Abstract
BACKGROUND
New high-performance liquid chromatography/ tandem mass spectrometry (LC-MS/MS) methods are among the most successful approaches to improve specificity problems inherent in many immunoassays.
CONTENT
We emphasize problems with immunoassays for the measurement of steroids and review the emerging role of LC-MS/MS in the measurement of clinically relevant steroids. The latest generation of tandem mass spectrometers has superior limits of quantification, permitting omission of previously employed derivatization steps. The measurement of steroid profiles in the diagnosis and treatment of congenital adrenal hyperplasia, adrenal insufficiency, chronic pelvic pain and prostatitis, oncology (breast cancer), and athletes has important new applications.
CONCLUSIONS
LC-MS/MS now affords the specificity, imprecision, and limits of quantification necessary for the reliable measurement of steroids in human fluids, enhancing diagnostic capabilities, particularly when steroid profiles are available.
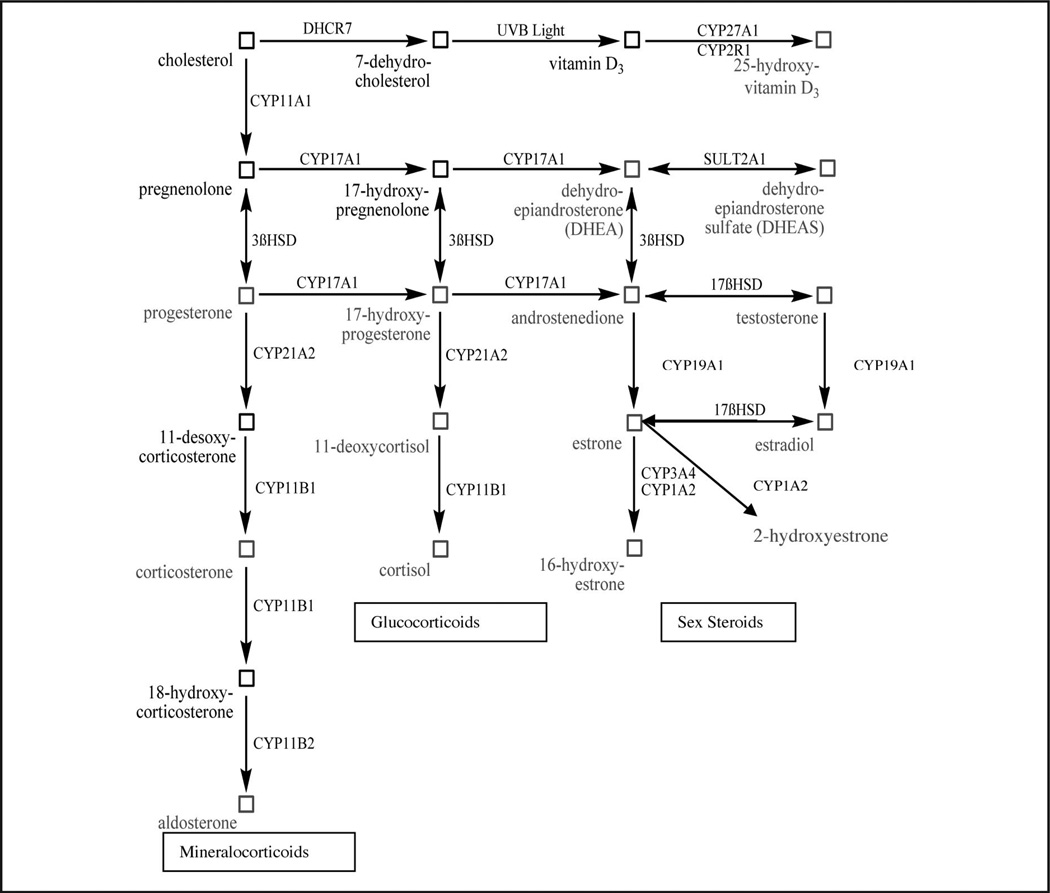
Steroid hormones are synthesized from cholesterol, and many are of great clinical importance (1). Synthesis occurs in the mitochondria and smooth endoplasmic reticulum of cells in the adrenal cortex, the gonads, and the placenta (Fig. 1). The adrenal gland is composed of the adrenal medulla and cortex. The latter is divided into 3 anatomic zones: the zona glomerulosa, which produces the mineralocorticoids such as corticosterone and aldosterone, and the zonae fasciculata and reticularis, which together produce the glucocorticoids (11-deoxycortisol, cortisol) and the adrenal androgens [dehydroepiandrostenedione (DHEA),5 DHEA-sulfate (DHEAS)], which in the adrenals are produced in far higher amounts than androstenedione and testosterone. Aromatase [cytochrome P (CYP)-450 19, CYP19A1] adds 2 double bonds to ring A of testosterone, yielding estradiol with an aromatic ring A, and it also aromatizes ring A of androstenedione to form estrone (E1).
Fig. 1.
Steroid hormone synthetic pathways.
One of the goals in the treatment of some breast cancers is reduction of estrogen concentrations. This can be achieved through the use of aromatase inhibitors (AIs), which block the conversion of androgens to estrogens (2). AIs do not sufficiently block estrogen synthesis by the ovaries, however, but do block other tissues from converting androgens to estrogens. For this reason, AIs are used mostly in women who have reached menopause, when the ovaries no longer synthesize steroids. E1 synthesized mainly by aromatase conversion of androstenedione is metabolized to several steroid metabolites, including CYP1A2 conversion to 2-hydroxyestrone (2-OHE1) and CYP3A4 and CYP1A2 to 16-α-hydroxyestrone (16-OHE1). The former is weakly estrogenic and inhibits breast cell proliferation (3–6), whereas the latter is carcinogenic and genotoxic, enhances breast cell growth, and increases DNAsynthesis and oncogene expression (3). Attempts to modify the 2-OHE1:16-OHE1 ratio in young women using the antidepressant fluoxetine are currently being explored (6). Fluoxetine is a known inhibitor of multiple P450 isoenzymes, including 3A4, 2C9, and 2D6, and known to affect estrogen concentrations.
Methods of Measurement
Endogenous steroids have been measured by immunoassay (IA) (7–12), GC-MS (13–15), and high-performance liquid chromatography–tandem mass spectrometry (LC-MS/MS) (16–21). An evaluation of the results for steroid measurement in the College of American Pathologists (CAP) proficiency testing (PT) program is very informative. The majority of laboratories participating in this program employ IA methods, each using a different antibody. For each method, CAP calculates the method mean, so it is possible to divide the mean value of the method giving the highest mean by the mean value of the method giving the lowest mean. Table 1 summarizes the IA results for one of the CAP challenges for 2008. For this particular challenge, laboratories using the method giving the highest results differed from those using the procedure giving the lowest results by a factor of 2.8, 9.0, and 3.3 for testosterone, estradiol, and progesterone, respectively.
Table 1.
Lack of specificity of steroid hormone immunoassays; data from CAP PT Program Y-A Survey 2008.
| Analyte | Lowest mean (L) |
Highest mean (H) |
Factor H/L |
|---|---|---|---|
| Testosterone, ng/dL | 52.6 | 148.7 | 2.8 |
| Estradiol, pg/mL | 25.4 | 229.0 | 9.0 |
| Progesterone, ng/mL | 0.83 | 2.72 | 3.3 |
This example illustrates vividly the magnitude of the IA problem, due to many factors, including lack of specificity of antibodies purported to measure a particular steroid. This poor IA performance for steroid measurement encompasses more than just the 3 steroids shown in Table 1 and is true for all steroids measured in the CAP PT program. This contrasts with the good IA performance for the measurement of drugs such as phenytoin, phenobarbital, carbamazepine, etc., where the mean values for drugs in the CAP PT program for different IAs are almost identical. Stanczyk et al. (22) also state that lack of standardization of steroid hormone assays is a major deficiency in epidemiologic studies. In postmenopausal women, the reported concentrations of estradiol are highly variable, with normal values differing by a factor of approximately 6 (22). This supports the CAP PT data reported above.
In contrast, Table 2 shows results for those laboratories using MS/MS for steroid measurement. The high/low ratio is much better, ranging from 1.0 to 1.4. It must be stated, however, that for testosterone, estradiol, and progesterone the number of laboratories reporting results by tandem mass spectrometry is small (9, 2, and 3, respectively, in this recent survey). This could partially account for the apparently superior performance of MS compared to IA. It also raises the question of whether all laboratories using MS/MS are complying with reporting their tandem mass spectrometry data in the CAP PT program.
Table 2.
MS/MS data of steroid hormones; CAP PT Program Y-A Survey 2008.
| Analyte | Lowest value (L) |
Highest value (H) |
Factor H/L |
|---|---|---|---|
| Testosterone, ng/dL | |||
| Testosterone no. 1 | 52 | 72 | 1.4 |
| Testosterone no. 2 | 182 | 225 | 1.2 |
| Estradiol, pg/mL | |||
| Estradiol no. 1 | 109 | 109 | 1.0 |
| Estradiol no. 2 | 628 | 630 | 1.0 |
| Progesterone, ng/mL | |||
| Progesterone no. 1 | 0.7 | 0.9 | 1.3 |
| Progesterone no. 2 | 8.1 | 8.6 | 1.1 |
FACTORS MERITING CONSIDERATION FOR MS/MS MEASUREMENT OF STEROIDS
Derivatization vs nonderivatization
This is currently an important issue, with advocates on both sides. Derivatization proponents claim that both enhanced limit of quantification and specificity can be achieved by adopting this approach. This is questionable, however; derivatization has its disadvantages, which include decreased precision due to the added derivatization steps (extraction, derivatization at an extreme of pH). Accuracy could be compromised as well through the possible hydrolysis of conjugates, which would clearly affect the accuracy of the assay by giving falsely increased results. An example of such a problem could occur with estradiol conjugates, which on hydrolysis would yield estradiol. While serving on the CAP PT committee, we compared the values obtained for testosterone, progesterone, and estradiol in 3 leading laboratories using tandem mass spectrometry. Although excellent agreement was found for testosterone and progesterone, the laboratories using derivatization at an extreme of pH for estradiol measurement had results approximately 10%–20% higher than the laboratory that avoided derivatization. Derivatization methods are also lengthy and therefore more time-consuming. Over the past 15 years, the detection limits with modern mass spectrometers have improved greatly and have made the derivatization of analytes unnecessary. For this reason, we have been able to avoid derivatization approaches for the measurement of steroids (17–19).
Type of ionization
Electrospray ionization (ESI) in the negative mode yields the best results for the estrogens (estradiol, estriol, E1, and 16-OHE1) (18). The method used, which avoids derivatization, has a lower limit of detection (LOD) of 1–2 pg/mL for all 4 estrogens when run on the API-5000 tandem mass spectrometer (Applied Biosystems). Total sample requirement is only 0.2 mL, and total chromatography time for each estrogen profile is 8 min. The use of C-8 analytical columns (Supelco LC-8-DB; 3.3 by 3.0 mm, 3 µm particle size) is preferred over C-18 columns, as they markedly reduce retention times of the analytes in question. Our experience, and that of others (14, 18, 22), has shown that IAs for estrogens have problems at low estrogen concentrations (<80 pg/mL), frequently reporting falsely increased results.
For DHEA, DHEAS, androstenedione, testosterone, progesterone, cortisol, 11-deoxycortisol, corticosterone, and aldosterone, we have found that atmospheric pressure photoionization (APPI) has potential advantages over ESI or APCI (atmospheric pressure chemical ionization) in that it is a soft ionization source that effectively ionizes these steroids fairly selectively, leading to cleaner chromatograms. Alary (23) compared APPI-MS/MS with APCI for the measurement of steroids in biological matrices and reported that the signal obtained using the APPI source was 3–10 times more intense than that obtained employing the APCI source. In our APPI method (19), the sample size is 0.2 mL serum or plasma. After protein precipitation with acetonitrile containing the deuterated internal standards, the solution is vortex-mixed and centrifuged, and the supernatant is injected directly onto a C-8 column, as with the estrogen profile assay. The column is washed with buffer, the switching valve is activated, and steroids are eluted into the tandem mass spectrometer using a methanol gradient. The total chromatography time for the steroid profile assay is 11 min. The LOD varies from 1.5 to 10.0 pg/mL depending on the analyte (19). Whereas ion suppression has limited the usefulness of many MS/MS methods, it is a rare occurrence with the method described. Use of deuterated internal standards and an online sample wash step are partly responsible for the good performance.
Profile vs single steroid testing
Large commercial reference laboratories have high daily volumes for many of the clinically important steroids discussed above. To accommodate this volume and meet the need for rapid turnaround time with short chromatography time, these laboratories have adopted a 1 tandem/1 steroid philosophy, with a different tandem mass spectrometer used for each steroid. Smaller teaching hospital laboratories with a less pressing workload can, on the other hand, evaluate the potential role of steroid profile testing. As LC-MS/MS allows the simultaneous measurement of several steroids, sample sizes can be reduced compared to IA platforms that require an additional sample for each steroid measured; this is particularly germane in the evaluation of infants, where specimen size is limited.
Multiple circumstances in which steroid profiles have been employed
1. Congenital adrenal hyperplasia (CAH) is an inborn error of steroid biosynthesis. CAH is a group of inherited diseases caused by defective activity of 1 of 5 enzymes in the adrenal cortex. The defective enzyme leads to decreased production of cortisol (causing an increased corticotropin) and excess production of hormones proximal to the defect. The 2 most common forms of CAH are caused by either 21-hydroxylase deficiency (defect in the P450c21 enzyme) or 11-β-hydroxylase deficiency (defect in P450c11 enzyme). Individuals with CAH due to 11- or 21-hydroxylase enzyme deficiency cannot produce adequate amounts of cortisol and, in some cases, are also aldosterone deficient. These hormones are essential in glucose metabolism and sodium reabsorption. Untreated CAH can lead to sudden adrenal insufficiency, with dehydration, shock, and even death. Steroids that have been recommended for the assessment of CAH are cortisol, androstenedione, and 17-hydroxyprogesterone (24–26). At Children’s National Medical Center, we routinely use a broad 11-steroid profile to improve the specificity of screening for CAH caused by either 21-hydroxylase or 11-hydroxylase deficiencies (19).
Routine newborn screening for congenital adrenal hyperplasia suffers from a high rate of false-positive and false-negative results when using an IA to measure 17-OH progesterone concentrations, especially in critically ill newborns and preterm neonates. Therefore, immediate reanalysis of all IA results above the cutoff using LC-MS/MS can allow a clear distinction of affected and nonaffected newborns (24, 26).
2. The evaluation of adrenal insufficiency is historically recommended by measuring cortisol at 0, 30, and 60 min after an adrenocorticotropic hormone (ACTH) stimulation test (27–29). We have improved the diagnostic reliability of this approach by measuring the 3 steroids aldosterone, cortisol, and most importantly, 11-deoxycortisol at 0, 30, and 60 min. Including aldosterone in the profile allows the differentiation of primary from secondary adrenal insufficiency. In primary adrenal insufficiency, no aldosterone response is observed, whereas an adequate response is found in secondary adrenal insufficiency (30). The concentration of 11-deoxycortisol increases 15- to 20-fold in controls after an ACTH stimulation test, which compares to an approximately 3-fold increase for the more traditionally measured analyte cortisol. Our study demonstrated greater diagnostic accuracy if these 3 steroids were measured instead of measuring only cortisol (30).
3. We have also assessed the role of steroid profiles in patients with chronic prostatitis/chronic pelvic pain syndrome. Our results suggest reduced activity of CYP21A2 (P450c21), which is the 21-hydroxylase enzyme that converts progesterone to 11-desoxycorticosterone and 17-hydroxyprogesterone to 11-deoxycortisol (31) (Fig. 1).
4. We assessed whether steroid profiles provided insight into the reasons for premature adrenarche and infants with genital hair. In both these groups, the concentrations of testosterone, androstenedione, DHEA, and DHEAS were somewhat higher than in age matched controls (32). We have also assessed the reference intervals for these steroids during pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry (33).
5. Sera from active smokers, passive smokers, and nonsmokers have been analyzed for 15 steroid hormones and thyroid hormones to examine the associations between smoke exposure and hormone concentrations (34, 35). Although we do not know whether the blood concentrations of the hormones reflect changes that parallel physiological variation in steroid hormone concentrations, the assumption is that differences reflect associations with tobacco smoke exposure.
6. LC-MS/MS after solid-phase extraction has been used in lipidomic profiling of some female steroid hormones in human urine, and can be potentially applied clinically and to metabolomic research (36).
7. Diabetes strongly affects neuroactive steroids in the nervous system. LC-MS/MS assessment of the concentrations of neuroactive steroids provides a basis for new therapeutic tools based on neuroactive steroids aimed at counteracting diabetic neuropathy (37).
8. Even in trace amounts, estrogens such as E2, E1, estriol, and 17α-ethinyl estradiol may have adverse effects on humans and the aquatic ecosystem. It is there-fore essential to be able to reliably determine trace amounts (at environmentally relevant concentrations) of steroid estrogens in water. Using LC-MS/MS, it is now possible to detect these chemicals in small samples of water at concentrations as low as 0.04 ng/L (38–41).
9. Finally, steroid profiling has been used to assess changes in adrenal steroids before and after a 56-km ultramarathon race (42). Concentrations of the mineralocorticoids corticosterone and aldosterone increased significantly, as did concentrations of the glucocorticoids cortisol, 11-deoxycortisol, and 17-OH progesterone and the adrenal steroids DHEA, DHEAS, and androstenedione (P < 0.0001 for all).
Some future areas for research include the assessment of the role of neurosteroids in epilepsy, particularly in pubertal girls in whom a marked increase in seizure activity has been found (43–45), and analysis of androgens in males, patients with benign prostatic hyperplasia, and prostate cancer (46, 47).
In conclusion, LC-MS/MS now affords the specificity, imprecision, and limits of quantification necessary for the reliable measurement of steroids in human fluids, thereby enhancing diagnostic capabilities, particularly when steroid profiles are available. Major advantages of tandem mass spectrometry include small sample size, the simultaneous measurement of many analytes, and enhanced specificity compared to IA methods. Mass spectrometric methods are still fairly labor intensive, and certainly require a higher level of laboratory expertise than do IA platforms. Occasional interferences when using mass spectrometric methods have been described, such as prednisolone/prednisone metabolite interference in urinary free cortisol measurements (48). It should be noted that currently reimbursement for steroid profile testing is not yet approved by Medicare (with the exception of the CAH steroid profile), nor are steroid profiles ordered frequently by clinicians. This could well change as steroid hormone profiling becomes more appreciated in the years ahead. Although mass spectrometric assays are not always more precise than IAs, they are more specific for measuring the analyte of interest. By omitting extraction and derivatization steps, the steroid tandem mass spectrometric procedures described here have good intrarun and interrun imprecision (18, 19). Drug interference has been tested and found not to be a problem for the steroid and estrogen profiles reported in this review (18, 19). These two methods are also relatively free of ion suppression.
Acknowledgments
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
Nonstandard abbreviations: DHEA, dehydroepiandrostenedione; DHEAS, DHEA-sulfate; CYP, cytochrome P; E1, estrone; AI, aromatase inhibitor; 2-OHE1, 2-hydroxyestrone; 16-OHE1, 16-α-hydroxyestrone; IA, immunoassay; LC-MS/MS, liquid chromatography–tandem mass spectrometry; CAP, College of American Pathologists; PT, proficiency testing; ESI, electrospray ionization; LOD, limit of detection; APPI, atmospheric pressure photoionization; APCI, atmospheric pressure chemical ionization; CAH, congenital adrenal hyperplasia; ACTH, adrenocorticotropic hormone.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: S.J. Soldin, NIH, and NMS.
Stock Ownership: None declared.
Honoraria: S.J. Soldin, AACC, CSCC, and CAMB.
Research Funding: S.J. Soldin, NMS, Applied Biosystems, and NIH.
Expert Testimony: None declared.
References
- 1.Holst JP, Soldin OP, Guo T, Soldin SJ. Steroid hormones: relevance and measurement in the clinical laboratory. Clin Lab Med. 2004;24:105–118. doi: 10.1016/j.cll.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swain SM. Aromatase inhibitors: a triumph of translational oncology. N Engl J Med. 2005;353:2807–2809. doi: 10.1056/NEJMe058273. [DOI] [PubMed] [Google Scholar]
- 3.Bradlow HL, Davis DL, Lin G, Sepkovic D, Tiwari R. Effects of pesticides on the ratio of 16 alpha/ 2-hydroxyestrone: a biologic marker of breast cancer risk. Environ Health Perspect. 1995;103(Suppl 7):147–150. doi: 10.1289/ehp.95103s7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Zheng W, Dunning LM, Anderson KG, Parrish RS, Holtzman JL. Within-person variability of the ratios of urinary 2-hydroxyestrone to 16alpha-hydroxyestrone in Caucasian women. Steroids. 1999;64:856–859. doi: 10.1016/s0039-128x(99)00073-2. [DOI] [PubMed] [Google Scholar]
- 5.Shou M, Korzekwa KR, Brooks EN, Krausz KW, Gonzalez FJ, Gelboin HV. Role of human hepatic cytochrome P450 1A2 and 3A4 in the metabolic activation of estrone. Carcinogenesis. 1997;18:207–214. doi: 10.1093/carcin/18.1.207. [DOI] [PubMed] [Google Scholar]
- 6.Thompson DS, Kirshner MA, Klug TL, Kastango KB, Pollock BG. A preliminary study of the effect of fluoxetine treatment on the 2:16-alpha-hydroxyestrone ratio in young women. Ther Drug Monit. 2003;25:125–128. doi: 10.1097/00007691-200302000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004;50:2195–2197. doi: 10.1373/clinchem.2004.040683. [DOI] [PubMed] [Google Scholar]
- 8.Dorgan JF, Fears TR, McMahon RP, Aronson Friedman L, Patterson BH, Greenhut SF. Measurement of steroid sex hormones in serum: a comparison of radioimmunoassay and mass spectrometry. Steroids. 2002;67:151–158. doi: 10.1016/s0039-128x(01)00147-7. [DOI] [PubMed] [Google Scholar]
- 9.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. Quantification of circulating 1,25- dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996;42:586–592. [PubMed] [Google Scholar]
- 10.Hsing AW, Stanczyk FZ, Belanger A, Schroeder P, Chang L, Falk RT, et al. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2007;16:1004–1008. doi: 10.1158/1055-9965.EPI-06-0792. [DOI] [PubMed] [Google Scholar]
- 11.Poulson K, Sancho J, Haber E. A simplified radioimmunoassay for plasma aldosterone employing an antibody of unique specificity. Clin Immunol. 1974;2:373–380. doi: 10.1016/0090-1229(74)90055-5. [DOI] [PubMed] [Google Scholar]
- 12.Schioler V, Thode J. Six direct radioimmunoassays of estradiol evaluated. Clin Chem. 1988;34:949–952. [PubMed] [Google Scholar]
- 13.Andrew R. Clinical measurement of steroid metabolism. Best Pract Res Clin Endocrinol Metab. 2001;15:1–16. doi: 10.1053/beem.2001.0116. [DOI] [PubMed] [Google Scholar]
- 14.Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Wolthers BG, Kraan GP. Clinical applications of gas chromatography and gas chromatographymass spectrometry of steroids. J Chromatogr A. 1999;843:247–274. doi: 10.1016/s0021-9673(99)00153-3. [DOI] [PubMed] [Google Scholar]
- 16.Draisci R, Palleschi L, Ferretti E, Lucentini L, Cammarata P. Quantitation of anabolic hormones and their metabolites in bovine serum and urine by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2000;870:511–522. doi: 10.1016/s0021-9673(99)01293-5. [DOI] [PubMed] [Google Scholar]
- 17.Guo T, Chan M, Soldin SJ. Steroid profiles using liquid chromatography-tandem mass spectrometry with atmospheric pressure photoionization source. Arch Pathol Lab Med. 2004;128:469–475. doi: 10.5858/2004-128-469-SPULCM. [DOI] [PubMed] [Google Scholar]
- 18.Guo T, Gu J, Soldin OP, Singh RJ, Soldin SJ. Rapid measurement of estrogens and their metabolites in human serum by liquid chromatographytandem mass spectrometry without derivatization. Clin Biochem. 2008;41:736–741. doi: 10.1016/j.clinbiochem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo T, Taylor RL, Singh RJ, Soldin SJ. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin Chim Acta. 2006;372:76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 22.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev. 2007;16:1713–1719. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- 23.Alary JF. Comparative study: LC/MS/MS analysis of four steroid compounds using a new photoionization source and a conventional APCI source. In: Proceedings of the 49th ASMS Conference on Mass Spectrometry and Allied Topics; 2001 May 27–31; Chicago. [Santa Fe (NM)]: American Society for Mass Spectrometry; 2001. Abstract nr A010942. [Google Scholar]
- 24.Lacey JM, Minutti CZ, Magera MJ, Tauscher AL, Casetta B, McCann M, et al. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin Chem. 2004;50:621–625. doi: 10.1373/clinchem.2003.027193. [DOI] [PubMed] [Google Scholar]
- 25.Minutti CZ, Lacey JM, Magera MJ, Hahn SH, McCann M, Schulze A, et al. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89:3687–3693. doi: 10.1210/jc.2003-032235. [DOI] [PubMed] [Google Scholar]
- 26.Janzen N, Peter M, Sander S, Steuerwald U, Terhardt M, Holtkamp U, et al. Newborn screening for congenital adrenal hyperplasia: additional steroid profile using liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2007;92:2581–2589. doi: 10.1210/jc.2006-2890. [DOI] [PubMed] [Google Scholar]
- 27.Crowley S, Hindmarsh PC, Honour JW, Brook CG. Reproducibility of the cortisol response to stimulation with a low dose of ACTH(1–24): the effect of basal cortisol levels and comparison of lowdose with high-dose secretory dynamics. J Endocrinol. 1993;136:167–172. doi: 10.1677/joe.0.1360167. [DOI] [PubMed] [Google Scholar]
- 28.Grinspoon SK, Biller BM. Clinical review 62: laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79:923–931. doi: 10.1210/jcem.79.4.7962298. [DOI] [PubMed] [Google Scholar]
- 29.May ME, Carey RM. Rapid adrenocorticotropic hormone test in practice: retrospective review. Am J Med. 1985;79:679–684. doi: 10.1016/0002-9343(85)90517-0. [DOI] [PubMed] [Google Scholar]
- 30.Holst JP, Soldin SJ, Tractenberg RE, Guo T, Kundra P, Verbalis JG, et al. Use of steroid profiles in determining the cause of adrenal insufficiency. Steroids. 2007;72:71–84. doi: 10.1016/j.steroids.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimitrakov J, Joffe HV, Soldin SJ, Bolus R, Buffington CA, Nickel JC. Adrenocortical hormone abnormalities in men with chronic prostatitis/ chronic pelvic pain syndrome. Urology. 2008;71:261–266. doi: 10.1016/j.urology.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplowitz P, Soldin SJ. Steroid profiles in serum by liquid chromatography-tandem mass spectrometry in infants with genital hair. J Pediatr Endocrinol Metab. 2007;20:597–605. doi: 10.1515/jpem.2007.20.5.597. [DOI] [PubMed] [Google Scholar]
- 33.Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005;84:701–710. doi: 10.1016/j.fertnstert.2005.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soldin OP, Loffredo CA, Shields PG, Marion C, Ressom H, Landy H. Hormone changes in women of reproductive age associations with genetics and tobacco smoke exposure. From: Flight Attendant Medical Research Institute’s Sixth Scientific Symposium; 2007 May 14–16; Miami, Fla. [Google Scholar]
- 35.Soldin OP, Soldin SJ, Ressom H, Landy HJ. Soc Gynecol Invest Annu Meeting. San Diego, CA: 2008. Hormone changes in women of reproductive age associated with tobacco smoke exposure using metabolomics. [Google Scholar]
- 36.Alvarez Sanchez B, Capote FP, Jimenez JR, Luque de Castro MD. Automated solid-phase extraction for concentration and clean-up of female steroid hormones prior to liquid chromatography-electrospray ionization-tandem mass spectrometry: an approach to lipidomics. J Chromatogr A. 2008;1207:46–54. doi: 10.1016/j.chroma.2008.08.085. [DOI] [PubMed] [Google Scholar]
- 37.Caruso D, Scurati S, Maschi O, De Angelis L, Roglio I, Giatti S, et al. Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: effect of diabetes. Neurochem Int. 2008;52:560–568. doi: 10.1016/j.neuint.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Lin YH, Chen CY, Wang GS. Analysis of steroid estrogens in water using liquid chromatography/ tandem mass spectrometry with chemical derivatizations. Rapid Commun Mass Spectrom. 2007;21:1973–1983. doi: 10.1002/rcm.3050. [DOI] [PubMed] [Google Scholar]
- 39.Reddy S, Iden CR, Brownawell BJ. Analysis of steroid conjugates in sewage influent and effluent by liquid chromatography-tandem mass spectrometry. Anal Chem. 2005;77:7032–7038. doi: 10.1021/ac050699x. [DOI] [PubMed] [Google Scholar]
- 40.Schlusener MP, Bester K. Determination of steroid hormones, hormone conjugates and macrolide antibiotics in influents and effluents of sewage treatment plants utilising high-performance liquid chromatography/tandem mass spectrometry with electrospray and atmospheric pressure chemical ionisation. Rapid Commun Mass Spectrom. 2005;19:3269–3278. doi: 10.1002/rcm.2189. [DOI] [PubMed] [Google Scholar]
- 41.Stanford BD, Weinberg HS. Isotope dilution for quantitation of steroid estrogens and nonylphenols by gas chromatography with tandem mass spectrometry in septic, soil, and groundwater matrices. J Chromatogr A. 2007;1176:26–36. doi: 10.1016/j.chroma.2007.10.085. [DOI] [PubMed] [Google Scholar]
- 42.Hew-Butler T, Jordaan E, Stuempfle KJ, Speedy DB, Siegel AJ, Noakes TD, et al. Osmotic and nonasmotic regulation of arginine vasopressin during prolonged endurance exercise. J Clin Endocrinol Metab. 2008;93:2072–8. doi: 10.1210/jc.2007-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higashi T, Nagahama A, Otomi N, Shimada K. Studies on neurosteroids XIX. Development and validation of liquid chromatography-tandem mass spectrometric method for determination of 5alpha-reduced pregnane-type neurosteroids in rat brain and serum. J Chromatogr. 2007;848:188–199. doi: 10.1016/j.jchromb.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 44.Hill M, Bicikova M, Parizek A, Havlikova H, Klak J, Fajt T, et al. Neuroactive steroids, their precursors and polar conjugates during parturition and postpartum in maternal blood: 2. Time profiles of pregnanolone isomers. J Steroid Biochem Mol Biol. 2001;78:51–57. doi: 10.1016/s0960-0760(01)00073-5. [DOI] [PubMed] [Google Scholar]
- 45.Hill M, Parizek A, Bicikova M, Havlikova H, Klak J, Fait T, et al. Neuroactive steroids, their precursors, and polar conjugates during parturition and postpartum in maternal and umbilical blood: 1. Identification and simultaneous determination of pregnanolone isomers. J Steroid Biochem Mol Biol. 2000;75:237–244. doi: 10.1016/s0960-0760(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 46.Higashi T, Yamauchi A, Shimada K, Koh E, Mizokami A, Namiki M. Determination of prostatic androgens in 10 mg of tissue using liquid chromatography-tandem mass spectrometry with charged derivatization. Anal Bioanal Chem. 2005;382:1035–1043. doi: 10.1007/s00216-005-3233-1. [DOI] [PubMed] [Google Scholar]
- 47.Zhao M, Baker SD, Yan X, Zhao Y, Wright WW, Zirkin BR, et al. Simultaneous determination of steroid composition of human testicular fluid using liquid chromatography tandem mass spectrometry. Steroids. 2004;69:721–6. doi: 10.1016/j.steroids.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Kushnir MM, Rockwood AL, Nelson GJ, Terry AH, Meikle AW. Liquid chromatography tandem mass spectrometry analysis of urinary free cortisol. Clin Chem. 2003;49:965–967. doi: 10.1373/49.6.965. [DOI] [PubMed] [Google Scholar]