Abstract
A new variant rat myogenic cell line, ts485, was isolated by subcloning the cell line ts3b2 (H. T. Nguyen, R. M. Medford, and B. Nadal-Ginard, Cell 34:281-293, 1983). Unlike the progenitor cell line, ts485 was thermosensitive for differentiation. Experiments with conditioned medium suggested that diffusible extracellular factors were not involved in dictating the differential phenotypes of ts485 cells cultured at the permissive and nonpermissive temperatures. Temperature shift experiments performed on cultures of ts485 cells indicated that the temperature-sensitive lesion was in a factor active during the growth phase and required to trigger a cascade of events leading to terminal differentiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J., Fee F., Balmain A. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988 Jan 28;331(6154):363–365. doi: 10.1038/331363a0. [DOI] [PubMed] [Google Scholar]
- Bader D., Masaki T., Fischman D. A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982 Dec;95(3):763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin E., Kayalar C. Metalloendoprotease inhibitors that block fusion also prevent biochemical differentiation in L6 myoblasts. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8029–8033. doi: 10.1073/pnas.83.21.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton P. J., Robert B., Fiszman M. Y., Leader D. P., Buckingham M. E. The same myosin alkali light chain gene is expressed in adult cardiac atria and in fetal skeletal muscle. J Muscle Res Cell Motil. 1985 Aug;6(4):461–475. doi: 10.1007/BF00712583. [DOI] [PubMed] [Google Scholar]
- Basilico C. Temperature-sensitive mutations in animal cells. Adv Cancer Res. 1977;24:223–266. doi: 10.1016/s0065-230x(08)61016-7. [DOI] [PubMed] [Google Scholar]
- Bendori R., Salomon D., Geiger B. Contact-dependent regulation of vinculin expression in cultured fibroblasts: a study with vinculin-specific cDNA probes. EMBO J. 1987 Oct;6(10):2897–2905. doi: 10.1002/j.1460-2075.1987.tb02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfield P. A., Zivin R. A., Miller L. S., Sowder R., Smythers G. W., Henderson L., Oroszlan S., Pearson M. L. Isolation and sequence analysis of cDNA clones coding for rat skeletal muscle creatine kinase. J Biol Chem. 1984 Dec 10;259(23):14979–14984. [PubMed] [Google Scholar]
- Blau H. M., Chiu C. P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Minty A., Robert B., Montarras D., Weydert A., Cohen A., Daubas P., Buckingham M. Regulation of muscle gene expression. The accumulation of messenger RNAs coding for muscle-specific proteins during myogenesis in a mouse cell line. J Mol Biol. 1982 Sep;160(1):59–76. doi: 10.1016/0022-2836(82)90131-0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu C. P., Blau H. M. Reprogramming cell differentiation in the absence of DNA synthesis. Cell. 1984 Jul;37(3):879–887. doi: 10.1016/0092-8674(84)90423-9. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Couch C. B., Strittmatter W. J. Rat myoblast fusion requires metalloendoprotease activity. Cell. 1983 Jan;32(1):257–265. doi: 10.1016/0092-8674(83)90516-0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Goeddel D. V. The murine transforming growth factor-beta precursor. J Biol Chem. 1986 Apr 5;261(10):4377–4379. [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Parfett C. L., Denhardt D. T. Transcriptional regulation of two serum-induced RNAs in mouse fibroblasts: equivalence of one species to B2 repetitive elements. Mol Cell Biol. 1985 Nov;5(11):3280–3288. doi: 10.1128/mcb.5.11.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Transcriptional and posttranscriptional control of c-myc during myogenesis: its mRNA remains inducible in differentiated cells and does not suppress the differentiated phenotype. Mol Cell Biol. 1986 May;6(5):1412–1421. doi: 10.1128/mcb.6.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone G., Tatò F., Alemà S. Distinctive effects of the viral oncogenes myc, erb, fps, and src on the differentiation program of quail myogenic cells. Proc Natl Acad Sci U S A. 1985 Jan;82(2):426–430. doi: 10.1073/pnas.82.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeman E. C., Chiu C. P., Minty A., Blau H. M. The pattern of actin expression in human fibroblast x mouse muscle heterokaryons suggests that human muscle regulatory factors are produced. Cell. 1986 Oct 10;47(1):123–130. doi: 10.1016/0092-8674(86)90373-9. [DOI] [PubMed] [Google Scholar]
- Hastings K. E., Emerson C. P., Jr cDNA clone analysis of six co-regulated mRNAs encoding skeletal muscle contractile proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1553–1557. doi: 10.1073/pnas.79.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., Chamberlain J. S., Buskin J. N., Johnson J. E., Hauschka S. D. Transcriptional regulation of the muscle creatine kinase gene and regulated expression in transfected mouse myoblasts. Mol Cell Biol. 1986 Aug;6(8):2855–2864. doi: 10.1128/mcb.6.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr 5-Azacytidine induction of stable mesodermal stem cell lineages from 10T1/2 cells: evidence for regulatory genes controlling determination. Cell. 1984 Oct;38(3):791–800. doi: 10.1016/0092-8674(84)90274-5. [DOI] [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr Complex regulation of the muscle-specific contractile protein (troponin I) gene. Mol Cell Biol. 1987 Sep;7(9):3065–3075. doi: 10.1128/mcb.7.9.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr Differentiation, not determination, regulates muscle gene activation: transfection of troponin I genes into multipotential and muscle lineages of 10T1/2 cells. Mol Cell Biol. 1985 Sep;5(9):2423–2432. doi: 10.1128/mcb.5.9.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J., Bushar G., Kakunaga T., Hamada H., Hirakawa T., Goldman D., Merril C. Variations in expression of mutant beta actin accompanying incremental increases in human fibroblast tumorigenicity. Cell. 1982 Feb;28(2):259–268. doi: 10.1016/0092-8674(82)90344-0. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Wahrmann J. P., Luzzati D. Temperature-sensitive variants of an established myoblast line. Proc Natl Acad Sci U S A. 1973 Feb;70(2):425–429. doi: 10.1073/pnas.70.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford R. M., Wydro R. M., Nguyen H. T., Nadal-Ginard B. Cytoplasmic processing of myosin heavy chain messenger RNA: evidence provided by using a recombinant DNA plasmid. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5749–5753. doi: 10.1073/pnas.77.10.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko A. S., Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987 Oct 9;51(1):51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Caravatti M., Buckingham M. E. A fetal skeletal muscle actin mRNA in the mouse and its identity with cardiac actin mRNA. Cell. 1982 Aug;30(1):185–192. doi: 10.1016/0092-8674(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Minty A., Blau H., Kedes L. Two-level regulation of cardiac actin gene transcription: muscle-specific modulating factors can accumulate before gene activation. Mol Cell Biol. 1986 Jun;6(6):2137–2148. doi: 10.1128/mcb.6.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
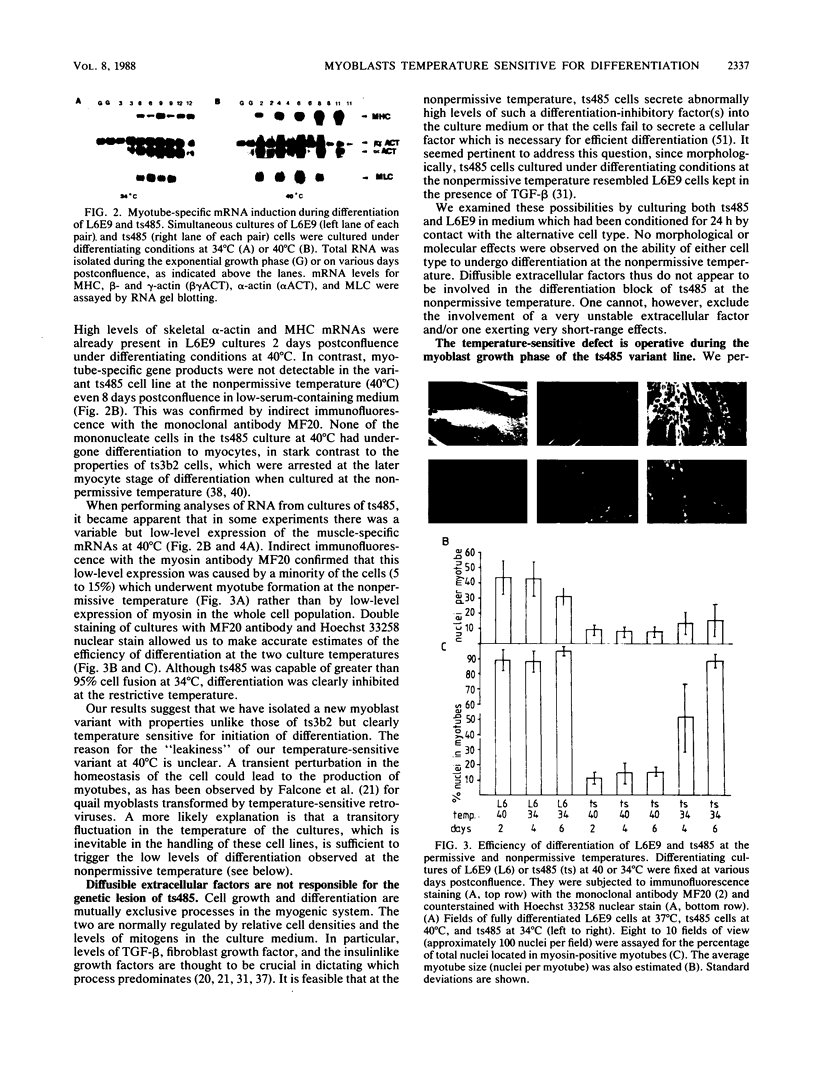
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Nameroff M., Munar E. Inhibition of cellular differentiation by phospholipase C. II. Separation of fusion and recognition among myogenic cells. Dev Biol. 1976 Mar;49(1):288–293. doi: 10.1016/0012-1606(76)90275-x. [DOI] [PubMed] [Google Scholar]
- Nguyen H. T., Medford R. M., Nadal-Ginard B. Reversibility of muscle differentiation in the absence of commitment: analysis of a myogenic cell line temperature-sensitive for commitment. Cell. 1983 Aug;34(1):281–293. doi: 10.1016/0092-8674(83)90159-9. [DOI] [PubMed] [Google Scholar]
- Nomura S., Yamagoe S., Kamiya T., Oishi M. An intracellular factor that induces erythroid differentiation in mouse erythroleukemia (Friend) cells. Cell. 1986 Feb 28;44(4):663–669. doi: 10.1016/0092-8674(86)90275-8. [DOI] [PubMed] [Google Scholar]
- Pinset C., Whalen R. G. Manipulation of medium conditions and differentiation in the rat myogenic cell line L6. Dev Biol. 1984 Apr;102(2):269–277. doi: 10.1016/0012-1606(84)90192-1. [DOI] [PubMed] [Google Scholar]
- Podleski T. R., Greenberg I., Schlessinger J., Yamada K. M. Fibronectin delays the fusion of L6 myoblasts. Exp Cell Res. 1979 Sep;122(2):317–326. doi: 10.1016/0014-4827(79)90308-2. [DOI] [PubMed] [Google Scholar]
- Razin A., Webb C., Szyf M., Yisraeli J., Rosenthal A., Naveh-Many T., Sciaky-Gallili N., Cedar H. Variations in DNA methylation during mouse cell differentiation in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2275–2279. doi: 10.1073/pnas.81.8.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Control of myogenesis in vitro by Ca 2 + concentration in nutritional medium. Exp Cell Res. 1969 Nov;58(1):163–167. doi: 10.1016/0014-4827(69)90127-x. [DOI] [PubMed] [Google Scholar]
- Shani M., Admon S., Yaffe D. The methylation state of 2 muscle-specific genes: restriction enzyme analysis did not detect a correlation with expression. Nucleic Acids Res. 1984 Sep 25;12(18):7225–7234. doi: 10.1093/nar/12.18.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M., Zevin-Sonkin D., Saxel O., Carmon Y., Katcoff D., Nudel U., Yaffe D. The correlation between the synthesis of skeletal muscle actin, myosin heavy chain, and myosin light chain and the accumulation of corresponding mRNA sequences during myogenesis. Dev Biol. 1981 Sep;86(2):483–492. doi: 10.1016/0012-1606(81)90206-2. [DOI] [PubMed] [Google Scholar]
- Silberstein G. B., Daniel C. W. Reversible inhibition of mammary gland growth by transforming growth factor-beta. Science. 1987 Jul 17;237(4812):291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Ginty C. A. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983 Dec;35(3 Pt 2):657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- Tarikas H., Schubert D. Regulation of adenylate kinase and creatine kinase activities in myogenic cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2377–2381. doi: 10.1073/pnas.71.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsipouras P., Myers J. C., Ramirez F., Prockop D. J. Restriction fragment length polymorphism associated with the pro alpha 2(I) gene of human type I procollagen. Application to a family with an autosomal dominant form of osteogenesis imperfecta. J Clin Invest. 1983 Oct;72(4):1262–1267. doi: 10.1172/JCI111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]