Abstract
Background:
Atherosclerosis is a complex disease that is associated with a variety of etiologic factors such as hyperlipidemia and inflammation. Aloe vera (Liliaceae family) has been used traditionally as an anti-inflammatory drug. The aims of this survey were to define the beneficial effects of Aloe vera leaf gel on some of the atherosclerosis risk factors, and also fatty streak formation in hypercholesterolemic rabbits.
Materials ans Methods:
32 white male rabbits were randomly divided into four experimental groups (n = 8, each). During the study, the animals had a standard diet (control group), high cholesterol diet (HC group), high cholesterol diet with Aloe vera leaf gel (3.2%v/v) (HC+ Aloe group) and Aloe vera leaf gel (Aloe group) for 30 days. Fasting blood samples were collected from all animals at the beginning and end of the study. Then total cholesterol (TC), fasting blood sugar (FBS), triglyceride (TG) and CRP were measured before and after experimental periods. By the end of the study, the aortas were removed and investigated for atherosclerosis plaque formation.
Results:
Significant differences were observed in TC and CRP levels of the high-cholesterol diet with Aloe vera and the high-cholesterol diet alone (p < 0.05). The formation of fatty streaks in the aorta was also significantly lower in the same animals under the influence of dietary Aloe vera(p < 0.05). The control and Aloe group did not show any evidence of atherosclerosis. No significant difference was found between the groups in TG and FBS.
Conclusions:
The data suggests that Aloe vera has beneficial effects on the prevention of fatty streak development; it may reduce the development of atherosclerosis through modification of risk factors. However, further studies are needed to understand the mechanisms whereby this plant exerts its anti-atherosclerotic effects.
Keywords: Atherosclerosis, Aloe vera, C-Reactive Protein, Fatty Streak, Inflammation
INTRODUCTION
Evidence have shown that inflammation plays an essential role in the development, progression, and outcome of atherosclerosis.[1] Many studies have shown that atherosclerosis is not only a simply lipid disorder, but also a chronic vascular inflammation.[2] Therefore, using anti-inflammatory drugs can be useful in the prevention of the atherosclerosis.[3] Because of the cost of therapy and vast variety of side effects of chemical drugs in the treatment of atherosclerosis, finding drugs from natural sources and without side-effects is highly desirable.[4]
There is increasing interest in medicinal plants for lipid lowering and reduction of the risk of cardiovascular disease.[5] Aloe vera is a popular plant which has a wide range of medicinal application,[6] It was used as a traditional treatment for its anti-inflammatory, immunostimulant, antiseptic, wound and burn healing, anti-tumoral and laxative effects.[7–9] Aloe vera is one of the few substances known to effectively decrease inflammation.[10] However, the effects of Aloe vera on the development of atherosclerosis and its relative risk factors have not yet been reported. The present study was conducted to evaluate the effect of this plant on the development and progression of atherosclerosis.
MATERIALS AND METHODS
Animals and experimental design:
This study was reviewed and approved by the Ethics Committee of Isfahan University of Medical Sciences. Thirty two white male rabbits with an average body weight of 2000 ± 150 g were purchased from the Pasteur Institute of Iran and were kept in standard temperature and light conditions. They had free access to water and a standard powdered and purified diet (PARS Co, Iran), which consisted of 15% protein, 40-50% carbohydrates, 2% vegetable fat and 15-25% fiber. The rabbits were randomly divided into four groups of eight animals. The first and second groups were given a high cholesterol diet. The second group was given a high cholesterol diet supplemented with Aloe vera leaf gel (Aloe vera gel (3.2%v/v) per day in their drinking water ad libitum); (forever living product, USA, it has certification by the International Aloe Science Council), third group was given a normal diet supplemented with 3.2% Aloe vera leaf gel per day, and the fourth had a normal diet.
Measurement of biochemical factors in rabbits:
Blood samples were collected from overnight fasted rabbits before starting the experiment and at the end of study. Samples were centrifuged at 3500 rpm for 20 minutes to obtain serum. For lipid measurements, before and after receiving the diet, total cholesterol kit (Pars Azmoon Inc., Tehran, Iran) was used. Triglyceride (TG) and FBS was assayed using enzymatic colorimetric assay kit (Pars Azmoon-co, Iran). CRP was measured using an ELISA kit (IBL, Hamburg, Germany).
Assessment of the severity of atherosclerotic lesions:
At the end of the 30-day period, the rabbits were anesthetized using ketamine-xylazine (0.7cc/kg body wt) and following a chest incision, the animals’ aorta was excised to study fatty streaks. After slicing and staining with hematoxylin, atherosclerotic thickness was assessed in hematoxylin stained sections on an arbitrary scale 1-4.[11]
Statistical analysis:
The data are reported as mean ± SD. All data were compared using one-way variance analysis or the kruskal-Wallis test. The Duncan test was applied to assess the difference between the groups. P values less than 0.05 (p < 0.05) were considered meaningful. The Mann–Whitney test was used to assess the difference between the groups as a post hoc test after the kruskal-Wallis test for non-parametric variables analysis.
RESULTS
Determination of some physiochemical factors in rabbits:
Statistical analysis of biochemical factors before the 30-day period did not show any significant difference between the four groups in respect to any of the biochemical factors.
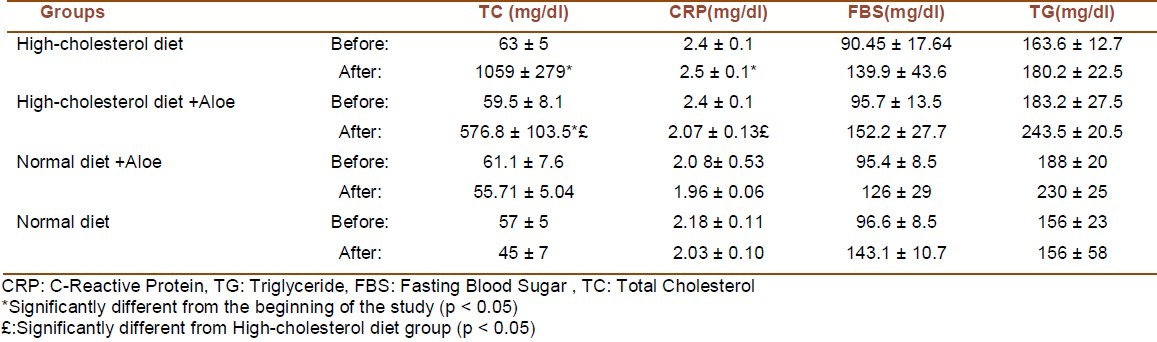
Assessment of biochemical factors at the end of the 30-day period showed that total cholesterol and CRP levels were significantly reduced in the group which received cholesterol diet supplemented with Aloe vera (p < 0.05). FBS and TG levels were not significantly affected by the intake of Aloe vera (Table 1).
Table 1.
Comparison of biochemical variables in 4 groups at the beginning and by the end of the 30 days dietary course
Fatty streak formation:
The scores of fatty streak formation in aorta of hypercholesterolemic rabbits were 1.16 ± 0.4. This score was 0.28 ± 0.1 in the hypercholesterolemic rabbits treated with Aloe vera. With the influence of dietary Aloe vera, fatty streak formation was significantly decreased (Fig. 1). In the control group rabbits and animals fed with normal diet supplemented with Aloe vera the same score was 0 ± 0.
Figure 1.
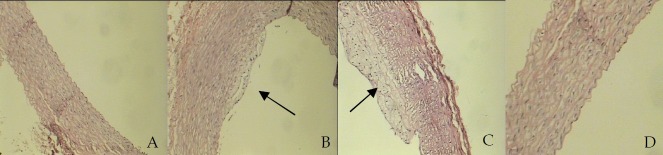
The development of atherosclerosis in rabbit's aorta: A (Normal diet), B(High-cholesterol diet +Aloe:IMT=0.21), C(High-cholesterol diet: IMT=0.62).D(Normal diet+ Aloe)
DISCUSSION
In this study Aloe vera leaf gel significantly decreased TC in rabbits receiving a high-cholesterol diet. Furthermore, our results indicate that oral supplementation of Aloe vera significantly reduced formation of atherosclerotic lesions in the aorta, as well as serum level of CRP in animals fed on a high cholesterol diet.
Atherosclerosis is the most common pathological process that leads to cardiovascular diseases (CVD), a disease of large- and medium-sized arteries that is characterized by a formation of atherosclerotic plaques.[12] Recent studies have demonstrated that atherosclerosis is an inflammatory disease.[3] Many cross-sectional studies have reported correlations between levels of serum lipids and various inflammatory markers, however, there is little information on temporal and causal relationships.[13] Proinflammatory cytokines are involved in the regulation of several enzymes of the lipid metabolism, for example, lecithin: cholesterol acyltransferase, lipoprotein lipase, cholesteryl ester transfer protein, and endothelial lipase.[14–19] Therefore, inflammatory state may lead to dyslipidemia and consequently fatty streak formation. Conversely, anti-inflammatory interventions may result in reduced atherosclerosis development. Several studies have reported diminished atherosclerotic lesion develop-ment in apolipoprotein E–deficient mice fed a high fat diet.[20–22] Interestingly some of these studies have shown that disruption of the proinflammatory cytokines gene reduces atherosclerosis development in apolipoprotein E–deficient mice without affecting serum cholesterol.[23,24]
Studies have shown that CRP is within atherosclerotic plaques close to LDL and macrophages.[25] Recently, Pepys et al. demonstrated that injection of contaminate-free human CRP in rats increases myocardial infarct size through a complement dependent mechanism.[26] CRP receptors have been identified on human aortic endothelial cells.[27] Elevated CRP levels could be a marker for the extent of atherosclerosis, or inflammatory activity and vulnerability of atherosclerotic plaques.[28–30] However, CRP may directly promote atherosclerosis through endothelial activation/dysfunction, smooth muscle cell proliferation and neointimal damage, and by changing of the monocyte/macrophage and matrix metalloproteinase function.[31]
The exact underlying mechanisms responsible for the Aloe-induced changes in the CRP level are not known. However, the anti-inflammatory effects of Aloe vera have been previously studied.[32–34] Aloe vera contains 75 potentially active constituents: vitamins, enzymes, minerals, sugars, lignin, saponins, salicylic acids, and amino acids,[35] some of which have anti-inflammatory effects. For example It has been shown that the polysaccharides in Aloe vera gel such as Mannose-6-phosphate and C-glucosyl chromone have anti-inflammatory and anti-oxidant effects.[34,7] On the other hand, two maloyl glucans of Aloe vera, namely veracylglucan B and C, also have the same effects.[37] Moreover, Bradykinase, one of the Aloe enzymes, helps to reduce excessive inflammation.[38] Therefore, it can be suggested that Aloe vera reduces the amount of CRP through its anti-inflammatory compounds.
In conclusion, oral administration of Aloe vera provides a new and effective approach to attenuating inflammation, and hyperlipidemia. Therefore, in view of its anti-inflammatory properties Aloe vera may be a good candidate to prevent the progression of atherosclerosis.
ACKNOWLEDGMENTS
This study was funded by Isfahan University of Medical Sciences (IUMS) grant number: 287242.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004;116(Suppl 6A):9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6(7):508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 3.Asgary S, Naderi G, Dashti G, Paknahad Z. Effect of Amirkabiria odorastissima mozaffarian on the development and progression of fatty streaks in hypercholesterolemic rabbits. Phytother Res. 2004;18(5):370–2. doi: 10.1002/ptr.1423. [DOI] [PubMed] [Google Scholar]
- 4.Setorki M, Asgary S, Eidi A, Rohani AH, Khazaei M. Acute effects of vinegar intake on some biochemical risk factors of atherosclerosis in hypercholesterolemic rabbits. Lipids Health Dis. 2010;9:10. doi: 10.1186/1476-511X-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kritchevsky D. Dietary protein, cholesterol and atherosclerosis: a review of the early history. J Nutr. 1995;125(3 Suppl):589S–93S. doi: 10.1093/jn/125.suppl_3.589S. [DOI] [PubMed] [Google Scholar]
- 6.Paulsen E, Korsholm L, Brandrup F. A double-blind, placebo-controlled study of a commercial Aloe vera gel in the treatment of slight to moderate psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2005;19(3):326–31. doi: 10.1111/j.1468-3083.2004.01186.x. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds T, Dweck AC. Aloe vera leaf gel: a review update. J Ethnopharmacol. 1999;68(1-3):3–37. doi: 10.1016/s0378-8741(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 8.Heggers JP, Kucukcelebi A, Listengarten D, Broemel KF. Wound healing effects of Aloe gel and other topical antibacterial agents in rat skin. Phytotherapy Res. 1995;9(6):455–7. [Google Scholar]
- 9.Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol. 1998;59(3):195–201. doi: 10.1016/s0378-8741(97)00124-4. [DOI] [PubMed] [Google Scholar]
- 10.Davis RH, Leitner MG, Russo JM, Byrne ME. Wound healing. Oral and topical activity of Aloe vera. J Am Podiatr Med Assoc. 1989;79(11):559–62. doi: 10.7547/87507315-79-11-559. [DOI] [PubMed] [Google Scholar]
- 11.Javanmard Sh, Nematbakhsh M, Mahmoodi F, Mohajeri MR. l-Arginine supplementation enhances eNOS expression in experimental model of hypercholesterolemic rabbits aorta. Pathophysiology. 2009;16(1):9–13. doi: 10.1016/j.pathophys.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 13.Engstrom G, Hedblad B, Janzon L, Lindgarde F. Long-term change in cholesterol in relation to inflammation-sensitive plasma proteins: a longitudinal study. Ann Epidemiol. 2007;17(1):57–63. doi: 10.1016/j.annepidem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Skretting G, Gjernes E, Prydz H. Regulation of lecithin:cholesterol acyltransferase by TGF-beta and interleukin-6. Biochim Biophys Acta. 1995;1255(3):267–72. doi: 10.1016/0005-2760(94)00240-y. [DOI] [PubMed] [Google Scholar]
- 15.Feister HA, Auerbach BJ, Cole LA, Krause BR, Karathanasis SK. Identification of an IL-6 response element in the human LCAT promoter. J Lipid Res. 2002;43(6):960–70. [PubMed] [Google Scholar]
- 16.Greenberg AS, Nordan RP, McIntosh J, Calvo JC, Scow RO, Jablons D. Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res. 1992;52(15):4113–6. [PubMed] [Google Scholar]
- 17.Hardardottir I, Moser AH, Fuller J, Fielding C, Feingold K, Grunfeld C. Endotoxin and cytokines decrease serum levels and extra hepatic protein and mRNA levels of cholesteryl ester transfer protein in syrian hamsters. J Clin Invest. 1996;97(11):2585–92. doi: 10.1172/JCI118707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feingold KR, Hardardottir I, Grunfeld C. Beneficial effects of cytokine induced hyperlipidemia. Z Ernahrungswiss. 1998;37(Suppl 1):66–74. [PubMed] [Google Scholar]
- 19.Broedl UC, Jin W, Rader DJ. Endothelial lipase: a modulator of lipoprotein metabolism upregulated by inflammation. Trends Cardiovasc Med. 2004;14(5):202–6. doi: 10.1016/j.tcm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Peppel K, Sivashanmugam P, Orman ES, Brian L, Exum ST, et al. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(5):1087–94. doi: 10.1161/ATVBAHA.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF, Bayard F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97(3):242–4. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- 22.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23(3):454–60. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 23.Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, et al. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180(1):11–7. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(11):2137–42. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 25.Bisoendial RJ, Kastelein JJ, Stroes ES. C-reactive protein and atherogenesis: from fatty streak to clinical event. Atherosclerosis. 2007;195(2):e10–e18. doi: 10.1016/j.atherosclerosis.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 26.Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440(7088):1217–21. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 27.Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006;113(17):2135–50. [PubMed] [Google Scholar]
- 28.Arroyo-Espliguero R, Avanzas P, Cosin-Sales J, Aldama G, Pizzi C, Kaski JC. C-reactive protein elevation and disease activity in patients with coronary artery disease. Eur Heart J. 2004;25(5):401–8. doi: 10.1016/j.ehj.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89(9):763–71. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 30.Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, et al. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105(17):2019–23. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49(21):2129–38. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 32.Lim BO, Seong NS, Choue RW, Kim JD, Lee HY, Kim SY, et al. Efficacy of dietary Aloe vera supplementation on hepatic cholesterol and oxidative status in aged rats. J Nutr Sci Vitaminol (Tokyo) 2003;49(4):292–6. doi: 10.3177/jnsv.49.292. [DOI] [PubMed] [Google Scholar]
- 33.Eamlamnam K, Patumraj S, Visedopas N, Thong-Ngam D. Effects of Aloe vera and sucralfate on gastric microcirculatory changes, cytokine levels and gastric ulcer healing in rats. World J Gastroenterol. 2006;12(13):2034–9. doi: 10.3748/wjg.v12.i13.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutter JA, Salman M, Stavinoha WB, Satsangi N, Williams RF, Streeper RT, et al. Antiinflammatory C-glucosyl chromone from Aloe barbadensis. J Nat Prod. 1996;59(5):541–3. doi: 10.1021/np9601519. [DOI] [PubMed] [Google Scholar]
- 35.Langmead L, Makins RJ, Rampton DS. Anti-inflammatory effects of Aloe vera gel in human colorectal mucosa in vitro. Aliment Pharmacol Ther. 2004;19(5):521–7. doi: 10.1111/j.1365-2036.2004.01874.x. [DOI] [PubMed] [Google Scholar]
- 36.Atherton P. Aloe vera revisited. Br J Phytotherapy. 1998;4(4):176–83. [Google Scholar]
- 37.Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13(8):1599–616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol. 2008;53(4):163–6. doi: 10.4103/0019-5154.44785. [DOI] [PMC free article] [PubMed] [Google Scholar]


