Abstract
Background:
Hypertension and obesity are risk factors of cardiovascular disease. The association between C-reactive protein, homocysteine, microalbuminuria and cardiovascular risk have been debated for decades. Resistin is a newly discovered adipocyte derived cytokine. In the current study we planned to investigate the relation of resistin to these probable cardiovascular risk factors and obesity in hypertensive patients.
Materials and Methods:
The study population consisted of 42 non-obese and 42 obese hypertensive females. After making comparisons between C-reactive protein, homocysteine, microalbuminuria and resistin in the two groups, we also sought correlations between all parameters in non-obese and obese groups.
Results:
In our obese hypertensive group, resistin levels were higher than in the non-obese hypertensive group (p < 0.001), but we did not find any difference in other parameters. We found a positive correlation between resistin and C-reactive protein in both non-obese and obese hypertensive groups (in non-obese hypertensives p < 0.05, and in obese hypertensives p < 0.001).
Conclusions:
We showed that in female obese hypertensive patients resistin levels were higher than in the non-obese patients. We also think that resistin may be associated with C-reactive protein levels but not with homocysteine or microalbuminuria in both non-obese and obese hypertensive patients.
Keywords: Hypertension, Obesity, Resistin, C-reactive protein, Homocysteine, Microalbuminuria
INTRODUCTION
Resistin is a newly discovered adipocyte derived protein. Although many research papers on resistin have been published since its initial description in 2001, knowledge about it has not resulted in a concensus about its biological role.[1–3] It has been linked to obesity, insulin resistance, type 2 diabetes mellitus (T2DM), inflammation and atherosclerosis, but the reports of animal and human studies have been at variance.[4–7] Insulin resistance is a major contributor to the pathogenesis of T2DM and plays a role in numerous other metabolic disorders including hypertension, obesity, dyslipidemia, atherosclerosis and cardiovascular disease.[8,9]
It is well known that excess obesity and hyper-tension are associated to the development of cardiovascular disease. Recently some novel markers have been proposed for assessing the risk of cardiovascular diseases such as C-reactive protein (CRP), homocysteine (Hcy) or micro-albuminuria.
Keeping in mind the complex relationship of cardiovascular disease, obesity, hypertension and cardiovascular risk factors, we planned to seek the difference in resistin levels between the two groups and investigate the association of resistin with CRP, homocysteine and microalbuminuria in patients who had obesity plus hypertension and only hypertension.
MATERIALS AND METHODS
Patients
In a cross-sectional study, a total of 84 hypertensive patients, 42 with body mass index (BMI) <30, 42 with BMI ≥ 30, aged 30-80 years, were recruited from the outpatient Clinic of Ankara Education and Research Hospital from April 2009 to July 2009. As resistin serum and messenger ribonucleic acid resistin levels were significantly higher in females than males at all ages, only females were included to obtain an homogenous group. Subjects with diabetes mellitus, glucose intolerance, hyper-lipidemia, conditions which may affect metabolic parameters (such as history thyroid dysfunctions), chronic diseases, infection and coronary artery disease were excluded.
After detailed physical examination, body weight and height were measured. Waist was calculated when fasting, in standing position half-way between costal edge and iliac crest, whereas hip was measured at the greatest circumference around the buttocks, by a non elastic measure. Waist to hip ratio (WHR) were calculated. Body mass index (BMI) was also calculated as weight in kilograms divided by the square of height in meters (kg/m2). Subjects were classified as BMI <30 and BMI ≥ 30. Body fat were estimated by Tanita body composition analayser (TBF-300) after the subjects rested for 30 minutes.
Blood was withdrawn after 12 hour of overnight fasting, at 08.30 a.m. for fasting plasma glucose, serum total and high density lipoprotein (HDL-C) cholesterol, triglyceride (TG), homocysteine, CRP and resistin levels. Another blood sample was taken for postprandial plasma glucose (PPBG) 2 hour after breakfast. Microalbuminuria was examined in spot urine.
Systolic and diastolic blood pressure (SBP and DBP) were measured after 5 minute rest in the semi-sitting position with a sphygmomanometer. Blood pressure was determined at least three times at the right upper arm, and the mean was used in the analysis. The patients who were taking antihypertensive drugs or patients whose determined mean blood pressure levels ≥140/90 mmHg were diagnosed as hypertensive.
This study was performed according to the Helsinki decleration 2008. The local ethics comitee approved this clinical trial in 24.01.2007 with the number 0217. All the subjects gave written informed consent.
Laboratory methods
Plasma glucose, total and HDL cholesterol, TG concentrations were determined by enzymo-calorimetric spectrophotometric method in a Roche/ Hitachi molecular PP autoanalyser. Low density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald Formula ( LDL = Total cholesterol - HDL - TG/5).
High sensitivity C-reactive protein (CRP) was measured by immunnofolometric tests by Beckman- Cutler device. Homocysteine concentrations were determined according to the method of HPLC using Agilend 1100 device. Microalbuminuria was determined with nepholometric method.
For the measurements of resistin, after fasting blood samples were drawn, they were put into a dry tube and were santrifuged 5000 cycle / min in 10 minutes. Serum was then separated and transferred to another dry tube before storing at -80°C. Serum resistin levels were assayed by a commercial resistin ELISA kit.
Statistical analysis
Calculations were performed using SPSS version 11.5 (Customer ID 30000105 930). Data are presented as mean ± SD. Student's t-test was used to compare the groups in a parametric way. A p value of < 0.05 was considered as statistically significant. Pearson correlation coofficient was used for the correlation analysis.
RESULTS
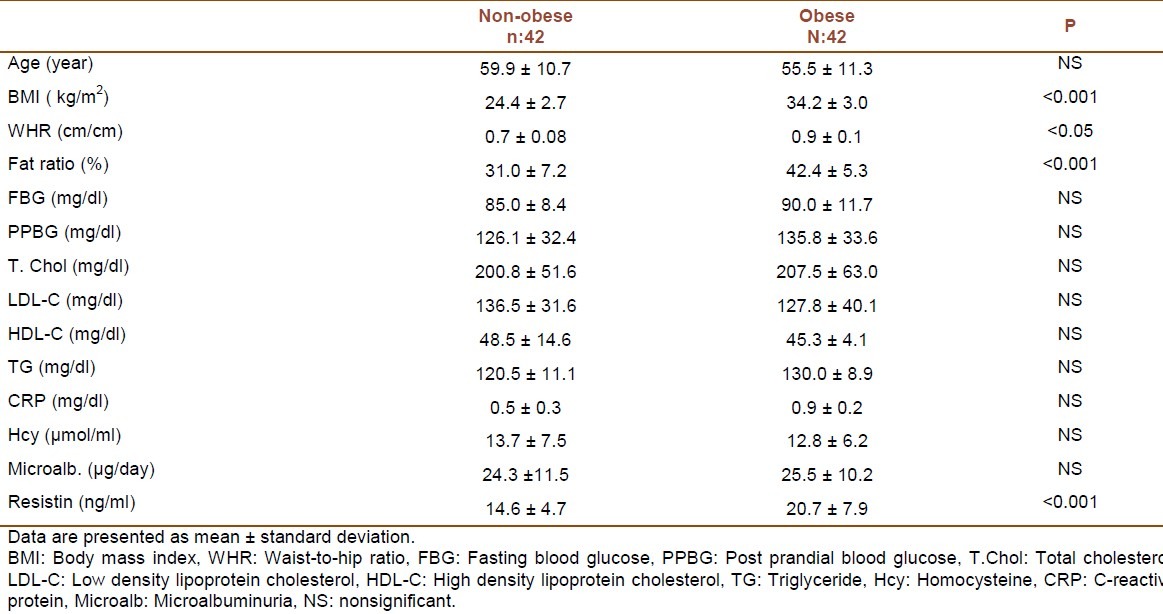
Findings of non-obese and obese hypertensive patients were presented in table 1. Resistin levels were found to be statistically higher in obese hypertensive group than non-obese hypertensive group (p < 0.001). CRP, homocysteine and microalbuminuria levels did not differ in the two groups (Table 1).
Table 1.
Characteristics of non-obese and obese hypertensive patients
When we made correlation analysis in both non-obese and obese hypertensives we only found positive correlations between resistin and CRP levels (In non-obese hypertensives p < 0.05, r = 0.433 and in obese hypertensives p < 0.001, r = 0.692, respectively).
DISCUSSION
Adipokines are now known to be associated with insulin resistance, inflammation, obesity, metabolic syndrome and cardiovascular diseases.[10–15] Resistin is one of those adipokines. Controversy still exists regarding the role of resistin in metabolic disturbances in human.[4–9]
Cardiovascular disease accounts for nearly 40% of worldwide all deaths each year.[16,17] The factors that make up the Framingham risk score (age, sex, blood pressure, serum total cholesterol or LDL-C, HDL-C levels, smoking and diabetes) account for most of the excess risk for incident cardiovascular disease (CVD).[18] However, these factors do not explain all of the excess risk.[19] Several lines of evidence have suggested some markers that have received much attention as emerging risk factors that could account for some of the unexplained variability in CVD risk.[19]
CRP, a plasma protein synthesized by liver, is a sensitive and dynamic systemic marker of inflammation.[20] It is also considered a predictor of cardiovascular events including myocardial infarction, stroke, peripheral arterial disease and sudden cardiac death.[21,22] CRP may also act directly on atherosclerosis, promoting endothelial cell activation and adhesion molecule expression, resulting in endothelial dysfunction.[23] Recently, correlation of resistin with inflammatory and endothelial markers has been widely investigated,[24] and resistin was shown to be associated with CRP in patients with obesity and inflammation.[25] Among our hypertensive patients, resistin levels were found to be higher in obese ones, than non-obese patients. CRP levels were not statistically different in non-obese and obese hypertensive patients. Both in non-obese and obese hypertensive groups, resistin levels were demonstrated to be correlated with CRP levels. These results made us speculate that resistin was associated with CRP in hypertensives whether they were non-obese or obese. Our thought was supported by the correlation analysis and by the indifference of CRP levels in non-obese and obese hypertensives.
Hcy is a sulphur containing amino acid that is an intermediatery product in methionine metabolism. After Hcy mediated vascular disease was first established in the 1960's, it was demonstrated that Hcy is an independent risk factor for CVD.[26–28] It was documented that high blood Hcy levels possibly contribute to insulin resistance through the induction of resistin expression in mouse adipose tissue.[29,30] Keeping in mind the relation of resistin and Hcy we examined the association of these two parameters, but there was no correlation between resistin and Hcy in non-obese and obese hypertensives, although resistin levels were higher in obese hypertensives than non-obese ones. We think that the reason why we could not find a relation between our patients may be due to normal Hcy levels in both groups.
An increased albumin excretion, known as microalbuminuria is associated with a range of diseases, most frequently diabetes mellitus and hypertension.[31,32] Microalbuminuria tends to be an indicator of the level of cardiovascular risk,[33–35] as an increased albumin excretion is considered to be a renal symptom of generalized endothelial dysfunction.[35,36] Recently association of resistin with urinary albumin excretion in nondiabetic patients with essential hypertension was shown.[37] In our study both our obese and non-obese patients with hypertension did not have statistically different microalbuminuria levels. Likewise, in our obese and non-obese hypertensives, we could not determine any correlation between microalbuminuria and resistin. This finding leads us to the conclusion that as hypertension exists obesity does not add any negative effect considering albumin excretion; and resistin levels are not related to microalbuminuria levels in hypertensives if they are obese or not. In conclusion, we may speculate that resistin may represent a link between obesity in hypertensive patients and resistin levels may be associated with serum CRP levels in hypertensives.
Our study had some limitations. Resistin was not evaluated in different ages, different BMI and different blood pressures. We did not seperated our patients according to their ages and their blood pressure. A real defect of our study was not mentioning the drugs and the duration of hypertension. Additionaly, increase in sample size of the groups were needed.
ACKNOWLEDGMENTS
We thank the patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 2.Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem. 2001;276(14):11252–6. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19(15):4046–55. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makimura H, Mizuno TM, Bergen H, Mobbs CV. Adiponectin is stimulated by adrenalectomy in ob/ob mice and is highly correlated with resistin mRNA. Am J Physiol Endocrinol Metab. 2002;283(6):E1266–E1271. doi: 10.1152/ajpendo.00227.2002. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303(5661):1195–8. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Nyomba BL. Glucose intolerance and resistin expression in rat offspring exposed to ethanol in utero: modulation by postnatal high-fat diet. Endocrinology. 2003;144(2):500–8. doi: 10.1210/en.2002-220623. [DOI] [PubMed] [Google Scholar]
- 7.Bhalla V, Kalogeropoulos A, Georgiopoulou V, Butler J. Serum resistin: physiology, pathophysiology and implications for heart failure. Biomark Med. 2010;4(3):445–52. doi: 10.2217/bmm.10.17. [DOI] [PubMed] [Google Scholar]
- 8.Mojiminiyi OA, Abdella NA. Associations of resistin with inflammation and insulin resistance in patients with type 2 diabetes mellitus. Scand J Clin Lab Invest. 2007;67(2):215–25. doi: 10.1080/00365510601032532. [DOI] [PubMed] [Google Scholar]
- 9.Salazar MR, Carbajal HA, Espeche WG, Dulbecco CA, Aizpurua M, Marillet AG, et al. Relationships among insulin resistance, obesity, diagnosis of the metabolic syndrome and cardio-metabolic risk. Diab Vasc Dis Res. 2011;8(2):109–16. doi: 10.1177/1479164111403170. [DOI] [PubMed] [Google Scholar]
- 10.Kýrnap NG, Gürsoy G, Eþbah O, Acar Y, Akçayöz S, Demirbaþ B, et al. The relationship between obesity and plasma omentin levels in newly diagnosed and untreated type 2 diabetic patients. Turkiye Klinikleri J Endocrin. 2010;5(2):68–73. [Google Scholar]
- 11.Gürsoy G, Kýrnap NG, Eþbah O, Acar Y, Demirbaþ B, Akçayöz S, et al. The relationship between plasma omentin-1 levels and insulin resistance in newly diagnosed type 2 diabetic women. Clinical Review and Opinions. 2010;2(4):49–54. [Google Scholar]
- 12.Gürsoy G, Akçayöz S, Acar Y, Demirbas B. Visfatin in hyperlipidemic female patients. J Med Med Sci. 2010;1(4):120–5. [Google Scholar]
- 13.Gürsoy G, Alagöz S, Acar Y, Demirbaþ B, Çetiner H, Kýlýç Z. Osteopontin a new probable marker for atherosclerosis in obese women? Clinical Review and Opinions. 2010;2(3):35–40. [Google Scholar]
- 14.Schutte AE, Huisman HW, Schutte R, van Rooyen JM, Malan L, Fourie CM, et al. Adipokines and cardiometabolic function: How are they interlinked? Regul Pept. 2010;164(2-3):133–8. doi: 10.1016/j.regpep.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Ferdinand KC. Coronary artery disease in minority racial and ethnic groups in the United States. Am J Cardiol. 2006;97(2A):12A–9A. doi: 10.1016/j.amjcard.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. Creactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):483–95. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 18.Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290(7):891–7. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 19.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 20.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109(23):2818–25. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 22.Schulze MB, Rimm EB, Li T, Rifai N, Stampfer MJ, Hu FB. C-reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care. 2004;27(4):889–94. doi: 10.2337/diacare.27.4.889. [DOI] [PubMed] [Google Scholar]
- 23.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106(8):913–9. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Curhan GC, Forman JP. Plasma resistin levels associate with risk for hypertension among nondiabetic women. J Am Soc Nephrol. 2010;21(7):1185–91. doi: 10.1681/ASN.2009101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straburzynska-Lupa A, Nowak A, Pilaczynska-Szczesniak L, Straburzynska-Migaj E, Romanowski W, Karolkiewicz J, et al. Visfatin, resistin, hsCRP and insulin resistance in relation to abdominal obesity in women with rheumatoid arthritis. Clin Exp Rheumatol. 2010;28(1):19–24. [PubMed] [Google Scholar]
- 26.He L, Zeng H, Li F, Feng J, Liu S, Liu J, et al. Homocysteine impairs coronary artery endothelial function by inhibiting tetrahydrobiopterin in patients with hyperhomocysteinemia. Am J Physiol Endocrinol Metab. 2010;299(6):E1061–E1065. doi: 10.1152/ajpendo.00367.2010. [DOI] [PubMed] [Google Scholar]
- 27.Yan TT, Li Q, Zhang XH, Wu WK, Sun J, Li L, et al. Homocysteine impaired endothelial function through compromised vascular endothelial growth factor/Akt/endothelial nitric oxide synthase signalling. Clin Exp Pharmacol Physiol. 2010;37(11):1071–7. doi: 10.1111/j.1440-1681.2010.05438.x. [DOI] [PubMed] [Google Scholar]
- 28.Abraham JM, Cho L. The homocysteine hypothesis: still relevant to the prevention and treatment of cardiovascular disease? Cleve Clin J Med. 2010;77(12):911–8. doi: 10.3949/ccjm.77a.10036. [DOI] [PubMed] [Google Scholar]
- 29.Jiang C, Zhang H, Zhang W, Kong W, Zhu Y, Zhang H, et al. Homocysteine promotes vascular smooth muscle cell migration by induction of the adipokine resistin. Am J Physiol Cell Physiol. 2009;297(6):C1466–C1476. doi: 10.1152/ajpcell.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Jiang C, Xu G, Wang N, Zhu Y, Tang C, et al. Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes. 2008;57(4):817–27. doi: 10.2337/db07-0617. [DOI] [PubMed] [Google Scholar]
- 31.Szanto MV, Ilyes I, Rurik I. Prevalence of microalbuminuria and its clinical correlation with other risk factors of cardiovascular diseases. Orv Hetil. 2010;151(35):1418–22. doi: 10.1556/OH.2010.28873. [DOI] [PubMed] [Google Scholar]
- 32.Salles GF, Cardoso CR, Fiszman R, Muxfeldt ES. Prognostic importance of baseline and serial changes in microalbuminuria in patients with resistant hypertension. Atherosclerosis. 2011;216(1):199–204. doi: 10.1016/j.atherosclerosis.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 33.Rein P, Vonbank A, Saely CH, Beer S, Jankovic V, Boehnel C, et al. Relation of albuminuria to angiographically determined coronary arterial narrowing in patients with and without type 2 diabetes mellitus and stable or suspected coronary artery disease. Am J Cardiol. 2011;107(8):1144–8. doi: 10.1016/j.amjcard.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Monhart V. Microalbuminuria. From diabetes to cardiovascular risk. Vnitr Lek. 2011;57(3):293–8. [PubMed] [Google Scholar]
- 35.Drury PL, Ting R, Zannino D, Ehnholm C, Flack J, Whiting M, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2011;54(1):32–43. doi: 10.1007/s00125-010-1854-1. [DOI] [PubMed] [Google Scholar]
- 36.Ariceta G. Clinical practice: proteinuria. Eur J Pediatr. 2011;170(1):15–20. doi: 10.1007/s00431-010-1334-0. [DOI] [PubMed] [Google Scholar]
- 37.Tsioufis C, Dimitriadis K, Selima M, Miliou A, Toutouzas K, Roussos D, et al. Association of resistin with urinary albumin excretion in nondiabetic patients with essential hypertension. Am J Hypertens. 2010;23(6):681–6. doi: 10.1038/ajh.2010.34. [DOI] [PubMed] [Google Scholar]


