Abstract
Objective
To determine if dobutamine induced abnormal stress induce changes in left ventricular (LV) stroke volume (SV) and aortic stiffness predict future pulmonary edema.
Background
Heightened aortic stiffness that reduces LV stroke volume during adrenergic stress may serve as a marker for future pulmonary edema (P edema).
Methods
We measured LVSV, ventriculo-vascular stiffness (pulse pressure/LVSVi), and aortic distensibility (AoD) at rest and during intravenous dobutamine using cardiovascular magnetic resonance (DCMR). Personnel blinded to DCMR followed participants longitudinally over time to identify those admitted to the hospital with P edema. Data from 44 participants who experienced a hospital admission for P edema were compared to data from 72 participants of similar age, gender, and resting LV ejection fraction who remained free of P edema.
Results
Expressed as median and interquartile range, participants with versus without P edema exhibited a reduced ratio of stress/rest LVSV (0.9 [0.7,1.1] versus 1.0 [0.9,1.2], respectively, p= 0.002); an increased ventriculo-vascular stiffness stress/rest ratio (1.4 [1.0,1.6] versus 1.0 [0.8,1.3], respectively, p= ≤ 0.001); and a reduced stress induced measure of AoD (0.8 [0.3,1.3] versus 1.6 [1.2,3.2] mmHg−3, respectively, p=0.002). After accounting for age, gender, LVEF, risk factors for P edema and the presence of dobutamine induced ischemia, LVSV reserve and the stress/rest ventriculo-vascular stiffness ratio remained different (p<0.008 for both) between those with and without P edema.
Conclusions
In patients without inducible ischemia during dobutamine stress, in whom one might otherwise assume a favorable prognosis, the failure to increase LV stroke volume, or an increase in ventriculo-vascular stiffness indicates patients at risk for subsequent P edema.
Keywords: Stroke volume, heart failure, dobutamine cardiovascular magnetic resonance
Introduction
Acute myocardial ischemia or infarction may limit the ability of the left ventricle to augment its stroke volume (SV) in response to stress (1). In this situation, the right ventricle may displace blood into the lungs, elevate left atrial (LA) pressure, and produce pulmonary edema (P edema). (2,3). The onset of P edema (often accompanied by arterial hypertension) can occur in the absence of a fall in left ventricular (LV) ejection fraction or the development of new regional wall motion abnormalities (4). As shown by Kawaguchi, et al (5), heightened vascular stiffness can adversely affect LV performance in patients with P edema that do not exhibit myocardial ischemia (5).
Accordingly, we hypothesized that in the absence of ischemia, an inability of the left ventricle to augment SV due to an abnormal increase in arterial stiffness may predispose patients to develop future P edema. To evaluate this hypothesis, we measured stress/rest LVSV, ventriculo-vascular stiffness (pulse pressure/LVSV indexed for body surface area), and aortic distensibility during intravenous dobutamine in patients who subsequently developed P edema. We compared their data to a group of individuals that also underwent dobutamine stress but did not develop P edema. Stratified analyses were performed to address the association of LVSV with future P edema in participants with and without dobutamine induced LV wall motion abnormalities indicative of ischemia.
Methods
Study Design and Population
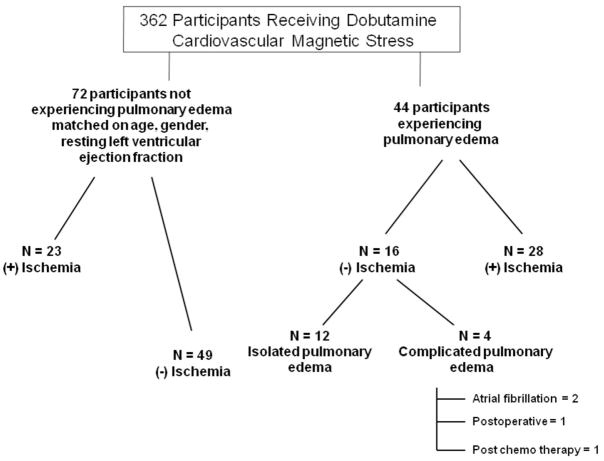
The Institutional Review Board of the Wake Forest University School of Medicine approved the study including the review of medical records. In addition, study participants provided informed consent for the dobutamine stress imaging procedure, and post-testing analysis of the imaging data. We utilized a study design in which participants experiencing P edema were selected from a patient population that previously (>1 month) had undergone dobutamine cardiovascular magnetic resonance (DCMR) stress testing. From 362 DCMR stress exams that were consecutively performed between April 1997, to April 2003, we identified all 44 individuals that subsequently experienced an inpatient hospitalization for P edema over a 6-year period of follow-up within our medical center. P edema was defined as an acute onset of dyspnea in the presence of rales on the physical exam recorded by the managing physician, evidence of pulmonary congestion on the chest radiogram, and subsequent receipt of intravenous diuretic therapy to relieve pulmonary congestion. This definition was commensurate with selection of criteria used to adjudicate P edema due to heart failure in the I-PRESERVE study (6). Patients with lung cancer or moderate to severe valvular heart diseases were excluded from analyses. Our comparison population was selected from the same 362 individuals who had undergone a DCMR stress exam and did not develop future P edema within the same follow-up time frame (Figure 1). Blinded to the cardiac and vascular imaging study results, subjects for the control group were selected to have a similar distribution of gender, age and resting LV ejection fraction to the 44 individuals that experienced P edema.
Figure 1. Study population.
Diagram segregating participants receiving DCMR stress that developed pulmonary edema (n=44) and an age, gender and resting LV ejection fraction matched distribution of participants (n=72) that did not develop pulmonary edema.
Personnel performing analyses were blinded to other aspects of the study. For example, those unaware of stress testing results reviewed the medical charts to identify P edema outcomes of the participants. Likewise, those assessing DCMR stress data were blinded to participant outcomes.
Dobutamine/Atropine CMR protocol
Images were acquired with a 1.5 T Horizon(General Electric Medical Systems, Milwaukee, Wisconsin) whole body imaging system using a phased array cardiac surface coil according to previously published techniques (7,8). Dobutamine was infused incrementally from low dose (7.5 micrograms kg−1min−1), to high dose (20 to 40 micrograms kg−1min−1), and atropine was infused (up to 1.5 mg) to achieve 85% of the maximum predicted heart rate response for age, the heart rate response associated with a maximal test (7,8). Images were acquired at rest, at low and high dose infusion, and then after 10 minutes of recovery (7,8). K-space segmentation was adjusted to achieve a temporal resolution of 20 msec for determining LV end-systolic dimensions at peak stress.
Image Analysis
Left ventricular volumes were determined according to previously published techniques using a biplane area-length technique (8–10) from the 4 and 2 chamber views of left ventricle. Cardiovascular stiffness was assessed using previously published methods using measurements of aortic distensibility (11) and the brachial pulse pressure/LV stroke volume index (12). Aortic distensibility was defined as the (maximum aortic area – the minimum aortic area ÷ minimum aortic area x brachial pulse pressure(13,14).
Statistical analysis
Categorized data were summarized by percentages. Since many of the continuous data were skewed, the central value and spread of the distribution of values were presented by the median and the interquartile range (IQR). When box and whisker plots were given, the box demonstrated the 25th, 50th, and 75th percentiles and the whiskers represented the largest values within 1.5 times the interquartile rage from the median. Comparison of proportions between groups was tested for significance using Fisher’s exact test. The association between measures was estimated and tested using Spearman’s rank correlation. Comparison of continuous data between groups was tested for significance using Wilcoxon’s rank sum test. Comparisons of change in measures during the stress test was tested for significance using Wilcoxon’s rank test for paired comparisons. Analysis of covariance, where other factors are included in the model, was conducted using rank based nonparametric methods. The estimates and tests of the association of increased risk of P edema by DCMR data were estimated and tested for significance using Cox’s proportional hazards model for case control studies.15 The estimate of group effect was made after controlling for known risk factors of P edema (age, gender, diabetes, hypertension, prior coronary artery revascularization or myocardial infarction (MI), body mass index). The statistical comparisons were 2-tailed and p-values < 0.05 were considered statistically significant.
Results
The average follow-up times for those with and without future P edema were similar, 6±2 years and 6±2 years, respectively (p= 0.41). Isolated PE occurred in 29 cases; in other cases, P edema occurred along with M (n=4), acute renal failure (n=3), post-operatively (n=3), during atrial fibrillation with a rapid ventricular response (n=3), post-chemotherapy (n=1), and after cardiac arrest (n=1). As shown in Figure 1, we performed analyses on our entire study population and additional stratified analyses on only those with isolated P edema.
Demographic data of the study participants are displayed in Table 1. The age and gender of both the participant groups were similar. Patients with future P edema exhibited more diabetes, but the prevalence of hypertension, prior history of MI, hypercholesterolemia, smoking, and medication use were similar between the groups. Body mass index trended higher in the P edema participants.
Table 1.
Baseline characteristic of participants with or without pulmonary edema (median and interquartile range).
| ALL (116) | Pulmonary edema | p-value | ||
|---|---|---|---|---|
| Yes(44) | No(72) | |||
| Demographics | ||||
| Age (years) | 67 (60,74) | 68 (60,76) | 67 (59,73) | 0.60 |
| Gender, male (%) | 54 | 52 | 55 | 0.85 |
| BMI | 29 (26,34) | 30 (27,35) | 29 (25,32) | 0.13 |
| Historical data (%) | ||||
| Prior MI | 42 | 48 | 39 | 0.44 |
| HTN | 72 | 70 | 72 | 0.84 |
| DM | 36 | 48 | 29 | 0.05 |
| Hypercholesterolemia | 57 | 59 | 55 | 0.85 |
| Smoking | 41 | 48 | 36 | 0.25 |
| Medication (%) | ||||
| β-blocker | 40 | 39 | 42 | 0.85 |
| Calcium channel blocker | 30 | 36 | 26 | 0.30 |
| Nitrate | 28 | 36 | 22 | 0.14 |
| ACE inhibitor/ARB | 37 | 43 | 33 | 0.33 |
| Statin | 30 | 32 | 43 | 0.83 |
| Rest PP | 63 (50,74) | 62 (47,77) | 63 (51,74) | 0.52 |
| Rest HR | 72 (64,81) | 69 (64,82) | 74 (65,81) | 0.49 |
| Rest SBP | 137 (124,154) | 134(121,153) | 139 (126,159) | 0.37 |
| Rest DBP | 76 (66,86) | 74 (65,84) | 76 (66,87) | 0.25 |
| Stress PP | 71 (56,88) | 70 (54,88) | 71 (59,86) | 0.66 |
| Stress HR | 130 (119,135) | 125(105,131) | 130 (120,135) | 0.02 |
| %MPHR | 85(79,87) | 84 (67,86) | 85 (81,88) | 0.01 |
| Stress SBP | 144 (126,168) | 143(128,168) | 144 (126,169) | 0.91 |
| Stress DBP | 73 (65,85) | 70 (63,81) | 74 (65,86) | 0.18 |
| Stress-Rest PP | 10 (−6,21) | 12 (2,23) | 5 (−8,19) | 0.08 |
| Dobutamine(mg/kg/min) | 20 (20,30) | 20 (20,30) | 20 (20,30) | 0.51 |
| Atropine(mg) | 0 (0,0.4) | 0 (0,0.4) | 0 (0,0.3) | 0.64 |
Abbreviations: BMI, body mass index; MI, myocardial infarction; HTN, hypertension; DM, diabetes; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; PP, pulse pressure; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MPHR, maximum predicted heart rate response for age.
Hemodynamic data from the subject’s dobutamine studies are also displayed in Table 1. Those with future P edema received 20 (20,30) mcg/kg/min of dobutamine and 0 (0.0,0.3) mg of atropine, and those without future P edema received 20 (20;30) mcg/kg/min of dobutamine and 0 (0.0,0.3) mg of atropine during testing (p=0.51 and 0.64, respectively). Participants without future P edema exhibited higher peak stress heart rate responses than those with P edema (p=0.02).
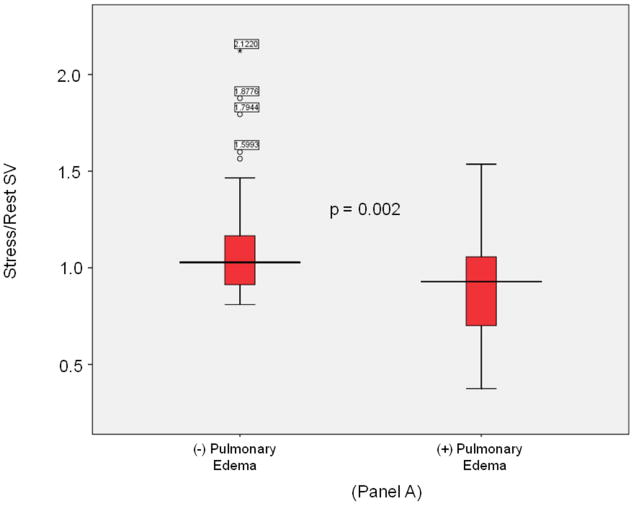
LV volumes, EF, cardiac output, and vascular stiffness data are displayed in Table 2. Patients with P edema had a higher prevalence of dobutamine induced LV wall motion abnormalities indicative of ischemia (63% vs. 32%, p = 0.001). Rest measures of LV volumes, EF and ventricular-vascular stiffness were similar between the groups. At peak stress, LV end-diastolic volume (LVEDV) was similar between those with and without P edema (101 [79,154] versus 104 [73,122] ml, p=0.29), the LV end systolic volume (LVESV) was lower in those without versus with P edema (41 [29,61] versus 58 [43,97] ml, respectively; p=0.007). Those without versus with P edema exhibited higher LVSV reserve measurements (1.0 [0.9,1.5] versus 0.9 [0.7,1.0], respectively, p=0.002 [Figure 2, Panel A]). After adjustment for age, gender, diabetes, hypertension, prior coronary artery revascularization, body mass index and the presence of prior MI, LVSV reserve (stress/rest LVSV) remained different in those with and without future P edema (p=0.002). Participants with a LVSV reserve of < 1.0 were 50% more likely to experience P edema (p< 0.001) than those with a LVSV reserve ≥ 1.0.
Table 2.
DCMR findings in participants with or without pulmonary edema. (expressed as median [interquartile range])
| ALL (116) | Pulmonary edema | p- value | ||
|---|---|---|---|---|
| Yes(44) | No(72) | |||
| Rest EF | 50 (40,55) | 48 (36,54) | 52 (44,55) | 0.08 |
| Rest EDV | 110 (91,147) | 116 (89,179) | 109 (91,131) | 0.43 |
| Rest ESV | 59 (43,83) | 66 (42,114) | 57 (43,72) | 0.38 |
| Rest SV | 52 (41,63) | 54 (37,72) | 51 (44,58) | 0.61 |
| Stress EDV | 103 (76,134) | 101 (79,154) | 104 (73,122) | 0.29 |
| Stress ESV | 46 (31,75) | 58 (43,97) | 41 (29,61) | 0.007 |
| Stress SV | 54 (41,62) | 47 (34,58) | 55 (48,65) | 0.02 |
| Rest EDVi | 55 (45,73) | 57 (43,87) | 54 (46,70) | 0.68 |
| Rest ESVi | 29 (16,35) | 31 (21,54) | 29 (23,38) | 0.56 |
| Rest SVi | 26 (21,32) | 27 (19,34) | 25 (21,31) | 0.77 |
| Stress EDVi | 52 (40,65) | 53 (40,78) | 52 (40,61) | 0.41 |
| Stress ESVi | 24 (16,35) | 26 (21,48) | 22 (15,32) | 0.02 |
| Stress SVi | 26 (21,32) | 23 (17,31) | 27 (24,33) | 0.02 |
| Rest PP/SV | 1.2 (0.9,1.7) | 1.1 (0.8,1.8) | 1.2 (0.9,1.5) | 0.56 |
| Rest PP/SVi | 2.5 (1.8,3.2) | 2.3 (1.7,3.5) | 2.5 (1.9,3.0) | 0.63 |
| Stress PP/SV | 1.3 (1.0,1.7) | 1.5 (1.1,2.3) | 1.3 (1.0,1.6) | 0.04 |
| Stress PP/SVi | 2.6 (2.0,3.7) | 2.8 (2.2,4.3) | 2.5 (1.9,3.3) | 0.04 |
| Stress-Rest SV | 0.6 (−6.5,6.7) | −3.7 (−12.5,2.5) | 1.5 (−5.2,8.9) | 0.002 |
| Stress-Rest SVi | 0.3 (−3.3,3.4) | −2.1 (−6.0,1.3) | 0.7 (−2.7,4.2) | 0.002 |
| Stress-Rest PP/SV | 0.1 (−0.2,0.5) | 0.3 (−0.0,0.8) | −0.0 (−0.2,0.3) | <0.001 |
| Stress-Rest PP/SVi | 0.2 (−0.4,0.9) | 0.6 (−0.0,1.4) | −0.0 (−0.5,0.6) | <0.001 |
| Stress/Rest PP/SV | 1.1 (0.8,1.5) | 1.4 (1.0,1.6) | 1.0 (0.8,1.3) | <0.001 |
| Rest CO | 3.6 (3.0,4.8) | 3.7 (2.5,5.1) | 3.6 (3.0,4.6) | 0.69 |
| Stress CO | 6.7 (4.9,7.7) | 5.3 (4.1,7.2) | 7.0 (5.9,7.8) | 0.002 |
| Stress/Rest CO | 1.7 (1.4,2.1) | 1.4 (1.2,1.8) | 1.9 (1.5,2.2) | <0.001 |
| Rest CI | 1.8 (1.5,2.4) | 1.9 (1.5,2.5) | 1.8 (1.4,2.4) | 0.89 |
| Stress CI | 3.2 (2.6,3.9) | 2.8 (2.0,3.7) | 3.4 (2.8,4.1) | 0.001 |
| Diff CO | 2.6 (1.9,3.9) | 1.6 (0.9,2.9) | 3.0 (2.2,4.2) | <0.001 |
| Diff CI | 1.3 (0.7,1.9) | 0.7 (0.4,1.4) | 1.5 (1.1,2.1) | <0.001 |
Abbreviations: CO, cardiac output; CI, cardiac index; DCMR, dobutamine cardiovascular magnetic resonance; EDV, end diastolic volume; EDVi end diastolic volume index; EF, ejection fraction; ESV, end systolic volume; ESVi, end systolic volume index; PP/SV, pulse pressure/stroke volume; PP/SVi, pulse pressure/stroke volume index; SV, stroke volume; SVi, stroke volume index.
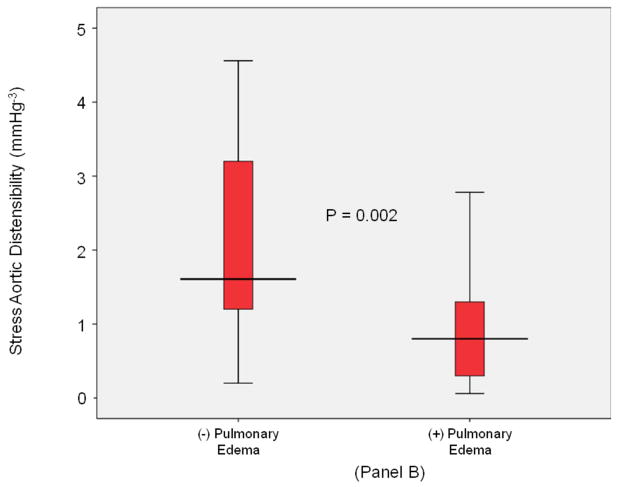
Figure 2. LV sroke volume and aortic stiffness (all).
Box-and-whisker plots of stress/rest left ventricular stroke volume (y-axis, Panel A) and peak stress aortic distensibility (y-axis, Panel B) measured during dobutamine cardiovascular magnetic resonance imaging. Pulmonary edema- and pulmonary edema+ groups are shown on the x-axis. LVSV reserve was reduced and stress induced aortic stiffness was elevated in pulmonary edema+ participants. In these plots, lines within boxes represent median values; the top and bottom lines of the boxes represent the 25th and 75th percentiles, respectively; the top and bottom whiskers outside the boxes represent the smallest data value that is ≥ quartile (Q) 1–1.5 of the interquartile range (IQR), and the largest data value that ≤ Q 3+1.5 (IQR), respectively.
Measures of total vascular stiffness (PP/LVSVi) were similar between the groups at rest. At peak stress, those with P edema exhibited an increased PP/LVSVi (2.8 [2.2,4.3] versus 2.5 [1.9,3.3], respectively, p=0.04), and stress/rest ratios of PP/LVSVi (1.4 [1.0,1.6] versus 1.0 [0.8,1.3], respectively, p=0.001). As shown in Table 2 and Figure 2, Panel B, the stress/rest ratios of PP/LVSVi were higher while stress induced AoD was lower in participants with versus without future P edema.
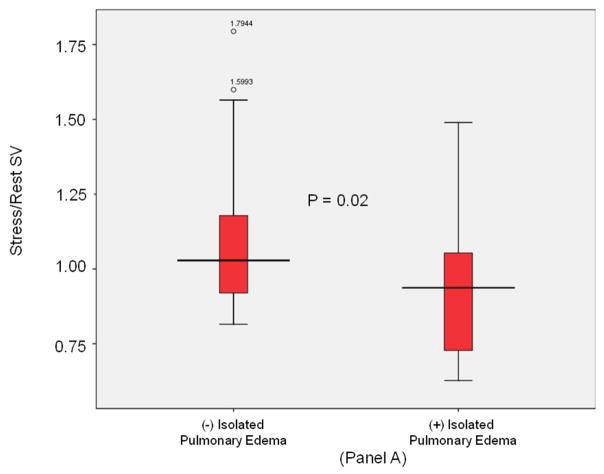
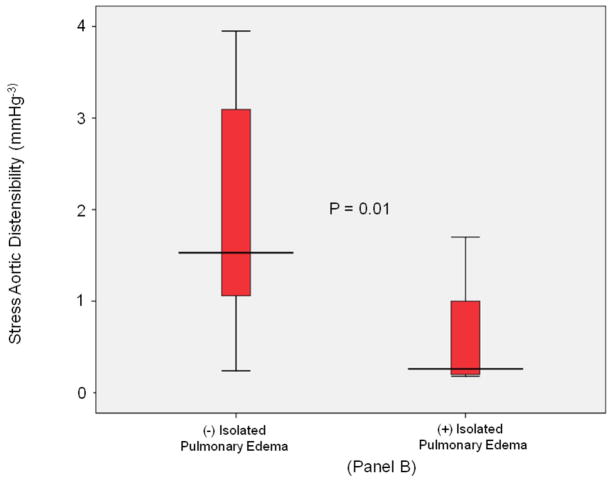
Stratified analyses were performed in participants without inducible LV ischemia during dobutamine stress. As displayed in Figure 1 and shown in Table 3, there were 12 subjects with isolated P edema and no ischemia during dobutamine stress. These 12 subjects were compared to the 49 subjects without P edema that also had no inducible ischemia during dobutamine stress. In these analyses, those with versus without P edema exhibited impaired LVSV reserve, (p = 0.02); Figure 3, Panel A), and a decrease in stress induced aortic distensibility (p=0.01; Figure 3, Panel B). Importantly, after adjustment for demographic and historical comorbidities associated with the future risk of P edema, stress/rest ratios of LVSV reserve (p=0.03), cardiovascular stiffness (p=0.02), and stress aortic distensibility (p=0.05) remained significantly different in those with versus without P edema.
Table 3.
DCMR findings in participants with or without isolated pulmonary edema and no inducible ischemia. (expressed as median [interquartile range])
| All (61) | Pulmonary edema | p- value | p-value adjusted for age, gender | p-value adjusted for all factors * | ||
|---|---|---|---|---|---|---|
| Yes (12) | No (49) | |||||
| Rest EF | 53 (45,56) | 51 (41,54) | 54 (47,56) | 0.10 | 0.02 | 0.01 |
| Rest EDV | 109 (92,126) | 113 (96,162) | 107 (91,125) | 0.26 | 0.09 | 0.10 |
| Rest ESV | 57 (43,72) | 66 (37,113) | 57 (43,69) | 0.53 | 0.24 | 0.27 |
| Rest SV | 51 (44,58) | 55 (46,61) | 50 (44,55) | 0.23 | 0.11 | 0.10 |
| Stress EDV | 99 (76,116) | 110 (95,140) | 99 (73,113) | 0.10 | 0.16 | 0.07 |
| Stress ESV | 42 (30,61) | 63 (44,101) | 40 (28,58) | 0.008 | 0.005 | 0.007 |
| Stress SV | 55 (47,59) | 50 (35,58) | 55 (49,59) | 0.18 | 0.28 | 0.22 |
| Rest EDVi | 53 (45,69) | 58 (47,90) | 52 (45,66) | 0.32 | 0.21 | 0.16 |
| Rest ESVi | 28 (22,38) | 32 (20,63) | 28 (22,35) | 0.64 | 0.42 | 0.40 |
| Rest SVi | 25 (21,30) | 30 (21,33) | 25 (21,30) | 0.42 | 0.45 | 0.30 |
| Stress EDVi | 50 (40,60) | 59 (44,78) | 50 (40,58) | 0.15 | 0.15 | 0.12 |
| Stress ESVi | 23 (16,31) | 29 (22,55) | 20 (15,28) | 0.02 | 0.02 | 0.02 |
| Stress SVi | 26 (23,31) | 23 (18,31) | 26 (25,31) | 0.21 | 0.21 | 0.21 |
| Rest PP/SV | 1.2 (0.9,1.7) | 1.1 (0.9,1.8) | 1.2 (1.0,1.7) | 0.67 | 0.34 | 0.28 |
| Rest PP/SVi | 2.6 (1.9,3.2) | 2.4 (1.7,3.3) | 2.6 (2.1,3.2) | 0.68 | 0.41 | 0.31 |
| Stress PP/SV | 1.3 (1.0,1.6) | 1.4 (1.2,2.1) | 1.2 (0.9,1.6) | 0.06 | 0.15 | 0.31 |
| Stress PP/SVi | 2.5 (2.0,3.3) | 2.8 (2.4,4.0) | 2.5 (1.9,3.2) | 0.10 | 0.28 | 0.39 |
| Stress/Rest SV | 1.3 (1.0,1.6) | 0.9 (0.7,1.1) | 1.0 (0.9,1.2) | 0.04 | 0.06 | 0.07 |
| Stress-Rest SV | 1.3 (−5.6,6.5) | −3.0 (−16.2,2.2) | 1.5 (−5.0,7.9) | 0.009 | 0.04 | 0.05 |
| Stress-Rest SVi | 0.6 (−3.0,3.3) | −1.6 (−7.5,1.3) | 0.8 (−2.8,4.0) | 0.04 | 0.06 | 0.08 |
| Stress-Rest PP/SV | −0.0 (−0.2,0.3) | 0.5 (0.1,0.8) | −0.1 (−0.6,0.5) | 0.004 | 0.01 | 0.02 |
| Stress-Rest PP/SVi | −0.0 (−0.5,0.7) | 0.8 (0.3,1.6) | −0.2 (−0.6,0.5) | 0.003 | 0.008 | 0.01 |
| Stress/Rest PP/SV | 1.0 (0.8,1.3) | 1.4 (1.1,1.6) | 0.9 (0.8,1.2) | 0.002 | 0.004 | 0.008 |
Abbreviations: DCMR, dobutamine cardiovascular magnetic resonance; EDV, end diastolic volume; EF, ejection fraction; EDVi, end diastolic volume index; ESV, end systolic volume; ESVi, end systolic volume index; PP/SV, pulse pressure/stroke volume; PP/SVi, pulse pressure/stroke volume index; SV, stroke volume; SVi, stroke volume index;
Factors included in the multivariate model include: age, gender/male, body mass index (BMI), prior myocardial infarction (MI), hypertension (HTN), diabetes mellitus (DM), hypercholesterolemia, smoking.
Figure 3. LV stroke volume and aortic stiffness (isolated pulmonary edema).
Box-and-whisker plots of stress/rest left ventricular stroke volume (y-axis, Panel A) and peak stress aortic distensibility (y-axis, Panel B) measured during dobutamine cardiovascular magnetic resonance imaging. Pulmonary edema- and pulmonary edema+ groups are shown on the x-axis. In participants with isolated pulmonary edema and no inducible left ventricular motion abnormalities indicative of ischemia during dobutamine, LVSV reserve was reduced and stress induced aortic distensibility was elevated relative to control subjects. In these plots, lines within boxes represent median values; the top and bottom lines of the boxes represent the 25th and 75th percentiles, respectively; the top and bottom whiskers outside the boxes represent the smallest data value that is ≥ Q1- 1.5 (IQR) and the largest data value that is ≤ Q3+1.5 (IQR), respectively.
Since we desired to identify associations of our DCMR measures with P edema in individuals with a preserved LVEF (often termed heart failure and preserved LVEF), we performed additional stratified analyses on the 59 participants with a LVEF ≥ 50% and found that the 18 participants with P edema respectively exhibited lower LVSV reserve of (0.9 [0.7,0.9] versus 1.0 [0.9,1.1], p=0.001), a higher stress/rest ratio of a PP/SV (1.4 [1.0,1.7] versus 1.1 [0.8,1.3], p=0.02), and a trend toward a lower stress induced AoD (0.8 [0.3, 0.9] versus 1.3 [0.8,2.7], p=0.08) than the 41 individuals without future P edema. Sixteen participants with a LVEF ≥ 50% exhibited inducible LV wall motion abnormalities indicative of ischemia. When we analyzed just participants with LVEF ≥ 50% and no inducible LV wall motion abnormalities indicative of ischemia, the results demonstrated that LVSV reserve, and stress/rest ratios of a PP/SV stiffness remained different in those with and without future P edema (p=0.02 and 0.04, respectively).
Overall, LVEDV decreased by 10 (1,19) ml from rest to peak stress (p<0.001) with a 12 (1,20) ml versus 7 (1,16) ml decrease in those without versus with P edema respectively, p=0.20. There was a small correlation of 0.30 (p=0.001) between LVSV reserve and change in LVEDV. Systolic blood pressure (SBP) and pulse pressure (PP) increased 8 (−8,23) mmHg and 10 (−6,21) mmHg, respectively (p≤0.001 for both) for all participants with 61% of the participants having an increase in their SBP during the stress test. For the participants without versus with P edema, SBP increased by 5 (−10,22) mmHg and 14 (−1,23) mmHg, respectively p=0.19 for the difference), and PP increased by 4 (−8,19) mmHg and 12 (1,23) mmHg, respectively, (p=0.08 for the difference). For those without ischemia during testing, SBP increased in subjects ithout versus with P edema by −1 (−10,14) mmHg and 9 (1,21) mmHg, respectively (p=0.12 for difference) in those without versus with future P edema. Similarly, pulse pressure ncreased by 3 (−10,16) mmHg and 10 (1,25) mmHg, respectively, (p=0.05 for the difference) in those without versus with future P edema.
Discussion
The results of this study indicate that: (1) impaired augmentation of LVSV during dobutamine stress testing may serve as a marker for the future development of P edema: an LVSV reserve of <1 was associated with a 2- fold increase in the risk of future P edema relative to those with a LVSV reserve ≥ 1; (2) reduced LVSV reserve was associated with P edema independent of the presence of age, gender, LVEF, inducible LV wall motion abnormalities, and other risk factors for P edema or CHF (p= 0.002); and (3) an increase in the stress/rest ratio of ventriculo-vascular stiffness (measured as brachial pulse pressure/the left ventricular stroke volume indexed for body surface area [PP/LVSVi], or stress induced aortic distensibility) is associated with future P edema in the absence of dobutamine induced LV wall motion abnormalities indicative of ischemia. This increase in cardiovascular stiffening during intravenous dobutamine may contribute to the mechanism by which LVSV could be limited during stress in the absence of inducible LV wall motion abnormalities indicative of ischemia.
P edema can occur in individuals with either a reduced or preserved LVEF (4,16,17). To this end, we assessed individuals with LVEF ranging from 33% to 77%, and found that impaired LV stroke volume reserve during dobutamine stress was associated with future P edema in those with a LVEF < or ≥ 50% (p=0.002). To address whether inducible LV wall motion abnormalities observed during dobutamine accounted for the reduction in LVSV reserve, we performed stratified analyses (Table 3) in individuals without dobutamine induced LV wall motion abnormalities indicative of ischemia. In those participants without inducible LV wall motion abnormalities indicative of ischemia during intravenous dobutamine, impaired augmentation of LVSV reserve remained associated with P edema when compared to controls (p=0.02, Figure 3). Importantly, the results of these stratified analyses indicate that impaired LVSV reserve serves as a marker for future P edema in the absence of conventional clinical markers of inducible ischemia used during intravenous dobutamine.
While our participants with P edema exhibited a greater frequency of diabetes and hypertension, LVSV reserve was associated with P edema independent of the presence of these conditions (p<0.001). We recognize that clinical conditions such as renal failure, acute MI, chemotherapy induced cardiomyopathy, valvular heart disease or lung cancer may cause individuals to experience P edema due to established causes. However, our analyses demonstrated that limits of LVSV reserve as well as increased ventriculo-vascular and aortic stiffness were predictive of future P edema in the presence or absence of these conditions. In addition, a DCMR LVSV reserve of >1 was highly predictive of those that would remain free from P edema 2 years after stress testing.
Why would reduced LVSV reserve serve as a marker for future P edema? In patients with normal cardiovascular performance, cardiac output increases during stress due to a rise in heart rate, enhanced venous return to the right heart due to recruitment of blood from peripheral veins, and an increase in LVSV due to a decrease in LV end-systolic volume and maintenance or elevation of LV end-diastolic volume (18). We found that patients who subsequently developed acute P edema had a reduced ability to augment LVSV in response to pharmacologic stress. In this study, we do not have information regarding RV stroke volume; however, if LVSV failed to increase during exercise or volume challenge, an augmentation of right ventricular stroke volume could result in displacement of blood into the lungs, elevation of left atrial pressure and the development of P edema (19–21).
It is important to note that we utilized intravenous dobutamine rather than exercise to induce cardiovascular stress. We selected intravenous dobutamine because of its ease of clinical implementation, and one can collect images for measuring LV volumes simultaneously with the intravenous infusion. The stress produced by intravenous dobutamine differs from exercise in 2 major respects (22). First, walking, running, or biking stimulates lower extremity muscle contraction and facilitates recruitment of venous blood into the central circulation. This, in turn, elevates right ventricular and LV end diastolic volume (23). Intravenous dobutamine does not exhibit this effect, and as shown in Table 2, we did not appreciate maintenance or an increase in LV end-diastolic volume in our subjects. In fact, LVEDV decreased in our participants and thus the majority of our differences in LVSV reserve were due to differences in stress induced change in LV end-systolic volume. We note, however, our study was not powered to identify differences in the LVEDV with stress in those with and without P edema, and while the standard deviation in change in LVEDV with stress was large in the study, it is interesting that the median change in LVEDV was 12ml versus 7ml in those without as opposed with P edema (p=0.20). Perhaps a study of more participants would identify an association of stress induced change in LVEDV and future pulmonary disease.
Second, intravenous dobutamine relaxes arterial tone which is often manifest by a reduction in systolic blood pressure during initial infusions of the drug (24). Exercise, however, often increases systolic blood pressure (25), and in patients with noncompliant vasculature, can augment systolic blood pressure further (19,21). Both our group and others have shown that proximal aortic distensibility is reduced in patients with heart failure with or without a reduced LVEF. In participants without inducible LV wall motion abnormalities, there was a strong trend (p=0.08) toward an increase in pulse pressure (a surrogate for aortic stiffness) in patients with (12mmHg) versus without (4mmHg) future P edema. Although intravenous dobutamine may not increase pulmonary blood flow (LV preload) or systolic blood pressure (LV afterload ) to the same degree as exercise, our data demonstrate that the failure to augment left ventricular stroke volume during dobutamine infusion is associated with future pulmonary congestion.
Why was LVSV impaired during intravenous dobutamine? Functionally apparent myocardial ischemia was not the cause of those with P edema in our stratified analyses shown in Table 3 and Figure 3. It appears that increased arterial stiffness did contribute (Figure 3B, Table 3). Several recent studies have identified a relationship between increased aortic stiffness and LV function (5,13,26). Abnormal vascular stiffness raises LV afterload (27,28) and stimulates LV hypertrophy (29); both factors that may reduce LVSV during stress (5) respectively by reducing LV ejection (19) and relaxation (30). Chattopahyay, et al., have recently reported on an association of abnormal dobutamine induced parameters of LV diastolic function and heart failure in the setting of a preserved LVEF (16). Similar to the associations we appreciated with impaired LVSV reserve and future P edema, our data show that patients that developed P edema displayed increased stress/rest ratios of vascular stiffness and heightened stress distensibility measures compared to patients that did not develop P edema.
Limitations
We recognize the following limitations of our study. First, our design, similar to a case/control study, may not readily identify a variable within the study population that could influence our primary outcomes of LVSV reserve or cardiovascular stiffness. To this end, we matched our “control” population to include those of similar age, gender, and LVEF to our study (or case) population. In addition, we performed multivariable analyses that accounted for other clinical variables, such as hypertension or diabetes that could influence the study results. Importantly, after accounting for these co-variables LVSV, reserve and vascular stiffness remained associated with future P edema.
Second, while our DCMR method of biplane LV volume analysis are similar to those used during dobutamine stress echocardiography, newer, 3-dimensional techniques can measure LV volumes more accurately and in future studies reduce potential variance in volume derived measures. Third, our measures of vascular stiffness and aortic distensability incorporated brachial rather than central aortic pulse pressure assessments. It has been shown in some individuals that the brachial cuff pressure estimate of central aortic pressure may not accurately reflect central aortic pressure in those with stiff aortas or advancing heart rates (31,32). Although large patient studies, such as the Multiethnic Study of Atherosclerosis [MESA] (33) or the Dallas Heart Study (34) have used aortic distensibility as an outcome measure in >3,000 participants, other techniques, such as pulse wave velocity, are less dependent on brachial pulse pressure assessments. Fourth, our retrospective review of hospital records may have inadvertently omitted episodes of P edema managed by physicians using oral (rather than intravenous) diuretics in an outpatient office setting. Other study designs could be implemented to capture these types of events and determine the association of P edema with our measures of ventriculo-vascular stiffness. Finally, we wish to recognize that our stratified analyses were performed on relatively small samples and there may be other variables, in addition to our measures of ventriculo-vascular stiffness, that could be significantly associated with further P edema events.
In conclusion, impaired left ventricular stroke volume reserve measured during dobutamine cardiac stress may predict an episode of future P edema. Increased arterial stiffness may impair LVSV augmentation during stress and therefore serve as a mechanism to induce P edema in the absence of inducible LV wall motion abnormalities indicative of ischemia. As a result, stress induced changes in LV stroke volume and vascular stiffness measured during noninvasive stress testing may identify those at risk for future P edema.
Acknowledgments
Research supported in part by the following grants from the National Institutes of Health: R01 HL076438, R33 CA1219601, T32HL091824, P30AG21332 and MO1-RR07122.
Abbreviations and acronyms
- AoD
aortic distensibility
- DCMR
dobutamine cardiovascular magnetic resonance
- LVSV
left ventricular stroke volume
- LVEDP
left ventricular end diastolic pressure
- EF
ejection fraction
- PE
P edema
- PP
pulse pressure
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tanimoto M, Pai RG, Jintapakorn W, Shah PM. Mechanisms of hypotension during dobutamine stress echocardiography in patients with coronary artery disease. Am J Cardiol. 1995;76:26–30. doi: 10.1016/s0002-9149(99)80795-4. [DOI] [PubMed] [Google Scholar]
- 2.Dodek A, Kassebaum DG, Bristow JD. Pulmonary edema in coronary-artery disease without cardiomegaly: Paradox of the stiff heart. N Engl J Med. 1972;286:1347–50. doi: 10.1056/NEJM197206222862507. [DOI] [PubMed] [Google Scholar]
- 3.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi SK, Powers JC, Nomeir AM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17–22. doi: 10.1056/NEJM200101043440103. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–20. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 6.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton CA, Link KM, Salido TB, Epstein FH, Hundley WG. Is imaging at intermediate doses necessary during dobutamine stress magnetic resonance imaging? J Cardiovasc Magn Reson. 2001;3:297–302. doi: 10.1081/jcmr-100108582. [DOI] [PubMed] [Google Scholar]
- 8.Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–33. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 9.Lawson MA, Blackwell GG, Davis ND, Roney M, Dell’Italia LJ, Pohost GM. Accuracy of biplane long-axis left ventricular volume determined by cine magnetic resonance imaging in patients with regional and global dysfunction. Am J Cardiol. 1996;77:1098–104. doi: 10.1016/s0002-9149(96)00140-3. [DOI] [PubMed] [Google Scholar]
- 10.Cranney GB, Lotan CS, Dean L, Baxley W, Bouchard A, Pohost GM. Left ventricular volume measurement using cardiac axis nuclear magnetic resonance imaging. Validation by calibrated ventricular angiography. Circulation. 1990;82:154–63. doi: 10.1161/01.cir.82.1.154. [DOI] [PubMed] [Google Scholar]
- 11.Honda T, Yano K, Matsuoka H, Hamada M, Hiwada K. Evaluation of aortic distensibility in patients with essential hypertension by using cine magnetic resonance imaging. Angiology. 1994;45:207–12. doi: 10.1177/000331979404500305. [DOI] [PubMed] [Google Scholar]
- 12.Palmieri V, Bella JN, Roman MJ, et al. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J Hypertens. 2003;21:781–7. doi: 10.1097/00004872-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 14.Stefanadis C, Stratos C, Boudoulas H, Kourouklis C, Toutouzas P. Distensibility of the ascending aorta: Comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J. 1990;11:990–6. doi: 10.1093/oxfordjournals.eurheartj.a059639. [DOI] [PubMed] [Google Scholar]
- 15.Cox DR, Oakes D. Analysis of Survival Data. London: Chapman and Hall Ltd; 1984. [Google Scholar]
- 16.Chattopadhyay S, Alamgir MF, Nikitin NP, Rigby AS, Clark AL, Cleland JGF. Lack of diastolic reserve in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2010;3:35–43. doi: 10.1161/CIRCHEARTFAILURE.108.824888. [DOI] [PubMed] [Google Scholar]
- 17.Kramer K, Kirkman P, Kitzman D, Little WC. Flash pulmonary edema: Association with hypertension and reoccurrence despite coronary revascularization. Am Heart J. 2000;40:451–5. doi: 10.1067/mhj.2000.108828. [DOI] [PubMed] [Google Scholar]
- 18.Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955;35:123–129. doi: 10.1152/physrev.1955.35.1.123. [DOI] [PubMed] [Google Scholar]
- 19.Rodeheffer RJ, Gerstenblith G, Becker LC, Reg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: Cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984;69:203–13. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- 20.Kass DA. Age-related changes in venticular-arterial coupling: Pathophysiologic implications. Heart Fail Rev. 2002;7:51–62. doi: 10.1023/a:1013749806227. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Nakayama M, Nevo E, Fetics Bl, Maughan WL, Kass DA. Coupled systolic ventricular and vascular stiffening with age: Implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–7. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 22.Cnota IF, Mays WA, Knecht SK, et al. Cardiovascular physiology during supine cycle ergometry and dobutamine stress. Med Sci Sports Exerc. 2003;35:1503–10. doi: 10.1249/01.MSS.0000084436.15808.52. [DOI] [PubMed] [Google Scholar]
- 23.Weiss JL, Weisfeldt ML, Mason SJ, Garrison JB, Livengood SV, Fortuin NJ. Evidence of Frank-Starling effect in man during severe semisupine exercise. Circulation. 1979;59:655–61. doi: 10.1161/01.cir.59.4.655. [DOI] [PubMed] [Google Scholar]
- 24.Leier CV, Heban PT, Huss P, Bush CA, Lewis RP. Comparative systemic and regional hemodynamic effects of dopamine and dobutamine in patients with cardiomyopathic heart failure. Circulation. 1978;58:466–38. doi: 10.1161/01.cir.58.3.466. [DOI] [PubMed] [Google Scholar]
- 25.Beleslin BD, Ostojic M, Stepanovic J, et al. Stress echocardiography in the detection of myocardial ischemia. Head-to-head comparison of exercise, dobutamine, and dipyridamole tests. Circulation. 1994;90:1168–76. doi: 10.1161/01.cir.90.3.1168. [DOI] [PubMed] [Google Scholar]
- 26.Rerkpattanapipat P, Hundley WG, Link KM, et al. Relation of aortic distensibility determined by magnetic resonance imaging in patients> or =60 years of age to systolic heart failure and exercise capacity. Am J Cardiol. 2002;90:1221–5. doi: 10.1016/s0002-9149(02)02838-2. [DOI] [PubMed] [Google Scholar]
- 27.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245:H773–H780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 28.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–21. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 29.Pearson AC, Gudipati C, Nagelhout D, Sear J, Cohen JD, Labovitz AJ. Echocardiographic evaluation of cardiac structure and function in elderly subjects with isolated systolic hypertension. J Am Coll Cardiol. 1991;17:422–30. doi: 10.1016/s0735-1097(10)80109-3. [DOI] [PubMed] [Google Scholar]
- 30.Fouad-Tarazi PM. Left ventricular diastolic dysfunction in hypertension. CUIT Opin Cardiol. 1994;9:551–60. doi: 10.1097/00001573-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Verdecchia P, Angeli F. Atherosclerosis, Large Arteries and Cardiovascular Risk: Does brachial pulse pressure predict coronary events? Adv Cardiol. 2007;44:150–159. doi: 10.1159/000096727. [DOI] [PubMed] [Google Scholar]
- 32.Haluska BA, Jeffriess L, Fathi RB, Mottram PM, Carlier SG, Marwick TH. Pulse pressure vs. total arterial compliance as a marker of arterial health. Eur J of Clin Invest. 2005;35:438–443. doi: 10.1111/j.1365-2362.2005.01513.x. [DOI] [PubMed] [Google Scholar]
- 33.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 34.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]