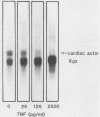
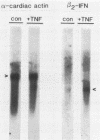
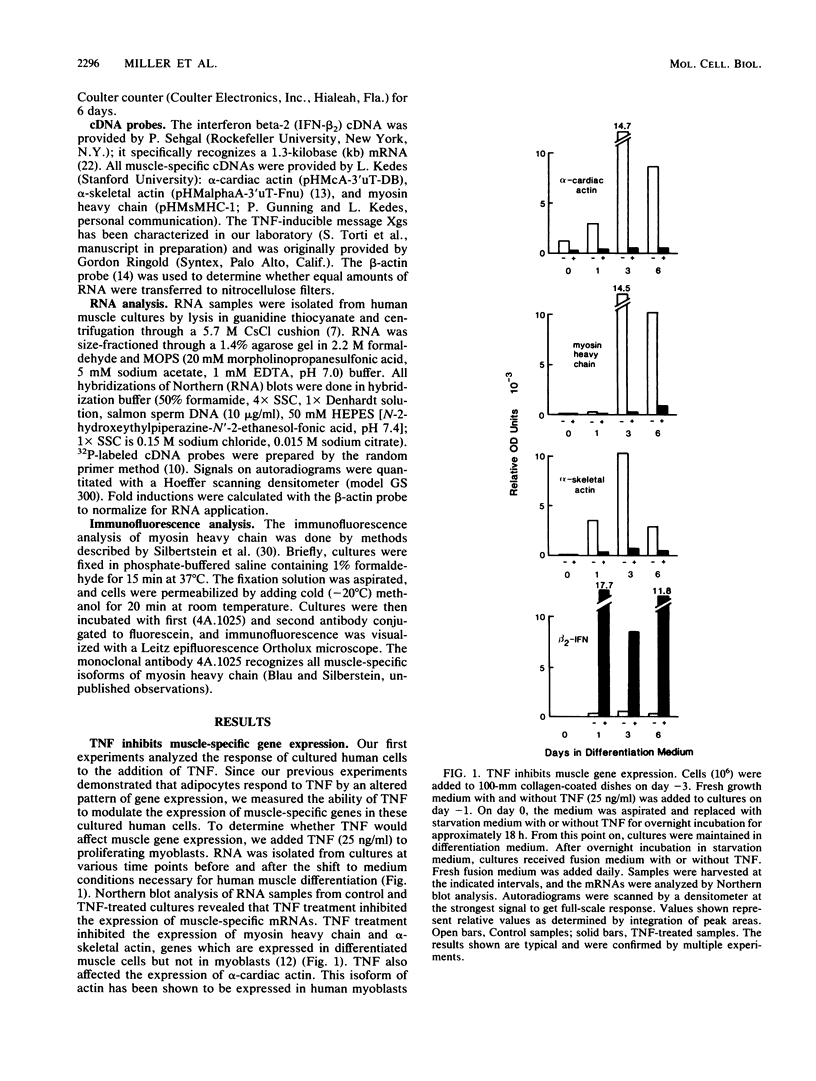
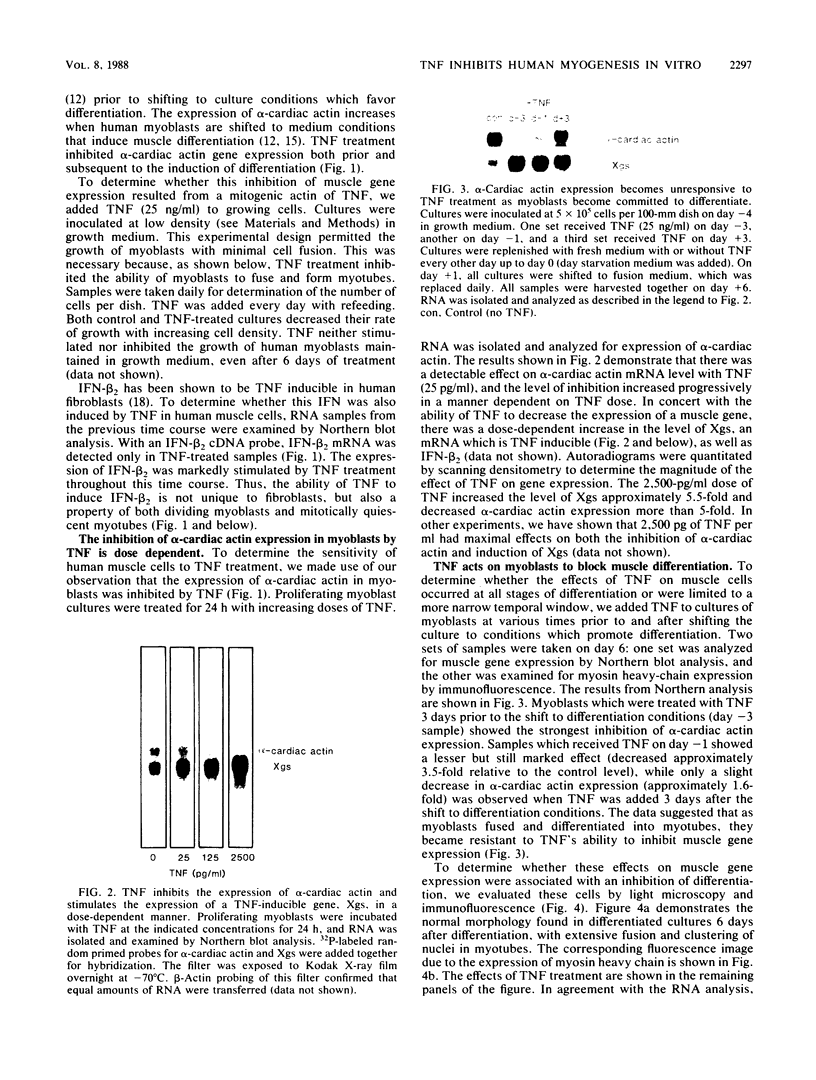
Abstract
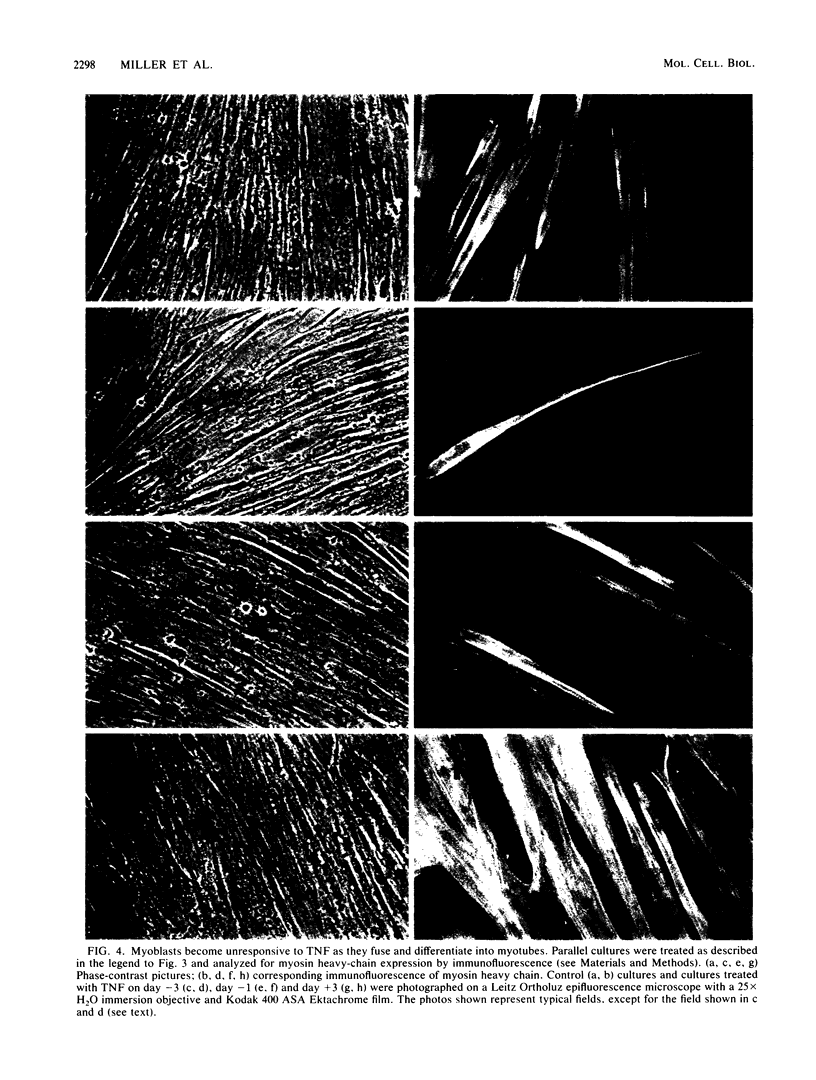
We examined the effects of human recombinant tumor necrosis factor-alpha (TNF) on human primary myoblasts. When added to proliferating myoblasts, TNF inhibited the expression of alpha-cardiac actin, a muscle-specific gene whose expression is observed at low levels in human myoblasts. TNF also inhibited muscle differentiation as measured by several parameters, including cell fusion and the expression of other muscle-specific genes, such as alpha-skeletal actin and myosin heavy chain. Muscle cells were sensitive to TNF in a narrow temporal window of differentiation. Northern (RNA) blot and immunofluorescence analyses revealed that human muscle gene expression became unresponsive to TNF coincident with myoblast differentiation. When TNF was added to differentiated myotubes, there was no effect on muscle gene expression. In contrast, TNF-inducible mRNAs such as interferon beta-2 still responded, suggesting that the signal mediated by TNF binding to its receptor had no effect on muscle-specific genes after differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bains W., Ponte P., Blau H., Kedes L. Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol Cell Biol. 1984 Aug;4(8):1449–1453. doi: 10.1128/mcb.4.8.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin E., Kayalar C. Metalloendoprotease inhibitors that block fusion also prevent biochemical differentiation in L6 myoblasts. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8029–8033. doi: 10.1073/pnas.83.21.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Epstein C. J. Manipulation of myogenesis in vitro: reversible inhibition by DMSO. Cell. 1979 May;17(1):95–108. doi: 10.1016/0092-8674(79)90298-8. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Creasey A. A., Reynolds M. T., Laird W. Cures and partial regression of murine and human tumors by recombinant human tumor necrosis factor. Cancer Res. 1986 Nov;46(11):5687–5690. [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Three types of muscle-specific gene expression in fusion-blocked rat skeletal muscle cells: translational control in EGTA-treated cells. Cell. 1987 May 22;49(4):515–526. doi: 10.1016/0092-8674(87)90454-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Hardeman E., Wade R., Ponte P., Bains W., Blau H. M., Kedes L. Differential patterns of transcript accumulation during human myogenesis. Mol Cell Biol. 1987 Nov;7(11):4100–4114. doi: 10.1128/mcb.7.11.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Mohun T., Ng S. Y., Ponte P., Kedes L. Evolution of the human sarcomeric-actin genes: evidence for units of selection within the 3' untranslated regions of the mRNAs. J Mol Evol. 1984;20(3-4):202–214. doi: 10.1007/BF02104727. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeman E. C., Chiu C. P., Minty A., Blau H. M. The pattern of actin expression in human fibroblast x mouse muscle heterokaryons suggests that human muscle regulatory factors are produced. Cell. 1986 Oct 10;47(1):123–130. doi: 10.1016/0092-8674(86)90373-9. [DOI] [PubMed] [Google Scholar]
- Kettelhut I. C., Fiers W., Goldberg A. L. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4273–4277. doi: 10.1073/pnas.84.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Krönke M., Schlüter C., Pfizenmaier K. Tumor necrosis factor inhibits MYC expression in HL-60 cells at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1987 Jan;84(2):469–473. doi: 10.1073/pnas.84.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. D., Zentella A., Vine W., Pekala P. H., Cerami A. Effect of endotoxin-induced monokines on glucose metabolism in the muscle cell line L6. Proc Natl Acad Sci U S A. 1987 May;84(9):2590–2594. doi: 10.1073/pnas.84.9.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L. T., Helfgott D. C., Sehgal P. B. Anti-beta-interferon antibodies inhibit the increased expression of HLA-B7 mRNA in tumor necrosis factor-treated human fibroblasts: structural studies of the beta 2 interferon involved. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8957–8961. doi: 10.1073/pnas.83.23.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Stockdale F. E. Developmental regulation of the multiple myogenic cell lineages of the avian embryo. J Cell Biol. 1986 Dec;103(6 Pt 1):2197–2208. doi: 10.1083/jcb.103.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Blau H., Kedes L. Two-level regulation of cardiac actin gene transcription: muscle-specific modulating factors can accumulate before gene activation. Mol Cell Biol. 1986 Jun;6(6):2137–2148. doi: 10.1128/mcb.6.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. T., Medford R. M., Nadal-Ginard B. Reversibility of muscle differentiation in the absence of commitment: analysis of a myogenic cell line temperature-sensitive for commitment. Cell. 1983 Aug;34(1):281–293. doi: 10.1016/0092-8674(83)90159-9. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Holtzer H. Myogenesis: fusion, myosin synthesis, and the mitotic cycle. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1484–1490. doi: 10.1073/pnas.56.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Sternberg E., Hu J. S., Spizz G., Wilcox C. Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol. 1986 Nov;103(5):1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala P. H., Kawakami M., Angus C. W., Lane M. D., Cerami A. Selective inhibition of synthesis of enzymes for de novo fatty acid biosynthesis by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2743–2747. doi: 10.1073/pnas.80.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Silberstein L., Webster S. G., Travis M., Blau H. M. Developmental progression of myosin gene expression in cultured muscle cells. Cell. 1986 Sep 26;46(7):1075–1081. doi: 10.1016/0092-8674(86)90707-5. [DOI] [PubMed] [Google Scholar]
- Spizz G., Roman D., Strauss A., Olson E. N. Serum and fibroblast growth factor inhibit myogenic differentiation through a mechanism dependent on protein synthesis and independent of cell proliferation. J Biol Chem. 1986 Jul 15;261(20):9483–9488. [PubMed] [Google Scholar]
- Takeda K., Iwamoto S., Sugimoto H., Takuma T., Kawatani N., Noda M., Masaki A., Morise H., Arimura H., Konno K. Identity of differentiation inducing factor and tumour necrosis factor. 1986 Sep 25-Oct 1Nature. 323(6086):338–340. doi: 10.1038/323338a0. [DOI] [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Beutler B., Cerami A., Albert J. D., Shires G. T. Cachectin/tumor necrosis factor mediates changes of skeletal muscle plasma membrane potential. J Exp Med. 1986 Oct 1;164(4):1368–1373. doi: 10.1084/jem.164.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Ritter M. A. Surface antigen differentiation during human myogenesis in culture. Nature. 1981 Jan 1;289(5793):60–64. doi: 10.1038/289060a0. [DOI] [PubMed] [Google Scholar]
- Webster C., Filippi G., Rinaldi A., Mastropaolo C., Tondi M., Siniscalco M., Blau H. M. The myoblast defect identified in Duchenne muscular dystrophy is not a primary expression of the DMD mutation. Clonal analysis of myoblasts from five double heterozygotes for two X-linked loci: DMD and G6PD. Hum Genet. 1986 Sep;74(1):74–80. doi: 10.1007/BF00278789. [DOI] [PubMed] [Google Scholar]