Abstract
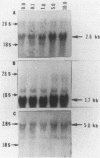
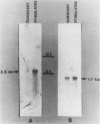
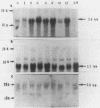
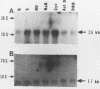
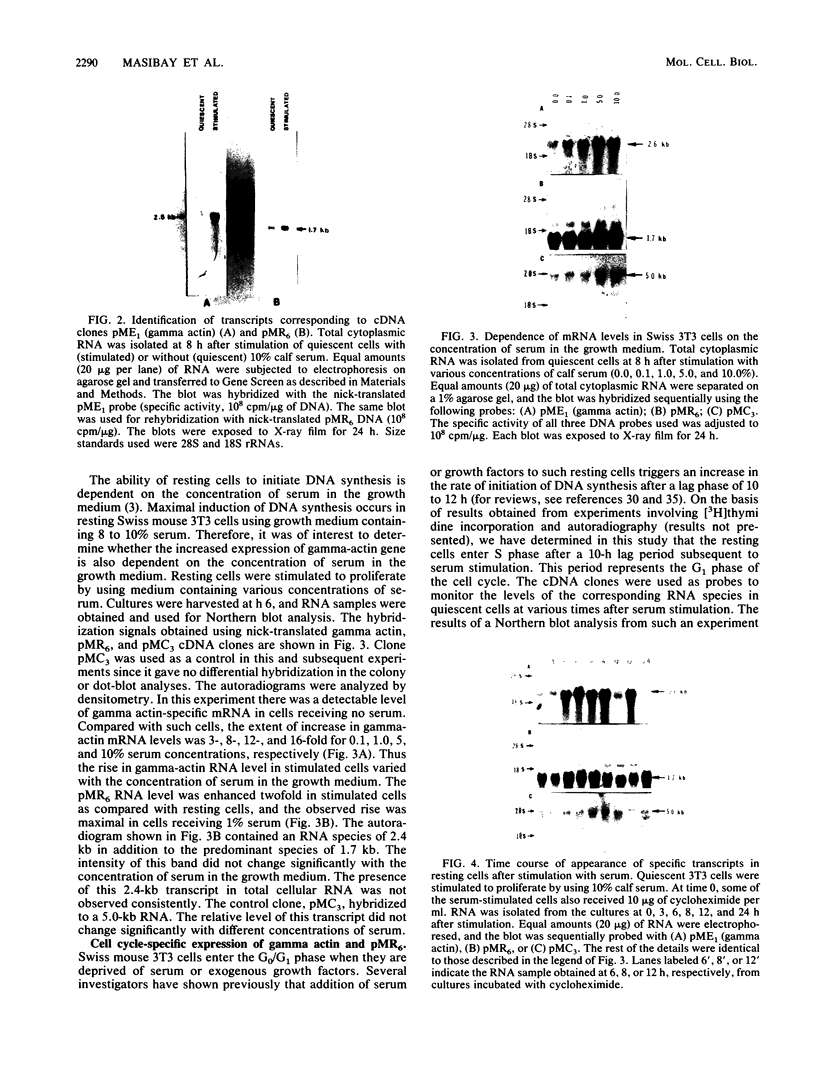
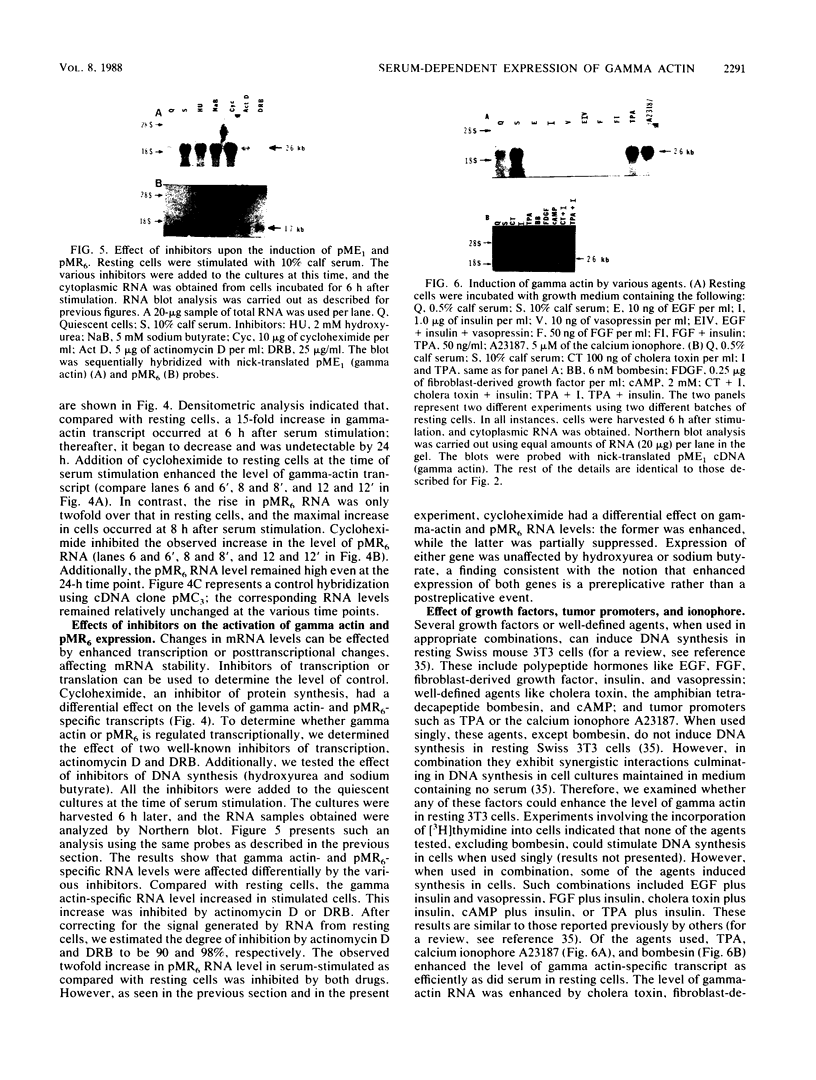
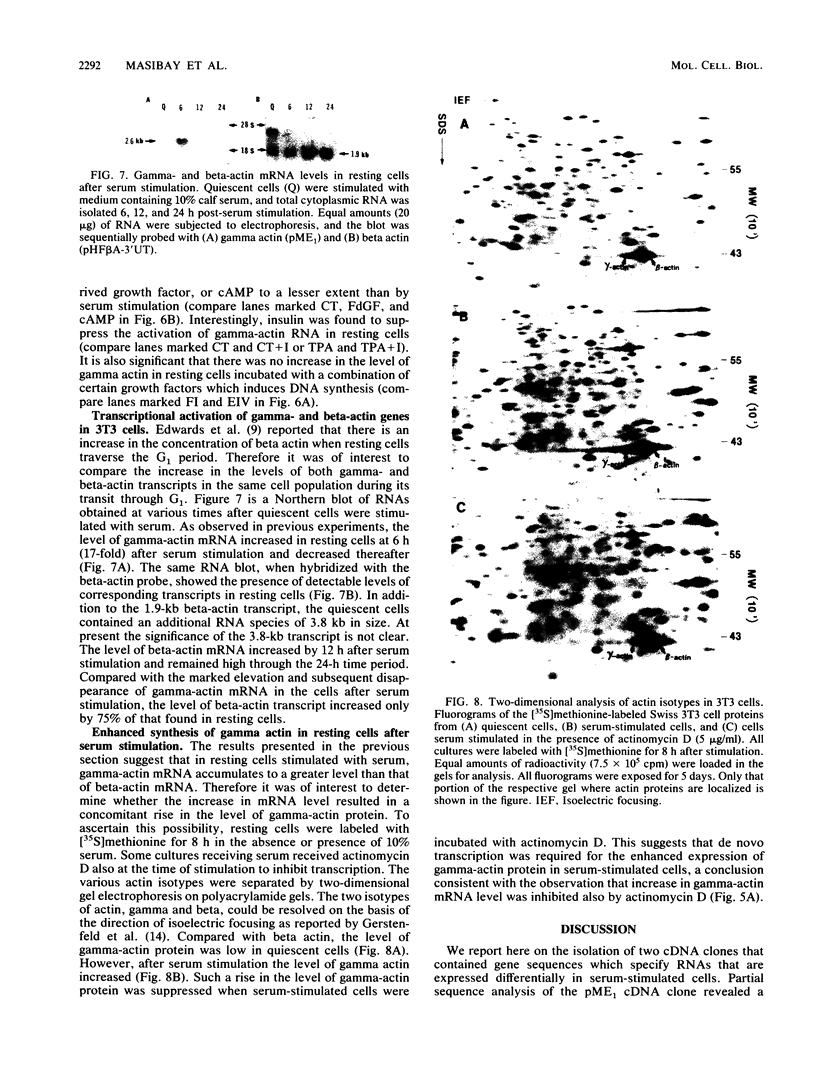
We isolated cDNA clones that represent genes whose expression is enhanced when resting Swiss mouse 3T3 cells are stimulated to proliferate with serum. Two clones (designated pME1 and pMR6) were analyzed further. A partial sequence analysis of the pME1 insert DNA indicated that it contained a 104-base-pair stretch with extensive homology to the 3' untranslated region of gamma actin. Similar analysis of the insert DNA from the pMR6 clone indicated that it did not correspond to any previously reported gene sequence. We used the pME1 clone as a probe to determine the level of gamma actin-specific transcript in 3T3 cells under a variety of conditions. The level of gamma actin-specific mRNA began to increase in resting cells upon serum stimulation and reached a peak at 6 h. Thereafter its level declined, and by 24 h it was hardly detectable. In contrast, pMR6-specific transcript was detectable in resting cells but remained elevated even at 24 h poststimulation. The level of gamma-actin mRNA was elevated in resting cells by 12-O-tetradecanoylphorbol-13-acetate, calcium ionophore A23187, and bombesin and to a lesser extent by cholera toxin, fibroblast-derived growth factor, and dibutyryl cyclic AMP. However, insulin, vasopressin, or epidermal growth factor failed to enhance gamma-actin mRNA levels in resting cells. Inhibitors of transcription diminished the induction of gamma-actin mRNA. Gamma-actin gene was superinduced in serum-stimulated cells by cycloheximide, an inhibitor of translation. Analysis of proteins from serum-stimulated cells by two-dimensional gel electrophoresis indicated that enhanced transcription of gamma-actin mRNA resulted in a concomitant increase in the corresponding actin protein. The possible role of gamma actin, a component of the cytoskeleton, in the regulation of cell growth is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R. F. The kinetics of serum-induced initiation of DNA synthesis in BHK 21/C13 cells, and the influence of exogenous adenosine. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):369–377. doi: 10.1002/jcp.1040860409. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou C. C., Davis R. C., Fuller M. L., Slovin J. P., Wong A., Wright J., Kania S., Shaked R., Gatti R. A., Salser W. A. Gamma-actin: unusual mRNA 3'-untranslated sequence conservation and amino acid substitutions that may be cancer related. Proc Natl Acad Sci U S A. 1987 May;84(9):2575–2579. doi: 10.1073/pnas.84.9.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Edwards D. R., Parfett C. L., Denhardt D. T. Transcriptional regulation of two serum-induced RNAs in mouse fibroblasts: equivalence of one species to B2 repetitive elements. Mol Cell Biol. 1985 Nov;5(11):3280–3288. doi: 10.1128/mcb.5.11.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Waterhouse P., Holman M. L., Denhardt D. T. A growth-responsive gene (16C8) in normal mouse fibroblasts homologous to a human collagenase inhibitor with erythroid-potentiating activity: evidence for inducible and constitutive transcripts. Nucleic Acids Res. 1986 Nov 25;14(22):8863–8878. doi: 10.1093/nar/14.22.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Battini R., Kaczmarek L., Rittling S., Calabretta B., de Riel J. K., Philiponis V., Wei J. F., Baserga R. Coding sequence and growth regulation of the human vimentin gene. Mol Cell Biol. 1986 Nov;6(11):3614–3620. doi: 10.1128/mcb.6.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedkin M., Legg A., Rozengurt E. Antitubulin agents enhance the stimulation of DNA synthesis by polypeptide growth factors in 3T3 mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3909–3912. doi: 10.1073/pnas.76.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates B. J., Friedkin M. Mid-G1 marker protein(s) in 3T3 mouse fibroblast cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4959–4961. doi: 10.1073/pnas.75.10.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenfeld L. C., Finer M. H., Boedtker H. Altered beta-actin gene expression in phorbol myristate acetate-treated chondrocytes and fibroblasts. Mol Cell Biol. 1985 Jun;5(6):1425–1433. doi: 10.1128/mcb.5.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R. R., Aller P., Yuan Z. A., Gibson C. W., Baserga R. Cell-cycle-specific cDNAs from mammalian cells temperature sensitive for growth. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6004–6008. doi: 10.1073/pnas.81.19.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader D. P., Gall I., Lehrach H. The structure of a cloned mouse gamma-actin processed pseudogene. Gene. 1985;36(3):369–374. doi: 10.1016/0378-1119(85)90193-3. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Lee S. J., Ogren L., Talamantes F., Nathans D. Identification of proliferin mRNA and protein in mouse placenta. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4356–4359. doi: 10.1073/pnas.82.13.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Nucleotide sequence of a growth-related mRNA encoding a member of the prolactin-growth hormone family. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4255–4259. doi: 10.1073/pnas.81.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness P. F., Walsh R. C., Jr Dihydrocytochalasin B disorganizes actin cytoarchitecture and inhibits initiation of DNA synthesis in 3T3 cells. Cell. 1982 Aug;30(1):253–262. doi: 10.1016/0092-8674(82)90031-9. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Edelman G. M. Density-dependent stimulation and inhibition of cell growth by agents that disrupt microtubules. Proc Natl Acad Sci U S A. 1980 May;77(5):2748–2752. doi: 10.1073/pnas.77.5.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto A. M., Zumbé A., Gibson L., Kubler A. M., Jimenez de Asua L. Cytoskeleton-disrupting drugs enhance effect of growth factors and hormones on initiation of DNA synthesis. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6435–6438. doi: 10.1073/pnas.76.12.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle V. G., Dubrow R., Pardee A. B. Changes in the synthesis of actin and other cell proteins after stimulation of serum-arrested cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1298–1302. doi: 10.1073/pnas.76.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rittling S. R., Gibson C. W., Ferrari S., Baserga R. The effect of cycloheximide on the expression of cell cycle dependent genes. Biochem Biophys Res Commun. 1985 Oct 15;132(1):327–335. doi: 10.1016/0006-291x(85)91026-5. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T., Rozengurt E., Taylor-Papadimitriou J., Burchell J. Differential effect of interferon on DNA synthesis, 2-deoxyglucose uptake and ornithine decarboxylase activity in 3T3 cells stimulated by polypeptide growth factors and tumor promotors. J Cell Physiol. 1980 Jul;104(1):1–9. doi: 10.1002/jcp.1041040102. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. H., Distel R. J., Hecht N. B. Mouse testes contain two size classes of actin mRNA that are differentially expressed during spermatogenesis. Mol Cell Biol. 1985 Jul;5(7):1649–1654. doi: 10.1128/mcb.5.7.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]