Frailty, as measured by the Veterans Aging Cohort Study Index, is an important predictor of fragility fracture in the context of established fracture risk factors. Anemia and increasing age drive this association in a male veteran population.
Keywords: HIV, frailty, fragility fractures, Veterans
Abstract
Background. The Veterans Aging Cohort Study (VACS) Index is associated with all-cause mortality in individuals infected with human immunodeficiency virus (HIV). It is also associated with markers of inflammation and may thus reflect physiologic frailty. This analysis explores the association between physiologic frailty, as assessed by the VACS Index, and fragility fracture.
Methods. HIV-infected men from VACS were included. We identified hip, vertebral, and upper arm fractures using ICD-9-CM codes. We used Cox regression models to assess fragility fracture risk factors including the VACS Index, its components (age, hepatitis C status, FIB-4 score, estimated glomerular filtration rate, hemoglobin, HIV RNA, CD4 count), and previously identified risk factors for fragility fractures.
Results. We included 40 115 HIV-infected male Veterans. They experienced 588 first fragility fractures over 6.0 ± 3.9 years. The VACS Index score (hazard ratio [HR], 1.15; 95% confidence interval [CI], 1.11–1.19), white race (HR, 1.92; 95% CI, 1.63–2.28), body mass index (HR, 0.94; 95% CI, .92–.96), alcohol-related diagnoses (HR, 1.65; 95% CI, 1.26–2.17), cerebrovascular disease (HR, 1.95; 95% CI, 1.14–3.33), proton pump inhibitor use (HR, 1.87; 95% CI, 1.54–2.27), and protease inhibitor use (HR, 1.25; 95% CI, 1.04–1.50) were associated with fracture risk. Components of the VACS Index score most strongly associated with fracture risk were age (HR, 1.40; 95% CI, 1.27–1.54), log HIV RNA (HR, 0.91; 95% CI, .88–.94), and hemoglobin level (HR, 0.82; 95% CI, .78–.86).
Conclusions. Frailty, as measured by the VACS Index, is an important predictor of fragility fractures among HIV-infected male Veterans.
Frailty represents a loss of homeostasis within an organism that leads to the decreased ability to recover from stressors [1]. It is associated with important outcomes such as disability, hospitalization, and mortality [2]. Research has linked frailty with fragility fractures among elderly persons in the general population [3], but whether it is also associated with fractures among individuals infected with human immunodeficiency virus (HIV) is as yet unexplored.
Frailty indices have been validated in the elderly population, but they have significant limitations. Many are based on subjective measures that are not captured in routine clinical practice [4]. In addition, frailty indices describe a frailty phenotype that, while applicable to the geriatric population, may not be appropriate for younger, HIV-infected individuals [4, 5].
The Veterans Aging Cohort Study (VACS) Index is a clinical risk index that possesses many characteristics of a frailty index: It is associated with absolute risk of all-cause mortality, hospitalization, medical intensive care unit admission, and functional performance [6–8]. It is also associated with markers of inflammation, specifically interleukin 6, D-dimer, and soluble CD14 [9], which is consistent with research from the geriatrics community linking the frailty phenotypes to chronic inflammation [10]. In contrast to other frailty indices, the VACS Index is based on objective data that are collected routinely for HIV-infected individuals: hepatitis C status; FIB-4, a reliable, noninvasive marker of hepatic fibrosis [11]; estimated glomerular filtration rate (eGFR); hemoglobin; CD4 count; HIV-RNA; and age.
Our previous work has identified specific risk factors for fragility fracture, an important outcome of frailty, among HIV-infected men, highlighting the multifactorial nature of fracture risk in this population. These risk factors include traditional risk factors found in the general population (white race, tobacco and alcohol abuse, low body mass index [BMI], and corticosteroid use) [12, 13], as well as HIV-specific risk factors (chronic inflammation and protease inhibitors [PIs]) [13, 14].
Because of the documented association between the VACS Index and outcomes associated with frailty, the current analysis seeks to explore the relationship between the VACS Index and fragility fracture risk among HIV-infected men from the VACS Virtual Cohort (VACS-VC) [12]. We also explored the association between the individual components of the Index and fragility fractures to identify those factors in the Index that most contribute to fracture risk.
METHODS
Sample
The VACS-VC is a prospective, observational cohort of HIV-infected and uninfected Veterans [15]. The HIV-infected subjects were identified by the presence of at least 2 outpatient or 1 inpatient International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for HIV [15]. The VACS-VC represents all HIV-infected Veterans receiving care in the Veterans Health Administration (VHA) system. Demographic, pharmacologic, and clinical data are available for these individuals. We restricted our analysis to include men enrolled for care in the VHA between 1997 and 2009. We excluded women because of the low prevalence of fractures.
Outcomes
Three fracture types were included in this study: hip, vertebral, and upper arm. These were selected as they typically result from a nontraumatic cause [16–18]. All 3 fracture types were combined into a composite outcome.
Fracture outcomes were defined using the following ICD-9-CM codes: hip—820.0X, 820.1X, 820.2X, 820.3X, 820.8, 820.9; vertebral—805.2, 805.3, 805.4, 805.5, 805.6, 805.7; and upper arm—812.0X, 812.1X, 812.2X, 812.3X, 812.4X, 812.5X. These codes have been validated by medical records review [12].
Observation Time
Observation time was calculated as the time between date of cohort entry (1997–2009) and date of first fracture for those with the event, or, for those who were censored, date of death or the midpoint between date of last clinic visit and the conclusion of this study (31 May 2009), as appropriate. Individuals whose entry into the cohort was on the same date as their last follow-up were excluded from this analysis. As this analysis included time-varying covariates, follow-up time was divided into 6-month intervals, beginning at the individual's entry into the cohort.
Covariates
The VACS Index utilizes demographic information and laboratory measures that are routinely assessed in the clinical care of HIV-infected individuals and that are associated with all-cause mortality: age, CD4 count, HIV-RNA, hemoglobin, FIB-4 [(age [years] × AST [IU/L]/platelet count [expressed as platelets × 109/L] × (ALT1/2 [IU/L])] [19], eGFR [(186.3 × (serum creatinine−1.154) × (age−0.203) × (1.21 if black)] [20] and hepatitis C status. These measures are used to generate a summary risk score that we included, assessed in 10-point increments, as a time-varying covariate.
We also explored the association of the components of the VACS Index with fracture risk among HIV-infected men. Age, CD4 count, HIV-RNA, and hemoglobin were included as continuous variables. The FIB-4 score was categorized as described in the literature: <1.45, 1.45–3.25, and >3.25 [19]. Estimated glomerular filtration rate was dichotomized at the 30 mL/minute cutoff as this indicates severe/end-stage renal disease. We also evaluated the impact of the VACS Index, CD4 count, HIV-RNA, and hemoglobin on fracture risk by quartiles for each of these covariates.
In addition, we included covariates that have been shown to be associated with increased fracture in our previous analyses: race/ethnicity, BMI, past or current smoking, inhaled/oral corticosteroid use, proton pump inhibitor (PPI) use, and comorbid conditions [12, 18, 21]. Body mass index was defined as the first available BMI from no more than 6 months prior to enrollment. Smoking status was evaluated at baseline. Proton pump inhibitor use and corticosteroid use were evaluated as time-varying covariates. Comorbid conditions were assessed at baseline and included diabetes mellitus, coronary artery disease, alcohol abuse, drug abuse, major depressive disorder, stroke, and cerebrovascular disease. HIV-specific variables included tenofovir use, PI use, and efavirenz use, all of which were assessed as time-varying covariates. Tenofovir and PI use were assessed because of the extensive literature suggesting an association with decreased bone mineral density and fracture [14]. We included efavirenz because of its association with central nervous system side effects and therefore possibly with falls. In addition, efavirenz has been associated with decreases in serum vitamin D levels [22], which are associated with an increased risk of falls [23].
To assess time-varying covariates, the data were organized as multiple records per patient, each record being defined as a 6-month interval, as described above. In each record a variable was used to note whether or not the individual was taking the medications of interest (corticosteroids, PPI, opiates, tenofovir, PIs, efavirenz) during the time interval (yes/no). As our previous analyses did not show an association between cumulative exposure to medication variables and fracture risk, we limited the current analysis to current exposure only. The VACS Index score was recalculated for each time period.
Ethics
VACS-VC was approved by the institutional review boards of the VHA Connecticut Healthcare System and Yale University School of Medicine, has been granted a waiver of informed consent, and is compliant with the Health Insurance Portability and Accountability Act [15].
Statistical Methods
Univariate statistics were used to describe the population. The percentage of missing data in our study ranged from <1% to 40% (for components of the VACS Index). By using multiple imputation, we were able to include all observations in our analysis. Imputation minimizes bias in the relationship between predictors and outcomes [24, 25]. We used multiple imputation via the SAS Procedure MI. The imputation model included hip, vertebral, and upper arm fractures as well as the composite outcome, the time variables for all 3 fracture types, and all covariates. Analyses of the imputed data sets were combined using the Rubin rule [25] as implemented in the SAS Procedure MIANALYZE.
We used an unadjusted Cox regression model to generate survival curves to evaluate fracture-free survival by VACS Index score quartiles (<18, 18–32.9, 33–52.9, ≥53).
Using Cox regression models, we adjusted both for covariates previously identified as risk factors for fragility fracture and for VACS Index score. We then used the same models, substituting the components of the VACS Index for the composite score, to assess the contribution of the individual components (age, hepatitis C status, FIB-4, eGFR, hemoglobin level, HIV-RNA, and CD4 count).
Analyses were repeated on complete cases, and the associations between the predictor variables and the outcome of interest were substantively similar to and in the same direction as those from the imputed analysis. We thus present only the results using multiple imputation.
RESULTS
Our analysis included 40 115 HIV-infected male Veterans who enrolled in VACS-VC between 1997 and 2009 (Table 1). They experienced 588 first fragility fractures (210 hip, 111 vertebral, 267 upper arm). Fracture incidence was 2.6 per 1000 person-years. Mean follow-up time was 6.0 ± 3.9 years. At baseline, median BMI was 25 m/kg2 (interquartile range [IQR], 22–28). Very few had a diagnosis of cerebrovascular disease/stroke (2%) or coronary artery disease/diabetes (7%). Twelve percent had a diagnosis of major depressive disorder. Sixteen percent had an alcohol-related diagnosis, and 19% had a diagnosis of drug use or abuse. More than half of the sample had a history of smoking (75%) or had used a PI (64%). More than one-third had ever been prescribed a PPI (36%), whereas only 21% had ever been prescribed an oral or inhaled corticosteroid. Tenofovir and efavirenz use were common: 41% of the sample population had ever been prescribed either of these medications. The median VACS Index Score at baseline was 33 (IQR, 18–53), a 5-year mortality risk of 14%. Median CD4 count was 280 cells/mm3 (IQR, 114–472 cells/mm3) and median HIV RNA was 9332 copies/mL (IQR, 400–74 666 copies/mL). Mean hemoglobin was 13.5 ± 2.1 g/dL, and median eGFR was 97 mL/minute (IQR, 82–115 mL/minute). Thirty-three percent of the sample had a FIB-4 score <1.45, whereas only 7% had a FIB-4 score >3.25. Twenty-seven percent of the sample was hepatitis C seropositive.
Table 1.
Sample Characteristics of HIV-Infected Male Veterans (N = 40 115)
| Variables | HIV+ Men in VACS |
|---|---|
| Follow-up timea, y | 6.0 ± 3.9 |
| Age at baselinea, y | 46 ± 10 |
| Age at time of fracturea, y | 53 ± 10 |
| White race | 37% |
| BMIb, m/kg2 at baseline | 25 (22–28) |
| Alcohol-related diagnoses | 16% |
| Cerebrovascular disease/stroke | 2% |
| Drug use/abuse | 19% |
| Smoking (past or current) | 75% |
| Major depressive disorder | 12% |
| Coronary artery disease/diabetes | 7% |
| Proton pump inhibitor usec | 36% |
| Corticosteroid usec | 21% |
| Protease inhibitor usec | 64% |
| Tenofovir usec | 41% |
| Efavirenz usec | 41% |
| VACS Index Score at baselineb | 33 (18–53) |
| CD4b, cells/mm3 | 280 (114–472) |
| HIV RNAb, copies/mL | 9332 (400–74 666) |
| Hemoglobina, g/dL | 13.5 ± 2.1 |
| FIB-4 score | |
| <1.45 | 33% |
| 1.45–3.25 | 19% |
| >3.25 | 7% |
| eGFRb, mL/min | 97 (82–115) |
| eGFR <30 mL/min | 5% |
| Hepatitis C positive | 27% |
| Bisphosphonate usec | 2% |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; VACS, Veterans Aging Cohort Study.
a Mean ± standard deviation.
b Median (interquartile range).
c Ever use of drug.
The survival curves relating VACS Index score quartiles to fracture (Figure 1) demonstrated a significant difference across strata (–2 log(likelihood ratio) χ2 31.65; P < .001). Those with scores in the highest quartile demonstrated less fracture-free time than any other group, and those with the lowest quartile demonstrated a longer time without fracture.
Figure 1.
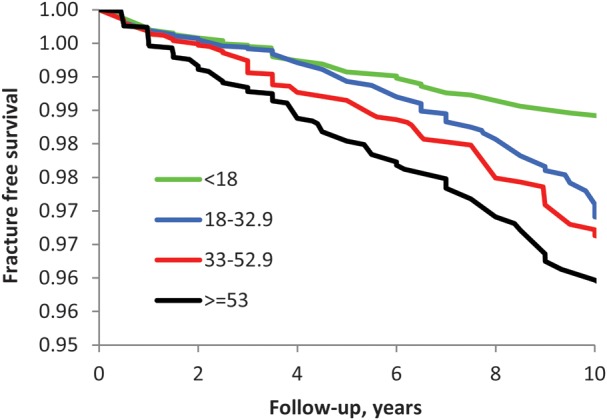
Fracture occurrence by Veterans Aging Cohort Study (VACS) Index Score divided into quartiles. A higher VACS Index Score indicates increased risk of fragility fracture.
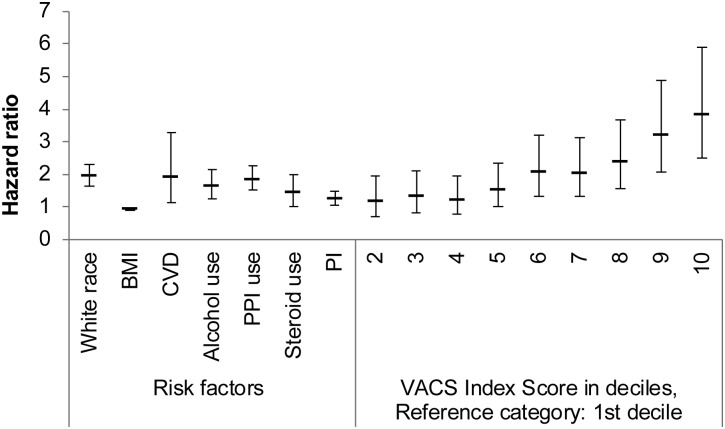
In the time-to-event analysis, the VACS Index score in 10-year increments (HR, 1.15; 95% CI, 1.11–1.19), white race (HR, 1.92; 95% CI, 1.63–2.28), BMI (HR, 0.94; 95% CI, .92–.96), alcohol-related diagnoses (HR, 1.65; 95% CI, 1.26–2.17), cerebrovascular disease (HR, 1.95; 95% CI, 1.14–3.33), PPI use (HR, 1.87; 95% CI, 1.54–2.27), and PI use (HR, 1.25; 95% CI, 1.04–1.50) were associated with fracture risk (Table 2). The strength of the association between the VACS Index and fracture risk was highlighted when we included the VACS Index score in deciles (Figure 2). Compared to those in the lowest 10% of VACS Index scores, those in the highest 10% had almost a 4-fold increased risk of fragility fracture (HR, 3.83; 95% CI, 2.49–5.90).
Table 2.
Hazard Ratios for Fracture Risk Using Veterans Aging Cohort Study (VACS) Index Score and VACS Index Components
| Variable | VACS Index Score | VACS Index Components |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| White race | 1.92 (1.63–2.28) | 2.04 (1.68–2.47) |
| BMI, m/kg2 | 0.94 (.92–.96) | 0.95 (.92–.97) |
| Alcohol-related diagnoses | 1.65 (1.26–2.17) | 1.64 (1.23–2.20) |
| Proton pump inhibitor use | 1.87 (1.54–2.27) | 1.63 (1.32–2.01) |
| Corticosteroid use | 1.45 (1.04–2.02) | 1.55 (1.10–2.18) |
| Protease inhibitor use | 1.25 (1.04–1.50) | 1.11 (.91–1.35) |
| Tenofovir use | 1.12 (.87–1.43) | 1.21 (.92–1.59) |
| Efavirenz use | 0.94 (.75–1.17) | 0.82 (.65–1.04) |
| Drug use/abuse | 1.10 (.84–1.44) | 1.15 (.85–1.54) |
| Smoking (past or current) | 1.12 (.92–1.37) | 1.17 (.94–1.47) |
| Major depressive disorder | 0.91 (.67–1.25) | 0.97 (.69–1.36) |
| Coronary artery disease/diabetes | 1.16 (.87–1.54) | 0.91 (.66–1.24) |
| Cerebrovascular disease/stroke | 1.95 (1.14–3.33) | 1.47 (.76–2.82) |
| VACS Index Score, per 10 units | 1.15 (1.11–1.19) | … |
| Age, 10-y increments | … | 1.40 (1.27–1.54) |
| Log HIV RNA | … | 0.91 (.88–.94) |
| Hemoglobin, g/dL | … | 0.82 (.78–.86) |
| FIB-4 <1.45 | … | Reference |
| FIB-4 between 1.45 and 3.25 | … | 1.07 (.85–1.34) |
| FIB-4 >3.25 | … | 1.24 (.94–1.63) |
| Hepatitis Cseropositive | … | 1.19 (.96–1.49) |
| CD4 count, per 100 cells/mm3 | … | 0.99 (.95–1.02) |
| eGFR <30 mL/min | … | 1.34 (.85–2.10) |
Boldface text indicates statistically significant associations.
Abbreviations: BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; HR, hazard ratio; VACS, Veterans Aging Cohort Study.
Figure 2.
Hazard ratios and 95% confidence intervals of statistically significant risk factors for fragility fractures with Veterans Aging Cohort Study Index Scores as deciles. Abbreviations: BMI, body mass index; CVD, cerebrovascular disease; PI, protease inhibitor; PPI, proton pump inhibitor; VACS, Veterans Aging Cohort Study.
When we explored the contributions of the VACS Index components, each 10-year increment in age was associated with an increased risk of fracture (HR, 1.40; 95% CI, 1.27–1.54). Decreased log HIV RNA was associated with an increase in fracture risk (HR, 0.91; 95% CI, .88–.94) as was decreased hemoglobin (HR, 0.82; 95% CI, .78–.86). Hepatitis C seropositivity, CD4 count, FIB-4 score, and eGFR were not significantly associated with fracture risk. Of note, FIB-4 as a continuous variable was significantly associated with fracture risk (HR, 1.01; 95% CI, 1.00–1.01). The model that included the VACS Index components was a better fit for the data than that which contained the VACS Index composite score (Akaike information criteria, 9727.668 vs 11381.371, respectively).
In the model using the VACS Index Score, other variables that were associated with an increased risk of fracture included white race (HR, 1.92; 95% CI, 1.63–2.28), lower BMI (HR, 0.94; 95% CI, .92–.96), alcohol-related diagnoses (HR, 1.65; 95% CI, 1.26–2.17), PPI use (HR, 1.87; 95% CI, 1.54–2.27), corticosteroid use (HR, 1.45; 95% CI, 1.04–2.02), PI use (HR, 1.25; 95% CI, 1.04–1.50), and cardiovascular disease/stroke (HR, 1.95; 95% CI, 1.14–3.33). In the model that included the VACS Index components, hazard ratios were similar to those in the model that included the VACS Index Score. However, cerebrovascular disease did not demonstrate a statistically significant association with increased fracture risk (HR, 1.47; 95% CI, .76–2.82), nor did PI use (HR, 1.11; 95% CI, .91–1.35).
DISCUSSION
Among HIV-infected men, physiologic frailty, as assessed by the VACS Index score, is strongly associated with risk of fragility fracture. The association, in this sample, was driven by increasing age, and anemia, but this may reflect the prevalent conditions in this sample. FIB-4 was likely not significant because hepatitis C virus (HCV) was included in the model. When we excluded HCV, those with the highest FIB-4 score (>3.25) were at greater risk for fracture compared to those with the lowest score (<1.45) (HR, 1.30; 95% CI, 1.00–1.70). The prevalence of severe kidney disease was quite low in this population. It is likely that in a population with higher rates of severe kidney disease, eGFR would also be an important predictor of fracture risk. Additional factors that contribute to fracture risk include those that we have identified previously: white race, low BMI, alcohol use, cerebrovascular disease or stroke, PPI use, corticosteroid use, and PI use [12].
This is the first time that anemia has been associated with fracture risk among HIV-infected individuals. Among older individuals in the general population, anemia is an established risk factor for fragility fractures [26]. Anemia may increase fracture risk through its association with decreased bone mass and density [27]. Hypoxemia and/or inflammation may drive this relationship [26, 28]. Prior work in VACS has established the independent inverse association of hemoglobin and inflammation [9]. Anemia may also increase the risk for fracture by increasing the risk for falls through its negative impact on physical performance and muscle strength [29, 30]. Effective treatments for anemia of chronic inflammation are scarce. However, given the association between anemia and falls, our results point to the importance of fall prevention interventions in this population.
Bone disease associated with liver damage is well established in the general population but has received little attention among HIV-infected individuals. The etiology of hepatic osteodystrophy is not well understood. Some studies suggest increased bone resorption as the primary defect [31], whereas others suggest decreased bone formation [32]. Lo Re and colleagues identified an association between HCV infection and hip fracture [33]. Even though HCV was not significantly associated with fracture in our model, the association was likely attenuated by the inclusion of FIB-4. When FIB-4 was excluded, the association between HCV and fracture risk was stronger (HR, 1.41; 95% CI, 1.17–1.71).
Our finding that HIV RNA was inversely associated with fracture risk was unexpected. One hypothesis is that the effect of HIV RNA was confounded by the presence of CD4 count in the model. However, when we excluded CD4 count, the direction of the association between HIV RNA and fracture risk remained the same. We hypothesize that this association is an example of competing risk: Individuals with a high viral load were sicker than those with lower viral loads and died before they experienced a fracture. This association requires further exploration.
Additional fracture risk factors identified in this study included low BMI, alcohol use, cerebrovascular disease or stroke, and specific medications (PPIs, corticosteroids, and PIs). The association between low BMI and decreased bone mineral density is well established. Low BMI may also be associated with sarcopenia, secondary to chronic inflammation [34]. This potential pathway, however, highlights the importance of exercise and falls prevention strategies among patients with low BMI. In the general population, problem drinking is associated with lower bone mineral density and increased fall risk [35], highlighting the importance of asking patients about alcohol intake and intervening to decrease consumption. As with BMI and alcohol intake, cerebrovascular disease and stroke may impact fracture risk both by increasing the risk of falls [36] and by decreasing bone mineral density [37]. Care needs to be taken for patients with stroke sequelae that impair movement, as they are highly susceptible to falls.
We have previously identified PIs as a risk factor for fragility fractures [12]. At the time, we speculated that PI use might be a marker for duration or severity of illness. The VACS Index, as a marker of physiologic frailty, is a good proxy for severity of illness. That PI use retained its association with fragility fractures even when controlling for severity of illness with the VACS Index is of interest and deserves further exploration.
In this analysis, the association between smoking and fragility fracture was not statistically significant. This may be mediated by the lower weight of smokers and the association between smoking and alcohol use. That we accounted for BMI and alcohol use may account for the weak association in this analysis. The absence of an important association between efavirenz and fragility fracture may result from the fact that low vitamin D levels are common in this population [38].
Our study has several strengths, including a large and diverse national sample, incident outcomes, and data on most major risk factors for fragility fracture [39]. We also explored risk factors for fragility fracture heretofore unexplored in this population: frailty, anemia, liver disease, and renal disease.
Missing data was an issue, possibly due to differences in follow-up protocols and adherence. We addressed this limitation by using multiple imputation as described earlier. In addition, the low prevalence of end-stage renal disease (eGFR <30 mL/minute) may have precluded our ability to thoroughly evaluate the role of eGFR on fragility fracture risk.
Our work is the first to explore the association between physiologic frailty and fracture risk among HIV-infected individuals. As women are more likely to be frail than men in the general population [40], a similar analysis should be conducted among HIV-infected women. The contribution of falls to fracture risk also needs to be explored. Although falls are difficult to capture in administrative datasets, creative approaches to identifying falls using electronic medical record–based datasets may be helpful. Research has focused on identifying specific medications and specific comorbid illnesses that contribute to fracture risk. A more fruitful direction may be to explore the contribution of polypharmacy and multimorbidity to fracture risk in this population and to target interventions to prevent falls and fractures in those at greatest risk. Finally, frailty may present differently among HIV-infected individuals than among geriatric populations. Whether the underlying pathophysiology is shared requires further investigation.
Notes
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the National Institutes of Health.
Financial support. This work was supported by the Veterans Administration Office, Academic Affiliations, Information Research and Development Medical Informatics Fellowship Program; the National Institute of Nursing Research of the National Institutes of Health (grant number 1K01NR013437–01); CTSA Grant Number UL1 RR024139 from the National Center for Research Resources and the National Center for Advancing Translational Science, components of the National Institutes of Health, and NIH roadmap for Medical Research; the Veterans Aging Cohort Study funded by the National Institute on Alcohol Abuse and Alcoholism (U10 AA 13566) and VHA Public Health Strategic Health Core Group.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–16. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–8. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–33. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 6.Tate JP, Justice AC. Vancouver, British Columbia, Canada: 2010. Change in a prognostic index for survival in HIV infection after one year on cART by level of adherence; pp. 21–24. 48th Annual Meeting of the Infectious Diseases Society of America, [Google Scholar]
- 7.Akgun KM, Pisani MA, Fried TR, et al. Risk factors for medical intensive care unit admission in HIV infected Veterans. American Thoracic Society International Conference, New Orleans, LA, 14–19 May 2010. Poster 204. [Google Scholar]
- 8.Oursler KK, Goulet JL, Crystal S, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: results from the Veterans Aging Cohort Study. AIDS Patient Care STDS. 2011;25:13–20. doi: 10.1089/apc.2010.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Justice AC, Freiberg MS, Tracy R, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–94. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133:456–66. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–12. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Womack JA, Goulet JL, Gibert C, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male Veterans. PloS One. 2011;6:e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin MT, Zhang CA, McMahon DJ, et al. Higher rates of bone loss in postmenopausal HIV-infected women: a longitudinal study. J Clin Endocrinol Metab. 2012;97:554–62. doi: 10.1210/jc.2011-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedimo RJ, McGinnis KA, Dunlap M, Rodriguez-Barradas MC, Justice AC. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr. 2009;52:203–8. doi: 10.1097/QAI.0b013e3181b033ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 16.Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS. 2007;21:617–23. doi: 10.1097/QAD.0b013e3280148c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajcsar EE, Hawker G, Bogoch ER. Investigation and treatment of osteoporosis in patients with fragility fractures. CMAJ. 2000;163:819–22. [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TV, Center JR, Sambrook PN, Eisman JA. Risk factors for proximal humerus, forearm, and wrist fractures in elderly men and women: the Dubbo Osteoporosis Epidemiology Study. Am J Epidemiol. 2001;153:587–95. doi: 10.1093/aje/153.6.587. [DOI] [PubMed] [Google Scholar]
- 19.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 20.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. New Engl J Med. 2006;354:2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–42. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 22.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15:425–9. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 23.Murad MH, Elamin KB, Abu Elnour NO, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2997–3006. doi: 10.1210/jc.2011-1193. [DOI] [PubMed] [Google Scholar]
- 24.Crawford SL, Tennstedt SL, McKinlay JB. A comparison of analytic methods for nonrandom missingness of outcome data. J Clin Epidemiol. 1995;48:209–19. doi: 10.1016/0895-4356(94)00124-9. [DOI] [PubMed] [Google Scholar]
- 25.Rubin D. Inference and missing data. Biometrika. 1976;63:581–92. [Google Scholar]
- 26.Jorgensen L, Skjelbakken T, Lochen ML, et al. Anemia and the risk of non-vertebral fractures: the Tromso Study. Osteoporos Int. 2010;21:1761–8. doi: 10.1007/s00198-009-1131-7. [DOI] [PubMed] [Google Scholar]
- 27.Cesari M, Pahor M, Lauretani F, et al. Bone density and hemoglobin levels in older persons: results from the InCHIANTI study. Osteoporos Int. 2005;16:691–9. doi: 10.1007/s00198-004-1739-6. [DOI] [PubMed] [Google Scholar]
- 28.Paganelli M, Albanese C, Borrelli O, et al. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:416–23. doi: 10.1002/ibd.20039. [DOI] [PubMed] [Google Scholar]
- 29.Dharmarajan TS, Avula S, Norkus EP. Anemia increases risk for falls in hospitalized older adults: an evaluation of falls in 362 hospitalized, ambulatory, long-term care, and community patients. J Am Med Dir Assoc. 2006;7:287–93. doi: 10.1016/j.jamda.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Penninx BW, Pluijm SM, Lips P, et al. Late-life anemia is associated with increased risk of recurrent falls. J Am Geriatr Soc. 2005;53:2106–11. doi: 10.1111/j.1532-5415.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Larramona G, Lucendo AJ, Gonzalez-Castillo S, Tenias JM. Hepatic osteodystrophy: an important matter for consideration in chronic liver disease. World J Hepatol. 2011;3:300–7. doi: 10.4254/wjh.v3.i12.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallego-Rojo FJ, Gonzalez-Calvin JL, Munoz-Torres M, Mundi JL, Fernandez-Perez R, Rodrigo-Moreno D. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology. 1998;28:695–9. doi: 10.1002/hep.510280315. [DOI] [PubMed] [Google Scholar]
- 33.Lo Re V, 3rd, Volk J, Newcomb CW, et al. Risk of hip fracture associated with hepatitis C virus infection and hepatitis C/HIV coinfection. Hepatology. 2012;56:1688–98. doi: 10.1002/hep.25866. ; : –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lima RM, Bezerra LM, Rabelo HT, et al. Fat-free mass, strength, and sarcopenia are related to bone mineral density in older women. J Clin Densitom. 2009;12:35–41. doi: 10.1016/j.jocd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Cawthon PM, Harrison SL, Barrett-Connor E, et al. Alcohol intake and its relationship with bone mineral density, falls, and fracture risk in older men. J Am Geriatr Soc. 2006;54:1649–57. doi: 10.1111/j.1532-5415.2006.00912.x. [DOI] [PubMed] [Google Scholar]
- 36.Lim JY, Jung SH, Kim WS, Paik NJ. Incidence and risk factors of poststroke falls after discharge from inpatient rehabilitation. PM R. 2012;4:945–53. doi: 10.1016/j.pmrj.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Yavuzer G, Ataman S, Suldur N, Atay M. Bone mineral density in patients with stroke. Int J Rehabil Res. 2002;25:235–9. doi: 10.1097/00004356-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Mueller NFC, Ledergerber B, Elzi L, et al. and Swiss HIV Cohort Study. San Francisco, CA: 2010. High prevalence of severe vitamin D deficiency in cART-naive and successfully treated Swiss HIV patients; pp. 16–19. In: 17th Conference on Retroviruses and Opportunistic Infections, Paper 752. [DOI] [PubMed] [Google Scholar]
- 39.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–9. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]