Abstract
Background:
Atherosclerosis is the leading cause of death in hemodialysis patients. These patients are also very prone to L-carnitine deficiency due to kidney disease. In this clinical trial, we investigated the effect of oral L-carnitine on endothelial function of these patients.
Materials ans Methods:
We studied 31 adult chronic hemodialysis patients in our center and divided them into two groups. The first group (n = 20) received 1500 mg/dialysis interval (every other day) oral L-carnitine. The control group (n = 11) received placebo for one month. Ultrasonographic measurements of flow mediated dilation and carotid intima-media thickness were performed before and after one month of L-carnitine and placebo therapy.
Results:
This study showed that after one month of L-carnitine or placebo therapy there was no significant improvement in flow mediated dilation (p = 0.80 and p = 0.59, respectively) or decrease in carotid intima-media thickness (p = 0.12 and p = 0.50, respectively).
Conclusions:
Our study revealed that one month of oral L-carnitine therapy did not improve endothelial function in hemodialysis patients. Long-term studies with large sample size using intravenous form and higher doses of the drug are required to clarify the questionable role of L-carnitine in hemodialysis patients.
Keywords: Flow Mediated Dilation, Carotid Intima-Media Thickness, Endothelial Dysfunction, Chronic Renal Failure, L-Carnitine
INTRODUCTION
Atherosclerosis advances in renal failure and develops early in the course of renal dysfunction. Therefore, the consequences of atherosclerosis especially cardiovascular events represent a major clinical problem in these patients.[1] Cardiac death accounts for approximately 40-50% of all deaths and is the leading cause of death in these patients. Death from cardiovascular causes is up to 20 times more common in uremic patients than in the general population with the risk being even higher than in patients with diabetes mellitus.[1]
Endothelial dysfunction (ED) seems to be the underlying cause of all vascular diseases. Accumulating evidence suggests that chronic renal failure (CRF) is associated with impaired endothelial cell function. ED is recognized as a key process in acute and chronic renal failure as well as end stage renal disease (ESRD) of all causes.[2]
ED and intima-media thickness (IMT) are predictors for the development and progression of atherosclerosis.[3] The central role of endothelium in the development of vascular disease has led to identification of new relevant methods to estimate endothelial function and injury. Flow-mediated dilatation (FMD) is the most widely used noninvasive ultrasound method to assess endothelial function.[4]
Measurement of ultrasound-based FMD in the brachial artery is noninvasive and relatively repeatable and reproducible, reflects important biology, and is useful in serial studies of disease reversibility. Brachial artery endothelium-dependent dilatation is also significantly correlated with findings in the coronary circulation in the same patients.[5,6]
L-carnitine is a naturally occurring hydrophilic amino acid derivative, produced endogenously in the kidneys and liver and derived from meat and dairy products in the diet. It plays an essential role in the transfer of long-chain fatty acids into the mitochondria for beta-oxidation.[7]
L-carnitine and short-chain acylcarnitines (esters of L-carnitine), such as acetyl-L-carnitine, are excreted by the kidneys. Renal reabsorption of L-carnitine is normally very efficient. In fact, an estimated 95% is thought to be reabsorbed by the kidneys.[7]
Patients with renal disease who undergo hemodialysis are at risk for secondary carnitine deficiency.
L-carnitine and many of its precursors are removed from the circulation during hemodialysis. Impaired L-carnitine synthesis by the kidneys may also contribute to the potential for carnitine deficiency in patients with end-stage renal failure undergoing hemodialysis.[7]
As Guarnieri et al. noted, carnitine supplementation has been approved by the US Food and Drug Administration not only for the treatment, but also for the prevention of carnitine depletion in dialysis patients.[8]
Previous studies have shown L-carnitine and its propionate to improve endothelial responses in animal models. L-carnitine has also been found to prevent the progression of atherosclerotic lesions. Endogenous carnitine depletion and/or carnitine deficiency should thus be viewed as an additional risk factor in atherogenesis.[9,10]
However, many of the previous studies were performed on small numbers of patients. They were not well-controlled and did not include patients with CRF, although atherosclerosis and its related complications are the leading cause of mortality in CRF.
To the best of our knowledge, this is the first study to evaluate the role of oral L-carnitine supplementation in improving endothelial function. For this purpose, FMD and carotid IMT in CRF patients were measured and compared with the values in a control group of patients with CRF who received placebo.
MATERIALS AND METHODS
In a randomized double-blind, placebo-controlled trial, patients were recruited from the maintenance HD (Hemodialysis) population at Shafa Hospital, and Javadolaemeh and Samenolaemeh centers (Kerman, Iran) in 2011. This study was approved by research and ethics board at Kerman University of Medical Sciences, Kerman, Iran. Informed written consents were also obtained from all subjects.
The number of cases was computed by NCSS statistical software using paired t-test. From previous studies, mean FMD changes before and after the intervention were considered as 7.5 and 6, respectively. Standard deviation (SD) was estimated as 2.5. Type I error probability (α) of 0.05, β of 20%, and power of 80% were also considered. Number of subjects in each group was thus calculated as 24 patients.
Patients had been on hemodialysis 3 times weekly and for a period of at least 3 months. We excluded patients with infection, inflammation, incomplete or irregular drug or placebo intake, or arteriovenous fistula (AV) fistula for dialysis in both upper extremities. Smokers and individuals who received any new vasodilator agent during the study were also excluded. During the study period, we followed the patients for effective dialysis as scheduled and for any changes in their prescribed medication. Predialysis FMD and carotid IMT measurement by ultrasound were performed by the same operator in the L-carnitine and placebo groups before starting therapy and after one month of therapy.
The patients were randomized to receive L-carnitine therapy or placebo. We administered 1500 mg oral L-carnitine or placebo between dialysis sessions in 3 divided doses in the mornings and nights except for the morning of days they underwent dialysis. All adverse effects during the course of the study in both case and control groups were recorded.
Measurement of Flow-Mediated Vasodilatation
Using the guidelines suggested by Corretti et al.,[11] ultrasound assessment of endothelial-dependent FMD of brachial artery was investigated one hour before starting scheduled dialysis. Briefly, the study was performed in a temperature-controlled room (22°C) with the subjects in a resting and supine position.
Brachial artery diameter was imaged using a 7.5-Mhz linear array transducer ultrasound system (Siemens Company). The brachial artery was imaged at a location 3-7 cm above the antecubital crease. To create a flow stimulus in the brachial artery, a sphygmomanometer cuff was placed on the forearm and the cuff was inflated 50 mmHg above systolic pressure to occlude artery flow for 5 minutes. All measurements were made at the end of diastole. The FMD was expressed as the percentage of change in post-stimulus diameter of the artery compared to the baseline diameter.
Measurement of carotid IMT
Longitudinal ultrasonographic scans of the carotid artery were obtained on the day of brachial artery reactivity assessment by FMD. The scans included the evaluation of the right common carotid artery 1 cm proximal to the carotid bulb. In each examination, the same operator used different scanning angles to identify the greatest IMT, defined as the distance between the junction of the lumen and intima and that of the media and adventitia in the far wall of the carotid artery. Finally, 3 measurements of carotid IMT were obtained from the right carotid artery and were averaged to determine the mean carotid IMT. Carotid IMT was expressed in millimeters.
Randomization and blinding
A person, not involved in the study, assigned codes to the study groups and randomly allocated the selected participants to receive oral placebo or L-carnitine (1500 mg/day). The randomization was carried out by a procedure based on a random numeric sequence. The authors were unaware of treatment allocation.
Statistical methods
All analyses were carried out using SPSS13.0 (SPSS Inc., Chicago, IL, US). Categorical variables are reported as counts (percentage) and continuous variables as means ± SD unless otherwise indicated.
To investigate the parameters in each group before and after therapy, t-test was used and to compare the data between different groups, analysis of variance (ANOVA) was performed. P values less than 0.05 were considered statistically significant.
RESULTS
The calculated sample size in each group was 24 patients. However, since this trial was interventional and there was the possibility of interrupting the course of the treatment by patients or due to medication side effects, we started the study with 60 adult chronic hemodialysis patients and divided them randomly into two equal groups of L-carnitine and placebo. Only 20 patients in the L-carnitine and 11 in the placebo group continued the medication for one month and the rest of the cases were excluded from the study. The compliance in carnitine group was higher than that in the placebo group.
The most common reason for discontinuing the drugs was their side effects, such as nausea and vomiting that were interestingly much more common in the placebo group (25% vs. 8%). The placebo was made exactly with the same basic ingredients and in the shape of L-carnitine tablets without including the L-carnitine component.
Almost all patients were under multi-drug therapy for different complications of CRF. Therefore, the second common reason of not completing the course of therapy was that they were just tired of taking too many tablets.
Finally, the data was obtained from 20 patients in the L-carnitine and 11 patients in the placebo groups. Sex and age distribution were comparable in the L-carnitine and control groups (male/female ratios of 16/4 and 7/4, respectively; p = 0.281 and 48.4 ± 13.8 and 51.2 ± 15.0 years old, respectively; p = 0.595). The duration of hemodialysis in the L-carnitine group was insignificantly longer than the placebo group (6.18 ± 6.73 vs. 2.95 ± 2.28 years; p = 0.394).
Antihypertensive agents, mostly angiotensin converting enzyme inhibitors, were used regularly by 60% of the patients in the L-carnitine group and 80% of the patients in the placebo group.
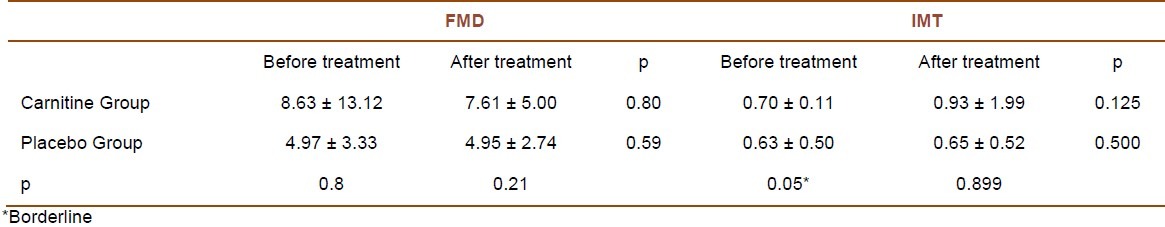
At the beginning of the study, FMD values measured in the L-carnitine and placebo groups were 8.63 ± 13.12 and 4.97 ± 3.33, respectively (p = 0.634). As Table 1 shows, FMD and carotid IMT values at the beginning and one month after the treatment were comparable, i.e. there was no statistically significant improvement in FMD or carotid IMT after 1 month of therapy. The difference between the two groups in the measured carotid IMT values at the beginning of the study was borderline (p = 0.05). Mann-Whitney test on FMD changes after one month of L-carnitine or placebo therapy showed the changes in the L-carnitine and placebo groups to be -1.019 and -0.0195, respectively (p = 0.71).
Table 1.
Flow mediated dilation (FMD) and carotid intima-media thickness (IMT) changes in L-carnitine and placebo groups, before and after treatment
Diabetics constituted 25% and 63% of the L-carnitine and placebo groups, respectively. In the beginning of the trial, hemoglobin A1c (HbA1c) was measured in diabetic patients. Considering 7% or less as normal HbA1c levels, only 41% of the patients had well-controlled diabetes mellitus. The influences of L-carnitine therapy on FMD in diabetic patients and also in nondiabetic patients were evaluated separately. The changes were not statistically significant in either diabetic patients (p = 0.625) or in nondiabetics (p = 0.678). Although at the beginning of the study, the measured FMD in the diabetic patients was lower than nondiabetics, the difference was not statistically significant (4.15 ± 3.38 vs. 6.84 ± 4.29, p = 0.108). In addition, there was no statistically significant difference in response after the therapy.
Likewise, the chronicity of hemodialysis did not affect FMD changes by L-carnitine, as it was studied in patients with L-carnitine treatment who were under dialysis for less than or equal to 2 years (9.93 ± 18.25 and 6.43 ± 4.48; p = 0.508), and those with more than two years of initiating dialysis (6.09 ± 3.79 and 6.77 ± 4.58; p = 0.815).
While 25% of the patients in the L-carnitine group reported a sense of well being following taking the medication, no patient in the placebo group had such a feeling.
Loffredo et al. measured FMD in 40 normal patients with a mean age of 64.2 ± 11.2 years. They indicated the mean percentage changes of FMD as 10.34 ± 2.14.[12] We compared it with the mean percentage changes of FMD measured in CRF patients (5.57 ± 4.58) at the beginning of our study (with removing just one value that seemed to be out of the normal range) and the difference was statistically significant (p < 0.005). In other words, FMD was impaired in CRF patients compared to the normal population.
DISCUSSION
This study demonstrated that one month of oral L-carnitine supplementation was not associated with improvement in endothelial function as measured by FMD and carotid IMT in CRF patients on chronic hemodialysis. In fact, our data indicated that the disease had a progressive course and L-carnitine could not influence on it significantly.
A number of studies have been conducted over the past years to assess the efficacy of supplemental L-carnitine on improving some clinical complaints of CRF patients. However, there has been no trial regarding the role of L-carnitine on the endothelial function in these patients. Considering the high prevalence of atherosclerosis and related complications in CRF patients, we conducted our study to investigate this concept.
Stasi et al.[13] and McMackin et al.[14] demonstrated that propionyl-L-Carnitine accelerated blood flow recovery and the restoration of vascular function in animal models. Loffredo et al. showed that propionyl-L-carnitine infusion was associated with increased FMD in patients with peripheral vascular disease.[12] In another study, Volek et al. reported that L-carnitine supplementation improved FMD in healthy individuals after high fat meals (which has been shown to cause impairment of vascular health by reducing FMD).[15] Silvestro et al. concluded that in patients with intermittent claudication, propionyl-L-carnitine administration provided a protective effect against deterioration of FMD and increase of soluble vascular cell adhesion molecule-1 (sVCAM-1) induced by exercise.[16]
Previous studies in animal models have shown that L-carnitine and its propionate improved endothelial responses by decreasing O2 production and increasing nitric oxide (NO) availability. They also suggested that L-carnitine prevents the progression of atherosclerotic lesions and endogenous carnitine depletion and/or deficiency should thus be viewed as an additional risk factor in atherogenesis.[9,10]
There has been no previous study to investigate the role of L-carnitine therapy on improvement of endothelial function in CRF patients. In this clinical trial, we found that L-carnitine did not change FMD or carotid IMT in CRF patients after one month of therapy. Some explanations can justify this finding. Considering the risk of accumulation of toxic metabolites of L-carnitine such as trimethylamine (TMA) in CRF patients, we prescribed 1500 mg of oral L-carnitine supplementation between dialysis sessions and only for one month. However, the bioavailability of oral L-carnitine is about 15%.[17,18] Though the recommended form of L-carnitine in ESRD is the intravenous form, unfortunately it does not exist in the country of study (Iran). Therefore, the oral form and the relatively short duration of prescription of L-carnitine might have influenced on our results. Further clinical trials with intravenous form of L-carnitine should be performed to increase not only the bioavailability of the drug, but also the compliance of the patients. There will be also no risk of toxic metabolites accumulation with prescribing the intravenous form.
On the other hand, most CRF patients receive antihypertensive agents, especially angiotensin converting enzyme inhibitors that mediate NO-related vasodilatation and increase baseline vessel diameter which in turn leads to smaller estimates of FMD including the brachial artery FMD. This might have effects on the percentage of changes of FMD after therapy.[19]
Moreover, since most of the time a chronic insidious disease such as diabetes mellitus leads to CRF, well-established atherosclerosis is usually encountered at the time of starting dialysis and this might have a marked influence on patients’ response to L-carnitine.
Based on Stephens et al., carbohydrate rich foods, which induce a state of hyperinsulinemia, appear to improve carnitine transport into the skeletal muscles.[20] Bloomer et al. suggested that this may also be true as related to uptake into endothelial cells.[21] If approved, considering the fact that the most common cause of CRF in adults is diabetes mellitus, abnormal insulin secretion and resistance to insulin, may minimize the effect of L-carnitine in the patients.
We conclude that the role of L-carnitine in chronic hemodialysis patients is still questionable. Our clinical trial demonstrated no significant improvement in endothelial function in these patients after one month of therapy. Additional randomized controlled clinical trials with larger sample sizes and sufficient power may lead to a better understanding of the possible beneficial effects of L-carnitine on hemodialysis patients.
ACKNOWLEDGMENT
We are very grateful to Roya Safari Faramani and Dr Jafar Ahmadi for statistical analysis of the study. We wish to thank Shahr-e-Daroo Company and Maghsoud Jafarzadeh for providing us with both L-carnitine and the placebo. Many thanks also go to Pooran Bahreiny, Alireza Eslami, Maryam Asgari, and Maryam Foroutan for coordination and laboratory work.
Footnotes
Source of Support: This study was funded by Kerman University of Medical Sciences, Kerman, Iran (research project number: 90189).
Conflict of Interest: None declared.
REFERENCES
- 1.Campean V, Neureiter D, Varga I, Runk F, Reiman A, Garlichs C, et al. Atherosclerosis and vascular calcification in chronic renal failure. Kidney Blood Press Res. 2005;28(5-6):280–9. doi: 10.1159/000090182. [DOI] [PubMed] [Google Scholar]
- 2.Segal MS, Baylis C, Johnson RJ. Endothelial health and diversity in the kidney. J Am Soc Nephrol. 2006;17(2):323–4. doi: 10.1681/ASN.2005121296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kocaman O, Oflaz H, Yekeler E, Dursun M, Erdogan D, Demirel S, et al. Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2004;43(5):854–60. doi: 10.1053/j.ajkd.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Santoro D, Bellinghieri G, Conti G, Pazzano D, Satta E, Costantino G, et al. Endothelial dysfunction in chronic renal failure. J Ren Nutr. 2010;20(5 Suppl):S103–S108. doi: 10.1053/j.jrn.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109(16):1981–6. doi: 10.1161/01.CIR.0000126599.47470.BE. [DOI] [PubMed] [Google Scholar]
- 6.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–41. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 7.Scaglia F. Carnitine deficiency [Online] 2010. [cited 2010 Apr 13]. Available from: URL: http://emedicine.medscape.com/article/942233-overview .
- 8.Guarnieri G, Situlin R, Biolo G. Carnitine metabolism in uremia. Am J Kidney Dis. 2001;38(4 Suppl 1):S63–S67. doi: 10.1053/ajkd.2001.27408. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez de SM, Bueno R, Perez-Guerrero C, Herrera MD. Effect of L-carnitine and propionyl-L-carnitine on endothelial function of small mesenteric arteries from SHR. J Vasc Res. 2007;44(5):354–64. doi: 10.1159/000102303. [DOI] [PubMed] [Google Scholar]
- 10.Sayed-Ahmed MM, Khattab MM, Gad MZ, Mostafa N. L-carnitine prevents the progression of atherosclerotic lesions in hypercholesterolaemic rabbits. Pharmacol Res. 2001;44(3):235–42. doi: 10.1006/phrs.2001.0852. [DOI] [PubMed] [Google Scholar]
- 11.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 12.Loffredo L, Marcoccia A, Pignatelli P, Andreozzi P, Borgia MC, Cangemi R, et al. Oxidative-stress-mediated arterial dysfunction in patients with peripheral arterial disease. Eur Heart J. 2007;28(5):608–12. doi: 10.1093/eurheartj/ehl533. [DOI] [PubMed] [Google Scholar]
- 13.Stasi MA, Scioli MG, Arcuri G, Mattera GG, Lombardo K, Marcellini M, et al. Propionyl-L-carnitine improves postischemic blood flow recovery and arteriogenetic revascularization and reduces endothelial NADPH-oxidase 4-mediated superoxide production. Arterioscler Thromb Vasc Biol. 2010;30(3):426–35. doi: 10.1161/ATVBAHA.109.201533. [DOI] [PubMed] [Google Scholar]
- 14.McMackin CJ, Widlansky ME, Hamburg NM, Huang AL, Weller S, Holbrook M, et al. Effect of combined treatment with alpha-Lipoic acid and acetyl-L-carnitine on vascular function and blood pressure in patients with coronary artery disease. J Clin Hypertens (Greenwich) 2007;9(4):249–55. doi: 10.1111/j.1524-6175.2007.06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volek JS, Judelson DA, Silvestre R, Yamamoto LM, Spiering BA, Hatfield DL, et al. Effects of carnitine supplementation on flow-mediated dilation and vascular inflammatory responses to a high-fat meal in healthy young adults. Am J Cardiol. 2008;102(10):1413–7. doi: 10.1016/j.amjcard.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Silvestro A, Schiano V, Bucur R, Brevetti G, Scopacasa F, Chiariello M. Effect of propionylcarnitine on changes in endothelial function and plasma levels of adhesion molecules induced by acute exercise in patients with intermittent claudication. Angiology. 2006;57(2):145–54. doi: 10.1177/000331970605700203. [DOI] [PubMed] [Google Scholar]
- 17.Evans AM, Fornasini G. Pharmacokinetics of L-carnitine. Clin Pharmacokinet. 2003;42(11):941–67. doi: 10.2165/00003088-200342110-00002. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber B. Safety of oral carnitine in dialysis patients. Semin Dial. 2002;15(1):71–2. doi: 10.1046/j.1525-139x.2002.0020a.x. [DOI] [PubMed] [Google Scholar]
- 19.Shahin Y, Khan JA, Samuel N, Chetter I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta-analysis of randomised controlled trials. Atherosclerosis. 2011;216(1):7–16. doi: 10.1016/j.atherosclerosis.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. Insulin stimulates L-carnitine accumulation in human skeletal muscle. FASEB J. 2006;20(2):377–9. doi: 10.1096/fj.05-4985fje. [DOI] [PubMed] [Google Scholar]
- 21.Bloomer RJ, Smith WA, Fisher-Wellman KH. Glycine propionyl-L-carnitine increases plasma nitrate/nitrite in resistance trained men. J Int Soc Sports Nutr. 2007;4:22. doi: 10.1186/1550-2783-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]


