Two 30-μg doses of unadjuvanted pH1N1 vaccine were moderately immunogenic in human immunodeficiency virus–infected pregnant women, and no serious vaccine-related adverse events were observed. Seroprotection persisted in most women postpartum. Efficient transplacental antibody transfer occurred, but seroprotection in infants waned rapidly.
Keywords: pH1N1, vaccine, HIV-infected, pregnancy, immunogenicity
Abstract
Background. Pregnant women infected with human immunodeficiency virus (HIV) may have particular vulnerability to 2009 pandemic H1N1 influenza (pH1N1) infection. The safety and immunogenicity of pH1N1 vaccination in HIV-infected pregnant women are unknown.
Methods. HIV-infected women 18–39 years of age and 14–34 weeks’ gestation on antiretroviral therapy received two 30-μg doses of unadjuvanted, inactivated pH1N1 vaccine 21 days apart. Hemagglutination inhibition titers were measured at entry, 21 days after dose 1, and 10 and 21 days after dose 2, and, in mothers and infants, at delivery and 3 and 6 months postdelivery.
Results. No severe vaccine-related adverse events were observed among 127 subjects. At entry, 21% had seroprotective (≥1:40) titers. Seroprotection and seroresponse (≥4-fold rise) occurred in 73% and 66% after dose 1 and 80% and 72% after dose 2, respectively. Of women lacking seroprotection at entry, 66% attained seroprotection after dose 1 and 75% after dose 2. Seroprotective titers were present in 67% of mothers and 65% of infants at delivery (median 66 days after dose 2), 60% of mothers and 26% of infants at 3 months postdelivery, and 59% of mothers and 12% of infants at 6 months postdelivery.
Conclusions. Two 30-μg doses were moderately immunogenic in HIV-infected pregnant women. No concerning vaccine-related safety signals were observed. Seroprotection persisted in most women postpartum. Efficient transplacental antibody transfer occurred, but seroprotection in infants waned rapidly. Vaccination to protect HIV-infected pregnant women and their newborns from new influenza strains is feasible, but more immunogenic platforms should be evaluated.
Clinical Trials Registration. NCT00992017.
Early in the 2009 pH1N1 pandemic, it was recognized that pregnant women were at high risk, with excess hospital and intensive care unit admissions and an estimated 5-fold relative risk of death. Risk was particularly associated with infection in the second and third trimesters, extending to the second week postpartum [1–6]. Similar patterns observed in previous influenza pandemics and epidemics were thought to reflect immunological and physiological changes associated with pregnancy [2]. Additionally, maternal infection with pH1N1 or seasonal influenza has been associated with preterm labor, preterm delivery, and adverse fetal/neonatal outcomes, and infection in young infants with an increased risk of severe illness and death [2, 3, 5, 7, 8].
A second group that had increased risk of hospitalization, intensive care unit admission, and death in the 2009 pH1N1 pandemic was patients with medical comorbidities, including immunocompromising conditions, which conferred an estimated 16-fold relative risk of fatality [4, 9]. Although some reports did not link human immunodeficiency virus (HIV) infection to excess pH1N1 morbidity or death [10], others demonstrated increased hospital admission rates among HIV-infected populations [11, 12].
Women who are pregnant and HIV infected lie at the intersection of 2 high-risk conditions that, in combination, may pose particular vulnerability to pH1N1 infection. This group represents a high priority for vaccination to prevent maternal morbidity and mortality, avert pregnancy complications, and provide protection to infants in the first months of life before they are eligible for vaccination. Although information is available on the immune response to pH1N1 vaccine in pregnancy [5, 7, 8], none exists for HIV-infected pregnant women. Study P1086 of the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) group was designed to evaluate safety and immunogenicity of a pH1N1 vaccination regimen in HIV-infected pregnant women.
METHODS
Study Population
HIV-infected women 18–39 years of age and between 14–34 weeks of gestation were enrolled at 31 US IMPAACT sites in October–November 2009. Subjects were required to be on antiretroviral therapy prior to or concomitantly with the first visit. Exclusion criteria included allergy to eggs or other vaccine components; history of severe reaction to seasonal inactivated influenza vaccine; previous polymerase chain reaction (PCR)–documented pH1N1 infection or positive influenza test between June 2009 and screening; previous pH1N1 vaccination; any recent vaccination, nonlicensed medication, or blood product; acute illness or fever; neoplastic or immunosuppressive disease (other than HIV infection); immunosuppressive treatment including corticosteroids in the preceding 3 months; personal or family history of Guillain-Barré syndrome; and onset of neurologic disease in the preceding 6 months. Informed consent was obtained in accordance with the guidelines of the US Department of Health and Human Services and participating institutions.
Vaccine Protocol
Unadjuvanted, inactivated pH1N1 monovalent vaccine (Fluvirin, Novartis, Basel, Switzerland), 30 μg/dose (2 standard 15 μg/0.5 mL injections, intramuscularly, 1 per upper extremity or at least 2 inches apart in the same upper extremity), was given at entry. Subjects who did not experience a grade ≥3 adverse event or develop any condition that would have led to study exclusion received a second 30-μg dose on day 21 (+7 days). A 2 double-dose regimen was chosen to optimize immunogenicity. The second dose was temporarily deferred for acute illness, recent infection, or steroid treatment. Subjects who delivered prior to the second dose received the second dose after delivery. Inactivated seasonal influenza vaccine (not containing pH1N1) was permitted ≥14 days prior to the first dose or ≥21 days after the second dose of pH1N1 vaccine. Adverse events were assessed by a symptom diary and telephone contact or clinic visits on days 2, 10, and 21 after each vaccination; at delivery; and at 3 and 6 months after delivery. Clinic visits occurred within 72 hours of fever or symptoms of an influenza-like illness and within 24 hours of onset of lower extremity weakness, hand/feet tingling, or difficulty walking. Respiratory specimens were obtained at fever/influenza-like illness visits.
Immunogenicity Assessments
Maternal pH1N1 hemagglutination inhibition (HAI) titers were measured as previously described [13], at entry, 21 days after dose 1; 10 and 21 days after dose 2; at delivery; and at 3 months after delivery. Infant HAI titers were measured at birth (cord blood or infant blood obtained within 7 days of delivery) and at 3 months. Maternal and infant titers were measured at 6 months after delivery in a subset of mother–infant pairs who received both doses of vaccine and had complete sets of samples prior to delivery. HAI titers were expressed as the reciprocal of the endpoint titer. Titers <10 were considered undetectable and were assigned values of 5. Seroprotection, defined as titer ≥40; geometric mean titers (GMTs); seroresponse, defined as a ≥4-fold rise in titer from baseline following vaccination; and complete response, defined as attaining seroprotection and seroresponse, were characterized.
Sample Size and Statistical Analysis
The accrual target was 130 subjects to obtain at least 100 mothers who received 2 vaccine doses and had complete sets of specimens prior to delivery, which would provide ≥95% probability of detecting an adverse event with a population incidence of ≥3% and confidence intervals of ±9% or less for vaccine response rates. Antibody responses after the first vaccination were analyzed for subjects with available data who received the first vaccine dose and had the day 21 postvaccine dose 1 visit prior to delivery. Responses 10 and 21 days after the second vaccination were analyzed for women with available data who received both doses of vaccine and had the respective visits prior to delivery. Exact McNemar test was used for comparisons of rates of seroprotection, seroresponse, and complete response among timepoints. Changes from baseline in maternal log10 HAI titers and from delivery in infant titers and between subsequent timepoints were analyzed with the sign test. Wilcoxon signed-rank test was used for comparisons of titers among groups. Correlations between maternal and infant titers were done with the Spearman correlation test. Univariate and multivariable logistic regression analyses were performed to identify predictors of complete response.
RESULTS
Study Population
Of the 127 women analyzed, 123 received both vaccinations, at a median interval of 22 days (range, 21–34 days; Figure 1; Table 1). One hundred sixteen women delivered subsequent to the 10-day postvaccine dose 2 timepoint, at a median of 66 days (range, 9–155 days) following dose 2.
Figure 1.
Flow diagram of subjects enrolled and analyzed. Numbers of vaccinated subjects, delivery timing, and numbers of maternal hemagglutination inhibition results at each time point are described. Abbreviation: HAI, hemagglutination inhibition.
Table 1.
Characteristics of the Analyzed Population (N = 127)
| Characteristic | Median (Range) or Percentage |
|---|---|
| Age at entry, y | 29 (18–38) |
| Race | |
| Black | 61 |
| White | 30 |
| Other or unknown | 9 |
| Ethnicity | |
| Hispanic | 34 |
| Non-Hispanic | 63 |
| Unknown | 3 |
| CD4 count at entry, cells/mm3 | 472 (28–1383) |
| CD4 percentage at entry | 31 (1–53) |
| CD8 count at entry, cells/mm3 | 680 (247–1951) |
| CD8 percentage at entry | 46 (18–75) |
| HIV load at entrya | |
| HIV load at entry, copies/mL | 75 (40–294 200) |
| Percentage with ≤50 copies/mL | 35 |
| Percentage with ≤400 copies/mL | 79 |
| Antiretroviral therapyb | |
| HAARTc | 94 |
| Non-HAART | 6 |
| Gestational age at entry, wk | 26 (14–34) |
| Seasonal influenza vaccination | 31d |
| Delivery characteristic (n = 125) | |
| Cesarean | 54 |
| Preterm (26–36 wk) | 14 |
| Vertical transmission of HIV infection | 1e |
Abbreviations: HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus.
a HIV load values below the limit of detection were replaced with the lower limit of the assay used for each subject.
b Prior to or at the initial vaccine visit.
c HAART is defined as ≥3 medications from ≥2 antiretroviral classes.
d Thirty-five subjects received inactivated seasonal influenza vaccine 14–30 days before study vaccine dose 1, and 5 between 21 days after study vaccine dose 2 and study end.
e One infant was confirmed to be HIV-infected during the period of infant follow-up.
Safety
There were no vaccine-related grade ≥3 events. Four subjects had grade 1 local reactions (pain, tenderness, erythema, and/or pruritis) and 4 had grade 1 systemic events (headache, rhinorrhea, and/or chills) temporally related to vaccination. One fetal death of undetermined etiology at 26 weeks’ gestation (32 days after dose 1; 7 days after dose 2) was judged unrelated to vaccine by site investigators. Six subjects had influenza-like illness visits; respiratory specimen influenza A virus PCR was negative in 5, and 1 did not have testing.
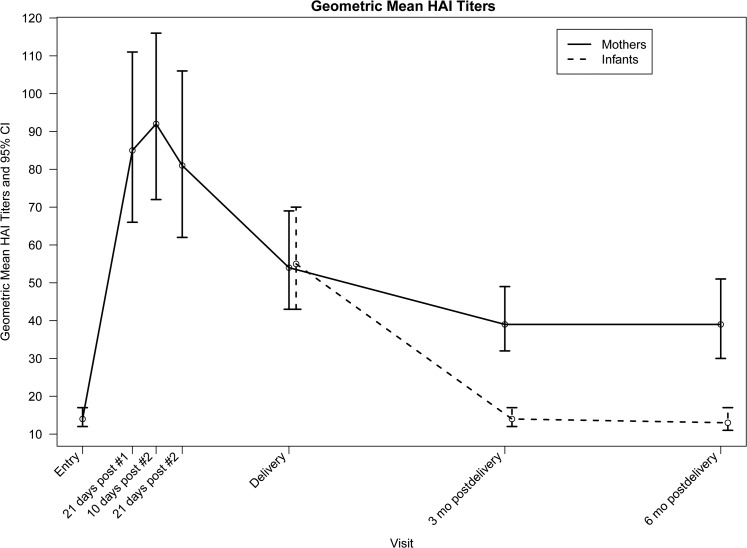
Maternal pH1N1 Antibody Responses
Seroprotection rates and GMTs increased, respectively, from 21% and 14 at entry to 73% and 85 following vaccine dose 1 and 80% and 92 at 10 days after vaccine dose 2 (Table 2; Figure 2). Among subjects assessed after vaccine dose 1, all 25 with baseline titers ≥40 retained seroprotective titers, and 62 of 94 (66%) with baseline titers <40 attained seroprotective titers (P < .0001). Among subjects who received both doses and were assessed after dose 1 and 10 days after dose 2, all with titers ≥40 after vaccine dose 1 had seroprotective titers after vaccine dose 2, and 8 of 29 (28%) with titers <40 after vaccine dose 1 achieved titers ≥40 after vaccine dose 2 (P = .008). Overall, after vaccine dose 2, 63 of 84 (75%) subjects with baseline titers <40 had seroprotective titers.
Table 2.
Maternal Hemagglutination Inhibition Antibody Responses
| Time Point | Subjects, No. | Seroprotectiona (%) (95% CI) | Geometric Mean Titer (95% CI) | Seroresponseb (%) (95% CI) | Complete Responsec (%) (95% CI) |
|---|---|---|---|---|---|
| Entry | 127 | 21 (15–29) | 14 (12–17)d | … | … |
| 21 d post–dose 1 | 119 | 73 (64–81)e | 85 (66–111)f | 66 (57–75) | 61 (51–69) |
| 10 d post–dose 2 | 107 | 80 (72–87)e,g | 92 (72–116)f | 72 (62–80) | 65 (56–74) |
| 21 d post–dose 2 | 94 | 77 (67–85)e | 81 (62–106)f,h | 67 (57–76) | 63 (52–73) |
| Delivery | 115 | 67 (58–75)e,g | 54 (43–69)f,h | 57 (48–67)i | 51 (42–61)j |
| 3 mo postdelivery | 105 | 60 (50–69)e | 39 (32–49)f,h | 51 (41–61) | 46 (36–56) |
| 6 mo postdelivery | 66 | 59 (46–71)e | 39 (30–51)f | 58 (45–70) | 48 (36–61) |
Abbreviation: CI, confidence interval; HAI, hemagglutination inhibition.
Entry antibody titers were analyzed for subjects who received at least the first vaccination. Antibody responses after the first vaccination were analyzed for subjects with available data who received the first vaccine dose and had the day 21 postvaccine dose 1 visit prior to delivery. Responses 10 and 21 days after the second vaccination were analyzed for women with available data who received both doses of vaccine and had the respective visits prior to delivery. Maternal antibody levels at delivery and 3 and 6 months postdelivery were analyzed for those women with available data who received both doses of vaccine and delivered subsequent to the 10-day postvaccine dose 2 timepoint.
a Seroprotection defined as HAI titer ≥40.
b Seroresponse defined as ≥4-fold rise in HAI titer from entry.
c Complete response defined as HAI titer ≥40 and ≥4-fold rise in HAI titer from entry.
d Baseline titers did not differ according to whether seasonal influenza vaccine had been received prior to entry (P = .7).
e P < .0001 (McNemar test) for the comparison to seroprotection at entry.
f P < .0001 (sign test) for the comparison to geometric mean titer at entry.
g P ≤ 008 (McNemar test) for the comparison to seroprotection at previous timepoint.
h P ≤ .04 (sign test) for the comparison to geometric mean titer at previous timepoint.
i P = .007 (McNemar test) for the comparison to seroresponse at previous timepoint.
j P = .007 (McNemar test) for the comparison to complete response at previous timepoint.
Figure 2.
Geometric mean hemagglutination inhibition titers of pregnant women and their infants. Titers and 95% confidence intervals at study entry; 21 days after the first dose of pH1N1 vaccine; 10 and 21 days after the second dose of vaccine; delivery; and 3 and 6 months postpartum are shown. Abbreviations: CI, confidence interval; HAI, hemagglutination inhibition.
Seroresponse was observed in 66% after vaccine dose 1 and 72% 10 days after vaccine dose 2. Complete responses (seroprotection and seroresponse) were observed in 61% after vaccine dose 1, with no difference between complete responders and nonresponders in baseline median titer (10 in both groups; P = .7). After vaccine dose 2, the complete response rate was 65%, including 7 of 41 (17%) nonresponders after vaccine dose 1 who achieved complete response and 3 of 66 (5%) complete responders after dose 1 who no longer met criteria after dose 2.
Seroprotection rates and GMTs declined to 67% and 54 at delivery, 60% and 39 three months postpartum, and 59% and 39 six months postpartum, respectively. Of subjects who had seroprotective titers 10 days after dose 2 and had subsequent assessments, 65 of 85 (76%) had titers ≥40 at delivery, 52 of 78 (67%) at 3 months postpartum, and 35 of 53 (66%) at 6 months postpartum. Subsequent to the 10-day postvaccine dose 2 visit, ≥4-fold declines in titers occurred in 26 of 106 (25%) subjects by delivery, 42 of 98 (43%) by 3 months postpartum, and 22 of 66 (33%) by 6 months postpartum. Eight subjects had ≥4-fold increases subsequent to the 10-day postvaccine dose 2 visit.
Infant pH1N1 Antibody Concentrations
Cord and neonatal blood specimen titers were not statistically different (median, 40 and 80, respectively; P = .4), allowing these to be combined. Infant seroprotection rates and GMTs were 65% and 55 at birth, 26% and 14 at 3 months, and 12% and 13 at 6 months, respectively (Table 3; Figure 2). There was no difference between maternal and infant delivery titers (median difference, 0 [interquartile range {IQR}, −30 to 5]; median ratio, 1 [IQR], .5–2]). Infant birth titers strongly correlated with maternal delivery titers (Spearman correlation coefficient = .86; P < .0001), but not with the interval between maternal vaccine dose 2 and delivery (Spearman correlation coefficient = −.08; P = .4). There was a weak negative association between gestational age at birth and infant birth titers (Spearman correlation coefficient = −.17; P = .08).
Table 3.
Infant Hemagglutination Inhibition Antibody Levels
| Timepoint | Subjects, No. | Seroprotectiona (%) | Geometric Mean Titer |
|---|---|---|---|
| (95% CI) | (95% CI) | ||
| Birth | 106 | 65 (55–74) | 55 (43–70) |
| 3 mo | 97 | 26 (17–36)b | 14 (12–17)c |
| 6 mo | 59 | 12 (5–23)b | 13 (11–17)c |
Abbreviation: CI, confidence interval.
Infant antibody levels at birth, 3 months, and 6 months were analyzed for those infants with available data whose mothers received both doses of vaccine and delivered subsequent to the 10-day postvaccine dose 2 timepoint.
a Seroprotection defined as hemagglutination inhibition titer ≥40.
b P < .0001 (McNemar test) for the comparison to seroprotection at birth.
c P < .0001 (sign test) for the comparison to geometric mean titer at birth.
Predictors of Maternal Complete Response
Greater nadir and entry CD4% and CD4 count were associated with complete response to both vaccinations and lower entry CD8% with response to the first vaccination (Table 4). In multivariable logistic regression models, nadir CD4% and entry CD8% did not add significantly to entry CD4% for the first vaccine dose (χ2 = .1 and .8, respectively) and nadir CD4% did not add significantly to entry CD4% for the second dose (χ2 = .08). Viral load was not associated with response.
Table 4.
Predictors of Maternal Complete Response to Study Vaccinations (Univariate Analyses)
| Characteristic | Dose 1 |
Dose 2 |
||||
|---|---|---|---|---|---|---|
| No.a | OR (95% CI) | P Value | No.a | OR (95% CI) | P Value | |
| Age | 119 | 1.062 (.992–1.136)b | .09 | 107 | 1.056 (.982–1.136)b | .1 |
| Black race | 112 | .989 (.449–2.175) | 1.0 | 100 | 1.364 (.589–3.158) | .5 |
| Hispanic ethnicity | 115 | 1.546 (.691–3.455) | .3 | 103 | 1.268 (.533–3.020) | .6 |
| Nadir CD4 percentage | 113 | 1.071 (1.031–1.113)c | <.001 | 102 | 1.054 (1.014–1.096)c | .01 |
| Nadir CD4 count | 113 | 1.002 (1.000–1.004)d | .02 | 102 | 1.003 (1.001–1.005)d | .01 |
| P1061s CD4 percentage | 119 | 1.068 (1.028–1.109)c | .001 | 107 | 1.043 (1.004–1.083)c | .03 |
| P1061s CD4 count | 116 | 1.002 (1.000–1.004)d | .02 | 105 | 1.002 (1.000–1.004)d | .03 |
| P1061s CD8 percentage | 119 | .963 (.931–.996)c | .03 | 107 | .976 (.941–1.011)c | .2 |
| P1061s CD8 count | 109 | .999 (.998–1.000)d | .2 | 98 | .999 (.998–1.001)d | .4 |
| P1061s CD19 percentage | 108 | .983 (.912–1.059)c | .7 | 98 | .983 (.909–1.063)c | .7 |
| P1061s CD19 count | 97 | .999 (.996–1.002)d | .6 | 88 | .999 (.996–1.002)d | .6 |
| P1061s HIV viral load <400 copies/mL | 119 | 1.253 (.570–2.759) | .6 | 107 | 1.047 (.446–2.459) | .9 |
| HAART | 119 | .596 (.111–3.205) | .5 | 107 | .743 (.137–4.028) | .7 |
| Gestational age at entry | 119 | 1.017 (.954–1.083)e | .6 | 107 | 1.010 (.943–1.080)e | .8 |
| Seasonal influenza vaccination prior to entry | 119 | .907 (.403–2.039) | .8 | 107 | .945 (.394–2.268) | .9 |
| pH1N1 HAI titer ≥40 at entry | 119 | .974 (.396–2.397) | 1.0 | 107 | .616 (.240–1.581) | .3 |
| pH1N1 log10 HAI titer at entry | 119 | 1.054 (.396–2.803)f | .9 | 107 | .695 (.249–1.944)f | .5 |
Complete responses after dose 2 were based on antibody titers measured at the 10 days post–dose 2 timepoint.
Abbreviations: CI, confidence interval; HAART, highly active antiretroviral therapy; HAI, hemagglutination inhibition; OR, odds ratio.
a No. of participants with available data.
b Odds ratio for every year increase.
c Odds ratio for every 1% increase.
d Odds ratio for every cell/mm3 increase.
e Odds ratio for every week increase.
f Odds ratio for every log10 increase in titer.
DISCUSSION
No serious vaccine-related reactions were observed with two 30-μg doses (twice the standard amount of hemagglutinin per dose administered for seasonal and pH1N1 vaccination) of unadjuvanted pH1N1 vaccine in HIV-infected women in the second or third trimester of pregnancy. Previous reports provide evidence of safety of unadjuvanted and adjuvanted monovalent pH1N1 vaccines during pregnancy [5, 14–19] and in HIV-infected, nonpregnant adults [20] and children [13, 21]. Although our study was not powered to detect uncommon events, it adds to the safety record supporting influenza vaccination during pregnancy.
The 2 double-dose regimen was moderately immunogenic, yielding seroprotective titers in 73% after 1 dose, including 66% of those lacking seroprotective titers at baseline, and seroresponse in 66%. After the second dose, 80% achieved seroprotective titers, including 75% of those lacking seroprotective titers at baseline and 28% of those lacking seroprotective titers after the first dose, and 72% demonstrated seroresponse. GMTs attained were relatively modest. Seroprotective titers were present in 67% of mothers and 65% of infants at delivery; however, rates of seroprotection in infants dropped rapidly over the first 3–6 months of life.
Several studies demonstrated that pH1N1 vaccines are immunogenic in HIV-uninfected pregnant women, producing high levels of seroprotection (89%–100%) and seroresponse (89%–93%) after a single, standard dose of unadjuvanted or adjuvanted vaccine [5, 8, 19, 22, 23], and maternal titers at delivery similar to those of women infected with pH1N1 virus during pregnancy [7]. In contrast, one small, unpublished study suggested that a standard 15-μg dose of unadjuvanted vaccine induced lower rates of seroprotection (85%) and seroresponse (69%) compared with a 30-μg dose (96% seroprotection and 93% seroresponse) in pregnant women or compared with a standard dose in nonpregnant women (93% seroprotection and 86% seroresponse) [24]. Nonetheless, findings of adequate immunogenicity of pH1N1 vaccines during pregnancy in most studies are consistent with studies of seasonal influenza vaccination in pregnancy [8, 19].
Nonpregnant, HIV-infected populations generally had inferior responses to standard doses of unadjuvanted pH1N1 vaccines compared with immunocompetent cohorts, with seroprotection and/or seroresponse rates after 1 dose between 31%–76% vs rates of 56%–97% in HIV-uninfected adult vaccinees and lower GMTs among HIV-infected vaccinees [20, 21, 25–29]. A higher (30 μg) dose increased seroprotection, seroresponse, and GMTs, but responses were still poorer than in HIV-uninfected populations [30]. A second dose improved responses in some studies, but not others [25, 26, 30]. Among HIV-infected children, a single, standard dose induced seroprotection in 54%–94% and seroresponse in 49%–63%, increasing to 68%–100% and 65%–68%, respectively, after a second dose, compared with seroprotection in 62%–100% and 98%–100% of HIV-uninfected children after 1 and 2 standard doses, respectively. Two 30-μg vaccinations produced a 80%–85% seroprotection and 80%–84% seroresponse in HIV-infected children, with incremental gains after the second vaccine, but seroprotection was lower than rates of 88%–100% in HIV-uninfected children [13, 21, 31] (R. Pass, S. Nachman, P. Flynn, et al, unpublished data). Poorer responses of HIV-infected persons to influenza vaccines, even with preserved CD4 counts, have been attributed to immune dysregulation affecting T- and B-cell quality and function, immune activation, immunosenescence, and high regulatory T-cell numbers [20, 32]. The responses we observed are lower than those in HIV-uninfected pregnant women and more similar to those described in other HIV-infected populations [32].
Response rates and GMTs in nonpregnant, HIV-infected cohorts were improved with adjuvanted pH1N1 vaccines [10]. Seroprotection and seroresponse rates after 1 ASO3-adjuvanted vaccine dose were 70%–93% and 68%–89%, increasing to 94%–98% and 86%–97%, respectively, after 2 doses and approaching >95% response rates in HIV-uninfected populations [26, 33–35]. With MF59-adjuvanted vaccine, seroprotection rates among HIV-infected adults were 88% after 1 dose and 91% after 2 doses, similar to HIV-uninfected controls, although GMTs of the HIV-infected group were lower. In HIV-infected children, seroprotection rates were 94%–100% after 1 MF59-adjuvanted vaccine dose and 100% after 2 doses [13, 36]. These observations are consistent with greater responses to adjuvanted seasonal influenza vaccines in HIV-infected populations and adjuvanted pH1N1 vaccines in immunocompetent adults and children [26, 35]. The safety and immunogenicity of adjuvanted pH1N1 vaccines in HIV-infected pregnant women have not been described.
Goals of influenza immunization during pregnancy include inducing a sufficiently durable antibody response to protect maternal health through the postpartum period, reduce exposure of the neonate to maternal influenza, and provide passive immunization to protect the newborn before his/her eligibility for vaccination at 6 months of life. In HIV-uninfected pregnant women vaccinated with various pH1N1 regimens in the second or third trimesters, protective titers were maintained in 62%–100% of women at delivery [5, 8, 19, 22, 24], with protective titers persisting in 90%–100% at 2–3 months [8, 19] and 100% at 5 months after delivery [8]. We found at delivery that, despite a drop in GMT, protective titers were present in 67% of women, including 76% who had achieved seroprotective titers after the second vaccine dose, and protective titers persisted in 60% and 59% at 3 and 6 months postpartum, respectively. Thus, the majority of HIV-infected pregnant women maintained protective titers at delivery and postpartum.
Efficient transplacental antibody delivery occurs following pH1N1 vaccination of HIV-uninfected pregnant women, yielding neonatal seropositivity rates of 77%–96%. Neonatal titers exceeded maternal titers in some studies, consistent with active transplacental immunoglobulin G transport, and were lower than maternal titers in others [5, 8, 19, 22, 24, 37]. In one study of infants born to HIV-uninfected women who received MF59-adjuvanted vaccine during the third trimester, seroprotective antibody levels were present in 96% of neonates at delivery and were still present in 96% at 2 months and 81% at 5 months [8]. Transplacental pH1N1 antibody likely confers clinical protection similar to that conferred by transplacental antibody following maternal seasonal influenza vaccination during gestation [5, 8, 19, 38, 39]. In the current study, maternal pH1N1 vaccination was associated with efficient transplacental antibody transfer; the 65% level of seroprotection in neonates and the neonatal GMT were virtually identical to those of mothers at delivery, and there was a strong correlation between maternal delivery titers and neonatal titers. However, because GMTs were modest, with the expected decline of passively acquired antibody, infant seroprotection rates dropped rapidly to 26% at 3 months and 12% at 6 months; infant protection against severe influenza infection was therefore likely short-lived.
Twenty-one percent of subjects had seroprotective titers at entry, consistent with baseline seroprotection rates of 25%–70% in other studies of pH1N1 vaccination in HIV-infected populations [13, 21, 25, 27, 28, 36]. Because our study was performed in the fall of 2009, well into the pandemic which began in the preceding spring, baseline seroprotection may reflect prior pH1N1 infection and/or cross-reactivity with prior seasonal influenza immunization or infection. Similarly, ≥4-fold antibody rises observed in small numbers of subjects subsequent to the postvaccination dose 2 visit may have been due to subclinical or mild pH1N1 infection that did not trigger clinical evaluation.
Nadir and entry CD4% and CD4 count were the primary predictors of maternal vaccine response, whereas HIV RNA load was not predictive. In studies of pH1N1 vaccination of HIV-infected adults and children, predictors included younger age, shorter duration of HIV infection, absence of AIDS-associated conditions, highly active antiretroviral therapy, higher nadir CD4 count, higher current CD4 count, CD8 count (lower in some studies, higher in others) and lower HIV RNA load [20, 21, 27–29, 30, 33, 36]. In some studies, a higher baseline antibody titer was associated with greater responses [13, 21, 27, 30], whereas the opposite was found in other studies [25, 33]. Our study population may have lacked sufficient variation in parameters such as receipt of highly active antiretroviral therapy and HIV RNA load to demonstrate an impact of these factors.
In this first study examining pH1N1 vaccination in HIV-infected pregnant women, a 2 double-dose vaccination regimen in the second and third trimesters had moderate immunogenicity and no concerning vaccine-related safety signals. Seroprotection persisted in a majority through delivery and postpartum, and efficient transplacental transfer of antibody was demonstrated, although seroprotection in infants waned rapidly. These results demonstrate feasibility of vaccination to protect HIV-infected pregnant women and their newborns from new influenza viruses. Nevertheless, pH1N1 vaccination was less immunogenic than in HIV-uninfected pregnant women. Although lack of a comparator group precludes definite conclusions about the optimal vaccination regimen in HIV-infected pregnant women, gains after a second vaccine dose were marginal. Alternative vaccine platforms, including adjuvanted vaccines, should be evaluated in HIV-infected pregnant women to improve upon protection that vaccination can provide to this highly vulnerable group against novel influenza strains.
Notes
Additional members of the P1086 Protocol Team. George Siberry, MD, MPH, Maternal and Pediatric Infectious Diseases Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland; Judi Miller, RN, BSN, Division of AIDS, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland; Wende Levy, RN, MS, Social and Scientific Systems, Silver Spring, Maryland; Barbara Heckman, BS, Frontier Science and Technology Research Foundation, Buffalo, New York; Ruth Ebiasah, PharmD, MS, RPh, Division of AIDS, National Institute of Allergy and Infectious Diseases, Bethesda, MD; Paul Palumbo, MD, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire; Joan Dragavon, MLM, University of Washington, Seattle; Lori Donelson, RN, BSN, CCRC, Westat, Rockville, Maryland; Andrea Jurgrau, MSRN, CPNP, Columbia-Presbyterian Medical Center, New York, New York; David Garry, DO, Jacobi Medical Center, Bronx, New York; Anthony Bloom, MS, Frontier Science and Technology Research Foundation, Amherst, New York.
Participating sites and site personnel. University of Miami Pediatric/Perinatal HIV/AIDS CRS (Amanda Cotter, MD; Gwendolyn B. Scott, MD; Erika Lopez-Bertiery, MD; Safia Khan, MD); University of Puerto Rico Pediatric HIV/AIDS Research Program CRS (Irma L. Febo, MD; Carmen D. Zorrilla, MD; Vivian Tamayo-Agrait, MD; Ruth Santos, RN, MPH); University of Southern California, LA NICHD CRS (Alice Stek, MD; Michael Neely, MD; LaShonda Spencer, MD; Andrea Kovacs, MD); Washington Hospital Center NICHD CRS (Sara Parker, MD; Patricia Tanjutco, MD; Vanessa Emmanuel, BA; Liv Thulin); Texas Children's Hospital CRS (Shelly Buschur, RN, CNM; Mary Paul, MD; Filiz Seeborg, MD; Kathy Pitts, PhD); Chicago Children's CRS (Jessica Shore, RN; Sarah Sutton, MD); UCSD Maternal, Child, and Adolescent HIV CRS (Stephen A. Spector, MD; Andrew Hull, MD; Mary Caffery, RN, MSN; Jean Manning, RN, BSN); DUMC Pediatric CRS (Margaret Donnelly, PA; Mary Jo Hassett, RN; Elizabeth Livingston, MD; Julieta Giner, RN); Rush University Cook County Hospital, Chicago NICHD CRS (Mariam Aziz, MD; Latania Logan, MD; Julie Schmidt, MD; Helen Cejtin, MD); The Children's Hospital of Philadelphia IMPAACT CRS (Samuel Parry, MD; Rita Leite, MD); University of South Florida, Tampa NICHD CRS (Karen L. Bruder, MD; Gail Lewis, RN; Patricia Emmanuel, MD; Tampa General Hospital); San Juan City Hospital PR NICHD CRS (Elvia Perez, MPH, MA; Rodrigo Diaz, MD; Dalila Guzman, RPh; Midnela Acevedo-Flores, MD); South Florida CDC, Ft Lauderdale NICHD CRS; Johns Hopkins University, Baltimore NCHD CRS (Allison Agwu, MD, ScM; Todd Noletto, MPH; Jennifer Chang, BS; Andi Weiss, PharmD); Tulane University, New Orleans NICHD CRS (Chi Dola, MD; Thomas Alchediak, MD; Yvette Luster, RN; Sheila Bradford, RN); Bronx-Lebanon Hospital IMPAACT CRS (Jenny Gutierrez, MD; Mahboobullah Mirza Baig, MD; Stefan Hagmann, MD; Murli Purswani, MD); NJ Medical School CRS (Arlene D. Bardeguez, MD, MPH; Charmane Calilap-Bernardo, RN; Linda Bettica, RN); Columbia IMPAACT CRS (Andrea Jurgrau, CNP; Gina Silva, RN; Alice Higgins, RN; Marc Foca, MD); Metropolitan Hospital NICHD CRS (Mahrukh Bamji, MD; Santa Paul, MD; Siobhan Riley, MPH; Deepali Jain, MD); Children's Hospital of Boston NICHD CRS (Sandra K. Burchett, MD, MS; Ruth Tuomala, MD; Arlene Buck, RN; Catherine Kneut, RN, CPNP); University of Colorado, Denver NICHD CRS (Jennifer Dunn, FNP-C; Paul Harding, MS; Kay Kinzie, FNP-C; Jenna Wallace, MSW; supported by NIH/NCATS Colorado CTSI grant number UL1 TR000154); St Jude/UTHSC CRS (L. Jill Utech, RN, MSN, CCRP; Edwin Thorpe, Jr, MD; Nina Sublette, RN, FNP, PhD; Pam Finnie, MSN); WNE Maternal Pediatric Adolescent AIDS CRS; UCLA–Los Angeles/Brazil AIDS Consortium CRS (Jaime G. Deville, MD; Karin Nielsen-Saines, MD; Nicole Falgout, RN; Joseph Geffen); Boston Medical Center Pediatric HIV Program NICHD CRS; Jacobi Medical Center Bronx NICHD CRS; Seattle Children's Hospital CRS; SUNY Stony Brook NICHD CRS (Denise Ferraro, FNP; Erin Infanzon; Michele Kelly, NP; Jennifer Griffin, CNM); Miller Children's Hospital Long Beach, CA NICHD CRS (Audra Deveikis, MD; Janielle Jackson-Alvarez, RN; Tempe K. Chen, MD; Jagmohan S. Batra, MD); University of Florida College of Medicine, Jacksonville NICHD CRS (Mobeen Rathore, MD; Ayesha Mirza, MD; Nizar Maraqa, MD; Kathleen Thoma, MA, CCRP); University of California, San Francisco NICHD CRS (Diane Wara, MD; Nicole Tilton, PNP; Mica Muscat, PNP).
Acknowledgments. The authors appreciate the contributions of the women and infants who participated in this study and the assistance of research personnel at the study sites. The authors thank Dr Coleen Cunningham and Dr Stephen Spector for their reviews of the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Overall support for the IMPAACT study group was provided by the National Institute of Allergy and Infectious Diseases (NIAID; U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (AI068632). This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the NIAID cooperative agreement 5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group and 1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C); NIH/NCATS Colorado CTSI grant number UL1 TR000154; NIH/NCRR UCSF-CTSI (grant number UL1 RR024131); and General Clinical Research Centers/National Institutes of Health (grant M01 number RR00069). Pharmaceutical support was provided by Novartis, through the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, US Department of Health and Human Services.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bergman H, Livornese LL, Jr., Sambhara S, Santaro J, Dessain SK. Patients hospitalized with pH1N1 influenza in an academic community medical center. Open Respir Med J. 2011;5:19–23. doi: 10.2174/1874306401105010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolan GP, Myles PR, Brett SJ, et al. The comparative clinical course of pregnant and non-pregnant women hospitalized with influenza A (H1N1)pdm09 infection. PLoS One. 2012;7:e41638. doi: 10.1371/journal.pone.0041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubar G, Azria E, Tesnière A, et al. French experience of 2009 A/H1N1v influenza in pregnant women. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013112. pii: e13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein E, Lipsitch M. H1N1 vaccination and adults with underlying health conditions in the US. PLoS Curr. 2009;1:RRN1132. doi: 10.1371/currents.RRN1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson LA, Patel S, Swamy GK, et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J Infect Dis. 2011;204:854–63. doi: 10.1093/infdis/jir440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kay MK, Koelemay KG, Kwan-Gett TS, Cadwell BL, Duchin JS. 2009 pandemic influenza A vaccination of pregnant women: King County, Washington State, 2009–2010. Am J Prev Med. 2012;42:S172–S179. doi: 10.1016/j.amepre.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Fisher BM, Van Bockern J, Hart J, et al. Pandemic influenza A H1N1 2009 infection versus vaccination: a cohort study comparing immune responses in pregnancy. PLoS One. 2012;7:e33048. doi: 10.1371/journal.pone.0033048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuccotti G, Pogliani L, Pariani E, Amendola A, Zanetti A. Transplacental antibody transfer following maternal immunization with a pandemic 2009 influenza A(H1N1) MF-59-adjuvanted vaccine. JAMA. 2010;304:2360–1. doi: 10.1001/jama.2010.1729. [DOI] [PubMed] [Google Scholar]
- 9.Gilca R, De Serres G, Boulianne N, et al. Risk factors for hospitalization and severe outcomes of 2009 pandemic H1N1 influenza in Quebec, Canada. Influenza Other Respi Viruses. 2011;5:247–55. doi: 10.1111/j.1750-2659.2011.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper CL. Pandemic H1N12009 influenza and HIV: a review of natural history, management, and vaccine immunogenicity. Curr Opin Infect Dis. 2012;25:26–35. doi: 10.1097/QCO.0b013e32834ef56c. [DOI] [PubMed] [Google Scholar]
- 11.Peters PJ, Skarbinski J, Louie JK, et al. HIV-infected hospitalized patients with 2009 pandemic influenza A (pH1N1)—United States, spring and summer 2009. Clin Infect Dis. 2011;52(1):S183–8. doi: 10.1093/cid/ciq036. [DOI] [PubMed] [Google Scholar]
- 12.Sheth AN, Patel P, Peters PJ. Influenza and HIV: lessons from the 2009 H1N1 influenza pandemic. Curr HIV/AIDS Rep. 2011;8:181–91. doi: 10.1007/s11904-011-0086-4. [DOI] [PubMed] [Google Scholar]
- 13.Flynn PM, Nachman S, Muresan P, et al. Safety and immunogenicity of 2009 pandemic H1N1 influenza vaccination in perinatally HIV-1-infected children, adolescents, and young adults. J Infect Dis. 2012;206:421–30. doi: 10.1093/infdis/jis360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heikkinen T, Young J, van Beek E, et al. Safety of MF59-adjuvanted A/H1N1 influenza vaccine in pregnancy: a comparative cohort study. Am J Obstet Gynecol. 2012;207:177.e1–8. doi: 10.1016/j.ajog.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Kallen B, Olausson PO. Vaccination against H1N1 influenza with Pandemrix during pregnancy and delivery outcome: a Swedish register study. BJOG. 2012;119:1583–90. doi: 10.1111/j.1471-0528.2012.03470.x. [DOI] [PubMed] [Google Scholar]
- 16.Oppermann M, Fritzsche J, Weber-Schoendorfer C, et al. A(H1N1)v2009: a controlled observational prospective cohort study on vaccine safety in pregnancy. Vaccine. 2012;30:4445–52. doi: 10.1016/j.vaccine.2012.04.081. [DOI] [PubMed] [Google Scholar]
- 17.Pasternak B, Svanstrom H, Molgaard-Nielsen D, et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A (H1N1) vaccine during pregnancy. JAMA. 2012;308:165–74. doi: 10.1001/jama.2012.6131. [DOI] [PubMed] [Google Scholar]
- 18.Pasternak B, Svanstrom H, Molgaard-Nielsen D, et al. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ. 2012;344:e2794. doi: 10.1136/bmj.e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsatsaris V, Capitant C, Schmitz T, et al. Maternal immune response and neonatal seroprotection from a single dose of a monovalent nonadjuvanted 2009 influenza A(H1N1) vaccine: a single-group trial. Ann Intern Med. 2011;155:733–41. doi: 10.7326/0003-4819-155-11-201112060-00005. [DOI] [PubMed] [Google Scholar]
- 20.Crum-Cianflone NF, Eberly LE, Duplessis C, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults. Clin Infect Dis. 2011;52:138–46. doi: 10.1093/cid/ciq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phongsamart W, Sirisanthana V, Wittawatmongkol O, et al. Immunogenicity and safety of monovalent influenza A (H1N1) 2009 in HIV-infected Thai children. Vaccine. 2011;29:8705–11. doi: 10.1016/j.vaccine.2011.08.101. [DOI] [PubMed] [Google Scholar]
- 22.Horiya M, Hisano M, Iwasaki Y, et al. Efficacy of double vaccination with the 2009 pandemic influenza A (H1N1) vaccine during pregnancy. Obstet Gynecol. 2011;118:887–94. doi: 10.1097/AOG.0b013e31822e5c02. [DOI] [PubMed] [Google Scholar]
- 23.Ohfuji S, Fukushim W, Deguchi M, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine among pregnant women: lowered antibody response by prior seasonal vaccination. J Infect Dis. 2011;203:1301–8. doi: 10.1093/infdis/jir026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novartis H1N1 vaccine in pregnant women. Study NCT00992719. Available at: http://clinicaltrials.gov/ct2/show/results/NCT00992719. Accessed 15 October 2012. [Google Scholar]
- 25.Lagler H, Grabmeier-Pfistershammer K, Touzeau-Romer V, et al. Immunogenicity and tolerability after two doses of non-adjuvanted, whole-virion pandemic influenza A (H1N1) vaccine in HIV-infected individuals. PLoS One. 2012;7:e36773. doi: 10.1371/journal.pone.0036773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Launay O, Desaint C, Durier C, et al. Safety and immunogenicity of a monovalent 2009 influenza A/H1N1v vaccine adjuvanted with AS03A or unadjuvanted in HIV-infected adults: a randomized, controlled trial. J Infect Dis. 2011;204:124–34. doi: 10.1093/infdis/jir211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruszak H, Jeganathan S, Smith DE, Robertson P, Barnes T, Furner V. Improved serological response to H1N1 monovalent vaccine associated with viral suppression among HIV-1-infected patients during the 2009 influenza (H1N1) pandemic in the southern hemisphere. HIV Med. 2012;13:352–7. doi: 10.1111/j.1468-1293.2011.00987.x. [DOI] [PubMed] [Google Scholar]
- 28.Tebas P, Frank I, Lewis M, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–92. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 29.Tiu CT, Lin YS, Pagala M, et al. Antibody responses to inactivated influenza A (H1N1) 2009 monovalent vaccine in patients with and without HIV. J Acquir Immune Defic Syndr. 2011;58:e99–102. doi: 10.1097/QAI.0b013e318232b50e. [DOI] [PubMed] [Google Scholar]
- 30.El Sahly HM, Davis C, Kotloff K, et al. Higher antigen content improves the immune response to 2009 H1N1 influenza vaccine in HIV-infected adults: a randomized clinical trial. J Infect Dis. 2012;205:703–12. doi: 10.1093/infdis/jir837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hakim H, Allison KJ, Van De Velde L, Li Y, Flynn PM, McCullers JA. Immunogenicity and safety of inactivated monovalent 2009 H1N1 influenza A vaccine in immunocompromised children and young adults. Vaccine. 2012;30:879–85. doi: 10.1016/j.vaccine.2011.11.105. [DOI] [PubMed] [Google Scholar]
- 32.Richardson K, Weinberg A. Reduced immunogenicity of influenza vaccines in HIV-infected compared to uninfected pregnant women is associated with regulatory T cells. AIDS. 2011;25:595–602. doi: 10.1097/QAD.0b013e32834411a8. [DOI] [PubMed] [Google Scholar]
- 33.Bickel M, von Hentig N, Wieters I, et al. Immune response after two doses of the novel split virion, adjuvanted pandemic H1N1 influenza A vaccine in HIV-1-infected patients. Clin Infect Dis. 2011;52:122–7. doi: 10.1093/cid/ciq003. [DOI] [PubMed] [Google Scholar]
- 34.Cooper C, Klein M, Walmsley S, et al. High-level immunogenicity is achieved vaccine with adjuvanted pandemic H1N1(2009) and improved with boosting dosing in a randomized trial of HIV-infected adults. HIV Clin Trials. 2012;13:23–32. doi: 10.1310/hct1301-023. [DOI] [PubMed] [Google Scholar]
- 35.Manuel O, Pascual M, Hoschler K, et al. Humoral response to the influenza A H1N1/09 monovalent ASO3-adjuvanted vaccine in immunocompromised patients. Clin Infect Dis. 2011;52:248–56. doi: 10.1093/cid/ciq104. [DOI] [PubMed] [Google Scholar]
- 36.Soonawala D, Rimmelzwaan GJ, Gelinck LBS, Visser LG, Kroon FP. Response to 2009 pandemic influenza A (H1N1) vaccine in HIV-infected patients and the influence of prior seasonal influenza vaccination. PLoS One. 2011;6:e16496. doi: 10.1371/journal.pone.0016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puleston RL, Bugg G, Hoschler K, et al. Observational study to investigate vertically acquired passive immunity in babies of mothers vaccinated against H1N1v during pregnancy. Health Technol Assess. 2010;14:1–82. doi: 10.3310/hta14550-01. [DOI] [PubMed] [Google Scholar]
- 38.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 39.Steinhoff MC, Omer SB, Roy E, et al. Influenza immunization in pregnancy—antibody responses in mothers and infants. N Engl J Med. 2010;362:1644–6. doi: 10.1056/NEJMc0912599. [DOI] [PubMed] [Google Scholar]