Abstract
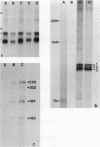
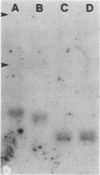
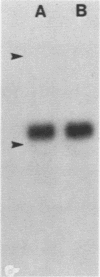
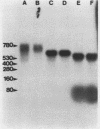
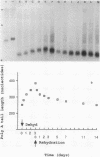
We developed a method, termed an H-blot, by which the poly(A) tract of any specific mRNA may be detected by RNA filter hybridization after its removal from the body of the mRNA by a RNase H-catalyzed endonucleolytic cleavage in the 3' untranslated region. Using this method, we studied the modulation of the length of the poly(A) tract of rat vasopressin mRNA in vivo during changes in the levels of this mRNA resulting from a physiologic stimulus, osmotic stress. The poly(A) tract of hypothalamic vasopressin mRNA in hydrated rats was, quite remarkably, approximately 250 nucleotides in length, in contrast to that of somatostatin mRNA, which was approximately 30 nucleotides long. Vasopressin mRNA poly(A) tail length increased progressively from approximately 250 to approximately 400 nucleotides with the application of the hyperosmotic stimulus and declined to base line after its removal; somatostatin mRNA poly(A) tail length did not change during osmotic stress. The poly(A) tract length of total hypothalamic mRNA was between 35 and 140 nucleotides and also did not change with osmotic stress. Modulation of poly(A) tract length of specific mRNAs during stimulation of gene expression may provide an additional level of genetic regulation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann I. E., Brawerman G. Control of breakdown of the polyadenylate sequence in mammalian polyribosomes: role of poly(adenylic acid)-protein interactions. Biochemistry. 1977 Jan 25;16(2):259–264. doi: 10.1021/bi00621a016. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Determinants of messenger RNA stability. Cell. 1987 Jan 16;48(1):5–6. doi: 10.1016/0092-8674(87)90346-1. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Russell J. T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan 25;207(4429):373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colot H. V., Rosbash M. Behavior of individual maternal pA+ RNAs during embryogenesis of Xenopus laevis. Dev Biol. 1982 Nov;94(1):79–86. doi: 10.1016/0012-1606(82)90070-7. [DOI] [PubMed] [Google Scholar]
- Danner D., Leder P. Role of an RNA cleavage/poly(A) addition site in the production of membrane-bound and secreted IgM mRNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8658–8662. doi: 10.1073/pnas.82.24.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. G., Arentzen R., Reid J. M., Manning R. W., Wolfson B., Lawrence K. L., Baldino F., Jr Glucocorticoid sensitivity of vasopressin mRNA levels in the paraventricular nucleus of the rat. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1145–1149. doi: 10.1073/pnas.83.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979 Sep 11;7(1):179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Cox R. A. Periodicity in the length of 3'-poly(A) tails from native globin mRNA of rabbit. Nucleic Acids Res. 1982 Jul 24;10(14):4173–4179. doi: 10.1093/nar/10.14.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowczynska A., Yenofsky R., Brawerman G. Regulation of messenger RNA stability in mouse erythroleukemia cells. J Mol Biol. 1985 Jan 20;181(2):231–239. doi: 10.1016/0022-2836(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Soreq H. The regulatory function of poly(A) and adjacent 3' sequences in translated RNA. Prog Nucleic Acid Res Mol Biol. 1982;27:53–83. doi: 10.1016/s0079-6603(08)60597-8. [DOI] [PubMed] [Google Scholar]
- Majzoub J. A., Carrazana E. J., Shulman J. S., Baker K. J., Emanuel R. L. Defective regulation of vasopressin gene expression in Brattleboro rats. Am J Physiol. 1987 May;252(5 Pt 1):E637–E642. doi: 10.1152/ajpendo.1987.252.5.E637. [DOI] [PubMed] [Google Scholar]
- Majzoub J. A., Rich A., van Boom J., Habener J. F. Vasopressin and oxytocin mRNA regulation in the rat assessed by hybridization with synthetic oligonucleotides. J Biol Chem. 1983 Dec 10;258(23):14061–14064. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. F., Wake S. A. An analysis of the rate of metallothionein mRNA poly(A)-shortening using RNA blot hybridization. Nucleic Acids Res. 1985 Nov 25;13(22):7929–7943. doi: 10.1093/nar/13.22.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. L., Sharp P. A. Accurate cleavage and polyadenylation of exogenous RNA substrate. Cell. 1985 Jul;41(3):845–855. doi: 10.1016/s0092-8674(85)80065-9. [DOI] [PubMed] [Google Scholar]
- Muschel R., Khoury G., Reid L. M. Regulation of insulin mRNA abundance and adenylation: dependence on hormones and matrix substrata. Mol Cell Biol. 1986 Jan;6(1):337–341. doi: 10.1128/mcb.6.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek I., Axel R. Glucocorticoids enhance stability of human growth hormone mRNA. Mol Cell Biol. 1987 Apr;7(4):1496–1507. doi: 10.1128/mcb.7.4.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik C. M., Storti R. V., Jacobson A. Partial purification of a developmentally regulated messenger RNA from Dictyostelium discoideum by thermal elution from poly(U)-sepharose. J Mol Biol. 1981 Aug 15;150(3):389–398. doi: 10.1016/0022-2836(81)90554-4. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Wilkins C., Jacobson A. Translational control during early Dictyostelium development: possible involvement of poly(A) sequences. Cell. 1984 Apr;36(4):1017–1025. doi: 10.1016/0092-8674(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. The end of the message and beyond. Nature. 1984 Feb 2;307(5950):412–413. doi: 10.1038/307412a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Tansey T. R., Ruderman J. V. Sequence-specific adenylations and deadenylations accompany changes in the translation of maternal messenger RNA after fertilization of Spisula oocytes. J Mol Biol. 1983 May 25;166(3):309–327. doi: 10.1016/s0022-2836(83)80087-4. [DOI] [PubMed] [Google Scholar]
- Sausville E., Carney D., Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem. 1985 Aug 25;260(18):10236–10241. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Puckett L., Darnell J. E. Possible relationship of poly(A) shortening to mRNA turnover. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1077–1081. doi: 10.1073/pnas.72.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman T. G., McKelvy J. F., Watson S. J. Vasopressin mRNA regulation in individual hypothalamic nuclei: a northern and in situ hybridization analysis. J Neurosci. 1986 Jun;6(6):1685–1694. doi: 10.1523/JNEUROSCI.06-06-01685.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Tavianini M. A., Hayes T. E., Magazin M. D., Minth C. D., Dixon J. E. Isolation, characterization, and DNA sequence of the rat somatostatin gene. J Biol Chem. 1984 Oct 10;259(19):11798–11803. [PubMed] [Google Scholar]
- Van Tol H. H., Voorhuis D. T., Burbach J. P. Oxytocin gene expression in discrete hypothalamic magnocellular cell groups is stimulated by prolonged salt loading. Endocrinology. 1987 Jan;120(1):71–76. doi: 10.1210/endo-120-1-71. [DOI] [PubMed] [Google Scholar]
- Vandesande F., Dierickx K. Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretroy system of the rat. Cell Tissue Res. 1975 Dec 2;164(2):153–162. doi: 10.1007/BF00218970. [DOI] [PubMed] [Google Scholar]
- Vary C. P., Vournakis J. N. RNase H-catalyzed site-specific deadenylylation of rabbit alpha- and beta- globin mRNAs. Secondary structure of 3'-noncoding regions. J Biol Chem. 1984 Mar 10;259(5):3299–3307. [PubMed] [Google Scholar]
- Wilt F. H. The dynamics of maternal poly(A)-containing mRNA in fertilized sea urchin eggs. Cell. 1977 Jul;11(3):673–681. doi: 10.1016/0092-8674(77)90084-8. [DOI] [PubMed] [Google Scholar]
- Zeevi M., Nevins J. R., Darnell J. E., Jr Newly formed mRNA lacking polyadenylic acid enters the cytoplasm and the polyribosomes but has a shorter half-life in the absence of polyadenylic acid. Mol Cell Biol. 1982 May;2(5):517–525. doi: 10.1128/mcb.2.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]