Abstract
Alzheimer’s disease begins about two decades before the onset of symptoms or neuron death, and is believed to be caused by pathogenic amyloid-β aggregates that initiate a cascade of molecular events culminating in widespread neurodegeneration. The microtubule binding protein tau may mediate the effects of amyloid-β in this cascade. Amyloid plaques comprised of insoluble, fibrillar amyloid-β aggregates are the most characteristic feature of Alzheimer’s disease. However, the correspondence between the distribution of plaques and the pattern of neurodegeneration is tenuous. This discrepancy has stimulated the investigation of other amyloid-β aggregates, including soluble amyloid-β oligomers. Different soluble amyloid-β oligomers have been studied in several mouse models, but not systematically in humans. Here, we measured three amyloid-β oligomers previously described in mouse models—amyloid-β trimers, Aβ*56 and amyloid-β dimers—in brain tissue from 75 cognitively intact individuals, ranging from young children to the elderly, and 58 impaired subjects with mild cognitive impairment or probable Alzheimer’s disease. As in mouse models, where amyloid-β trimers appear to be the fundamental amyloid-β assembly unit of Aβ*56 and are present in young mice prior to memory decline, amyloid-β trimers in humans were present in children and adolescents; their levels rose gradually with age and were significantly above baseline in subjects in their 70s. Aβ*56 levels were negligible in children and young adults, rose significantly above baseline in subjects in their 40s and increased steadily thereafter. Amyloid-β dimers were undetectable until subjects were in their 60s; their levels then increased sharply and correlated with plaque load. Remarkably, in cognitively intact individuals we found strong positive correlations between Aβ*56 and two pathological forms of soluble tau (tau-CP13 and tau-Alz50), and negative correlations between Aβ*56 and two postsynaptic proteins (drebrin and fyn kinase), but none between amyloid-β dimers or amyloid-β trimers and tau or synaptic proteins. Comparing impaired with age-matched unimpaired subjects, we found the highest levels of amyloid-β dimers, but the lowest levels of Aβ*56 and amyloid-β trimers, in subjects with probable Alzheimer’s disease. In conclusion, in cognitively normal adults Aβ*56 increased ahead of amyloid-β dimers or amyloid-β trimers, and pathological tau proteins and postsynaptic proteins correlated with Aβ*56, but not amyloid-β dimers or amyloid-β trimers. We propose that Aβ*56 may play a pathogenic role very early in the pathogenesis of Alzheimer’s disease.
Keywords: amyloid-β, Alzheimer, dimer, trimer, Aβ*56, oligomer
Introduction
In Alzheimer’s disease functional, structural and biochemical abnormalities appear one or two decades before the clinical symptoms of dementia develop (Jack et al., 2010; Sperling et al., 2011; Bateman et al., 2012). Longitudinal neuropsychological studies indicate that cognitive impairment begins approximately a decade before individuals report any symptoms (Amieva et al., 2008). These observations suggest that Alzheimer’s disease, like many common chronic diseases of ageing, consists of an asymptomatic followed by a symptomatic phase.
While the cause of Alzheimer’s disease is unknown, genetic studies suggest it is due to the formation of abnormal amyloid-β aggregates (reviewed in Ashe and Zahs, 2010). Neuropathological, genetic and molecular data indicate that the microtubule binding protein tau may mediate the disease process; the pattern of neurodegeneration correlates with tau neuropathology and tau mediates amyloid-β induced neurotoxicity and cognitive deficits (Gomez-Isla et al., 1997; Rapoport et al., 2002; Roberson et al., 2007). However, Alzheimer’s disease has never been linked to tau mutations, arguing against a role for tau in initiating the disease. The amyloid cascade hypothesis consolidates these observations (Hardy and Higgins, 1992), positing that specific aggregates of amyloid-β trigger molecular events leading to widespread neurodegeneration mediated by tau. An important focus of recent studies is to identify the specific amyloid-β aggregates involved in the amyloid cascade.
Although insoluble, fibrillar aggregates of amyloid-β constitute the chief neuropathological hallmark of Alzheimer’s disease, they correlate poorly with brain dysfunction in humans (Giannakopoulos et al., 2003; Bennett et al., 2004). Several soluble aggregates (oligomers) of amyloid-β have been studied in animal models (Lesne et al., 2006; Cheng et al., 2007; Shankar et al., 2008; Gandy et al., 2010; Reed et al., 2011). Three specific amyloid-β oligomers isolated directly from the brains of patients with Alzheimer’s disease or transgenic mouse models of Alzheimer’s disease have been assayed for their effects on cognition: amyloid-β dimers, amyloid-β trimers and Aβ*56. There is strong evidence that amyloid-β dimers and Aβ*56 impair brain function when injected into young, healthy rats (Lesne et al., 2006; Shankar et al., 2008; Reed et al., 2011). In contrast, the evidence for deleterious effects of amyloid-β trimers is less clear. Amyloid-β trimers appear to be the compositional assembly unit of Aβ*56 because Aβ*56 dissociates into trimers before monomers (Lesne et al., 2006). It has been difficult to conclude that amyloid-β trimers disrupt cognition, because they are present in mice before memory decline (Lesne et al., 2006), and do not convincingly impair cognition when injected into the cerebral ventricles of rats (Reed et al., 2011). These amyloid-β oligomers have been studied in animals, but we do not yet know their relevance to human disease. In particular, we know neither the order in which these amyloid-β oligomers appear nor the stages of Alzheimer’s disease in which they are present. Characterizing amyloid-β dimers, amyloid-β trimers and Aβ*56 in human brain tissue from cognitively intact individuals spanning a wide age range as well as from impaired subjects may provide clues about the temporal sequence and effects of amyloid-β oligomers in the amyloid cascade.
Our measurements of amyloid-β oligomers in human brain tissue used techniques that under some conditions artificially generate or degrade synthetic amyloid-β oligomers. There has, therefore, been concern that Aβ*56 and other oligomers found in the brain are methodological artefacts, related to exposing amyloid-β to non-native conditions (Benilova et al., 2012). However, this would not be the case if they were identified in CSF. Amyloid-β dimers were previously described in human CSF (Klyubin et al., 2008). In the present study, we identified Aβ*56 and amyloid-β trimers in human CSF.
Several studies of amyloid-β oligomers in human brain have focused on subjects with clinically diagnosed Alzheimer’s disease (Shankar et al., 2008; Noguchi et al., 2009; Mc Donald et al., 2010; Pham et al., 2010). Recent developments in the field suggest that therapeutic interventions may be more effective in the asymptomatic phase of Alzheimer’s disease (Zahs and Ashe, 2010; Golde et al., 2011); understanding how amyloid-β is involved very early in its pathogenesis may lead to better preventive treatments. One approach is to examine amyloid-β oligomers in cognitively intact individuals, some of whom may be in the asymptomatic phase of Alzheimer’s disease. Therefore, in addition to measuring amyloid-β dimers, amyloid-β trimers and Aβ*56 in 58 subjects with mild cognitive impairment or probable Alzheimer’s disease, and five subjects with non-Alzheimer dementia, we also studied 75 neurologically intact individuals between the ages of 1 and 96 years.
Materials and methods
Human brain tissue
Religious Orders Study
Brain tissue from the inferior temporal gyrus (Brodmann area 20) from 90 subjects underwent biochemical analyses. Cognitive status was assessed with the Mini-Mental Status Examination and 19 other tests summarized as a global measure of cognition and five cognitive domains (Boyle et al., 2006). Selected cases were chosen to ensure that the three groups would not differ significantly from the whole Religious Orders Study cohort (283 subjects at the start of this study). The characteristics of the three clinical diagnostic groups are summarized in Supplementary Table 1.
Each participant had undergone a uniform structured baseline clinical evaluation and annual follow-up evaluation until death (Bennett et al., 2012). Briefly, both dementia and an Alzheimer’s disease diagnosis required evidence of meaningful decline in cognitive function and impairment in at least two cognitive domains (for Alzheimer’s disease, one domain had to have been episodic memory), based on the results of 21 cognitive performance tests and their review by a clinical neuropsychologist and expert clinician. Mild cognitive impairment refers to participants with cognitive impairment as assessed by the neuropsychologist but without a diagnosis of dementia, as determined by the clinician. No cognitive impairment refers to those individuals without dementia or mild cognitive impairment. At death, a neurologist blinded to all post-mortem data reviewed all available clinical data and rendered a summary diagnostic opinion regarding the clinical diagnosis at the time of death (Bennett et al., 2006).
Following death, each case was assigned, by examiners blinded to all clinical data, a Braak score based on neuronal neurofibrillary tangle pathology (Braak and Braak, 1991), a neuritic plaque score based on the modified Consortium to Establish a Registry for Alzheimer Disease criteria (excluding age and clinical diagnosis), and an Alzheimer’s pathological diagnosis based on the National Institute on Aging–Reagan Institute Working Group criteria (Bennett et al., 2005). Neuritic plaques, diffuse plaques, and neurofibrillary tangles in the inferior temporal cortex were counted after Bielschowsky silver staining, as previously described (Bennett et al., 2003). Amyloid load and the densities of plaques and tangles were quantified in six brain regions (hippocampus, entorhinal cortex, midfrontal gyrus, inferior temporal gyrus, inferior parietal gyrus and calcarine cortex) (Bennett et al., 2004). For amyloid load, amyloid-β was labelled with an N-terminus directed monoclonal antibody (MO0872, Dako, 1:100), using diaminobenzidine as the reporter with 2.5% nickel sulphate to enhance immunoreaction product contrast. Video images of amyloid-β stained sections were captured for a random sample for quantitative analysis of amyloid deposition using StereoInvestigator software version 9 (MicroBrightfield Inc) and an Olympus BX-51 microscope with an attached motorized stage.
Exclusion criteria
Cases with post-mortem intervals >12 h (four with no cognitive impairment, one with Alzheimer’s disease) were excluded from the original selection, because after 12 h levels of the postsynaptic markers drebrin and NR2B declined with increasing post-mortem intervals. One study participant with a clinical diagnosis of mixed dementia (Alzheimer’s disease plus stroke) was also excluded. One tissue sample showed pronounced protein degradation of all proteins tested and was excluded from all analyses.
National Institute of Child Health and Human Development Brain and Tissue Bank for developmental disorders brain specimens
The inferior temporal gyrus (Brodmann area 20) from 49 individuals with post-mortem intervals <12 h was obtained from the Brain and Tissue Bank at the University of Maryland, Baltimore.
Human cerebrospinal fluid specimens
After the calvarium but before the brain was removed, a needle was inserted into the occipital horn of the lateral ventricle and ∼20 ml of ventricular CSF was removed, centrifuged and stored in cryogenic tubes at −80°C, as previously reported (Hensley et al., 2011).
Transgenic animals
Mice used in this study included heterozygous transgenic mice and non-transgenic littermates from Tg2576 (Hsiao et al., 1996) and J20 (Mucke et al., 2000), which express human amyloid precursor protein with the Swedish (Lys670Arg, Met671Leu) and Indiana (Val717Phe) mutations, respectively, driven by the prion protein and platelet-derived growth factor promoters, respectively. All Tg2576 mice were offspring of mice backcrossed successively to B6SJLF1 breeders while J20 mice were crossed for at least 10 generations with C57Bl/6J mice. Genotype was analysed by PCR and confirmed using immunoblots probed with 6E10 antibodies.
Protein extractions
We used two extraction protocols, as previously described (Lesne et al., 2006; Shankar et al., 2008; Sherman and Lesne, 2011). All supernatants were ultracentrifuged for 20 min at 100 000g. Protein amounts were determined using a bicinchoninic acid protein assay (BCA Protein Assay, Pierce). Finally, before analysis, fractions were immunodepleted by incubating for 1 h at room temperature with 50 µl of 1:1 slurry Protein A-Sepharose, Fast Flow® followed by 50 µl of 1:1 slurry Protein G-Sepharose, Fast Flow® (GE Healthcare Life Sciences).
Immunoprecipitation of amyloid-β from human cerebrospinal fluid
For immunoblots probed with 6E10 antibodies, aliquots of human CSF (500 µl) were pre-cleared with 50 µl of 1:1 slurry of Protein-G Fast Flow Sepharose® (GE Healthcare Life Sciences) for 1 h at 4°C, then centrifuged at 9300g for 5 min. Subsequently, 250 µl of immunoglobulin-depleted CSF was incubated with 5 µg 6E10 antibodies and 50 µl Protein-G coated magnetic beads (Life Technologies) overnight at 4°C. The beads were washed sequentially with immunoprecipitation buffer A [50 mM Tris-HCl, 300 mM NaCl, 0.1% Triton® X-100 (v/v), 1 mM EDTA, pH 7.4] and immunoprecipitation buffer B [50 mM Tris-HCl, 150 mM NaCl, 0.1% Triton® X-100 (v/v), 1 mM EDTA, pH 7.4] for 20 min under gentle agitation at 4°C and captured proteins were eluted by boiling in 30 μl of SDS-PAGE loading buffer.
For immunoblots probed with A11 antibodies, aliquots of human CSF (1 ml) were pre-cleared with 50 µl of 1:1 slurry Protein A-Sepharose, Fast Flow® (GE Healthcare Life Sciences) for 1 h at 4°C. Following centrifugation at 9300g for 5 min, supernatants were incubated with 5 µg of 6E10 antibodies and 50 µl of 1:1 slurry Protein A-Sepharose, Fast Flow® overnight at 4°C. The beads were washed sequentially with immunoprecipitation buffer A [50 mM Tris-HCl, 300 mM NaCl, 0.1% Triton® X-100 (v/v), 1 mM EDTA, pH 7.4] and immunoprecipitation buffer B [50 mM Tris-HCl, 150 mM NaCl, 0.1% Triton® X-100 (v/v), 1 mM EDTA, pH 7.4] for 20 min under gentle agitation at 4°C and captured proteins were eluted by boiling in 25 µl of SDS-PAGE loading buffer.
Western blotting and quantification
Gel electrophoresis
Depending upon the targeted protein, 2–100 µg of protein were aliquoted, resuspended with 4× Tricine loading buffer, and size fractionated by PAGE using pre-cast 10–20% SDS polyacrylamide Tris-Tricine gels, or 10.5–14% or 7.5% Tris-HCl gels (Bio-Rad).
Transfer
Proteins were transferred to a 0.45 µm polyvinylidene difluoride membrane (Immobilon P membrane, Millipore) or 0.2 µm nitrocellulose membrane (Bio-Rad).
Blotting
Nitrocellulose membranes were boiled twice in 50 ml PBS by microwaving first for 25 s and then, after 3 min, for 15 s. Membranes were blocked in Tris-buffered saline-0.1% Tween®20 containing 5% bovine serum albumin (Sigma) for 2 h at room temperature, and probed with appropriate antisera/antibodies diluted in blocking buffer. Primary antibodies were detected with anti-IgG immunoglobulins conjugated with either biotin or horseradish peroxidase. When biotin-conjugated secondary antibodies were used, horseradish peroxidase-conjugated Neutravidin® (Pierce) or ExtrAvidin® (Sigma) was added to amplify the signal. All blots were developed with an enhanced chemiluminescence western blotting detection system (Supersignal Pico Western system, Pierce).
Stripping
Membranes were stripped using Restore™ Plus Stripping buffer (Pierce) for 30–180 min at room temperature, depending on antibody affinity.
Quantification
Densitometry was performed using OptiQuant software (Packard Bioscience). Pilot experiments for each protein were run to determine the experimental conditions that produced signals within the linear range of detection. This method produced a dynamic range of ∼100-fold above the background level of 104 densitometry light units. The level of each protein was the mean of triplicate measurements. The 138 brain specimens were each extracted using two methods, yielding five soluble fractions or extracts per specimen; >600 samples were assayed for each protein studied. We did not normalize protein level values to α-tubulin or βIII-tubulin, because these tubulins correlated inversely with some amyloid-β species.
Antibodies
The following primary antibodies were used in this study: 6E10 (1:2500) against the amino-terminus of amyloid-β, 4G8 (1:2500) against the mid-region of amyloid-β, biotinylated-6E10 (1:2500) (Covance), A11 (1:2000) against large oligomers (generous gift of R. Kayed and C. Glabe), Aβ42 - and Aβ40-end specific monoclonal antibodies Mab2.1.3 and Mab13.1.1 (1:1000; generous gift of Pritam Das) and Aβ42 and Aβ40 carboxyl end-specific monoclonal antibodies 8G7 and 5C3 (1:1000; EMD Biosciences), amyloid-β amino end-specific monoclonal antibody 82E1 (1:2000; IBL), APPCter-C17 (1:5000) against APP C-terminus (generous gift of A. Delacourte and N. Sergeant), anti-α-tubulin (Sigma; 1:100 000), anti-βIII-tubulin (Sigma; 1:50 000), anti-drebrin (1:4000; Sigma), anti-NeuN (Invitrogen; 1:5000), anti-synaptophysin (Chemicon; 1:25 000), CP13/PG5/PHF1/Alz50 (generous gift of P. Davies; 1:1000), Tau-5 (Biosource; 1:2000) and T14 (Invitrogen; 1:2000), anti-PSD95 (1:200; Santa Cruz Biotechnology), anti-Fyn (1:1000; BD Biosciences) and anti-NR1CT (1:1000; Millipore).
Statistical analyses
When dependent variables were not normally distributed, non-parametric statistics were used (Spearman rank correlations, Kruskal-Wallis one-way ANOVA by ranks followed by Bonferroni-corrected two-group post hoc Mann-Whitney U tests). Relationships between amyloid-β trimers or amyloid-β dimers and age were determined using the Pearson correlation test. Curvilinear relationships between Aβ*56 levels and age were estimated by quadratic polynomial regression analysis.
To control for the increased probability of type I errors due to multiple comparisons, the criterion for statistical significance was set at P = 0.01. All analyses were performed using StatView software, version 5.0.1 (SAS Institute).
Results
Detection of amyloid-β dimers, amyloid-β trimers and Aβ*56 in human cerebrospinal fluid and brain tissue
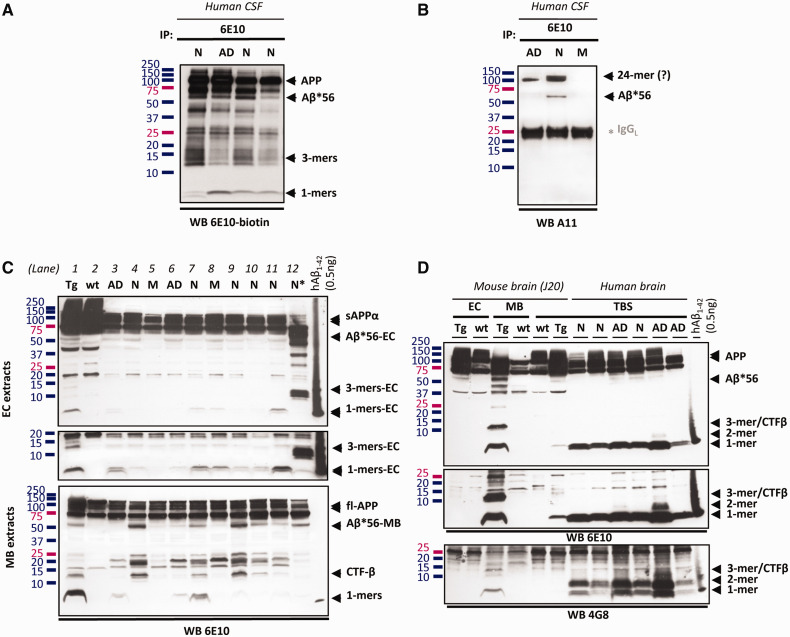
Our analysis focused on three oligomers—amyloid-β dimers, amyloid-β trimers and Aβ*56—whose effects on cognition have been studied in animals. To ascertain whether these oligomers exist under native conditions in humans, we immunocaptured proteins from human CSF with 6E10 antibodies, and found 6E10-immunoreactive bands with molecular masses consistent with amyloid-β trimers and Aβ*56 (Fig. 1A), and 4G8-immunoreactive bands with molecular masses consistent with amyloid-β dimers (not shown). Immunocaptured Aβ*56 also reacted with A11 antiserum (Fig. 1B), which specifically detects soluble amyloid-β oligomers larger than tetramers that are distinct from amyloid-β fibrils (Kayed et al., 2003). In a separate study (unpublished data), we obtained additional evidence supporting the existence of amyloid-β dimers, amyloid-β trimers and Aβ*56 in human CSF: (i) proteins with the expected molecular masses were immunocaptured with 4G8 antibodies and detected with 6E10 antibodies, excluding the possibility that they were fragments of soluble amyloid precursor protein; (ii) proteins with the expected molecular masses were not immunoprecipitated by amyloid precursor protein antibodies flanking amyloid-β, arguing against the possibility that they were cleavage or degradation products of the amyloid precursor protein recognized by 6E10; and (iii) 6E10-immunoreactive proteins with the expected molecular masses were separated under native conditions using size-exclusion chromatography, arguing against the possibility that they were artificially generated by detergents.
Figure 1.
Detection of amyloid-β dimers, amyloid-β trimers and Aβ*56 in CSF and brain. (A) 6E10-immunoreactive soluble amyloid-β and soluble amyloid precursor proteins in 6E10-immunocaptured proteins from human CSF. The middle band in the triplet running at molecular masses between 13–16 kDa is an amyloid-β oligomer; the upper and lower bands are fragments of the amyloid precursor protein (unpublished data). (B) A11-immunoreactive amyloid-β oligomers in 6E10-immunocaptured proteins from human CSF. (C) 6E10-immunoreactive proteins in extracellular (EC)- or membrane (MB)-enriched fractions from human and mouse brain. Lanes 1 and 2: J20 transgenic (Tg) and non-transgenic (wt) mice; Lanes 3–12: human brain from subjects with no cognitive impairment (N), mild cognitive impairment (M) or probable Alzheimer’s disease (AD). *The sample in Lane 12 shows protein degradation and was excluded from all analyses. The middle panel represents a longer exposure of the lower portion of the full panel above. (D) 6E10- and 4G8-immunoreactive proteins in extracellular-enriched and membrane-enriched fractions as well as Tris-buffered saline (TBS) extracts from human and mouse brain. Middle: A longer exposure of the lower portion of the full panel above. Amyloid-β dimers are better detected using 4G8 than 6E10 antibodies. A synthetic human amyloid-β1-42 loading standard (0.5 ng) was included on all blots.
To measure Aβ*56, amyloid-β trimers and amyloid-β dimers in brain tissue, we used two protein extraction protocols previously used to detect soluble amyloid-β oligomers in brains from humans and Tg2576 mice overexpressing human amyloid-β (Lesne et al., 2006; Shankar et al., 2008). One noteworthy difference between the two extraction protocols is that the extracellular- and intracellular-enriched fractions are devoid of membrane-associated proteins, while Tris-buffered saline extracts contain small amounts of membrane-associated proteins (Supplementary Fig. 1). The presence of membrane-associated proteins in Tris-buffered saline extracts precludes using these extracts to measure amyloid-β trimers, because they could potentially be confused with C-terminal fragments, which associate with membranes and have similar molecular masses. We estimate that in our brain extracts 50–100 µg protein contains 20–1000 pg of Aβ*56 or amyloid-β trimers, and may therefore escape detection using standard immunoblot protocols in which the limit of detection is ∼1000 pg of amyloid-β. By employing high-sensitivity, high-specificity immunoblots, which detect as few as ∼10 pg of amyloid-β, in combination with a broad panel of antibodies, we completed a biochemical analysis confirming that these proteins were comprised of amyloid-β.
We examined the inferior temporal gyrus (Brodmann area 20) because this brain region shows metabolic abnormalities in the asymptomatic phase of Alzheimer’s disease (Small et al., 2000; Reiman et al., 2004; Petrie et al., 2009), and its neuropathological characteristics, defined by plaque load and tangle density, were highly representative of the six regions sampled. In the inferior temporal gyrus, plaques correlated with the average plaque load of the six regions (Spearman rho = 0.95; P < 0.01) and tangles also correlated with the average tangle load of the six regions (Spearman rho = 0.77; P < 0.01). To assess the possibility that amyloid-β oligomer levels might be influenced by neuron loss, we performed measurements of the neuronal protein NeuN and a general cellular marker, α-tubulin (Supplementary Fig. 2A). We detected no significant changes in the levels of α-tubulin (not shown) or NeuN with respect to clinical status or Braak stage (Supplementary Fig. 2B). However, there was a trend toward lower levels of NeuN in subjects with mild cognitive impairment or Alzheimer’s disease [Kruskal-Wallis followed by Mann-Whitney U test with Bonferroni corrections, P(no cognitive impairment versus mild cognitive impairment) = 0.12 and P(no cognitive impairment versus Alzheimer’s disease) = 0.12].
We identified soluble Aβ*56 in extracellular- and membrane-enriched fractions (Fig. 1C), and also in Tris-buffered saline extracts (Fig. 1D). To confirm that Aβ*56 is not a cleavage product of the amyloid precursor protein, we showed that antibodies recognizing the amyloid precursor protein but not amyloid-β, including APPCter-C17 and 22C11, do not detect a 56 kDa protein (Supplementary Fig. 3). We also showed that Aβ*56 is recognized by the mid-region antibody 4G8 and another amino-region antibody, 82E1 (Supplementary Fig. 4). We could not detect Aβ*56 using Aβ40 or Aβ42 end-specific antibodies 5C3 and 8G7, presumably because its folding structure buries the carboxyl-terminus of amyloid-β (Supplementary Fig. 3). Importantly, the A11 antiserum detected Aβ*56 (Supplementary Fig. 5), providing additional evidence that it is an oligomer comprised of amyloid-β.
We found amyloid-β dimers in Tris-buffered saline extracts (Fig. 1D). Although both 6E10 and 4G8 detected amyloid-β dimers, we used 4G8 antibodies to quantify amyloid-β dimers, because 4G8 showed greater affinity for dimers than 6E10 antibodies, consistent with a previous report (Kawarabayashi et al., 2004). We also showed that some amyloid-β dimers are recognized by Aβ40 and Aβ42 end-specific antibodies (Supplementary Fig. 6). Thus, we detected amino-, mid- and carboxyl regions of amyloid-β, confirming the composition of amyloid-β dimers.
We measured amyloid-β trimers in extracellular-enriched extracts (Fig. 1C). We avoided membrane-enriched extracts because C-terminal fragments of the amyloid precursor protein can mask potential trimers, which have equivalent molecular masses. We detected amyloid-β trimers with 6E10, 4G8 and 8G7. Hence, we detected amino-, mid- and carboxyl regions of amyloid-β, confirming the composition of amyloid-β trimers.
In summary, using amyloid-β-specific antibodies, we detected the amino-, mid- and carboxyl-regions of amyloid-β in amyloid-β trimers and amyloid-β dimers. We detected the amino- and mid-regions of amyloid-β in Aβ*56, and showed that it was recognized by A11, an oligomer-specific antiserum. We found no immunoreactivity of these proteins using antibodies that detect regions of the amyloid precursor protein outside of the amyloid-β sequence. We eliminated the possibility that the amyloid-β oligomers were artificially generated through exposure to detergents by showing that they fractionated separately in CSF under non-denaturing conditions. Collectively, the data show that the oligomers are comprised of amyloid-β, and support the existence of amyloid-β dimers, amyloid-β trimers and Aβ*56 in the human CNS.
Amyloid-β oligomers increase with age in intact subjects
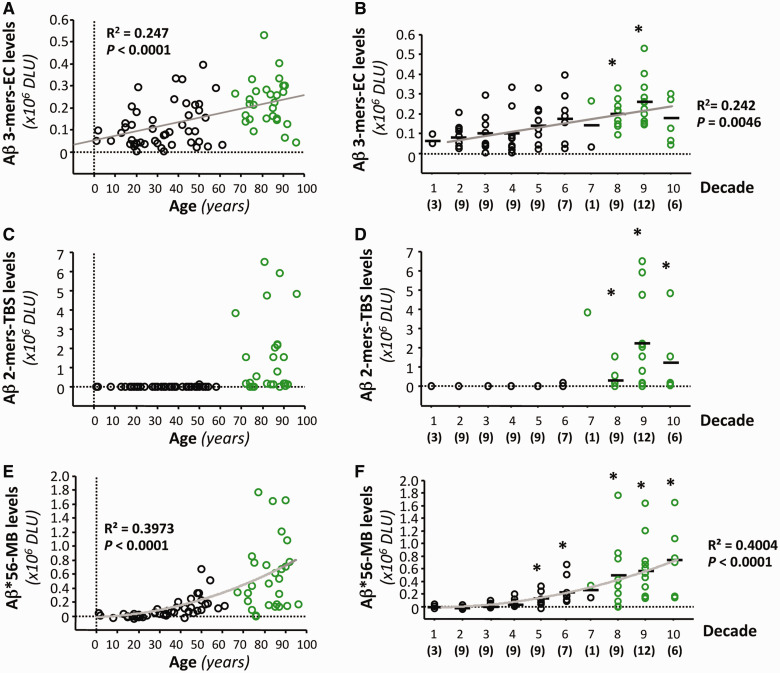
To study the accumulation of amyloid-β dimers, amyloid-β trimers and Aβ*56 in neurologically intact individuals, we measured these amyloid-β oligomers in the inferior temporal gyrus (Brodmann area 20) in 75 neurologically intact subjects between the ages of 1 and 96 years. Forty-nine specimens were obtained from the Brain and Tissue Bank for Developmental Disorders and 26 specimens from the Religious Orders Study Tissue Bank. All measurements were performed blind to the age of the subjects. Analyses were performed on the mean of triplicate measurements.
We detected amyloid-β trimers at all ages (Fig. 2A and B). This finding is consistent with previous observations in mice overexpressing amyloid-β, in which amyloid-β trimers appear to be the basic assembly unit and are present prior to memory decline (Lesne et al., 2006). Their levels rose gradually and became significantly higher relative to those in children and adolescents beginning in subjects in their 70s.
Figure 2.
Specific amyloid-β oligomers in the brain rise at different ages in cognitively intact subjects. (A–F) Forty-nine brain specimens from the Brain and Tissue Bank for Developmental Disorders and 26 brain specimens from the Religious Orders Study Tissue Bank (shown in black and green, respectively) were analysed as one group (n = 75) to evaluate soluble amyloid-β dimers, amyloid-β trimers and Aβ*56 in cognitively intact subjects ranging from 1 to 96 years of age. The relationships between age and amyloid-β trimers in extracellular (EC)-enriched fractions (A and B), amyloid-β dimers in Tris-buffered saline (TBS) extracts (C and D) and Aβ*56 in membrane (MB)-enriched fractions (E and F) expressed as a function of age (A, C and E) or grouped by decade (B, D and F). Curves show the best fit quadratic regression functions. *P < 0.01, compared with individuals<20 years of age, using Kruskal-Wallis test followed by Mann-Whitney U test with Bonferroni corrections. DLU = densitometry light units.
We found amyloid-β dimers in a single individual <60 years of age; their levels increased markedly thereafter (Fig. 2C and D).
We found that Aβ*56 rose with age in a curvilinear manner (Fig. 2E and F). Aβ*56 was undetectable in children and adolescents. It remained at low or undetectable levels until middle-age, increasing significantly above baseline levels in subjects in their 40s. It is noteworthy that on average, significant increases in Aβ*56 occurred at least two decades before the increases in amyloid-β dimers and amyloid-β trimers became significant. These observations raised the possibility that Aβ*56 may play a pathogenic role in the asymptomatic phase of Alzheimer’s disease.
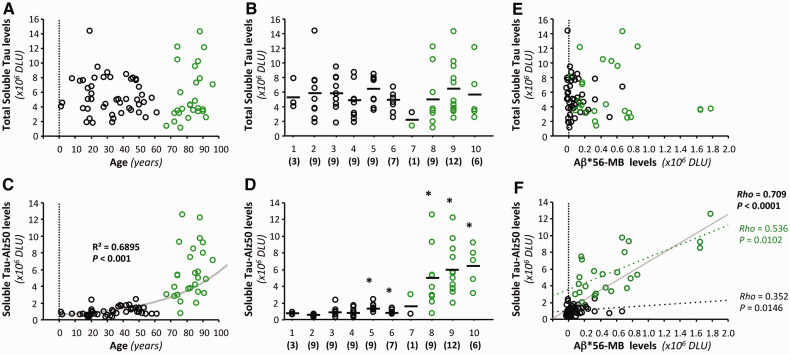
Aβ*56 correlates positively with pathological soluble tau proteins and negatively with postsynaptic proteins
To explore the possibility that Aβ*56 may play a pathogenic role in the asymptomatic phase of Alzheimer’s disease, we first examined its relationship to pathological tau molecules. Recent in vitro and in vivo studies have shown that insoluble tau in neurofibrillary tangles exerts negligible neurotoxicity compared with soluble tau (Santacruz et al., 2005; Oddo et al., 2006; Roberson et al., 2007; Hoover et al., 2010; Ittner et al., 2010). The recent finding that unspecified forms of soluble amyloid-β appear to induce pathological changes in soluble tau in mice (Chabrier et al., 2012) raises the questions of whether this also occurs in humans and which forms of amyloid-β are responsible. To address these questions, we measured two forms of soluble tau in membrane-enriched extracts, total tau and tau-Alz50, a pathological tau conformer (Wolozin et al., 1986). Total soluble tau remained stable (Fig. 3A and B), but soluble tau-Alz50 rose significantly with age (Fig. 3C and D). Interestingly, both soluble tau-Alz50 and Aβ*56 began to increase in subjects in their 40s (Figs 2F, 3C and D). Consistent with this coincidental increase in the levels of both proteins, we found strong correlations between Aβ*56 and soluble tau-Alz50 (Spearman rho = 0.71, P < 0.01; Fig. 3F), but not between Aβ*56 and total soluble tau (Fig. 3E). We also evaluated our data using a polynomial regression approach to control for the possibility that the significant associations between Aβ*56 and soluble tau-Alz50 occurred because both dependent variables correlated with age. Even after adjusting for age, we found a very strong relationship between Aβ*56 and soluble tau-Alz50 (adjusted multiple R2 = 0.74, ANOVA, P < 0.01; significance of Aβ*56 as a predictor of tau-Alz50, P < 0.01), indicating that Aβ*56 predicts soluble tau-Alz50, independent of age.
Figure 3.
Brain Aβ*56 correlates with pathological soluble tau in cognitively intact subjects. Forty-nine brain specimens from the Brain and Tissue Bank for Developmental Disorders and 26 brain specimens from the Religious Orders Study Tissue Bank (shown in black and green, respectively) were analysed as one group (n = 75) to evaluate total soluble tau and soluble tau-Alz50 as a function of age (A and C) or decade (B and D). (A and B) Total soluble tau, detected with Tau-5 antibodies, did not change with age. (C and D) The function that best defines the relationship between soluble tau-Alz50 and age is an exponential curve. Soluble tau-Alz50 increased with age, starting in subjects in their 40s, compared with individuals <20 years of age (Kruskal-Wallis followed by Mann-Whitney U test with Bonferroni corrections, *P < 0.01). (E and F) Relationships (Spearman rank correlations) between Aβ*56, total soluble tau and soluble tau-Alz50 in membrane (MB)-enriched fractions. We found no correlation between total soluble tau and Aβ*56 (E), but a strong correlation between Aβ*56 and soluble tau-Alz50 (F). The solid line is the regression for the pooled samples and the dotted lines are for the separate Religious Orders Study and Brain and Tissue Bank for Developmental Disorders samples, respectively. DLU = densitometry light units.
To obtain additional support for our hypothesis that Aβ*56 may play a pathogenic role in the asymptomatic phase of Alzheimer’s disease, we examined the relationships between Aβ*56 and two additional pathological forms of soluble tau, tau-CP13 and tau-PG5. These phospho-specific tau antibodies detect Ser202 and Ser409, respectively. In this study we focused on subjects in the Religious Orders Study without cognitive impairment. We found strong positive correlations between Aβ*56 and two pathological forms of soluble tau (tau-CP13 and tau-Alz50) (Table 1). The positive correlations with tau-CP13 and tau-Alz50 are consistent with Aβ*56 inducing specific pathological abnormalities in tau in humans. We also measured five synaptic proteins: the presynaptic protein, synaptophysin, and four postsynaptic proteins, PSD-95, drebrin, the NR1 subunit of NMDA receptors, and fyn kinase, because Aβ*56 appears to impair long-lasting synaptic plasticity (Chapman et al., 1999). We found strong negative correlations between Aβ*56 and two postsynaptic proteins (drebrin and fyn kinase) (Table 1). The negative correlations between Aβ*56 and the two postsynaptic proteins are consistent with the possibility that Aβ*56 disrupts synaptic function in humans. Since these relationships were found in unimpaired subjects, they might underlie the reductions in glucose utilization and neuropsychological test performance observed in subjects at risk of Alzheimer’s disease. Taken together, the data support our hypothesis that Aβ*56 may play a pathogenic role in the asymptomatic phase of Alzheimer’s disease.
Table 1.
Correlations between amyloid-β dimers, amyloid-β trimers, Aβ*56 and synaptic and soluble pathological tau proteins in cognitively intact elderly subjectsa
| Soluble amyloid-β speciesb | Amyloid-β dimers | Amyloid-β trimers | Aβ*56 |
|---|---|---|---|
| Synaptic proteins | |||
| Presynaptic | |||
| Synaptophysin | 0.01, 0.85 | −0.17, 0.40 | 0.02, 0.91 |
| Postsynaptic | |||
| PSD-95 | 0.28, 0.17 | −0.20, 0.33 | -0.14, 0.48 |
| Drebrin | −0.01, 0.97 | −0.30, 0.13 | −0.52, <0.01 |
| Fyn | 0.19, 0.34 | −0.25, 0.22 | −0.53, <0.01 |
| NMDAR1 | −0.10, 0.64 | 0.15, 0.46 | 0.02, 0.92 |
| Soluble pathological tau proteins | |||
| Tau-CP13 | −0.00, 0.99 | 0.35, 0.08 | 0.62, <0.01 |
| Tau-Alz50 | 0.08, 0.71 | 0.04, 0.85 | 0.44, <0.01 |
| Tau-PG5 | −0.06, 0.75 | −0.07, 0.75 | 0.10, 0.64 |
a26 subjects in the Religious Orders Study with no cognitive impairment.
bAmyloid-β dimers, amyloid-β trimers and Aβ*56 were measured in Tris-buffered saline, extracellular-enriched and membrane-enriched fractions, respectively.
Table entries show (rho, P-value) for Spearman rank correlations.
Significant correlations are indicated in bold.
In contrast, we found no correlations between amyloid-β dimers or amyloid-β trimers and either total soluble tau or soluble tau-Alz50 in the intact full cohort (Supplementary Fig. 7), and none between these two amyloid-β oligomers and the three pathological tau or five synaptic proteins we examined from subjects in the Religious Orders Study without cognitive impairment (Table 1). The lack of significant relationships between amyloid-β dimers or amyloid-β trimers and the tau or synaptic proteins attenuates the possibility that these oligomers are pathogenic in the asymptomatic phase of Alzheimer’s disease.
Differential expression patterns of amyloid-β oligomers between clinical groups
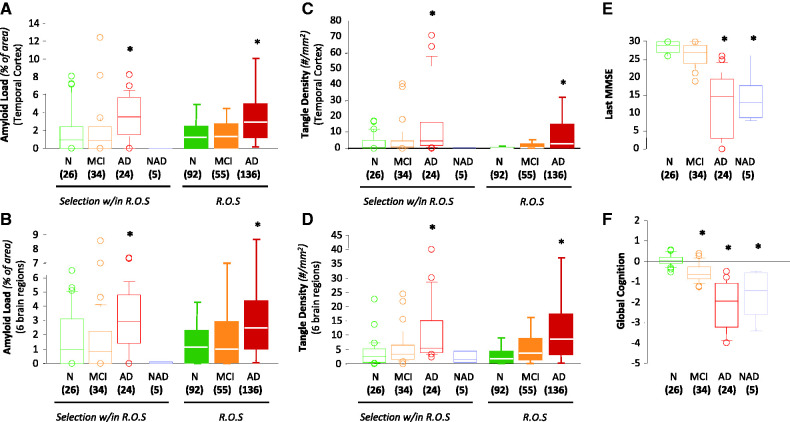
We also characterized amyloid-β dimers, amyloid-β trimers and Aβ*56 in brain tissue from impaired subjects from the Religious Orders Study with mild cognitive impairment, probable Alzheimer’s disease and non-Alzheimer’s dementia, and compared them with subjects without cognitive impairment. The clinical statuses of these individuals were assigned before autopsy, and the cases with non-Alzheimer dementia were confirmed by neuropathological inspection after autopsy. Education, post-mortem interval, gender, and interval of time between last exam and autopsy did not differ among the clinical diagnostic groups (Supplementary Table 1). Global cognitive status, episodic memory, plaque load, and neurofibrillary tangle density differed significantly between the clinical diagnostic groups (Fig. 4). The pathological characteristics of the clinical diagnostic groups selected for this study were similar to those of the entire cohort, whether assessed by plaque load or tangle density (Fig. 4). All measurements were performed blind to the clinical status of the subjects. Analyses were performed on the mean of triplicate measurements.
Figure 4.
Neuropathological features and cognitive profiles of the Religious Orders Study (R.O.S.) subjects in this study compared with those in the complete cohort. Amyloid-β plaques and neurofibrillary tangles were assessed using unbiased stereological methods. (A and B) Plaque loads were quantified in the inferior temporal gyrus (A) and as the mean of six brain regions (B). (C and D) Neurofibrillary tangle densities (Bielschowsky-positive tangles/mm2) were quantified in the inferior temporal gyrus (C) and as the mean of six brain regions (D). (E and F) Box plots illustrating last Mini-Mental Status Examination (MMSE) (E) and Global Cognition (F) among the clinical groups. Group sizes are shown in parentheses. Green indicates no cognitive impairment (N), orange mild cognitive impairment (MCI), red probable Alzheimer’s disease (AD) and blue non-Alzheimer’s dementia (NAD). Lines within box plots denote median values, upper and lower box boundaries represent the 75th and 25th percentiles, respectively, and short bars flanking boxes represent the 95th and 5th percentiles. *P < 0.01, compared with subjects with no cognitive impairment.
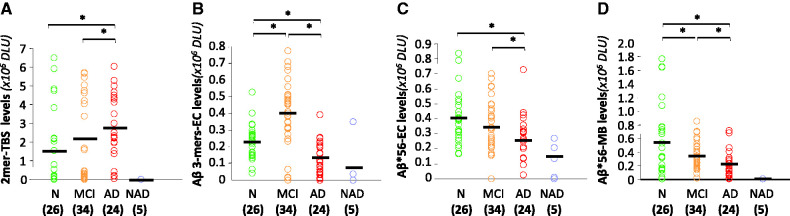
We measured amyloid-β dimers, amyloid-β trimers and Aβ*56 in each protein fraction, and found distinct but overlapping levels of these oligomers in the different clinical groups (Fig. 5). Amyloid-β dimers in Tris-buffered saline extracts were elevated in subjects with probable Alzheimer’s disease compared with subjects with no or mild cognitive impairment, but the latter two groups did not differ from each other (Fig. 5A).
Figure 5.
Association between clinical status and specific amyloid-β oligomers in the brain. (A) Amyloid-β dimer levels in Tris-buffered saline (TBS) extracts overlapped between groups but were significantly higher in subjects with probable Alzheimer’s disease (AD) than in subjects with mild cognitive impairment (MCI) or no cognitive impairment (N). (B) Amyloid-β trimer levels in extracellular (EC)-enriched fractions were highest in the group with mild cognitive impairment. (C and D) Aβ*56 levels in both in extracellular (EC)-enriched and membrane (MB)-enriched fractions were highest in subjects with no cognitive impairment. Group sizes are shown in parentheses. *P < 0.01; Kruskal-Wallis test followed by Mann-Whitney U test with Bonferroni corrections. Green symbols indicate no cognitive impairment, orange symbols mild cognitive impairment, red symbols probable Alzheimer’s disease, and blue symbols non-Alzheimer dementia. DLU = densitometry light units.
Amyloid-β trimers in extracellular-enriched fractions peaked in subjects with mild cognitive impairment and fell in probable Alzheimer’s disease. Amyloid-β trimers in subjects with probable Alzheimer’s disease were lower than those in subjects with either no or mild cognitive impairment (Fig. 5B).
Aβ*56 in extracellular-enriched fractions in subjects without cognitive impairment and mild cognitive impairment did not differ significantly, but was significantly lower in subjects with probable Alzheimer’s disease (Fig. 5C). Aβ*56 in membrane-enriched fractions was significantly lower in subjects with both mild cognitive impairment and probable Alzheimer’s disease compared with subjects without cognitive impairment (Fig. 5D).
Five brain specimens from individuals with non-Alzheimer’s dementia showed very low levels of all three amyloid-β oligomers. This is a small sample size, but nonetheless suggests that amyloid-β oligomers may play more important roles in Alzheimer’s disease than in other forms of dementia (Fig. 5).
Amyloid-β dimers reflect plaque load
Previous studies of amyloid-β oligomers have focused on amyloid-β dimers and on clinically and pathologically diagnosed Alzheimer’s disease. Shankar et al. (2008) found amyloid-β dimers specifically in Alzheimer’s disease but not in control brains devoid of Alzheimer-related pathology. However, we did not exclude subjects with plaques or tangles from the group without cognitive impairment. We found amyloid-β dimers in subjects with and without cognitive impairment. Although the discrepancy between previously published results and ours may stem from differences in the sensitivity of detection methods, we believe it is more likely to be related to the exclusion of Alzheimer-related pathology in the control brains in the former study.
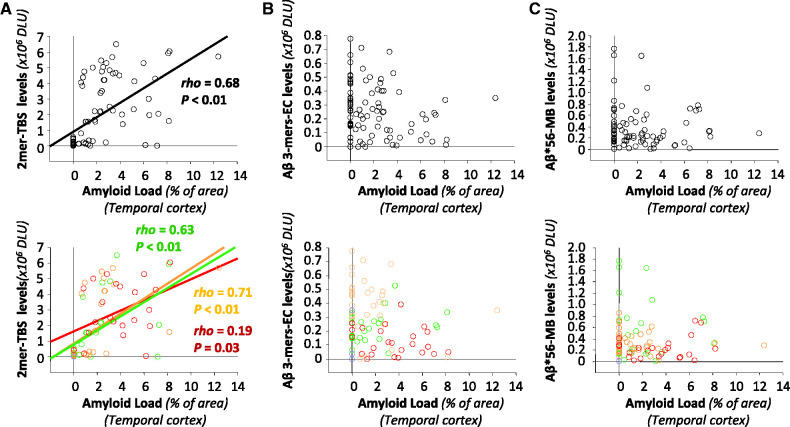
To explore the relationship between plaque load and the three amyloid-β oligomers, we calculated correlation coefficients between these variables. We found a strong correlation between amyloid-β dimers and plaque load in pooled samples (Spearman rho = 0.68, P < 0.01, Fig. 6A), consistent with a recent report (Villemagne et al., 2010), and significant correlations within each clinical group (Fig. 6A). In contrast, neither amyloid-β trimers nor Aβ*56 correlated with plaque load (Fig. 6B and C).
Figure 6.
Relationships between specific amyloid-β oligomers in the brain and amyloid plaque load. Top: The relationships (Spearman rank correlations) between plaque load and amyloid-β dimers, amyloid-β trimers or Aβ*56, and lower panels illustrate these relationships stratified by clinical status. (A) Positive correlations between amyloid load and amyloid-β dimers in Tris-buffered saline (TBS) extracts. (B) No correlations between amyloid load and amyloid-β trimers in extracellular (EC)-enriched fractions. (C) No correlations between amyloid load and Aβ*56 in membrane (MB)-enriched fractions. Green symbols indicate no cognitive impairment, orange symbols mild cognitive impairment, red symbols probable Alzheimer’s disease, and blue symbols non-Alzheimer dementia. DLU = densitometry light units.
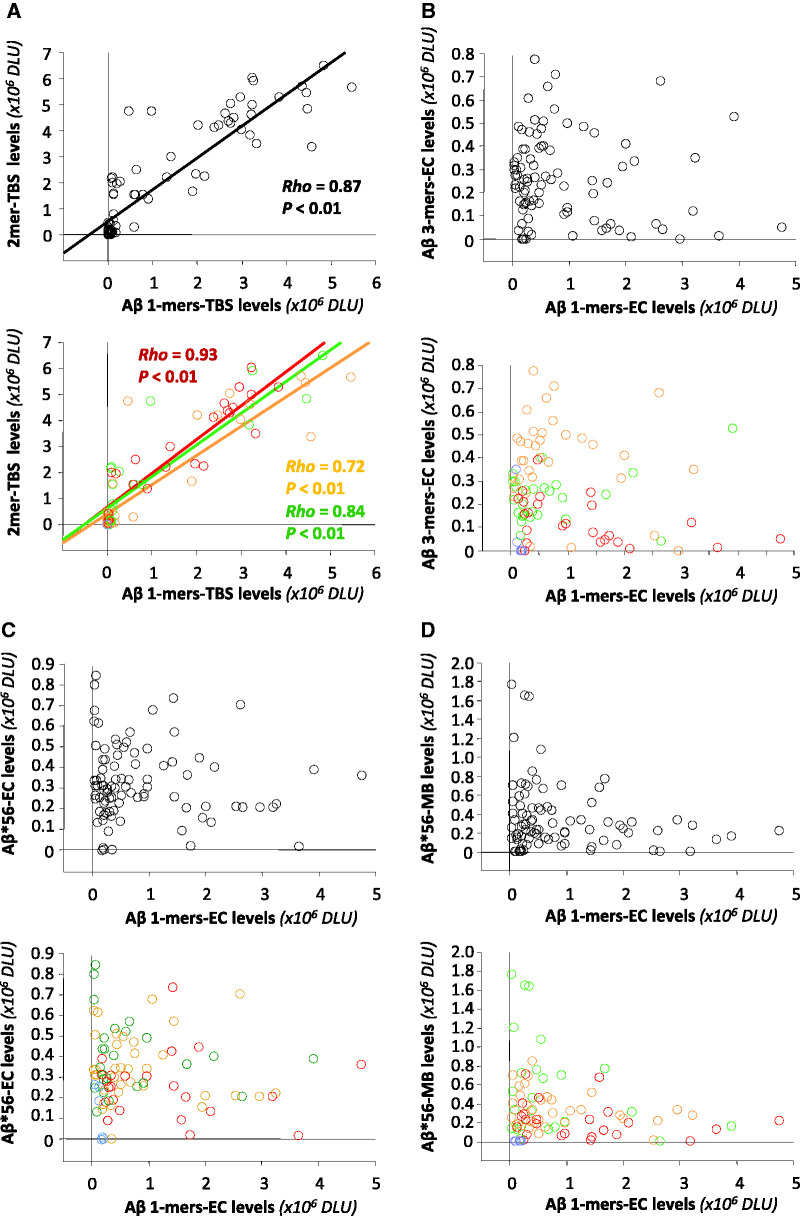
Amyloid-β monomers and dimers correlate
We also examined the relationships between amyloid-β monomers and amyloid-β dimers, amyloid-β trimers or Aβ*56. We found that amyloid-β monomers and amyloid-β dimers in Tris-buffered saline extracts correlated strongly (Spearman rho = 0.87, P < 0.01, Fig. 7A). We also found strong correlations between amyloid-β monomers and plaque load (Spearman rho = 0.74, P < 0.01), in keeping with the correlation between amyloid-β dimers and plaque load described above.
Figure 7.
Relationships between specific amyloid-β oligomers and amyloid-β monomers in the brain. In each panel, the upper graphs show the relationships (Spearman rank correlations) between amyloid-β monomers and amyloid-β dimers, amyloid-β trimers or Aβ*56, and the lower graphs show these relationships stratified by clinical status. (A) Very strong correlations between amyloid-β monomers and amyloid-β dimers in Tris-buffered saline (TBS) extracts. (B–D) No correlations between amyloid-β monomers and amyloid-β trimers in extracellular (EC)-enriched fractions (B), Aβ*56 in extracellular (EC)-enriched fractions (C) or Aβ*56 in membrane (MB)-enriched fractions (D). Green symbols indicate no cognitive impairment, orange symbols mild cognitive impairment, red symbols probable Alzheimer’s disease, and blue symbols non-Alzheimer dementia. DLU = densitometry light units.
In contrast, we found no significant associations between amyloid-β monomers and amyloid-β trimers in extracellular-enriched fractions (Fig. 7B) or Aβ*56 in extracellular-enriched fractions (Fig. 7C) or membrane-enriched fractions (Fig. 7D).
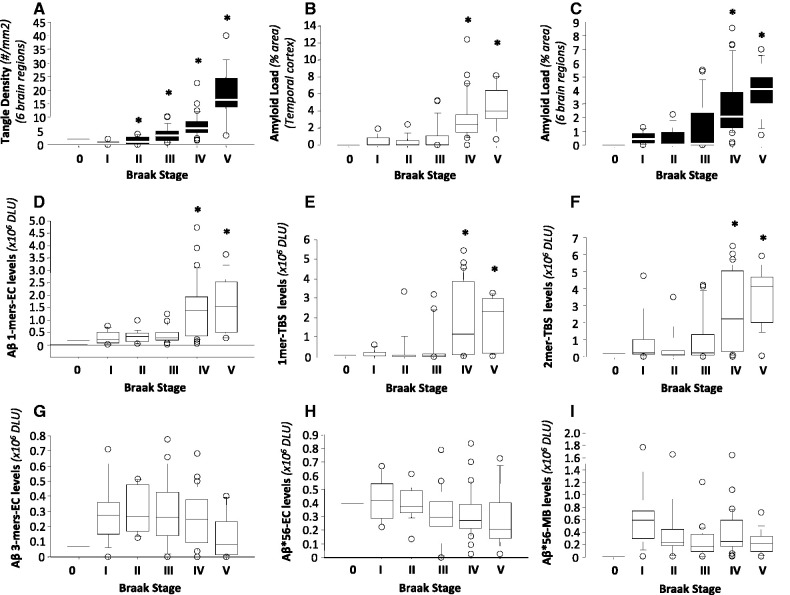
Amyloid-β and neurofibrillary tangle pathology
Finally, we searched for relationships between neurofibrillary tangle pathology and amyloid-β dimers, amyloid-β trimers and Aβ*56. We used Braak staging to catalogue the extent of neurofibrillary tangles (Braak and Braak, 1991). As expected, Braak stages represented tangle density well (Fig. 8A), but plaque load to a lesser extent (Fig. 8B and C). Amyloid-β monomers in both extracellular-enriched fractions and Tris-buffered saline extracts were elevated in Braak stages IV and V (Fig. 8D and E), as were amyloid-β dimers in Tris-buffered saline extracts (Fig. 8F). In contrast, amyloid-β trimers in extracellular-enriched fractions (Fig. 8G) and Aβ*56 in extracellular-enriched and membrane-enriched fractions (Fig. 8H and I) showed no significant changes across Braak stages.
Figure 8.
Relationships between specific amyloid-β oligomers in the brain and Braak stages. (A–C) The relationships between Braak stages and neurofibrillary tangle density (A), amyloid load in temporal cortex (B) and average amyloid load in six brain regions (C). (D–I) The relationships between Braak stages and the levels of various amyloid-β species, including amyloid-β monomers in extracellular (EC)-enriched fractions (D) and Tris-buffered saline (TBS) extracts (E), amyloid-β dimers in Tris-buffered saline extracts (F), amyloid-β trimers in extracellular-enriched fractions (G), and Aβ*56 in extracellular-enriched (H) and membrane (MB)-enriched (I) fractions. *P < 0.01; each of Braak stages II to V is compared with Braak stage I using Kruskal-Wallis test followed by Mann-Whitney U test with Bonferroni corrections. DLU = densitometry light units.
Discussion
Here we report the results of an extensive analysis of amyloid-β dimers, amyloid-β trimers and Aβ*56 in humans. In rodents, there is more evidence supporting the biological activity of amyloid-β dimers and Aβ*56 than amyloid-β trimers (Lesne et al., 2006; Shankar et al., 2008; Reed et al., 2011). In cognitively intact humans, all three amyloid-β oligomers increased with age and the levels of Aβ*56, amyloid-β dimers and amyloid-β trimers became significantly greater than those in children and adolescents in subjects in their 40s, 60s and 70s, respectively. In these subjects Aβ*56, but not amyloid-β dimers or amyloid-β trimers, correlated positively with soluble pathological tau proteins and negatively with the postsynaptic proteins, drebrin and fyn kinase. We speculated that the relationships we found between Aβ*56, tau and synaptic proteins may underlie the subtle cognitive deficits and impaired brain metabolism that have been observed in cognitively normal individuals who are at risk of Alzheimer’s disease (Amieva et al., 2008; Jack et al., 2010; Sperling et al., 2011; Bateman et al., 2012). Based upon the temporal sequence of increases in the levels of specific amyloid-β oligomers, their correlations with tau and synaptic proteins, and our previous knowledge from rodent studies about the biology of these amyloid-β oligomers, we placed Aβ*56 ahead of amyloid-β trimers and amyloid-β dimers in the molecular sequence of events in Alzheimer’s disease.
In mice, Aβ*56 appears to impair long-lasting synaptic plasticity and disrupt cognition independently of neuron loss or plaque deposition (Chapman et al., 1999; Lesne et al., 2006). Consistent with studies in rodents, the associations with Aβ*56 in humans were found in the absence of neuron loss and independently of plaque deposition.
We found significant increases in Aβ*56 in subjects in their 40s, when age-associated memory impairment, including subtle difficulties in declarative memory function, first appears (Youngjohn and Crook, 1993). However, we do not know whether the simultaneity of these events is coincidental or causal. Because Aβ*56 is sufficient to cause impairment on memory tasks in rodents, it is reasonable to speculate that the rising levels of this oligomer in middle and advanced ages might be associated with age-associated memory impairment. Another interesting question is whether Aβ*56 levels predict the development of dementia. The average age of our intact subjects in the Religious Orders Study was 83 years and the annual incidence of Alzheimer’s disease increases from ∼1% to ∼4% between the ages of 80 and 90 years (Rocca et al., 2011); therefore, some would probably have developed Alzheimer’s disease had they lived longer. We can now measure Aβ*56 and amyloid-β trimers in CSF from living subjects (Handoko et al., 2013), and are undertaking studies to define the relationships between these oligomers and both Alzheimer’s biomarkers and risk for Alzheimer’s disease.
The actual existence of Aβ*56 and other amyloid-β oligomers in the brain was eloquently called into question recently (Benilova et al., 2012). An important, legitimate cause for concern about quantifying the levels of specific amyloid-β oligomers is that currently no method exists that does not involve exposing proteins to non-native conditions, which could theoretically lead to the dissociation or self-assembly of amyloid-β molecules that would invalidate estimates of molecular mass. In one study, Aβ*56 in extracellular-enriched brain extracts fractionated by size-exclusion chromatography appeared at the interval that was appropriate for its molecular mass (Lesne et al., 2006), but a potential confound was the presence of detergents in the extraction buffer. The observations that Aβ*56 can be immunocaptured directly from CSF and that Aβ*56 elutes separately from other amyloid-β oligomers when CSF is fractionated by size-exclusion chromatography mitigate but do not fully overcome this objection. A finite possibility remains that our current estimates of molecular mass may not reflect the native state of some or all the Aβ*56 molecules in the brain or CSF. The resolution of this problem will require the development of new techniques, including highly specific capture reagents. That said, it was difficult for us to ascribe the definitive temporal and correlative associations that we found for Aβ*56 to an entirely fictitious molecule ‘in need of clothes’.
In this study we did not measure two other soluble amyloid-β oligomers that have been described in brain tissue, amylospheroids and annular protofibrils, both of which were first detected in synthetic preparations and later found in Alzheimer’s brains. Annular protofibrils exhibit structural and immunospecific properties distinct from amyloid-β fibrils and globular oligomers such as Aβ*56 (Kayed et al., 2009). Immunoblots of synthetic annular protofibrils reveal a band at ∼55 kDa that stains with antisera to annular protofibrils, but is A11-negative and therefore differs from Aβ*56. Amylospheroids are high molecular weight (>100 kDa), A11-negative assemblies concentrated in diffuse and compact plaques (Noguchi et al., 2009). Here, we noted with great interest a novel ∼110 kDa A11-immunoreactive band in 6E10-immunocaptured proteins in CSF (Fig. 1B) and brain (Supplementary Fig. 5), which was obscured by full-length or soluble amyloid precursor proteins in our standard protocol. Additional studies will be needed to determine its biological properties and relevance to Alzheimer’s disease, as well as whether there is a stoichiometric relationship between it and Aβ*56.
In our cross-sectional analysis of Religious Orders Study subjects, the mean levels of amyloid-β dimers, amyloid-β trimers and Aβ*56 were highest in subjects with probable Alzheimer’s disease, mild cognitive impairment and no cognitive impairment, respectively. However, their levels overlapped to such an extent that clinical status could not be predicted on the sole basis of any single amyloid-β species. Interestingly, Aβ*56 and amyloid-β trimers were lowest in subjects with probable Alzheimer’s disease, and exhibited distinctive fluctuating patterns (Larson and Lesne, 2012). Protein aggregates in neurodegenerative diseases are believed to accumulate as the neurological condition worsens. To our knowledge, our results are the first to show that some protein aggregates (Aβ*56 and amyloid-β trimers) decrease as clinical status worsens. We do not know the mechanism by which this occurs. There was a trend toward lower levels of NeuN in subjects with mild cognitive impairment and probable Alzheimer’s disease that might have contributed to lowering Aβ*56, but does not explain the increase in amyloid-β trimers in the group with mild cognitive impairment. The diversion of amyloid-β monomers from a shared substrate pool to form amyloid-β fibrils and amyloid plaques might provide another explanation. Evidence supporting this possibility was observed in two transgenic mouse models in which the levels of Aβ*56 and amyloid-β trimers fell when the rate of plaque deposition increased (Cheng et al., 2007; Lesne et al., 2008). A version of this mechanism has been invoked to explain the inverse relationship between Aβ42 in the CSF and amyloid-β deposition in the brain (reviewed in de Leon et al., 2007).
Our measurements of amyloid-β dimers, amyloid-β trimers and Aβ*56 enabled us to demonstrate a clear distinction between amyloid-β-derived diffusible ligands and Aβ*56. Amyloid-β-derived diffusible ligands are strikingly higher in subjects with Alzheimer’s disease than in age-matched control subjects (Lacor et al., 2004; Georganopoulou et al., 2005; Bao et al., 2012). In contrast, Aβ*56 is higher in control subjects than in subjects with Alzheimer’s disease. The pattern of amyloid-β-derived diffusible ligands matched those of plaque load and amyloid-β dimers in our study.
Our data about amyloid-β oligomers provide biochemical support for an emerging consensus about phase-specific responses to therapies in Alzheimer’s disease (Zahs and Ashe, 2010; Golde et al., 2011), and may guide future studies in humans, animals and cells. First, Aβ*56, but not amyloid-β dimers or amyloid-β trimers, correlated with two pathological soluble tau proteins in intact subjects. Understanding how Aβ*56 is linked to tau abnormalities is an important question that might be elucidated by delineating the molecular pathways that are specifically triggered by Aβ*56 in vitro and in vivo. Second, Aβ*56, but not amyloid-β dimers or amyloid-β trimers, correlated inversely with postsynaptic proteins in intact subjects. Determining how Aβ*56 induces synaptic dysfunction may reveal new drug targets. Finally, Aβ*56 levels peaked in cognitively normal older adults and is the only oligomer we found that appears to participate in the amyloid cascade before the onset of symptoms. A key practical implication of these observations is that anti-amyloid-β therapies administered in the asymptomatic phase of Alzheimer’s disease may be more likely to succeed if they block or reduce Aβ*56.
Funding
Supported by grants from the National Institutes of Health to S.E.L. (K99AG031293), K.H.A. (R01NS33249) and D.A.B. (P30AG10161, R01AG15819), and a gift from B. Grossman to K.H.A.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank C. Wilmot, M. Ramsden, K. Zahs. J. Cleary and M. Handoko for discussions, and L. Kotilinek, L. Kemper, M. Larson, J. Starks, and J. Paulson for technical help. We thank the participants and scientists contributing to the Minneapolis Veterans Administration hospital brain and cerebrospinal bank, the participants in the Religious Orders Study, and the families and scientists contributing to the National Institute for Child Health and Human Development Brain and Tissue Bank for Developmental Disorders at the University of Maryland.
References
- Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, et al. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–8. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- Ashe KH, Zahs KR. Probing the biology of Alzheimer's disease in mice. Neuron. 2010;66:631–45. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Wicklund L, Lacor PN, Klein WL, Nordberg A, Marutle A. Different beta-amyloid oligomer assemblies in Alzheimer brains correlate with age of disease onset and impaired cholinergic activity. Neurobiol Aging. 2012;33:825 e1–13. doi: 10.1016/j.neurobiolaging.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–57. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–76. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012;9:628–45. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–41. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–84. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, et al. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003;60:246–52. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–5. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Chabrier MA, Blurton-Jones M, Agazaryan AA, Nerhus JL, Martinez-Coria H, Laferla FM. Soluble abeta promotes wild-type tau pathology in vivo. J Neurosci. 2012;32:17345–50. doi: 10.1523/JNEUROSCI.0172-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–6. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–28. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Mosconi L, Blennow K, DeSanti S, Zinkowski R, Mehta PD, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:114–45. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- Gandy S, Simon AJ, Steele JW, Lublin AL, Lah JJ, Walker LC, et al. Days to criterion as an indicator of toxicity associated with human Alzheimer amyloid-beta oligomers. Ann Neurol. 2010;68:220–30. doi: 10.1002/ana.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer's disease. Proc Natl Acad Sci USA. 2005;102:2273–6. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron. 2011;69:203–13. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Handoko M, Grant M, Kuskowski M, Zahs KR, Wallin A, Blennow K, et al. Specific amyloid-β oligomers, but not amyloid-β(1-42), correlate with tau in cerebrospinal fluid from cognitively normal older adults. JAMA Neurol. 2013 doi: 10.1001/jamaneurol.2013.48. Advance Access published on March 11, 2013, doi: 10.1001/jamaneurol.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hensley K, Barnes LL, Christov A, Tangney C, Honer WG, Schneider JA, et al. Analysis of postmortem ventricular cerebrospinal fluid from patients with and without dementia indicates association of vitamin E with neuritic plaques and specific measures of cognitive performance. J Alzheimers Dis. 2011;24:767–74. doi: 10.3233/JAD-2011-101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–81. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–97. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T, Shoji M, Younkin LH, Wen-Lang L, Dickson DW, Murakami T, et al. Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2004;24:3801–9. doi: 10.1523/JNEUROSCI.5543-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–9. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kayed R, Pensalfini A, Margol L, Sokolov Y, Sarsoza F, Head E, et al. Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer. J Biol Chem. 2009;284:4230–7. doi: 10.1074/jbc.M808591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, et al. Amyloid β protein dimer-containing human CSF disrupts plasticity: prevention by systemic passive immunization. Journal of Neuroscience. 2008;28:4231–7. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci. 2004;24:10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ME, Lesne SE. Soluble Abeta oligomer production and toxicity. J Neurochem. 2012;120(Suppl 1):125–39. doi: 10.1111/j.1471-4159.2011.07478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lesne S, Kotilinek L, Ashe KH. Plaque-bearing mice with reduced levels of oligomeric amyloid-beta assemblies have intact memory function. Neuroscience. 2008;151:745–9. doi: 10.1016/j.neuroscience.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Donald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, et al. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133:1328–41. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–8. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi A, Matsumura S, Dezawa M, Tada M, Yanazawa M, Ito A, et al. Isolation and characterization of patient-derived, toxic, high mass amyloid beta-protein (Abeta) assembly from Alzheimer disease brains. J Biol Chem. 2009;284:32895–905. doi: 10.1074/jbc.M109.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–23. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, Peskind ER, et al. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66:632–7. doi: 10.1001/archneurol.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham E, Crews L, Ubhi K, Hansen L, Adame A, Cartier A, et al. Progressive accumulation of amyloid-beta oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010;277:3051–67. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to beta-amyloid-induced neurotoxicity. Proc Natl Acad Sci USA. 2002;99:6364–9. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Hofmeister JJ, Jungbauer L, Welzel AT, Yu C, Sherman MA, et al. Cognitive effects of cell-derived and synthetically derived Abeta oligomers. Neurobiol Aging. 2011;32:1784–94. doi: 10.1016/j.neurobiolaging.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci USA. 2004;101:284–9. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, et al. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7:80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–81. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MA, Lesne SE. Detecting abeta*56 oligomers in brain tissues. Methods Mol Biol. 2011;670:45–56. doi: 10.1007/978-1-60761-744-0_4. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA. 2000;97:6037–42. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Perez KA, Pike KE, Kok WM, Rowe CC, White AR, et al. Blood-borne amyloid-beta dimer correlates with clinical markers of Alzheimer's disease. J Neurosci. 2010;30:6315–22. doi: 10.1523/JNEUROSCI.5180-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin BL, Pruchnicki A, Dickson DW, Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986;232:648–50. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Youngjohn JR, Crook TH., III Learning, forgetting, and retrieval of everyday material across the adult life span. J Clin Exp Neuropsychol. 1993;15:447–60. doi: 10.1080/01688639308402570. [DOI] [PubMed] [Google Scholar]
- Zahs KR, Ashe KH. ‘Too much good news'—are Alzheimer mouse models trying to tell us how to prevent, not cure, Alzheimer's disease? Trends Neurosci. 2010;33:381–9. doi: 10.1016/j.tins.2010.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.