Abstract
The implantation of cardiac pacing devices in children and young adults can be challenging and different from the adult population due to their smaller size, their longer life expectancy, and anatomical variations associated with congenital heart defects. A knowledge of indications, pacing leads and devices, anatomical variations, and the technical skills are important for those who implant and care for children with pacemakers. In this review we attempt to discuss these specific points of cardiac pacing in children and young adults.
Keywords: Congenital heart defects, pacemakers, pacing, heart block
INTRODUCTION
Pacing in children is mainly performed in the setting of congenital or post-surgical complete heart block and less frequently in some surgical patients with sinus node dysfunction. The indications, anatomical variations, and the technical skills required for pacing children are different compared to those for adults. Also, current pacemakers are smaller sized, have longer battery life, multiple programming options, and therapeutic capabilities, and therefore provide greater options for pacing in children.
INDICATIONS FOR PACING
The most common indications for permanent pacemaker implantation in children are third-degree atrioventricular (AV) block, either congenital or post-surgical, and rarely symptomatic sinus node dysfunction.
Congenital AV block
The indications for pacing in children and young adults with congenital AV block include heart rate <55 beats/min in a neonate or <40 beats/min in a child or adolescent, heart rate <70 bpm in patients with associated congenital heart defects (CHD), symptomatic bradycardia in the form of long naps and nightmares, exercise intolerance, pauses >3 sec while awake or >5 sec while sleeping, wide QRS escape rhythm, ventricular dysfunction, prolonged QTc, complex ventricular ectopy (couplets or greater) regardless of escape rhythm, ventricular ectopy with exercise, and prolonged subsidiary pacemaker recovery time.[1]
Post-surgical AV block
The indications for pacing related to non-congenital/surgical AV block include advanced second- or third-degree AV block associated with symptomatic bradycardia, ventricular dysfunction, or low cardiac output and postoperative advanced second- or third-degree AV block persisting for at least 7-10 days or not expected to resolve.[2]
Sinus node dysfunction
Sinus node dysfunction (SND) severe enough to need pacing is rare except after cardiac operations such as atrial switch surgery, the Fontan operation, or surgery for sinus venosus atrial septal defect. The indications for pacing in patients with SND include symptomatic SND, sinus bradycardia with complex CHD with a resting heart rate less than 40 bpm or pauses in ventricular rate >3 sec and CHD, and impaired hemodynamics due to sinus bradycardia or loss of AV synchrony.[2] Atrial pacing may also help prevent paroxysmal supraventricular tachycardia in certain forms of congenital heart disease, but definitive proof for this effect is lacking.
IMPLANTATION OF PACING SYSTEMS IN CHILDREN
Pacing leads can be implanted via the transvenous (endocardial) or surgical (epicardial) route. The choice of route is dependent upon the size of the patient, anatomy, and surgical procedures performed that can affect the access to certain cardiac structures.
Epicardial
We recommend epicardial pacing in patients less than 15 kg, patients with intracardiac shunt lesions, patients with limited access to the atrium or the ventricle (e.g. patients with single ventricular physiology post Fontan palliation), and patients with prosthetic tricuspid valves. Epicardial lead implantation requires sternotomy or thoracotomy or subxiphoid approach, and is associated with higher chronic stimulation threshold, higher lead failures and fractures, and early depletion of battery life.[3–5] However, it preserves the venous access for future use. Steroid-eluting epicardial leads are preferred as they prevent threshold increase in the long term.[6] Even though the right ventricular (RV) epicardial surface is relatively easily accessible, left ventricular apical pacing is the best site for epicardial leads in children.[7] Dual chamber epicardial pacemakers can easily be implanted in children over 3 kg. Rate-responsive ventricular only pacing, as opposed to AV synchronous pacing, may be adequate in smaller infants with isolated congenital heart block. We have practiced using the St. Jude's Microny single chamber pacemaker in extremely small (<2.5 kg) or premature infants who need a pacemaker.
Endocardial
Transvenous route of lead implantation is preferred in most patients except for those situations referred above. Endocardial lead placement offers the advantages of avoidance of thoracotomy, lower pacing thresholds, and a lower incidence of lead fractures. However, its disadvantages include a greater risk of lead dislodgment, venous occlusion, embolic vascular events, and endocarditis.[8] The primary risk factor for venous obstruction after pacemaker lead implantation in children was found to be related to the ratio of cross-sectional lead area to the body surface area at implantation.[9] Our general approach is to implant only a single transvenous lead when the patient is less than 25 kg and a dual lead system once they are over 25 kg. Introduction of smaller 4-Fr diameter leads may allow implantation of endocardial leads in smaller children without the risk of venous obstruction. The model 3830, 4.1-Fr diameter pacing lead (Medtronic Inc., Minneapolis, MN, USA) had excellent initial success in smaller children and patients with CHD, but is currently not available.[10] The smallest diameter pacing leads currently available range between 5.1 and 5.7 Fr, and require a 6- or 7-Fr introducer. Magnetic resonance imaging (MRI) is generally contraindicated for patients with a pacemaker. However, newer pacemaker systems have been approved for MRI scanning under certain conditions.[11] Lead lengths can range from 45 to 75 cm. It is prudent to minimize lead redundancy in the pocket, yet leave extra slack in the heart to provide for future growth [Figure 1].
Figure 1.
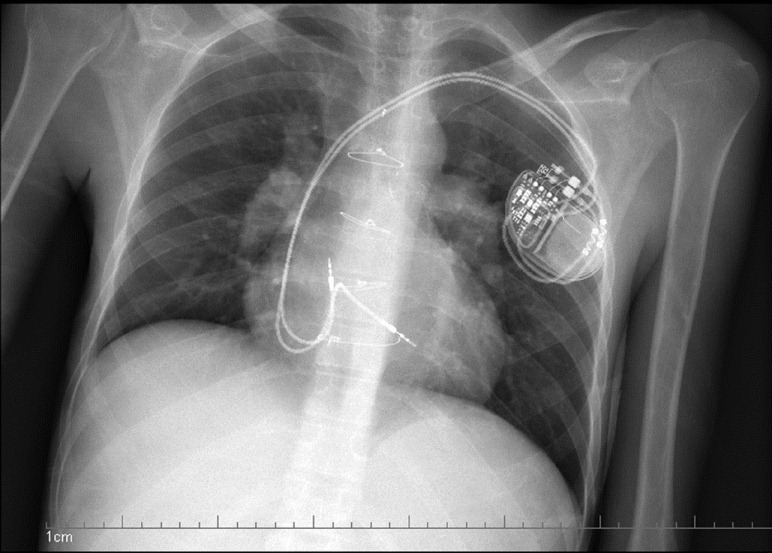
Dual chamber pacemaker showing implantation of the atrial lead in the high right atrium and septal implantation of the ventricular lead, with extra slack in the atrial and ventricular leads
TECHNIQUE OF ENDOCARDIAL LEAD IMPLANTATION
It is important to plan beforehand the mode of pacing and the number of leads to be implanted. Placement of a temporary transvenous pacemaker may be an option in patients that are pacemaker dependent and who are undergoing a generator or lead revision, but this is rarely indicated. Antibiotic coverage is provided during and immediately after the procedure.[12] We use intravenous Cefazolin or Clindamycin for the antibiotic coverage. A subcutaneous pocket appropriate for the size of the generator is created. However, in rare cases, a submuscular pocket is created for extremely thin individuals with minimal fat tissue to prevent device erosion and in patients with or at risk of Twiddler's syndrome. The target vein is accessed with modified Seldinger technique. The target veins are the axillary vein, innominate vein, or the subclavian vein. We attempt to access the axillary vein to avoid the complication of subclavian crush at the site of the ligament, reduce the risk of pneumothorax or hemothorax, and for potentially less cumbersome extraction, if necessary. The axillary vein is accessed by using standard landmarks, and creating a roadmap by performing a venogram from a peripheral venous line [Figure 2].
Figure 2.
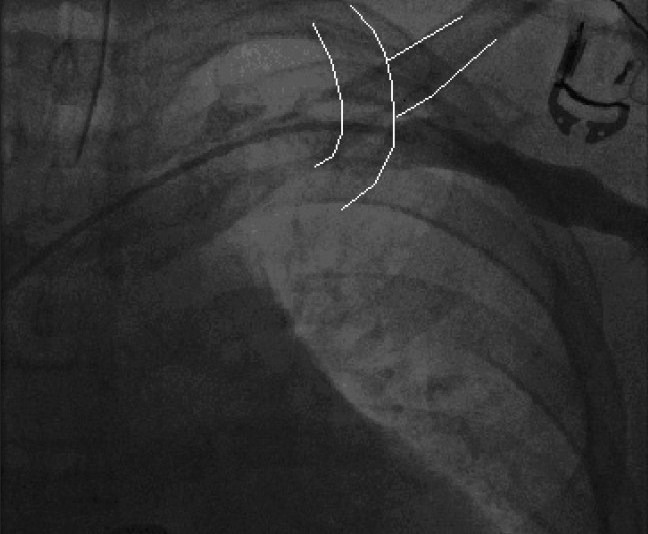
The axillary venogram shows the course of the vein as it courses the junction of the outer border of the first rib with the inferior margin of the clavicle
Placement of the lead tip at the right ventricular apex has historically been the standard approach. However, some studies have demonstrated that chronic pacing from such implant sites may cause myocardial dysfunction.[13] In our current practice, the RV septum is usually targeted. For the atrial lead, the right atrial appendage has historically been the optimal site for placement of the lead tip. Based on some recent data, we have been placing the atrial lead at the site of the Bachmann's bundle as it may be associated with lower far-field R-wave sensing, atrial synchronization, and prevention of atrial arrhythmias. The Bachmann's bundle is located in the posterior high right atrial septum near the superior vena cava [Figure 1].
Steroid-eluting active-fixation (screw-in) leads are preferred for both the atrium and the ventricle for better thresholds and ease of extraction in the future. Thresholds for capture below 1 V for both the atrium and the ventricle and sensing over 2 mV for the atrium and 5 mV for the ventricle are desirable at implant, but sometimes can be challenging in patients with scar tissue from previous surgeries. There is a good probability that thresholds for capture will improve after implant with steroid-eluting leads.
In patients with complete heart block, use of a single lead with capability of atrial sensing and ventricular pacing can be used in the VDD mode. The size of the model 2088 VDD lead at 8.1 Fr (St. Jude Inc., St. Paul, MN, USA), however, remains relatively large and requires a 9-Fr introducer. The larger size along with the availability of only a tine model has prevented more extensive use of this VDD lead in this population.
ENDOCARDIAL LEAD IMPLANTATION IN CHILDREN WITH STRUCTURAL HEART DEFECTS
Endocardial lead implantation can have unique challenges in children with structural heart defects, surgical repairs, synthetic septal patches, atrial baffles, conduits, absence of appendages, obstructed venous channels, persistent left superior vena cava, and extensive surgical fibrosis. For obstructed venous channels, recanalizing occluded veins should always be attempted initially as thin guide wires can often be threaded through the stenotic areas, allowing subsequent placement of sheaths over these wires and pacing wires through these sheaths [Figure 3]. However, often alternative venous access sites may need to be explored in these patients, including cephalic venous cutdown, supraclavicular access of the internal jugular vein, transhepatic and rarely transiliac or transfemoral routes [Figure 4].
Figure 3.
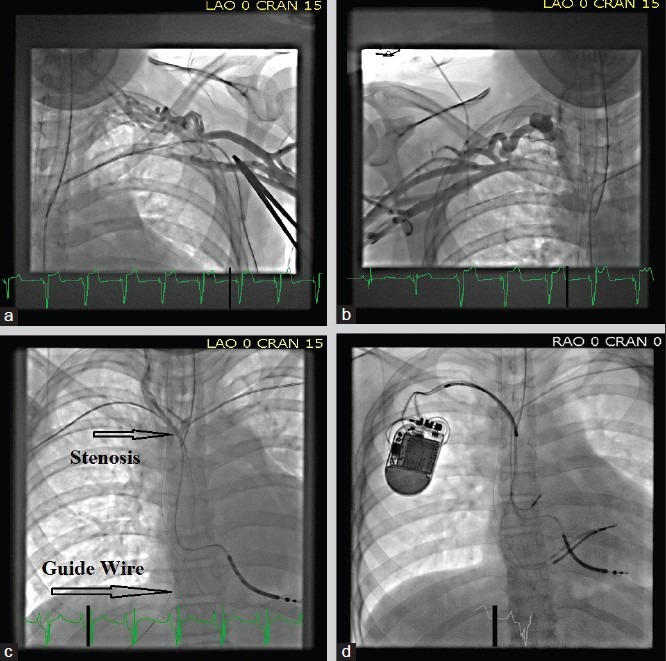
Venograms showing complete occlusion of the left subclavian (a) and proximal right subclavian (b) vein in a patient with an existing ICD lead that was fractured. (c) A guide wire (platinum plus 018, Boston scientific) was threaded from the right subclavian vein into the IVC through the stenotic site. (d) A dilator and sheath were then cannulated over the guide wire and used to pass two additional leads across this stenotic site
Figure 4.
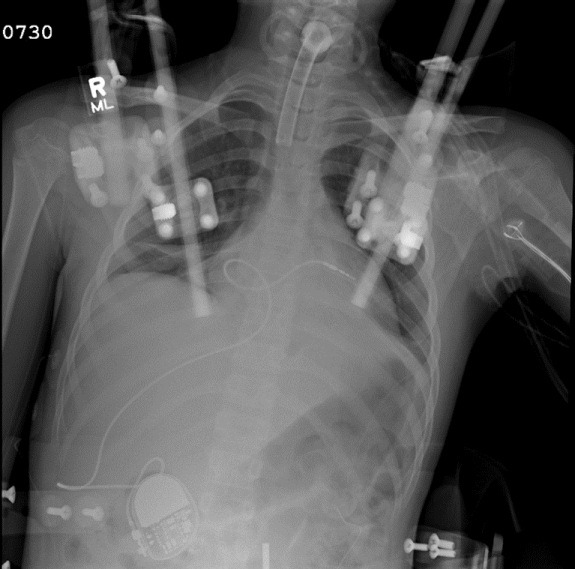
The RV lead is placed via a transhepatic approach in a 12-year-old girl for symptomatic recurrent asystolic pauses following a motor vehicular accident. She had multiple fractures of her vertebral column requiring placement of a cranial halo with traction. The access to the usual veins was limited, and therefore the lead was placed via transhepatic approach
In patients with D-transposition of the great arteries who have undergone an intratrial baffle repair (Mustard, Senning procedures) the atrial lead ideally passes through the baffle and secures to the superior aspect of the left atrium [Figure 5]. In those patients where ventricular pacing is also indicated, an active fixation lead is preferred as the systemic venous ventricle is a morphologic smooth-walled left ventricle. There is also a possibility of systemic venous baffle stenosis in these patients and there may be need for venous stents to enlarge the atrial baffle concomitantly. A selective venous angiogram is always recommended in these patients to identify vasculature and ascertain patency.
Figure 5.
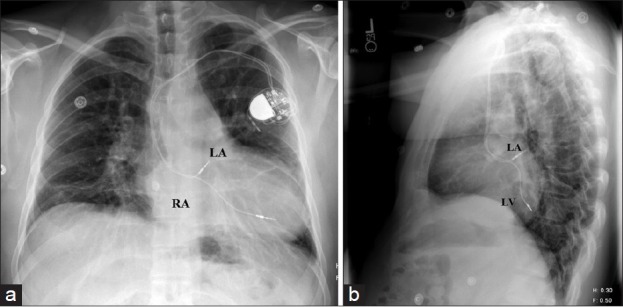
An AP and lateral view of a CXR showing a dual chamber pacemaker in a patient with Mustard procedure for D-TGA. Notice the placement of the atrial lead in the superior aspect of the left atrium and the ventricular lead in the posterior left ventricle
Patients with corrected transposition of the great arteries (L-TGA) are at a high risk of developing heart block from a displaced AV conduction system. As the systemic venous ventricle has a smooth-walled morphologic left ventricular morphology, an active fixation lead is recommended for pacing the ventricle.
In patients who develop heart block after a ventricular septal defect repair, the large septal patches may limit effective implant sites, and alternative sites away from the patch need to be explored.
Endocardial pacing is not an option in patients with single ventricles as access to the atria or ventricles is eliminated from the systemic veins after the extracardiac conduit Fontan procedure. Although atrial access can be obtained for pacing through the systemic veins in the classic form of the Fontan procedure, the sluggish venous pooling associated with this physiology increases the risk of thrombi and placement of endocardial atrial leads is not advocated.
Choice of stimulation mode
Knowledge of the AV node conduction is helpful in deciding the need for dual chamber pacemaker as it offers the ability to maintain constant AV synchrony and sinus-based chronotropy, but may increase the amount of ventricular pacing, which has been associated with adverse outcomes in adults.[14] Single ventricle patients do better with AV synchrony; however, patients with congenital AV block can tolerate single chamber ventricular pacing in the first few years of life.[15]
-
Single chamber pacing modes
- Ventricular pacing mode (VVI or VVIR): In small children and patients with structurally normal two ventricles, single chamber ventricular pacing is an acceptable mode of pacing as it requires a single lead and can achieve higher heart rates. Use of dual chamber pacemaker requires two leads, and access to the atrium via a limited surgical access may be difficult.
- Atrial pacing mode (AAI or AAIR): In patients with SND and an intact AV node function, atrial pacing mode is preferred.
Dual chamber modes (DDD or DDDR): This is the most common mode of pacing in the adults. AV synchrony can add up to 15% to the cardiac output.
PROGRAMMING OF THE DEVICE
Children have faster resting heart rates than adults and higher peak heart rates; it is not unusual for infants to have resting heart rates between 120 and 150 beats/min, and it is easy for children of all ages to attain sinus rates in excess of 200 beats/min during active play. Many pacemakers cannot pace at or track sinus rates beyond 180 beats/min. These limits to the maximum tracking rate can result in a substantial decrease in exercise performance, peak oxygen consumption, and anaerobic threshold.[16] In addition; higher heart rates result in increased battery utilization that can significantly impact the longevity of the pulse generators.
The heart rate ranges that are physiologically achievable decide the programming of the tracking and sensing rates as well as the activity of daily living (ADL) rates, the characteristics of the rate responsiveness, and the programming of the tachyarrhythmia parameters. Some pacing generators are capable of being custom programmed to such extended heart rate limits. Based on the patient size, the most appropriate-sized device is selected that caters to the requirements, as well as is not bulky.
The patient and the device are assessed prior to discharge, in 1-2 weeks for incision check, at 2-3 months to assess chronic pacing thresholds and cardiac function (because of the risk of pacing-induced cardiac dysfunction), and then every 6 months in infants and children. Patients are advised to transmit data via telephone line using the remote monitoring services on a 3-monthly basis or if any change in clinical status occurs.
COMPLICATIONS
Device implantation data using the Kids’ Inpatient database from 1997 to 2006 revealed specific complication rates for all device types were pneumothorax 2.2%, hematoma 3.3%, endocarditis/pericarditis 1.1%, surgical infection 2.4%, and death 1.7%. Pacemakers had patient-related complications in 11.2%.[17]
CONCLUSIONS
The clinical indications and scope of utility of cardiac devices in the young population is increasing due to improved survival of patients with CHDs, as well as advances in pacing technology. Patient size plays an important role in deciding the implantation technique. The implanting physician needs to be well versed with the anatomical variations as well as growth potential in customizing the device therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kertesz NJ, Fenrich AL, Friedman RA. Congenital complete atrioventricular block. Tex Heart Inst J. 1997;24:301–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the american college of cardiology/American Heart Association Task Force On Practice Guidelines (Writing Committee To Revise The ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers And Antiarrhythmia Devices) Developed in Collaboration With The American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:E1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MI, Bush DM, Vetter VL, Tanel RE, Wieand TS, Gaynor JW, et al. Permanent epicardial pacing in pediatric patients: Seventeen years of experience and 1200 outpatient visits. Circulation. 2001;103:2585–90. doi: 10.1161/01.cir.103.21.2585. [DOI] [PubMed] [Google Scholar]
- 4.Fortescue EB, Berul CI, Cecchin F, Walsh EP, Triedman JK, Alexander ME. Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and Congenital Heart Disease. Heart Rhythm. 2004;1:150–9. doi: 10.1016/j.hrthm.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Sachweh JS, Vazquez-Jimenez JF, Schondube FA, Daebritz SH, Dorge H, Muhler EG, et al. Twenty years experience with pediatric pacing: Epicardial and transvenous stimulation. Eur J Cardiothorac Surg. 2000;17:455–61. doi: 10.1016/s1010-7940(00)00364-x. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos N, Rouhollapour A, Kleine P, Moritz A, Bakhtiary F. Long-term follow-up after steroid-eluting epicardial pacemaker implantation in young children: A single centre experience. Europace. 2010;12:540–3. doi: 10.1093/europace/euq037. [DOI] [PubMed] [Google Scholar]
- 7.Vanagt WY, Verbeek XA, Delhaas T, Mertens L, Daenen WJ, Prinzen FW. The left ventricular apex is the optimal site for pediatric pacing: Correlation with animal experience. Pacing Clin Electrophysiol. 2004;27:837–43. doi: 10.1111/j.1540-8159.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 8.Silvetti MS, Drago F, De Santis A, Grutter G, Rava L, Monti L, et al. Single-centre experience on endocardial and epicardial pacemaker system function in neonates and infants. Europace. 2007;9:426–31. doi: 10.1093/europace/eum043. [DOI] [PubMed] [Google Scholar]
- 9.Figa FH, Mccrindle BW, Bigras JL, Hamilton RM, Gow RM. Risk factors for venous obstruction in children with transvenous pacing leads. Pacing Clin Electrophysiol. 1997;20:1902–9. doi: 10.1111/j.1540-8159.1997.tb03594.x. [DOI] [PubMed] [Google Scholar]
- 10.Khan A, Zelin K, Karpawich PP. Performence of the lumenless 4.1-Fr diameter pacing lead implanted at alternative pacing sites in congenital heart disease: A chronic 5-year experience. Pacing Clin Electrophysiol. 2010;33:1467–74. doi: 10.1111/j.1540-8159.2010.02884.x. [DOI] [PubMed] [Google Scholar]
- 11.Wilkoff BL, Bello D, Taborsky M, Vymazal J, Kanal E, Heuer H, et al. Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm. 2011;8:65–73. doi: 10.1016/j.hrthm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 12.De Oliveira JC, Martinelli M, Nishioka SA, Varejao T, Uipe D, Pedrosa AA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: Results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2:29–34. doi: 10.1161/CIRCEP.108.795906. [DOI] [PubMed] [Google Scholar]
- 13.Tse HF, Lau CP. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol. 1997;29:744–9. doi: 10.1016/s0735-1097(96)00586-4. [DOI] [PubMed] [Google Scholar]
- 14.Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The dual chamber and VVI implantable defibrillator (DAVID) Trial. JAMA. 2002;288:3115–23. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 15.Horenstein MS, Karpawich PP, Tantengco MV. Single versus dual chamber pacing in the young: Noninvasive comparative evaluation of cardiac function. Pacing Clin Electrophysiol. 2003;26:1208–11. doi: 10.1046/j.1460-9592.2003.t01-1-00170.x. [DOI] [PubMed] [Google Scholar]
- 16.Mathony U, Schmidt H, Gröger C, Francis DP, Konzag I, Müller-Werdan U, et al. Optimal maximum tracking rate of dual-chamber pacemakers required by children and young adults for a maximal cardiorespiratory performance. Pacing Clin Electrophysiol. 2005;28:378–83. doi: 10.1111/j.1540-8159.2005.09330.x. [DOI] [PubMed] [Google Scholar]
- 17.Czosek RJ, Meganathan K, Anderson JB, Knilans TK, Marino BS, Heaton PC. Cardiac rhythm devices in the pediatric population: Utilization and complications. Heart Rhythm. 2012;9:199–208. doi: 10.1016/j.hrthm.2011.09.004. [DOI] [PubMed] [Google Scholar]


