF = characteristic frequency.
In contrast, ribbon synapse dysfunction (Fig. 7) has been shown in experimental animals to have reduced output of neurotransmitter at stimulus onset that then declines further with continued stimulation (Pangrsic et al., 2010). In subjects with ribbon synapse disorder, abnormal loudness adaptation occurs to both low and high frequencies, consistent with the disorder affecting inner hair cell ribbon synapses at both basal and apical regions of the basilar membrane. In the adapted state, auditory N100 cortical potentials to changes of frequency but not to changes of intensity are larger in ribbon synaptic disorder than in control subjects (Dimitrijevic et al., 2011). The occurrence of abnormal adaptation in synaptic disorders is paradoxically accompanied by increased responsiveness of unadapted neurons with higher best frequencies than the adapting tone. We suggest that these data may be evidence of cortical modulation of signal intensity accompanying adaptation.
In neural disorders (Fig. 7), the loss of nerve fibres is equally distributed throughout the cochlea and disordered neural conduction is likely independent of the fibre’s site of origin along the basilar membrane (Starr et al., 2003). The finding of normal adaptation to low frequency tones in neural forms of auditory neuropathy was therefore unexpected and may reflect the engagement of central auditory structures involved in loudness perception that compensate for reduction of auditory nerve input in a frequency specific manner (Zeng and Shannon, 1994; Zeng, 2013).
Effect of abnormal adaptation on hearing
Temporal resolution for different auditory percepts occurs on different time scales. For instance, sound localization utilizes resolution of temporal differences, on the order of tens of microseconds, between neural signals originating from each ear; in contrast, defining the pitch of low frequency acoustic signals requires resolution of temporal cues on the order of milliseconds (Moore, 2012). Although temporal processing deficits have been widely accepted as a hallmark of auditory neuropathy, previous studies have concentrated on disorders of processing occurring in the range of microseconds or milliseconds (Zeng et al., 2005). The present study extends the upper limit of disordered temporal processing in auditory neuropathy by several orders of magnitude from milliseconds to a second-to-minute scale.
At present, little is known about the consequences on hearing of abnormal loudness adaptation over such large time scales despite the fact that continuous stimuli lasting seconds or minutes (e.g. air conditioning, road noise, speech babble in rooms) are present in everyday listening environments. In auditory neuropathy, abnormal loudness adaptation may be accompanied by depletion of both neurotransmitter and energy resources both in auditory nerves and in ribbon synapses similar to other types of neuropathic disorders (Park et al., 2011). This depletion accompanying adaptation to background sounds may exacerbate abnormal speech perception in auditory neuropathy (Zeng et al., 1999; Zeng and Liu, 2006; Rance et al., 2008). In our subjects with temperature-sensitive hearing loss, long-term sound exposure may be also associated with subtle elevation of temperature within the cochlea resulting in functional loss (Starr et al., 1998; Marlin et al., 2010). Anecdotally, two of those subjects reported sound sensations at the end of the continuous tone that were reminiscent of the sensation of ‘noise’ they experience accompanying increases of body temperature.
Relevance of adaptation for diagnosis and treatment of auditory neuropathy
The clinical criteria for diagnosis of auditory neuropathy use physiological tests that reveal disordered function of inner hair cells, ribbon synapses, or auditory nerve (see Starr et al., 1996). Clinical evaluations are used to suggest the site(s) of abnormal function causing auditory neuropathy. Thus, auditory neuropathy due to neural disorders is considered likely if there is evidence of other cranial or peripheral neuropathies. Disorders of inner hair cells and their ribbon synapses are considered likely by identifying the presence of particular genetic mutations (Manchaiah et al., 2011 for review). The ability to define the temporal changes of ABR to trains of clicks provided objective evidence that distinguished between auditory neuropathy due to neural or inner hair cell disorders. This information will be helpful both in supporting localization of auditory neuropathy based on clinical methods and in identifying the likely benefits of cochlear implantation.
Conclusion
Subjects with auditory neuropathy display abnormal adaptation of both subjective loudness to continuous tones and auditory brainstem responses to trains of clicks. Abnormal loudness adaptation accompanies both disordered inner hair cell ribbon synapse function and impaired auditory nerve function. Objective measures of adaptation in ribbon synapse and auditory nerve disorders have significant differences. However, these cochlear and neural differences do not fully account for the differences observed for subjective loudness adaptation in ribbon synapse and neural disorders. The experience of loudness adaptation must involve changes initiated in the auditory periphery and then modified further in central auditory sites. We suggest that these central changes might be accessed by physiological measures of auditory cortical and subcortical structures during adaptation.
Funding
This work was supported by the National Institutes of Health (R01 DC02618 to A.S., R01 DC008858 and P30 DC008369 to F.Z.).
Acknowledgements
The authors thank the study participants for their time and contribution. We also thank Len Kitzes and two anonymous reviewers for their comments on earlier drafts of the manuscript.
Glossary
Abbreviation
- ABR
auditory brainstem response
References
- Berlin CI, Hood LJ, Morlet T, Wilensky D, Li L, Mattingly KR, et al. Multi-site diagnosis and management of 260 patients with auditory neuropathy/dyssynchrony (Auditory Neuropathy Spectrum Disorder) Int J Audiol. 2010;49:30–43. doi: 10.3109/14992020903160892. [DOI] [PubMed] [Google Scholar]
- Bekesy G. Zur Theorie des Hörens: Über die Bestimmung des einem reinen Tonempfinden entsprechenden Erregungsgebietes der Basilarmembran vermittelst Ermüdungserscheinungen. Physik Zeits. 1929;30:115–25. [Google Scholar]
- Carhart R. Clinical determination of abnormal auditory adaptation. AMA Arch Otolaryngol. 1957;65:32–9. doi: 10.1001/archotol.1957.03830190034008. [DOI] [PubMed] [Google Scholar]
- Craik KJ. The effect of adaptation on differential brightness discrimination. J Physiol. 1938;92:406–21. doi: 10.1113/jphysiol.1938.sp003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic A, Starr A, Bhatt S, Michalewski HJ, Zeng FG, Pratt H. Auditory cortical N100 in pre- and post-synaptic auditory neuropathy to frequency or intensity changes of continuous tones. Clin Neurophysiol. 2011;122:594–604. doi: 10.1016/j.clinph.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don M, Allen AR, Starr A. Effect of click rate on the latency of auditory brain stem responses in humans. Ann Otol Rhinol Laryngol. 1977;86:186–95. doi: 10.1177/000348947708600209. [DOI] [PubMed] [Google Scholar]
- Dowling JE. Neural and photochemical mechanisms of visual adaptation in the rat. J Gen Physiol. 1963;46:1287–301. doi: 10.1085/jgp.46.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ. Analog modelling of cochlear adaptation. Kybernetik. 1973;14:117–26. doi: 10.1007/BF00288909. [DOI] [PubMed] [Google Scholar]
- Fukaya T, Hata Y, Komatuzaki A. Cochlear nerve conduction block restoration of hearing after removal of cerebellopontine tumors. Nihon Jibiinkoka Gakkai Kaiho. 1993;96:24–8. doi: 10.3950/jibiinkoka.96.24. [DOI] [PubMed] [Google Scholar]
- Goble AK, Hollins M. Vibrotactile adaptation enhances amplitude discrimination. J Acoust Soc Am. 1993;93:418–24. doi: 10.1121/1.405621. [DOI] [PubMed] [Google Scholar]
- Hellman R, Miskiewicz A, Scharf B. Loudness adaptation and excitation patterns: Effects of frequency and level. J Acoust Soc Am. 1997;101:2176–85. doi: 10.1121/1.418202. [DOI] [PubMed] [Google Scholar]
- Jang DP, Lee KM, Lee SY, Oh JH, Park CW, Kim IY, et al. Auditory adaptation to sound intensity in conscious rats: 2-[F-18]-fluoro-2-deoxy-D-glucose PET study. Neuroreport. 2012;23:228–33. doi: 10.1097/WNR.0b013e32835022c7. [DOI] [PubMed] [Google Scholar]
- Javel E. Long-term adaptation in cat auditory-nerve fiber responses. J Acoust Soc Am. 1996;99:1040–52. doi: 10.1121/1.414633. [DOI] [PubMed] [Google Scholar]
- Johnson EW. Results of auditory tests in acoustic tumor patients. In: House WF, Luetje CM, editors. Acoustic tumors: diagnosis and management. Baltimore: University Park Press; 1979. [Google Scholar]
- Kiang NY. Research monograph No. 35. Cambridge: M.I.T. Press; 1965. Discharge patterns of single auditory nerve fibers in the cat’s auditory nerve. [Google Scholar]
- Kim DO, Chang SO, Sirianni JG. A population-response study of auditory nerve fibers in unanesthetized decerebrate cats: response to pure tones. J Acoust Soc Am. 1990;87:1648–55. doi: 10.1121/1.399412. [DOI] [PubMed] [Google Scholar]
- Kim DO, Parham K. Auditory nerve spatial encoding of high-frequency pure tones: Population response profiles derived from d’ measure associated with nearby places along the cochlea. Hear Res. 1991;52:167–79. doi: 10.1016/0378-5955(91)90196-g. [DOI] [PubMed] [Google Scholar]
- Kovach MJ, Lin JP, Boyadjiev S, Campbell K, Mazzeo L, Herman K, et al. A unique point mutation in PMP22 gene is associated with Charcot-Marie-Tooth disease and deafness. Am J Hum Genet. 1999;64:1580–93. doi: 10.1086/302420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Lenarz T, Joseph G, Battmer RD, Patrick JF, Lenarz M. Effects of phase duration and pulse rate on loudness and pitch percepts in the first auditory midbrain implant patients: Comparison to cochlear implant and auditory brainstem implant results. Neuroscience. 2008;154:370–80. doi: 10.1016/j.neuroscience.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Manchaiah VK, Zhao F, Danesh AA, Duprey R. The genetic basis of auditory neuropathy spectrum disorder (ANSD) Int J Pediatr Otorhinolaryngol. 2011;75:151–8. doi: 10.1016/j.ijporl.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Marlin S, Feldmann D, Nguyen Y, Rouillon I, Loundon N, Jonard L, et al. Temperature-sensitive auditory neuropathy associated with an otoferlin mutation: Deafening fever! Biochem Biophys Res Comm. 2010;394:737–42. doi: 10.1016/j.bbrc.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Kanzaki J. Morphological evidence that impaired intraneural microcirculation is a possible mechanism of eighth nerve conduction block in acoustic neuromas. Eur Arch Otorhinolaryngol. 2000;257:412–17. doi: 10.1007/s004050000258. [DOI] [PubMed] [Google Scholar]
- Michaelis L, Menten ML. Die kinetik der invertinwirkung. Biochem Z. 1913;49:333–69. [Google Scholar]
- Michalewski HJ, Starr A, Zeng FG, Dimitrijevic A. N100 cortical potentials accompanying disrupted auditory nerve activity in auditory neuropathy (AN): effects of signal intensity and continuous noise. Clin Neurophysiol. 2009;120:1352–63. doi: 10.1016/j.clinph.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BC, editor. An introduction to the psychology of hearing. 6th edn. Bingley: Emerald Group Publishing Limited; 2012. [Google Scholar]
- Owens E. Tone decay in VIIIth nerve and cochlear lesions. J Speech Hear Disord. 1964;29:14–22. doi: 10.1044/jshd.2901.14. [DOI] [PubMed] [Google Scholar]
- Pangrsic T, Lasarow L, Reuter K, Takago H, Schwander M, Riedel D, et al. Hearing requires otoferlin-dependent efficient replenishment of synaptic vesicles in hair cells. Nat Neurosci. 2010;13:869–76. doi: 10.1038/nn.2578. [DOI] [PubMed] [Google Scholar]
- Park SB, Lin CS, Burke D, Kiernan MC. Activity-dependent conduction failure: molecular insights. J Peripher Nerv Syst. 2011;16:159–68. doi: 10.1111/j.1529-8027.2011.00358.x. [DOI] [PubMed] [Google Scholar]
- Pratt H, Polyakov A, Bleich N, Mittelman N. The combined effects of forward masking by noise and high click rate on monaural and binaural human auditory nerve and brainstem potentials. Hear Res. 2004;193:83–94. doi: 10.1016/j.heares.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Rance G. Auditory neuropathy/dys-synchrony and its perceptual consequences. Trends Amplif. 2005;9:1–43. doi: 10.1177/108471380500900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance G, Fava R, Baldock H, Chong A, Barker E, Corben L, et al. Speech perception in individuals with Friedreich ataxia. Brain. 2008;131:2002–12. doi: 10.1093/brain/awn104. [DOI] [PubMed] [Google Scholar]
- Reisinger E, Bresee C, Neef J, Nair R, Reuter K, Bulankina A, et al. Probing the functional equivalence of otoferlin and synaptotagmin 1 in exocytosis. J Neurosci. 2011;31:4886–95. doi: 10.1523/JNEUROSCI.5122-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Ballesteros M, Reynoso R, Olarte M, Villamar M, Morera C, Santarelli R, et al. A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with nonsyndromic hearing impairment and auditory neuropathy. Hum Mutat. 2008;29:823–31. doi: 10.1002/humu.20708. [DOI] [PubMed] [Google Scholar]
- Roosli C, Linthicum FH, Jr, Cureoglu S, Merchant SN. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: an underappreciated entity. Otol Neurotol. 2012;33:473–80. doi: 10.1097/MAO.0b013e318248ee02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillon I, Marcolla A, Roux I, Marlin S, Feldmann D, Couderc R, et al. Results of cochlear implantation in two children with mutations in the OTOF gene. Int J Pediatr Otorhinolaryngol. 2006;70:689–96. doi: 10.1016/j.ijporl.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, et al. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–89. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Santarelli R, Starr A, Michalewski HJ, Arslan E. Neural and receptor cochlear potentials obtained by transtympanic electrocochleography in auditory neuropathy. Clin Neurophysiol. 2008;119:1028–41. doi: 10.1016/j.clinph.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Sato T. Adaptation of primary gustatory nerve responses in the frog. Comp Biochem Physiol A Comp Physiol. 1972;43:1–12. doi: 10.1016/0300-9629(72)90462-8. [DOI] [PubMed] [Google Scholar]
- Scharf B. Loudness adaptation. In: Tobias JV, Schubert ED, editors. Hearing research and theory. New York: Academic Press; 1983. [Google Scholar]
- Shaia WT, Shapiro SM, Spencer RF. The jaundiced Gunn rat model of auditory neuropathy/dyssynchrony. Laryngoscope. 2005;115:2167–73. doi: 10.1097/01.MLG.0000181501.80291.05. [DOI] [PubMed] [Google Scholar]
- Smith RL. Short-term adaptation in single auditory nerve fibers: some poststimulatory effects. J Neurophysiol. 1977;40:1098–112. doi: 10.1152/jn.1977.40.5.1098. [DOI] [PubMed] [Google Scholar]
- Smith RL, Brachman ML. Adaptation in auditory-nerve fibers: a revised model. Biol Cybern. 1982;44:107–20. doi: 10.1007/BF00317970. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Optic cochleovestibular degenerations in hereditary ataxias. II. Temporal bone pathology in two cases of Friedreich's ataxia with vestibulo-cochlear disorders. Brain. 1974;97:41–8. doi: 10.1093/brain/97.1.41. [DOI] [PubMed] [Google Scholar]
- Starr A, McPherson D, Patterson J, Don M, Luxford W, Shannon R, et al. Absence of both auditory evoked potentials and auditory percepts dependent on timing cues. Brain. 1991;114:1157–80. doi: 10.1093/brain/114.3.1157. [DOI] [PubMed] [Google Scholar]
- Starr A, Michalewski HJ, Zeng FG, Fujikawa-Brooks S, Linthicum F, Kim CS, et al. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser) Brain. 2003;126:1604–19. doi: 10.1093/brain/awg156. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119:741–53. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger Y, Nguyen T, Michalewski HJ, Oba S, Abdala C. Cochlear receptor (microphonic and summating potentials, otoacoustic emissions) and auditory pathway (auditory brain stem potentials) activity in auditory neuropathy. Ear Hear. 2001;22:91–9. doi: 10.1097/00003446-200104000-00002. [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger Y, Winter M, Derebery MJ, Oba S, Michalewski HJ. Transient deafness due to temperature-sensitive auditory neuropathy. Ear Hear. 1998;19:169–79. doi: 10.1097/00003446-199806000-00001. [DOI] [PubMed] [Google Scholar]
- Tang Q, Liu S, Zeng FG. Loudness adaptation in acoustic and electric hearing. J Assoc Res Otolaryngol. 2006;7:59–70. doi: 10.1007/s10162-005-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerdahl M, Hester KD, Felix ER, Hollins M, Favorov OV, Quibrera PM, et al. Human vibrotactile frequency discrimination capacity after adaptation to 25 Hz or 200 Hz discrimination. Brain Res. 2005;1057:1–9. doi: 10.1016/j.brainres.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Varga R, Avenarius MR, Kelley PM, Keats BJ, Berlin CI, Hood LJ, et al. OTOF mutations revealed by genetic analysis of hearing loss families including a potential temperature sensitive auditory neuropathy allele. J Med Genet. 2006;43:576–81. doi: 10.1136/jmg.2005.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DY, Wang YC, Weil D, Zhao YL, Rao SQ, Zong L, et al. Screening mutations of OTOF gene in Chinese patients with auditory neuropathy, including a familial case of temperature-sensitive auditory neuropathy. BMC Med Genet. 2010;11:79. doi: 10.1186/1471-2350-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG. An active loudness model suggesting tinnitus as increased central noise and hyperacusis as increased nonlinear gain. Hear Res. 2013;295:172–9. doi: 10.1016/j.heares.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Kong YY, Michalewski HJ, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93:3050–63. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Liu S. Speech perception in individuals with auditory neuropathy. J Speech Lang Hear Res. 2006;49:367–80. doi: 10.1044/1092-4388(2006/029). [DOI] [PubMed] [Google Scholar]
- Zeng FG, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10:3429–35. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Shannon RV. Loudness-coding mechanisms inferred from electrical stimulation of the human auditory system. Science. 1994;264:564–6. doi: 10.1126/science.8160013. [DOI] [PubMed] [Google Scholar]