Abstract
Migrating partial seizures of infancy, also known as epilepsy of infancy with migrating focal seizures, is a rare early infantile epileptic encephalopathy with poor prognosis, presenting with focal seizures in the first year of life. A national surveillance study was undertaken in conjunction with the British Paediatric Neurology Surveillance Unit to further define the clinical, pathological and molecular genetic features of this disorder. Fourteen children with migrating partial seizures of infancy were reported during the 2 year study period (estimated prevalence 0.11 per 100 000 children). The study has revealed that migrating partial seizures of infancy is associated with an expanded spectrum of clinical features (including severe gut dysmotility and a movement disorder) and electrographic features including hypsarrhythmia (associated with infantile spasms) and burst suppression. We also report novel brain imaging findings including delayed myelination with white matter hyperintensity on brain magnetic resonance imaging in one-third of the cohort, and decreased N-acetyl aspartate on magnetic resonance spectroscopy. Putaminal atrophy (on both magnetic resonance imaging and at post-mortem) was evident in one patient. Additional neuropathological findings included bilateral hippocampal gliosis and neuronal loss in two patients who had post-mortem examinations. Within this cohort, we identified two patients with mutations in the newly discovered KCNT1 gene. Comparative genomic hybridization array, SCN1A testing and genetic testing for other currently known early infantile epileptic encephalopathy genes (including PLCB1 and SLC25A22) was non-informative for the rest of the cohort.
Keywords: early infantile epileptic encephalopathy, migrating partial seizures in infancy, epilepsy of infancy with migrating focal seizures, malignant migrating partial epilepsy of infancy, infantile seizures
Introduction
Migrating partial seizures of infancy (MPSI), also more recently termed ‘epilepsy of infancy with migrating focal seizures’, is a devastating infantile epileptic encephalopathy first described by Coppola et al. (1995). Key features include onset of focal seizures within the first 6 months of life associated with autonomic features, post-natally acquired microcephaly and developmental stagnation or delay. Interictal EEG shows multi-focal spikes and slowing and ictal EEG reveals shifting (migrating) foci of ictal onset. MPSI appears to be rare with 100 cases reported in the literature (Coppola et al., 1995, 2005, 2007; Okuda et al., 2000; Wilmshurst et al., 2000; Veneselli et al., 2001; Gross-Tsur et al., 2004; Marsh et al., 2005; Hmaimess et al., 2006; Hahn et al., 2007; Caraballo et al., 2008; Jocic-Jakubi and Lagae, 2008; Cilio et al., 2009; Bedoyan et al., 2010; Nabatame et al., 2010; Carranza Rojo et al., 2011; Djuric et al., 2011; Freilich et al., 2011; Gilhuis et al., 2011; Irahara et al., 2011; Lee et al., 2012; Poduri et al., 2011, 2012; Sharma et al., 2011; Vendrame et al., 2011; Barcia et al., 2012; Chien et al., 2012; Fasulo et al., 2012; Merdariu et al., 2013). The details of these cases are summarized in Table 1. The underlying disease mechanisms are poorly understood and a limited number of genetic aetiologies have been described.
Table 1.
Clinical features of 100 previously reported children with MPSI
| Feature | Incidence | Not stated |
|---|---|---|
| Males: females | 46% male: 54% female | 17 |
| Age < 6 months at onset | 97% cases | |
| Development normal before seizure onset (babies >28 days old at seizure onset) | 100% cases | 36 |
| Siblings affected | One report of two siblings* | |
| Family history of epilepsy | 8 cases | |
| Deaths | 18 cases | |
| SUDEP | 3 cases | |
| Seizure semiology: | ||
| Focal motor seizures at onset | 64% | |
| Focal seizures affecting alternating sides of body | 59% | |
| Secondary generalization | 17% | |
| Epileptic spasms | 7% | |
| Autonomic features | 43% | |
| Generalized tonic-clonic seizures | 8% | |
| Reduction in seizures with time | 23% | |
| Developed extrapyramidal signs | 3% | |
| Developed pyramidal signs | 20% | 21 |
| Hypotonia | 85% | 21 |
| Decline in OFC | 85% | 60 |
| leading to microcephaly | 75% | 60 |
| Initial interictal EEG normal | 29% | |
| Initial MRI/CT normal | 68% | 7 |
| Developed cerebral atrophy on MRI/CT | 46% | 19, only one scan 9, not stated |
| Developed mesial temporal sclerosis on MRI | 4 cases | |
| Muscle/liver biopsy for mitochondrial disorders | 18 cases | |
| CSF neurotransmitters tested (no.) | 6 cases | |
| SCN1A gene tested | 22 cases (3 have mutations) | |
| Microarray | 16 cases (2 abnormal) |
OFC = occipitofrontal circumference; SUDEP = sudden unexpected death in epilepsy.
*Subsequent pregnancy terminated at 32/40 gestation for progressive microcephaly in one additional patient.
A national surveillance study was undertaken in conjunction with the British Paediatric Neurology Surveillance Unit (BPNSU, www.bpnsu.co.uk) to investigate the prevalence of MPSI. We present the clinical, electrographic, radiological, pathological and genetic features of the 14 cases identified.
Materials and methods
A national surveillance study was performed in conjunction with the BPNSU over a 27 month period (February 2008 to May 2010). Study inclusion criteria were as follows: onset of seizure disorder before 9 months of age; focal motor seizures at onset; multifocal seizures intractable to conventional anti-epileptic drugs; EEG criteria: initial EEG may be normal but subsequent development of characteristic changes, including interictal multifocal spikes and ictal independent, unilateral and migrating involvement of different cortical areas with clinical-EEG correlation; delayed developmental progress or signs of psychomotor regression associated with seizure onset; and not meeting electroclinical criteria for any other International League against Epilepsy (ILAE)-classified syndrome. These criteria were adapted from the original definition of MPSI proposed by Coppola et al. (1995) with extension of the upper age limit in case older infants were identified who otherwise fulfilled the criteria. Members of the British Paediatric Neurology Association were contacted by e-mail once per month over 2 years to identify both new and historical cases of MPSI. Questionnaires were sent to responding clinicians (Supplementary material) and anonymized phenotypic information was collated on clinical presentation, disease course, EEG, neuroimaging and the results of neurometabolic and diagnostic genetic investigations undertaken within the clinical setting.
In addition, a number of early infantile epileptic encephalopathy genes were sequenced on a research basis in a number of patients in the cohort. The research genetic study was approved by the local research ethics committee and specific consent was given by individual parents for genetic studies to be undertaken on a research basis. The following genetic research investigations were undertaken.
Direct sequencing of KCNT1
Mutational analysis of KCNT1 was undertaken by direct Sanger sequencing. The genomic DNA sequence of the gene was taken from Ensembl (http://www.ensembl.org/index.html) and primer pairs for all exons (according to the NCBI reference sequence) were designed using primer3 software (http://fokker.wi.mit.edu/primer3/input.html). The exons were amplified by PCR using BioMix Red™ (Bioline Ltd). For the amplification of GC rich sequence of some exons, GC rich solution of FastStart Taq DNA Polymerase (Roche Diagnostic) was added to the PCR. Amplification conditions were an initial denaturation of 95°C for 5 min, followed by 35 cycles of 30 s denaturation at 95°C, 1 min annealing at 55–62°C (depending on fragment) and 1 min extension at 72°C with a final extension at 72°C for 5 min. PCR products were purified using MicroCLEAN (Web Scientific) and were directly sequenced by the BigDye® Terminator Cycle Sequencing System (Applied Biosystems Inc., Life Technologies Corporation). Sequencing reactions were run on an ABI PRISM 3730 DNA Analyzer (Applied Biosystems Inc.) and analysed with Chromas software (http://www.technelysium.com.au/chromas.html).
Multiple gene panel screening of genes associated with early infantile epileptic encephalopathy and severe neurodevelopmental delay
For a number of patients with MPSI, we undertook multiple gene testing using a gene panel. Twenty-eight known genes associated with early infantile epileptic encephalopathy and/or severe neurodevelopmental delay were analysed. Sequence analysis with custom Haloplex sequence capture (Agilent) and Illumina sequencing using a MiSeq platform and multiplexing (16 cases per lane) was undertaken. Copy number variant analysis was performed using a custom Roche Nimblegen oligonucleotide 135K aCGH (Roche Diagnostic Ltd.). Sequence data were analysed using NextGENe software (Softgenetics) and array data using CGH Fusion (InfoQuant). For any variants identified, we planned to use conventional sequencing to confirm point mutations and multiplex ligation-dependent probe amplification/quantitative PCR for confirmation of copy number abnormalities, in tandem with establishing mutation status in parents and/or unaffected siblings.
Whole exome sequencing
Whole exome sequencing was undertaken and analysed in four patients of our cohort. DNA was sheared to fragments of 100–400 bp for Illumina paired-end DNA library preparation, then enriched for target sequences according to the manufacturer’s recommendations. Enriched libraries were sequenced using the HiSeq platform (Illumina) according to standardized protocols. Realignment of binary sequence alignments and recalibration of base quality scores was undertaken using standard kits. Binary sequence alignments were merged to sample level and duplicates marked. Variants including single nucleotide polymoprhisms and insertions/deletions were called on each sample individually. A number of quality filters were applied to each data set separately. Calls were merged, then annotated with allele frequencies from 1000 Genomes and dbSNP132 unique rs identification numbers (if available). SIFT and PolyPhen data (on predicted functional impact of missense mutations) was also included. Filtered data were analysed to see if mutations in known genes were identified on whole exome sequencing.
Results
The web-based surveillance questionnaire asked clinicians whether or not they had seen a case of MPSI in the last month, and if so, how many cases they had seen. One hundred and ninety-six of the 285 members of the BPNA responded over the 2 year period, representing an overall response rate of 68.4% of members. Seventeen patients were initially referred suspected as having a diagnosis of MPSI, of which 14 (five male) fulfilled the criteria for an electroclinical diagnosis of MPSI; all were <6 months at seizure onset
Family history
In one patient there was a family history of foetal loss (a single stillbirth). There were no similarly affected siblings in any patients in this cohort. Three probands had a strong family history of epilepsy (adult-onset generalized or focal seizures) but none in a first degree relative. There was no history of parental consanguinity. Thirteen patients were Caucasian and one was African-Caribbean.
Early clinical history and presentation
All but one were born at term (one at 35 weeks) and the mean birth weight was 3.0 kg (range: 2.18–3.69 kg). Three children had feeding difficulties within the perinatal period; one of these had neonatal seizures. The median age at seizure-onset was 7 weeks (range: 4 days to 5 months).
Seizures
Seizure semiology is summarized in Table 2. The majority of patients had focal motor seizures at onset that were clearly migratory in 8 of 14 cases; adversive seizures with head turning and involvement of the eyes with eye flickering or rolling were also common. Twelve (88%) demonstrated autonomic features at presentation, most commonly facial flushing, followed by drooling or pupillary changes (either dilatation or fluctuation in size during the seizure). Five (36%) did not have focal motor seizures at presentation but presented with generalized stiffening or jerking; all showed autonomic features and all subsequently developed focal motor seizures by 6 months of age. Autonomic features did not arise later in the disease course if they were not a feature on initial clinical presentation.
Table 2.
Semiology of presenting and subsequent seizures in the 14 patients with MPSI
| Number of patients with this feature at presentation | Number of patients who developed this feature later in the disease course | |
|---|---|---|
| Focal motor seizures | 10 | 14 |
| Involving face | 4 | 3 |
| Involving eyes | 7 | 4 |
| Involving limbs | 8 | 12 |
| With head turning | 6 | 4 |
| Clinically migrating | 0 | 8 |
| Generalized seizures | 5 | 9 |
| Tonic | 3 | 5 |
| Clonic | 2 | 2 |
| Tonic-clonic | 0 | 5 |
| Epileptic spasms | 0 | 2 |
| Myoclonic | 0 | 2 |
| Autonomic features | 12 | |
| Flushing | 9 | |
| Drooling | 3 | |
| Pupillary changes | 3 | |
| Pallor | 2 | |
| Rash | 2 | |
| Apnoea | 2 | |
| Epiphora | 1 | |
| Convulsive status epilepticus | 0 | 4 |
| Non-convulsive status epilepticus | 0 | 4 |
All patients subsequently developed multiple seizure types (Table 2). Six patients experienced multifocal or clinically migrating seizures and two patients developed infantile spasms in association with hypsarrhythmia at 2 and 8 months of age, respectively (see below).
Seizures were frequent in all 14 patients; ranging from three to ∼100 each day. Seizures occurred in clusters and were most frequent on waking and falling asleep. Age at peak seizure frequency varied from 3 weeks to 3 years. In the seven patients where seizure frequency declined over time, the median age of peak seizure frequency was 4 months (range 3 weeks to 1 year).
Clinical features
Coarse facial features were reported in five patients. A post-natal decline in head growth was seen in all cases with resultant microcephaly in seven cases. Only three infants had abnormal neurological findings at presentation (central hypotonia in one patient, central hypotonia with peripheral limb spasticity in one patient and peripheral limb spasticity in one further patient). By 12 months from presentation, one further patient had developed pyramidal tract signs with spasticity and hyperreflexia and seven were noted to have axial hypotonia.
Three patients (Patients 2, 3 and 7) developed a severe gut motility disorder with intractable vomiting and weight loss between 2 and 3 years of age. Episodes of pain, distress and stiffening were associated with explosive diarrhoea in the most severe case (Patient 3). Two patients underwent fundoplication, but only had a minimal improvement in symptoms.
A movement disorder developed in Patients 2, 6, 7 and 11. Patient 7 showed a cervical dystonia from 19 months of age resulting in a scoliosis and required treatment with l-DOPA (with no response), baclofen, trihexyphenidyl and botulinum toxin. This patient had an abnormal MRI brain scan with bilateral basal ganglia changes (see ‘Neuroimaging’ section). Patient 2 developed generalized choreoathetosis from 30 months (normal brain MRI) which responded to treatment with haloperidol. Patient 6 had central hypotonia and peripheral hypertonia at presentation in the neonatal period that subsequently evolved to peripheral dystonia with early development of severe scoliosis. Patient 11 exhibited a period of limb dyskinesias between 5 and 7 months, which resolved spontaneously. Other causes of acute dyskinesia including iatrogenic causes were excluded in all of these patients.
Electrographic findings
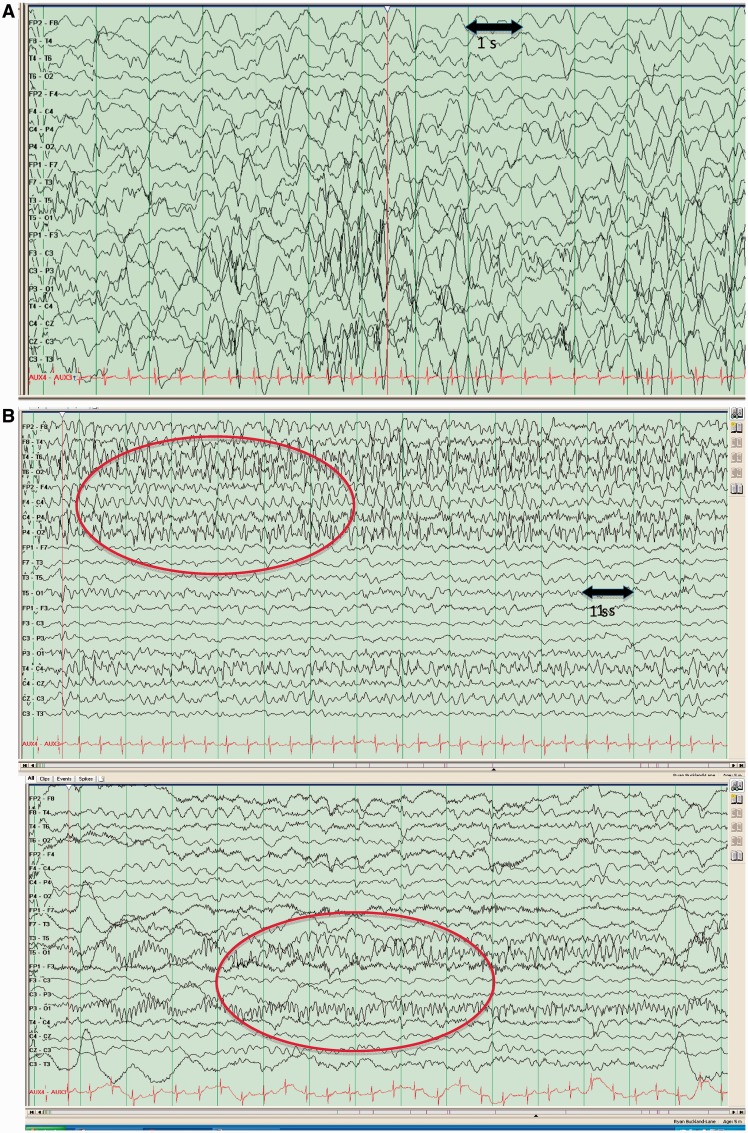
All patients underwent multiple EEG investigations with a minimum of two per patient (Supplementary material). The initial EEG was normal in three. In most patients, the inter-ictal EEG showed diffuse background slowing and multifocal epileptogenic foci within 6 months of presentation. Evolving or modified hypsarrhythmia was reported in Patient 1 at 2 and 6 months of age but this patient never experienced epileptic spasms. Two children demonstrated hypsarrhythmia associated with infantile spasms (Patients 5 and 9). Spasm onset in Patient 5 was at 3 months and persisted for 6 months until other seizure types emerged. A diagnosis of MPSI was made following the onset of clinical migratory seizures at 2 months of age and an EEG, which showed multiple independent ictal foci during the same EEG (in keeping with our electroclinical inclusion criteria). Epileptic spasms occurred at 2 months of age in Patient 9 associated with a modified (unilateral) hypsarrhythmia and resolved after 2 weeks. This patient subsequently developed daily focal motor seizures in addition to myoclonic and tonic seizures from 3 months of age. Repeat EEG at 5 months demonstrated migrating ictal foci (Fig. 1) and MPSI was diagnosed.
Figure 1.
(A) EEG recording from Patient 9 illustrating modified hypsarrhythmia at 2 months of age. (B) EEG recording from Patient 9 illustrating migrating epileptic foci within the same recording at 5 months of age.
Periods of relative suppression were noted in the EEGs of two patients and more marked suppression was seen in one (Table 3). This appearance evolved with time in all three patients and was not seen in later EEGs. Two of these patients had modified hypsarrhythmia, as described above.
Table 3.
Clinical, imaging and electrographic features of the 14 patients
| Patient | Age at seizure onset (weeks) | Age at diagnosis with MPSI (weeks) | Age at peak seizure frequency (months) | MRI findings | EEG findings other than characteristic changes (age at EEG finding) | Additional clinical features | Abnormal neurological examination at presentation | Abnormal neurological examination 12 months from presentation | Age at death | Current age |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 16 | Normal | Subtle burst suppression (6 weeks) | – | No | Axial hypotonia | 19 months | – |
| Normal MRS | Hypsarrhythmia (6 months) | |||||||||
| 2 | 10 | 52 | 12 | Atrophy | – | Gastrointestinal dysmotility | No | Axial hypotonia | 41 months | – |
| HM with WMI | Movement disorder | |||||||||
| MRS abnormal, low NAA | ||||||||||
| 3 | 2 | 16 | 36 | Atrophy | – | Gastrointestinal dysmotility | No | Axial hypotonia | 64 months | – |
| HM with WMI | ||||||||||
| MRS low NAA | ||||||||||
| 4 | 0.6 | 28 | 3 | Normal | Post-ictal slowing (4 weeks) | – | No | Axial hypotonia | – | 6 years |
| 5 | 8 | 8 | 16 | Atrophy | Hypsarrhythmia (3 months) | Infantile spasms | No | Peripheral hypertonia and spasticity | 8 years 10 months | – |
| Post-ictal slowing (2 months) | ||||||||||
| Brief periods of suppression (3 months) | ||||||||||
| 6 | 4 | 28 | 24 | Atrophy | Electrodecrementation (7.5 months) | Movement disorder | Yes, axial hypotonia, peripheral spasticity | Peripheral hypertonia and spasticity | – | 4 years |
| HM with WMI | ||||||||||
| 7 | 7 | 44 | 52 | Abnormal signal putamen and caudate | – | Gastrointestinal dysmotility | Yes, axial hypotonia | Axial hypotonia | 54 months | – |
| Atrophy | Movement disorder | |||||||||
| 8 | 7 | 12 | Not known | Atrophy | – | – | No | No | – | 7 years |
| 9 | 2 | 20 | 32 | Atrophy | Hypsarrhythmia (8 weeks) | Infantile spasms | No | Axial hypotonia | 25 months | – |
| HM with WMI | Electrodecrementation (8 weeks) | |||||||||
| MRS low NAA | ||||||||||
| 10 | 10 | 16 | 8 | HM | Electrodecrementation (5 months) | – | Yes, peripheral spasticity | No | – | 4 years |
| 11 | 20 | 28 | 24 | Normal | – | Movement disorder | No | No | – | 5 years |
| 12 | 7 | 36 | 30 | Normal | – | – | No | N/A | 9 months | – |
| 13 | 6 | 20 | 20 | Normal | – | – | No | Axial hypotonia | – | 2 years |
| 14 | 2 | 20 | 8 | Normal | Burst suppression (5 weeks) | – | No | N/A | 2 months | – |
HM = hypomyelination; WMI = white matter hyperintensity; GI = gastrointestinal; N/A = not applicable; MRS = magnetic resonance spectroscopy; NAA = N-acetyl aspartate.
Ictal EEG was recorded in all patients, with multifocal paroxysmal activity a uniform finding. All patients demonstrated either shifting areas of ictal onset (migrating spikes) between hemispheres within the same EEG recording or overlapping seizures with different areas of ictal onset in differing hemispheres. Additional ictal findings included electrodecrementation associated with seizures in three patients (epileptic spasms in one, focal motor seizures in the other two) and post-ictal slowing in two patients (Supplementary material).
Neuroimaging
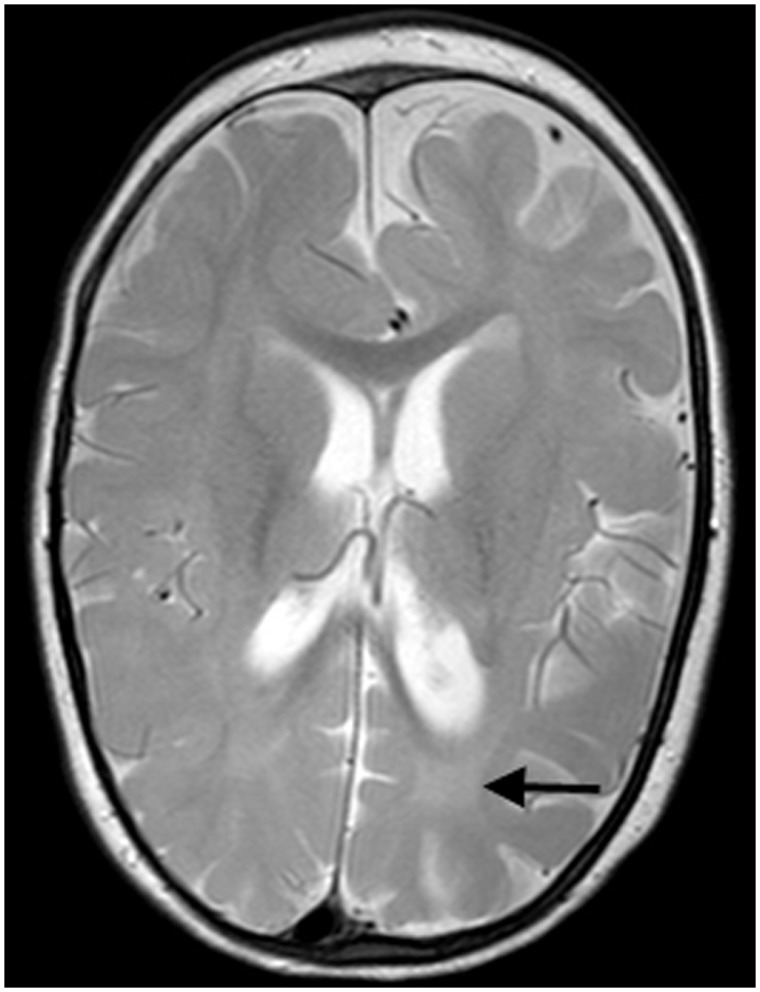
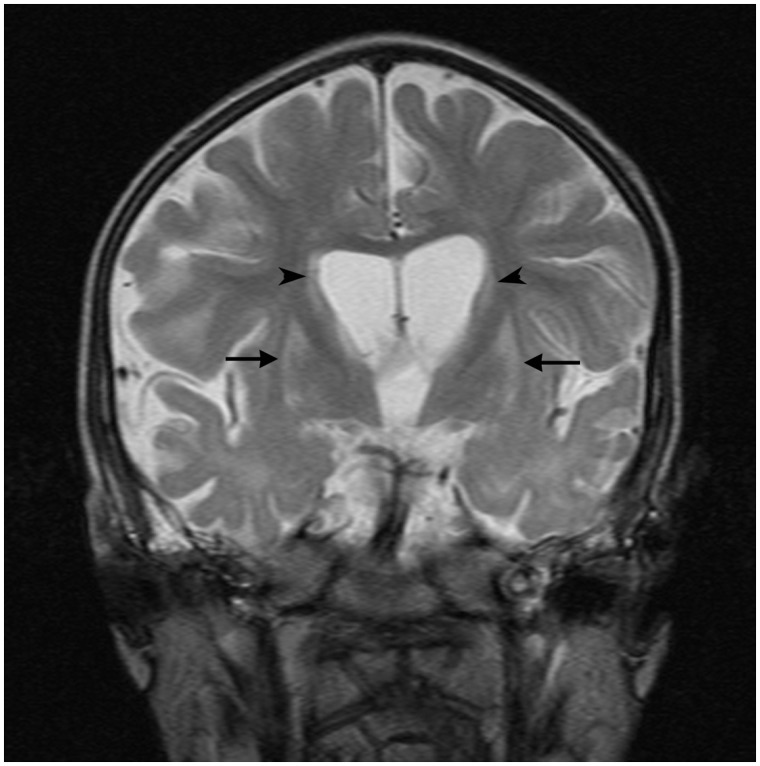
Eight (57%) had an abnormal brain MRI, although the initial scan was normal in four (28%) (Table 3). Three patterns were identified. Diffuse cerebral atrophy (with an increase in extra axial fluid) was seen in seven (50%) children between the ages of 4 months and 3.5 years. Five demonstrated delayed myelination with T2 hyperintensity of the deep white matter in four. Figure 2 illustrates the particularly striking deep white matter hyperintensity seen in Patient 9. In Patient 7, bilateral symmetrical signal abnormality was seen in the putamen and caudate nuclei in addition to cerebral atrophy (Fig. 3). Extensive metabolic investigations and a subsequent post-mortem did not determine an underlying aetiology in this patient.
Figure 2.
Axial T2-weighted MRI of Patient 10 at 16 months of age demonstrating relative hyperintensity of the deep white matter (arrow) suggesting delayed myelination.
Figure 3.
T2-weighted MRI in Patient 7 at 39 months of age reveals bilateral symmetrical signal abnormality of putamen (arrows) and caudate (arrow heads) in addition to cerebral atrophy.
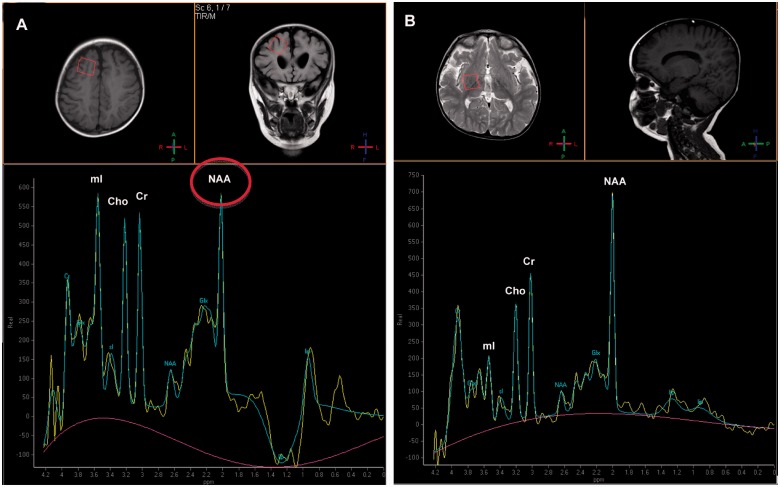
Single voxel magnetic resonance spectroscopy was abnormal in three of the four patients in whom this investigation was undertaken (Patients 2, 3 and 9). Magnetic resonance spectroscopy in Patient 2 showed a high myoinositol peak bilaterally in the white matter, with the N-acetyl aspartate peak similar in height to the choline and creatine peaks (Fig. 4). Similarly, in Patients 3 and 9 the N-acetyl aspartate peaks were reduced in comparison with the choline and creatine peaks. These would be the expected findings of proton spectroscopy in much younger children (early infancy) and are thus consistent with immaturity of neuronal development.
Figure 4.
Single voxel magnetic resonance spectroscopy. (A) With short echo time (35 ms) of the right frontal white matter from Patient 2 at 24 months of age demonstrating a relatively high myoinositol (mI) peak and a relatively low N-acetyl aspartate peak (NAA, circled) in relation to the creatine (Cr) and choline (Cho) peaks signifying immaturity. Magnetic resonance spectroscopy obtained with similar acquisition parameters of a normal 24-month-old child is shown (B) for comparison.
Six patients showed no abnormality on brain MRI. Five of these patients had only one scan between 2 weeks and 5 months of age; the remaining patient had the first scan at 4 weeks and the second at 5 months of age.
Other investigations
All patients underwent extensive metabolic investigations (Supplementary material) and some had genetic testing (Table 4). A number of patients in our cohort had genetic investigations including diagnostic screening of SCN1A, MECP2, CDKL5, ARX and SLC25A22 (Table 4); all were negative.
Table 4.
Genetic investigations undertaken in 14 patients with MPSI
| Patient | Diagnostic sequencing |
Multiple gene panel | Whole exome sequencing | Research sequencing | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ARX | CDKL5 | SCN1A | MECP2 | SLC25A22 | PLCB1 | SLC2A1 | STXBP1 | CGH | Multiple gene panel including exon-level CGH and next generation sequencing* | Analysis for variants in known genes* | KCNT1 | |
| 1 | – | – | N | N | – | – | N | – | – | – | Mutation exon 10 KCNT1 | c.G811T: p.V271F |
| 2 | N | N | N | N | – | – | – | – | – | – | – | – |
| 3 | – | N | N | N | – | – | – | – | – | – | Mutation exon 24 KCNT1 | c.G2800A, p.A934T |
| 4 | – | – | N | N | – | – | – | – | – | – | – | – |
| 5 | – | – | – | – | – | – | – | – | – | – | – | – |
| 6 | – | N | – | – | – | – | – | N | N | N | – | – |
| 7 | N | N | N | N | N | N | N | – | N | N | N | – |
| 8 | N | – | N | – | – | – | – | – | – | N | – | – |
| 9 | – | – | N | – | – | – | – | – | N | N | N | – |
| 10 | – | – | N | – | – | – | – | – | N | – | – | – |
| 11 | – | – | N | – | – | – | – | – | – | – | – | – |
| 12 | – | – | N | – | – | – | – | – | – | N | – | – |
| 13 | – | – | – | – | – | – | – | – | – | N | – | – |
| 14 | – | – | – | – | – | – | – | – | – | – | – | – |
– = investigation not undertaken; N = negative (no mutation identified); CGH = comparative genomic hybridization studies.
*CDKL5, MECP2, ARX, ATRX, SLC9A6, SLC16A2, ADSL, CNTNAP2, NRXN1, PNKP, KIAA1279, UBE3A, EHMT1, FOXG1, MEF2C, SIP1, ZEB2, TCF4, STXBP1, SLC25A22, PCDH19, SCN1A, PLCB1, SPTAN1, KCNQ2, ARHGEF9, SCN2A, MAGI2.
Variants in KCNT1 were identified in two patients. These were missense mutations (c.G2800A, p.A934T; c.G811T, p.V271F). Amino acid residues affected by these variants were highly conserved throughout species. PolyPhen scores were 0.994 (probably damaging) and 0.733 (possibly damaging), respectively. Where DNA was available and of sufficient quality, research testing using the multiple gene panel and whole exome sequencing was undertaken for a number of patients with MPSI. No mutations in currently known early infantile epileptic encephalopathy genes were identified using either multiple gene panel or whole exome platform (Table 4).
Neuropathology
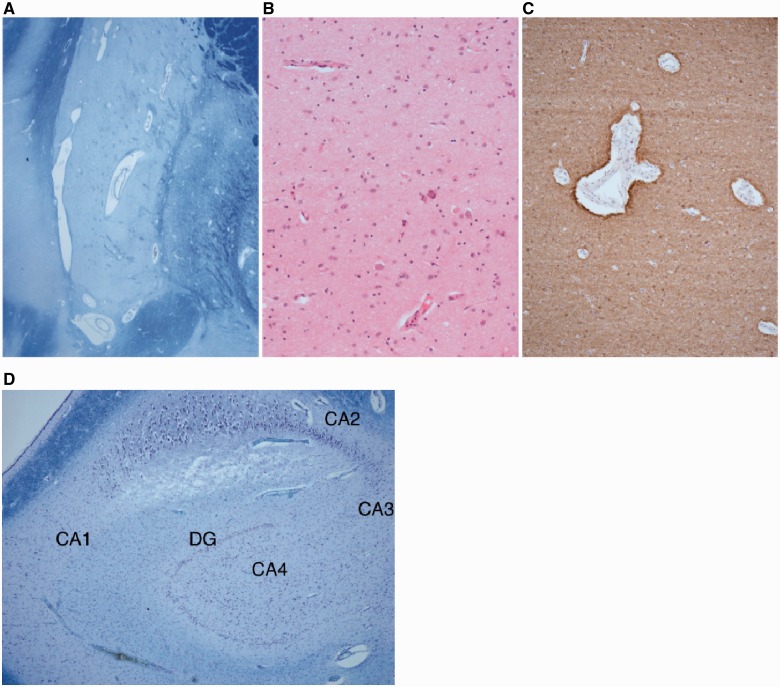
Two of the 14 patients (Patients 7 and 12) underwent post-mortem examinations. In Patient 7 the brain was small, weighing 781 g at almost 4 years of age. Externally, the temporal lobe and the orbito-frontal surface were particularly atrophic. The cerebral cortex was thin and the sulci widened. The caudate nuclei, putamina, globi pallidi, hippocampi and thalami were small. The most striking feature on microscopic examination was atrophy of the putamina with severe neuronal loss and gliosis (Fig. 5A). A similar, but less pronounced appearance was seen in the caudate nucleus. The thalamus, cerebral cortex and globus pallidus were relatively well preserved. The hippocampi showed neuronal loss in the CA4, CA3 and CA1 regions (Fig. 5B). The dentate nucleus was thin and depleted of neurons. A post-mortem muscle sample revealed severe type 2 atrophy and an excess of lipid but no specific features of a mitochondrial disorder.
Figure 5.
(A–C) The images show the abnormal putamen in Patient 7 demonstrated by (A) large perivascular spaces revealing neuronal loss in the putamen (Luxolfastblue/Nissl), (B) depletion of neurons and gliosis with focal calcification (haematoxylin and eosin) and (C) astrocytosis in putamen (GFAP). (D) CA1, CA3, CA4 sectors and the dentate gyrus (DG) of hippocampus show cell depletion, consistent with a form of hippocampal sclerosis in Patient 7.
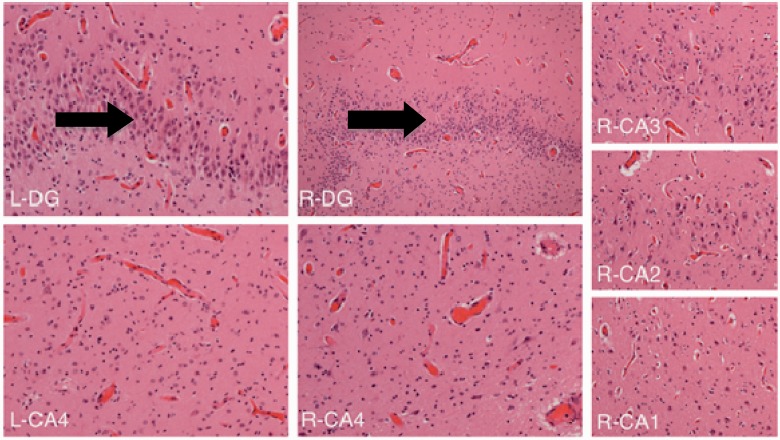
In Patient 12, macroscopic examination was made at another centre but was reported to demonstrate antero-posterior foreshortening with a reduction in the white matter volume but no evidence of a malformation. The thalami were unusually pale on both sides. The remainder of the deep grey matter was normal. There was grey discolouration of the basis pontis and medullary olives. Microscopy revealed subpial (Chaslin’s) gliosis but no cortical dysplasia. Both hippocampi showed neuronal loss and gliosis in the CA4 region on both sides and possibly extending into CA3, but CA2 and CA1 were well preserved. There were small foci of granule cell dispersion with areas of the dentate gyrus that were bilaminate (Fig. 6). Muscle histology revealed type II fibre atrophy with normal respiratory chain enzyme analysis.
Figure 6.
Hippocampal sections from Patient 12 revealing a pattern of hippocampal sclerosis. Right and left CA4 sectors show depletion of neurons and gliosis (i.e. end folial sclerosis). R-DG (right dentate gyrus) and L-DG (left dentate gyrus) show an extra layer of cells (arrow) due to granule cell dispersion. Right CA3/CA2/CA1 are relatively preserved.
Treatment
All children were treated with multiple anti-convulsants. No sustained remission was seen but partial responses (partial seizure reduction or brief and unsustained periods of seizure freedom) were seen with oxcarbazepine, phenytoin, bromides, topiramate, levetiracetam, vigabatrin, stiripentol and lacosamide. Bromide salts were used in three patients but were not particularly effective and were poorly tolerated in one case with excessive sedation. Nine patients were treated with corticosteroids. One patient had a good response to adrenocorticotrophic hormone injections whereas another responded to a course of oral prednisolone in combination with the ketogenic diet. The ketogenic diet was used in nine patients. It was partially effective in Patients 2 and 6 in combination with topiramate and prednisolone, respectively. Patient 7 was seizure-free from 1 year to 3 years 10 months of age. However, following discontinuation of the diet due to poor weight gain, seizures recurred and remained intractable. This patient had a normal fasting CSF: plasma glucose ratio and testing for a SLC2A1 mutation was negative.
Outcome
Eight (57%) children died at between 2 months and 8 years 10 months of age. There was no obvious relationship between the age at onset and age at death; those who died had a seizure onset at <10 weeks of age but some patients are still alive with seizure onset within 2 weeks of birth. Of the remaining six patients, the oldest surviving child at the time of writing is 7 years of age. The epilepsy seemed to ‘burn out’ in seven patients although none have become seizure free. Nine (64%) children lost previously acquired skills with the onset of seizures and the remaining five never made significant developmental progress. Patient 2 regained a ‘social smile’ at 31 months of age, corresponding with a period of reasonable seizure control. The best developmental stage achieved was in Patient 2 who could sit with support and babble; these skills were lost at ∼12 months following an exacerbation of seizures and did not return. The highest developmental level maintained beyond 1 year of age in all patients was partial head control, rolling and visual fixation.
Discussion
We describe the clinical, electrographic, radiological, neuropathological and genetic findings in a cohort of patients with MPSI. This is the first national case series of children with this condition. We have estimated the prevalence of MPSI in the UK to be 0.11 per 100 000 over the 2-year period of the study. The incidence was 0.26 cases and 0.55 cases per 100 000 live births in the first and second years of the study, respectively. MPSI thus appears to be a very rare disorder. Clearly, this figure may be an under-estimate for a number of reasons. First, the response rate to the BPNSU survey was only 68.8%; second, it may be that the condition is under-recognized though this seems unlikely, as most infants with a severe epilepsy would be referred to a tertiary care paediatric neurologist in the UK; third, it is possible that some infants who die relatively early are not recognized as having the condition as either seizure semiology, EEG or both may not have evolved to show the characteristic features of this electroclinical syndrome.
We have identified clinical, MRI, EEG and neuropathological features in this study which expand the previously reported phenotype. We describe atypical or modified hypsarrhythmia in three cases with the presence of infantile spasms in two of these cases. Infantile spasms have been described in seven patients with MPSI. One had onset at 9 months associated with ‘focal ictal EEG changes’ (Coppola et al., 1995). A child with Aicardi syndrome (Jocic-Jakubi and Lagae, 2008) had electroclinical migrating focal seizures from Day 1 of life followed by spasms from 6 months of age, associated with a ‘disorganised but not typically hypsarrhythmic’ background EEG. Three children with infantile spasms were noted in a large case series, two with hypsarrhythmia (Carranza Rojo et al., 2011). In addition, a recent report described hypsarrhythmia and spasms following initial presentation with migrating partial seizures (Lee et al., 2012). We also describe periods of EEG suppression in two patients and more marked burst suppression in one further patient. A recent single case report described an initial presentation with early myoclonic encephalopathy and suppression-burst pattern on EEG, followed by migrating partial seizures with typical EEG changes and subsequently by infantile spasms with hypsarrhythmia (Chien et al., 2012). Therefore this study adds to the growing evidence that MPSI and the other infantile electroclinical epileptic disorders are age-related disorders and that there may be clinical evolution from one syndrome to another, as often reported between Ohtahara and West syndromes.
Five children (Patients 2, 3, 6, 7 and 11) manifested novel clinical features. Three had severe gut dysmotility, which appeared after 18 months of age and in two cases contributed directly to their deaths. Despite many investigations a cause was not found. Gut dysmotility is frequently observed in children with a range of severe neurological disabilities (Sullivan, 2008). It is possible that the underlying cause of the MPSI, as well as the resulting neurological disability may both contribute to this clinical feature. Four patients developed a movement disorder that was not considered iatrogenic. These features have not been reported previously but movement disorders are recognized in a number of early infantile epileptic encephalopathies, particularly those caused by mutations in STXBP1 (Deprez et al., 2010) and ARX (Guerrini et al., 2007). Mutations in these genes were not detected in those tested from this cohort. One patient in this series had basal ganglia abnormalities on brain MRI but in the other two, no abnormality was found. All four children underwent extensive metabolic investigations (including measurement of neurotransmitters in the CSF in two patients) with normal results.
The imaging findings in our cohort include both novel and rarely reported features, such as delayed myelination with white matter hyperintensity and basal ganglia abnormalities. Previously, the most frequently reported imaging finding seen months or years after the onset of seizures has been the development of increased extra-axial fluid reflecting cerebral atrophy; this was reported in 35 of 77 earlier cases (45%). Other reported abnormalities include an absent corpus callosum in a child with a clinical diagnosis of Aicardi syndrome (Jocic-Jakubi and Lagae, 2008), hypoplasia of the corpus callosum in a child with 16p11.2 duplication and MPSI (Bedoyan et al., 2010) and four children with parenchymal lesions in one of the temporal lobes (Coppola et al., 2007; Caraballo et al., 2008). Recent reports have also described delayed myelination (Carranza Rojo et al., 2011; Freilich et al., 2011; Barcia et al., 2012) but not white matter hyperintensity. Nine of the 14 patients in this series had normal scans, although all were either single or undertaken before 6 months of age. Consequently, it is possible that if brain MRI had been performed later, after the age at which myelination should be complete, cerebral atrophy or delayed myelination, or both, would have been present.
We also report abnormal magnetic resonance spectroscopy in three of four patients, consistent with the delayed and abnormal myelination patterns. Magnetic resonance spectroscopy was abnormal with decreased N-acetyl aspartate peaks in four of seven children investigated in the literature, in keeping with the findings of this study (Gross-Tsur et al., 2004; Cilio et al., 2009; Freilich et al., 2011).
Previously reported autopsy data from patients with MPSI is limited to just five patients. Neuronal loss and accompanying gliosis within the hippocampi was described in two patients (Coppola et al., 1995) microcephaly in another case (Freilich et al., 2011) but no abnormalities were found in the fourth case (Wilmshurst et al., 2000). More recently a patient reported to have multiple cortical malformations with polymicrogyria, focal cortical dysplasia and hippocampal sclerosis has been described (Fasulo et al., 2012). Neuropathology was available in two of our cases. Neither patient demonstrated evidence of a malformation, but the feature common to both was bilateral hippocampal damage. In one patient, this showed a pattern of classical hippocampal sclerosis and in the other, there was bilateral severe end folial sclerosis with granule cell dispersion. In addition Patient 12 had marked putaminal injury which, so far, has not been reported in association with MPSI. Putaminal abnormalities on MRI scan and neuropathology are very unusual in MPSI. Patient 12’s electroclinical presentation did fit the criteria for MPSI but it is possible that this case may have an underlying neurometabolic or genetic syndrome in which MPSI is one of many neurological features which forms one part of the whole clinical picture.
The underlying cause or causes of most cases of MPSI are not known or understood. Mitochondrial cytopathy must be considered in children who present with developmental delay and intractable, multifocal epilepsy. In the 88 previously reported cases, 18 have undergone invasive investigation for mitochondrial disorders all with normal results. Six of our 14 patients underwent a muscle biopsy, with mild and non-specific abnormalities seen in three. Although it is possible that Patient 7 might have a mitochondrial cytopathy (in view of the pattern of basal ganglia injury) this was not confirmed on detailed metabolic studies (Supplementary material). The characteristics of cases reported to date suggest MPSI is likely to represent a heterogeneous age-related epileptic encephalopathy with multiple genetic, structural and metabolic causes similar to that seen in other early infantile epileptic encephalopathy syndromes such as West and Ohtahara syndromes. The description of an infant with Aicardi syndrome who was also considered to have MPSI would support the latter hypothesis (Jocic-Jakubi and Lagae, 2008).
It is likely that with the continual advances in gene discovery in early infantile epileptic encephalopathy, further patients with MPSI will be found to have a genetic disease basis. Early investigations focused on looking for abnormalities in the sodium (SCN1A, SCN2A), potassium (KCNQ2, KCNQ3) and chloride (CLCN2) ion channels with negative findings (Coppola et al., 2006). This focus on ion channel dysfunction having a role in MPSI has recently been illustrated by a very significant genetic finding of gain-of-function KCNT1 mutations in 50% of a cohort (6 of 12 probands) of infants with MPSI (Barcia et al., 2012). KCNT1 encodes a sodium-activated potassium channel, and genetic mutations lead to constitutive activation of the channel, mimicking the effects of phosphorylation of the C-terminal domain by protein kinase C. In addition to regulating ion flux, KCNT1 is also thought to have a non-conducting function, as its C terminus interacts with cytoplasmic proteins involved in developmental signalling pathways. Two patients with KCNT1 mutations were identified in this MPSI cohort (Table 4). Although it is likely that KCNT1 is a major MPSI gene, MPSI is genetically heterogeneous. Three cases of MPSI with abnormalities in the SCN1A gene have been reported. Two cases (Carranza Rojo et al., 2011) were from a cohort of 15 patients with MPSI who were also screened for CDKL5, STXBP1, PCDH19 and POLG, all of which were negative. The third case (Freilich et al., 2011) was found to have a missense mutation of the SCN1A gene, subsequently confirmed on post-mortem genetic testing. Consequently, of the 22 MPSI cases in the literature tested for SCN1A mutations, three (14%) were abnormal. Carranza Rojo et al. (2011) emphasized that MPSI does share some phenotypic features with severe infantile multifocal epilepsy. The first report of affected siblings in a consanguineous family from Saudi Arabia with two affected children was recently presented (Poduri et al., 2011). Whole exome sequencing revealed a novel mutation in SLC25A22, a gene previously found to be mutated in two cases of early infantile epileptic encephalopathy with suppression burst pattern on EEG (Molinari et al., 2005, 2009). Another reported patient was found to have a mutation in PLCB1 (Poduri et al., 2012), a recently described cause of infantile epileptic encephalopathy and West syndrome (Kurian et al., 2010).
Four of the patients in this study had diagnostic microarray studies performed, and all were normal. Comparative genomic hybridization array has been carried out in 16 patients in the literature and was normal in 13. One patient was found to have a 16p11.2 duplication (Bedoyan et al., 2010) and another had a deletion of chromosome 2 (q24.2q31.1) (Carranza Rojo et al., 2011), which included the entire SCN1A gene and other sodium channel genes.
The management of children with MPSI is challenging both in terms of seizure control and the frequent additional medical complications associated with severe neuro-disability. In the current series one patient showed a response to the ketogenic diet and another to a combination of adrenocorticotropic hormone and the diet. Seizures are difficult to control with only 21 of the 88 previously reported cases having an apparent sustained response to specific anti-epileptic drugs. These have included levetiracetam in four cases (Hmaimess et al., 2006; Caraballo et al., 2008; Cilio et al., 2009), a combination of bromides, stiripentol and levetiracetam in two cases (Djuric et al., 2011) and a combination of stiripentol and high dose clonazepam in two (Coppola et al., 1995). Most recently, a 12-month period of seizure control was achieved with a combination of clonazepam, stiripentol and levetiracetam (Merdariu et al., 2013). Successful treatment with rufinamide has recently been reported in two of five patients with MPSI (Vendrame et al., 2011). One patient (who did not have epileptic spasms) became seizure free with vigabatrin (Bedoyan et al., 2010). Finally, two patients with MPSI, whose primary seizure type was epileptic apnoea, responded to acetazolamide (Irahara et al., 2011). Bromides have been used in 12 of the 88 cases in the literature, being effective in seven (Okuda et al., 2000; Caraballo et al., 2008; Djuric et al., 2011; Fasulo et al., 2012), ineffective in two and poorly tolerated in one case (Hmaimess et al., 2006; Cilio et al., 2009). One patient developed bromoderma (Nabatame et al., 2010) despite non-toxic levels. In the current study bromides were effective but led to over-sedation (despite normal plasma levels) in one patient and were ineffective and poorly tolerated in one other patient. Therefore according to the literature, complete seizure control has been achieved only with clonazepam, stiripentol and levetiracetam in combination (n = 1), stiripentol and clonazepam (n = 2) and bromides (n = 1).
Over time, the natural course of MPSI seems to be associated with a general reduction of seizure frequency (Okuda et al., 2000; Hmaimess et al., 2006). Seizures are described as being ‘burnt-out’ in a number of patients with increasing age (Coppola et al., 1995), and again, this phenomenon is reflected in this current series.
All 14 patients in the current series demonstrated either minimal developmental progress or lost most or all of their previously acquired skills as the disorder progressed. The prognosis of MPSI is uniformly poor. A fifth of the previously reported cases have died; two were found dead, probably representing sudden unexplained death in epilepsy (SUDEP) and a further case died suddenly in hospital, also attributed to SUDEP. The prognosis for psychomotor development is extremely poor for those surviving infancy, although six cases had a relatively better outcome (Marsh et al., 2005). However, the details of these six patients were limited and all showed ‘significant’ developmental delay. The best reported developmental outcome has been in a 6-year-old child from this series who walked at 18 months, started to talk at 2 years and was described as ‘learning disabled’ without further details. Other patients have been reported who have regained developmental milestones with good seizure control (Okuda et al., 2000).
Conclusion
We report the first UK national series of patients with MPSI. Our findings have expanded the spectrum of the disease. MPSI should be considered early in the differential diagnosis of any child <6 months of age with medically intractable focal seizures, particularly if associated with autonomic features and in whom initial MRI demonstrates no abnormality. Identification of new clinical features in a syndrome may be controversial. However, a rational approach with identification of the key features (in this case the unusual electrographic hallmark of migrating electrographic seizure focus with clinical focal/multifocal/migratory seizures) but an open approach to report other, perhaps atypical features seen in cases of children with this electroclinical syndrome is most likely to result in further clinical and electrographic disease delineation, as well as elucidation of more pathogenic mechanisms that cause this type of early infantile epileptic encephalopathy.
The identification of an epilepsy syndrome is clinically useful because it gives prognostic information, drives elucidation of disease mechanisms (including the potential for genetic counselling in some cases) and therapeutic strategies and provides realistic expectations for both seizure control and cognitive outcome. Further study into the pathogenesis of MPSI will almost certainly improve our understanding of this severe and early infantile epileptic encephalopathy.
Funding
A.M. and R.K. received funding from the Neurology Charitable Fund (Alder Hey Children's Hospital) for the BPNSU study. M.A.K. is a Wellcome Trust appointed Intermediate Clinical Fellow. Genetic investigations undertaken on a research basis in this study were funded by the MPSI support group, Action Medical Research, the UK Childrens Neurological Research Campaign (UKCNRC), Roald Dahl's Marvellous Children's Charity and Great Ormond Street Hospital Children's Charities (GOSHCC).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We would like to thank all who provided clinical and pathological information (including Dr C. Adcock, Department of Paediatrics, Derriford Hospital, Plymouth, UK) and most importantly the children with MPSI and their families. We would like to thank Dr Richard Scott (Department of Genetics, Great Ormond Street Hospital, London, UK) for assistance with the multiple gene panel testing. We would like to thank the MPSI parental support group for their ongoing support for our research studies, as well as the British Paediatric Epilepsy Interest Group, British Paediatric Neurology Surveillance Unit, UK Children's Neurological Research Campaign and Roald Dahl Marvellous Children’s Charity.
Glossary
Abbreviation
- MPSI
migrating partial seizures of infancy
References
- Barcia G, Fleming MR, Deligniere A, Gazula VR, Brown MR, Nabbout R, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44:1255–9. doi: 10.1038/ng.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoyan JK, Kumar RA, Sudi J, Silverstein F, Ackley T, Iyer RK, et al. Duplication 16p11.2 in a child with infantile seizure disorder. Am J Med Genet. 2010;6:1567–74. doi: 10.1002/ajmg.a.33415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo RH, Fontana E, Darra F, Cassar L, Negrini F, Fiorini E, et al. Migrating focal seizures in infancy: analysis of the electro-clinical patterns in 17 patients. J Child Neurol. 2008;23:497–506. doi: 10.1177/0883073807309771. [DOI] [PubMed] [Google Scholar]
- Carranza Rojo D, Hamiwka L, McMahon JM, Dibbens LM, Arsov T, Suls A, et al. De novo SCN1A mutations in migrating partial seizures of infancy. Neurology. 2011;77:380–3. doi: 10.1212/WNL.0b013e318227046d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien YH, Lin MI, Weng WC, Du JC, Lee WT. Dextromethorphan in the treatment of early myoclonic encephalopathy evolving into migrating partial seizures in infancy. J Formos Med Assoc. 2012;111:290–4. doi: 10.1016/j.jfma.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Cilio MR, Bianchi R, Balestri M, Onofri A, Giovannini S, Di Capua M, et al. Intravenous levetiracetam terminates refractory status epilepticus in two patients with migrating partial seizures in infancy. Epilepsy Res. 2009;86:66–71. doi: 10.1016/j.eplepsyres.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Coppola G, Operto FF, Auricchio G, D'Amico A, Fortunato D, Pascotto A. Temporal lobe dual pathology in malignant migrating partial seizures in infancy. Epileptic Disord. 2007;9:145–8. doi: 10.1684/epd.2007.0106. [DOI] [PubMed] [Google Scholar]
- Coppola G, Plouin P, Chiron C, Robain O, Dulac O. Migrating partial seizures in infancy: a malignant disorder with developmental arrest. Epilepsia. 1995;36:1017–24. doi: 10.1111/j.1528-1157.1995.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Coppola G, Veggiotti P, Del Giudice EM, Bellini G, Longaretti F, Taglialatela M, et al. Mutational scanning of potassium, sodium and chloride ion channels in malignant migrating partial seizures in infancy. Brain Dev. 2006;28:76–9. doi: 10.1016/j.braindev.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Deprez L, Weckhuysen S, Holmgren P, Suls A, Van Dyck T, Goossens D, et al. Clinical spectrum of early-onset epileptic encephalopathies associated with STXBP1 mutations. Neurology. 2010;75:1159–65. doi: 10.1212/WNL.0b013e3181f4d7bf. [DOI] [PubMed] [Google Scholar]
- Djuric M, Kravljanac R, Kovacevic G, Martic J. The efficacy of bromides, stiripentol and levetiracetam in two patients with malignant migrating partial seizures in infancy. Epileptic Disord. 2011;13:22–6. doi: 10.1684/epd.2011.0402. [DOI] [PubMed] [Google Scholar]
- Fasulo L, Saucedo S, Caceres L, Solis S, Caraballo R. Migrating focal seizures during infancy: a case report and pathologic study. Pediatr Neurol. 2012;46:182–4. doi: 10.1016/j.pediatrneurol.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Freilich ER, Jones JM, Gaillard WD, Conry JA, Tsuchida TN, Reyes C, et al. Novel SCN1A mutation in a proband with malignant migrating partial seizures of infancy. Arch Neurol. 2011;68:665–71. doi: 10.1001/archneurol.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhuis HJ, Schieving J, Zwarts MJ. Malignant migrating partial seizures in a 4-month-old boy. Epileptic Disord. 2011;13:185–7. doi: 10.1684/epd.2011.0424. [DOI] [PubMed] [Google Scholar]
- Gross-Tsur V, Ben-Zeev B, Shalev RS. Malignant migrating partial seizures in infancy. Pediatr Neurol. 2004;31:287–90. doi: 10.1016/j.pediatrneurol.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Moro F, Kato M, Barkovich AJ, Shiihara T, McShane MA, et al. Expansion of the first PolyA tract of ARX causes infantile spasms and status dystonicus. Neurology. 2007;69:427–33. doi: 10.1212/01.wnl.0000266594.16202.c1. [DOI] [PubMed] [Google Scholar]
- Hahn A, Heckel M, Neubauer BA. Pronounced microcephaly in a patient with malignant migrating partial seizures in infancy. Epileptic Disord. 2007;9:94–7. doi: 10.1684/epd.2007.0055. [DOI] [PubMed] [Google Scholar]
- Hmaimess G, Kadhim H, Nassogne MC, Bonnier C, van Rijckevorsel K. Levetiracetam in a neonate with malignant migrating partial seizures. Pediatr Neurol. 2006;34:55–9. doi: 10.1016/j.pediatrneurol.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Irahara K, Saito Y, Sugai K, Nakagawa E, Saito T, Komaki H, et al. Effects of acetazolamide on epileptic apnea in migrating partial seizures in infancy. Epilepsy Res. 2011;96:185–9. doi: 10.1016/j.eplepsyres.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Jocic-Jakubi B, Lagae L. Malignant migrating partial seizures in Aicardi syndrome. Dev Med Child Neurol. 2008;50:790–2. doi: 10.1111/j.1469-8749.2008.03091.x. [DOI] [PubMed] [Google Scholar]
- Kurian MA, Meyer E, Vassallo G, Morgan NV, Prakash N, Pasha S, et al. Phospholipase C beta 1 deficiency is associated with early-onset epileptic encephalopathy. Brain. 2010;133:2964–70. doi: 10.1093/brain/awq238. [DOI] [PubMed] [Google Scholar]
- Lee EH, Yum MS, Jeong MH, Lee KY, Ko TS. A case of malignant migrating partial seizures in infancy as a continuum of infantile epileptic encephalopathy. Brain Dev. 2012;34:768–72. doi: 10.1016/j.braindev.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Marsh E, Melamed SE, Barron T, Clancy RR. Migrating partial seizures in infancy: expanding the phenotype of a rare seizure syndrome. Epilepsia. 2005;46:568–72. doi: 10.1111/j.0013-9580.2005.34104.x. [DOI] [PubMed] [Google Scholar]
- Merdariu D, Delanoe C, Mahfoufi N, Bellavoine V, Auvin S. Malignant migrating partial seizures of infancy controlled by stiripentol and clonazepam. Brain Dev. 2013;35:177–80. doi: 10.1016/j.braindev.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Molinari F, Kaminska A, Fiermonte G, Boddaert N, Raas-Rothschild A, Plouin P, et al. Mutations in the mitochondrial glutamate carrier SLC25A22 in neonatal epileptic encephalopathy with suppression bursts. Clin Genet. 2009;76:188–94. doi: 10.1111/j.1399-0004.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- Molinari F, Raas-Rothschild A, Rio M, Fiermonte G, Encha-Razavi F, Palmieri L, et al. Impaired mitochondrial glutamate transport in autosomal recessive neonatal myoclonic epilepsy. Am J Hum Genet. 2005;76:334–9. doi: 10.1086/427564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabatame S, Saito Y, Sakuma H, Komaki H, Nakagawa E, Sugai K, et al. Bromoderma in a patient with migrating partial seizures in infancy. Epilepsy Res. 2010;91:283–8. doi: 10.1016/j.eplepsyres.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Okuda K, Yasuhara A, Kamei A, Araki A, Kitamura N, Kobayashi Y, et al. Successful control with bromide of two patients with malignant migrating partial seizures in infancy. Brain Dev. 2000;22:56–9. doi: 10.1016/s0387-7604(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Poduri A, Heinzen EL, Salih MA, Chitsazzadeh V, Hill RS, Elhosary PC, et al. Whole exome sequencing implicates SLC25A22 as a gene for MMPEI. Epilepsia. 2011;52(Suppl 6):16. [Google Scholar]
- Poduri A, Chopra SS, Neilan EG, Elhosary PC, Kurian MA, Meyer E, et al. Homozygous PLCB1 deletion associated with malignant migrating partial seizures in infancy. Epilepsia. 2012;53:e14650. doi: 10.1111/j.1528-1167.2012.03538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Sankhyan N, Ramesh K, Gulati S. Child neurology: epilepsy of infancy with migrating focal seizures. Neurology. 2011;77:21–4. doi: 10.1212/WNL.0b013e3182267b4f. [DOI] [PubMed] [Google Scholar]
- Sullivan P. Gastrointestinal disorders in children with neurodevelopmental disabilities. Dev Disabil Res Rev. 2008;14:128–36. doi: 10.1002/ddrr.18. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Poduri A, Loddenkemper T, Kluger G, Coppola G, Kothare SV. Treatment of malignant migrating partial epilepsy of infancy with rufinamide: report of five cases. Epileptic Disord. 2011;13:18–21. doi: 10.1684/epd.2011.0406. [DOI] [PubMed] [Google Scholar]
- Veneselli E, Perrone MV, Di Rocco M, Gaggero R, Biancheri R. Malignant migrating partial seizures in infancy. Epilepsy Res. 2001;46:27–32. doi: 10.1016/s0920-1211(01)00197-8. [DOI] [PubMed] [Google Scholar]
- Wilmshurst JM, Appleton DB, Grattan-Smith PJ. Migrating partial seizures in infancy: two new cases. J Child Neurol. 2000;15:717–22. doi: 10.1177/088307380001501102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.