Abstract
Background
Recurrent glomerulonephritis (GN) remains an important cause of kidney allograft loss and whether rapid discontinuation of steroids (RDS) is associated with a higher risk of recurrence is not known.
Methods
We studied recurrence rate, and graft and patient survival in four groups of recipients: 216 recipients with GN transplanted under RDS (group 1), 978 concurrent non-GN recipients transplanted under RDS (group 2), 260 historic comparator group transplanted for GN between 1994 and 1999 with steroid maintenance (group 3), and 950 recipients who were also transplanted between 1994 and 1999 for non-GN and also maintained on steroids (group 4). Regression analysis adjusting for donor and recipient factors, steroid and sirolimus use, and also GN type was used to address factors associated with recurrent disease.
Results
The 1-, 5-, and 7-year recurrence rate in the GN group under RDS was 6.7%, 13.7%, and 19.2% and in historic GN recipients maintained on steroids it was 2.4%, 3.8%, and 5.3%, respectively (P<0.0001). RDS was associated with a higher adjusted risk of recurrent disease for all GN types (hazard ratio 4.86; 95% confidence interval 2.34 –10.07; P<0.0001). Graft and patient survival were similar in the two GN groups and both were highest among all groups. Notably, death-censored graft survival was not different among the groups.
Conclusion
Steroid avoidance may be associated with a higher rate of recurrent GN but no apparent increase in risk of graft loss. This group of recipients needs to be studied more carefully, in larger numbers, and for a longer time period.
Keywords: Kidney transplant, Recurrent glomerulonephritis, Steroid avoidance, Graft survival
Rapid discontinuation of steroid (RDS) protocols aimed at avoiding the adverse metabolic effects of steroids are being increasingly used. In fact, over a third of US kidney transplant recipients are discharged on steroid-free regimens (1, 2). The challenge, however, is to identify recipients who may not benefit or may be actually harmed by using steroid sparing protocols. One group of concern is patients with underlying glomerulonephritis (GN) as a cause of end-stage kidney disease (ESKD) because steroids are frequently used, alone or in combination with other agents, to treat some of these conditions (3–5).
We have previously reported that a RDS protocol in recipients with GN did not compromise the short-term (up to 4 years) patient and allograft survival nor increased the rate of recurrent disease (6). This has recently been confirmed by others (7). Herein, we report a longer follow-up in a larger number of recipients regarding the rate of recurrent disease and long-term graft and patient outcomes on and off steroids in kidney transplant recipients with GN and compare these outcomes with those in recipients for causes other GN.
RESULTS
Recipients Characteristics
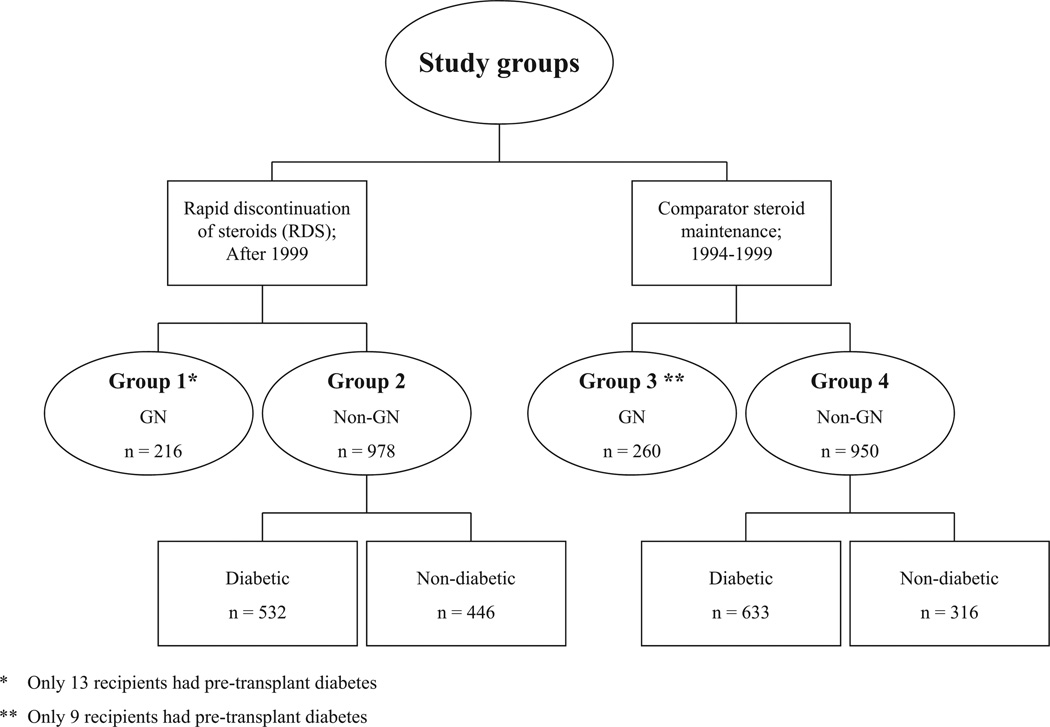
As of July 31, 2008, 1194 patients underwent kidney transplantation under RDS. Of those, 216 recipients (18%) carried the diagnosis of GN as a cause of ESKD (group 1) and 978 (82%) were transplanted; mainly for diabetes, polycystic kidney disease, and hypertension (group 2). Two historic comparator groups were used to address the question of our analysis; group 3 that consisted of 260 recipients transplanted for GN between 1994 and 1999 but maintained on steroids and group 4 that consisted of 950 recipients transplanted for non-GN-related ESKD between 1994 and 1999 and were also maintained on steroids (Fig. 1). The majority of the recipients in all groups were primary transplants and had comparable body mass index at transplantation (Table 1). Those transplanted for GN (groups 1 and 3) were more likely to be younger and of non-white ethnicity. Naturally, there were significantly more patients with type 1 or type 2 diabetes in non-GN groups. Those transplanted in recent era (groups 1 and 2) were less likely be sensitized at the time of the transplant and delayed graft function was more frequent in groups 3 and 4 (Table 1).
FIGURE 1.
Study groups. GN, glomerulonephritis.
TABLE 1.
Recipient characteristics
| Group 1 (GN: no steroids) |
Group 2 (non-GN: no steroids) |
Group 3 (GN: steroids) |
Group 4 (non-GN: steroids) |
P | |
|---|---|---|---|---|---|
| n | 216 | 978 | 260 | 950 | |
| Age at transplant (yr) | 43±15 | 47±15 | 44±13 | 45±12 | <0.0001 |
| Donor age (yr) | 41±13 | 40±13 | 39±14 | 38±14 | 0.03 |
| Men (%) | 63 | 58 | 59 | 41 | 0.64 |
| White (%) | 76 | 89 | 85 | 93 | <0.0001 |
| BMI at transplant >30 kg/m2 (%) | 23.9 | 21.5 | 24.1 | 18.3 | 0.13 |
| Pretransplant dialysis >1 yr | 43.6 | 46.5 | 48.5 | 43.5 | 0.03 |
| Pretransplant hypertension (%) | 92.7 | 87.2 | 89.2 | 86.6 | 0.001 |
| Pretransplant type 1 DM (%) | 1.4 | 38.1 | 0.4 | 55.9 | <0.0001 |
| Pretransplant type 2 DM (%) | 4.6 | 16.3 | 3.1 | 10.7 | <0.0001 |
| Male donor (%) | 40 | 50 | 48 | 48 | 0.1 |
| Donor race (% white) | 86 | 94 | 91 | 95 | <0.0001 |
| Deceased donor (%) | 34 | 37 | 41 | 55 | <0.0001 |
| Transplant number (%) | |||||
| First | 84 | 86 | 81 | 88 | 0.001 |
| Second | 14 | 12 | 19 | 12 | |
| PRA (0% at transplant) | 83 | 79 | 73 | 76 | 0.07 |
| DGF (%) | 7 | 10 | 13 | 13 | 0.01 |
BMI, body mass index; GN, glomerulonephritis; PRA, panel reactive antibody; DM, diabetes mellitus; DGF, delayed graft function.
Immunosuppressive Protocol
The immunosuppressive protocol for the two RDS groups consisted of Thymoglobulin (SangStat Corporation, Fremont, CA) at a dose of 1.25 to 1.5 mg/kg intravenously for five doses with the first dose given intraoperatively with methylprednisolone 500 mg. Prednisone was then given at 1 mg/kg on posttransplant day 1; 0.5 mg/kg on days 2 and 3; and 0.25 mg/kg on days 4 and 5. After day 5, prednisone was discontinued. Maintenance therapy for all recipients consisted of a calcineurin inhibitor (cyclosporine A [CsA] or tacrolimus) and an antimetabolite (mycophenolate mofetil [MMF], azathioprine [AZA], or sirolimus). In the GN-no steroid group, 55% received CsA + MMF, 18% received tacrolimus + MMF, 24% received tacrolimus + sirolimus, and the rest received other combinations. The immunosuppression protocol for the two historic groups consisted of induction with antithymocyte globulin, CsA or tacrolimus, antimetabolite and lifelong steroids. In the historic GN group 3, 68% received CsA + AZA, 22% received CsA + MMF, 8% received tacrolimus + AZA, and the rest received other combinations. Sirolimus was used in 53 (11.1%) of recipients with GN transplanted in the RDS era, as compared with 2 (0.42%) historical GN recipients. Target CsA levels in our center are as follows: 150 to 200 µg/L in the first 3 months posttransplant, 125 to 150 µg/L, 3 to 6 months posttransplant, 100 to 125 µg/L, 6 to 12 months, and 80 to 100 µg/L thereafter. Target levels for tacrolimus and sirolimus are as follows: 8 to 12 µg/L first 6 months posttransplant, 7 to 8 µg/L, 6 to 12 months posttransplant, and 5 to 7 µg/L thereafter. Tacrolimus, CsA and sirolimus levels were not different between the three groups at any time point (data not shown).
Recipients who experienced acute T-cell mediated rejection received oral steroid taper after initial treatment and continued on maintenance dose of prednisone; based on our experience of demonstrating a lower rate of subsequent rejection in those who remained on steroids after treatment of a rejection episode (8).
Recurrent Disease
Our program does not perform protocol biopsies. The rate of histologic recurrence of the original disease was therefore based on percutaneous allograft biopsy done for more than or equal to 25% increase in serum creatinine level or new onset proteinuria. Kidney tissue was routinely sent for light microscopy, immunofluorescence, and also electron microscopy.
For GN recipients under RDS, 85% had a native kidney biopsy-proven diagnosis. Of the 15% (n = 32) who did not have a biopsy, 12 were labeled as “chronic GN” based on the clinical presentation and the lack of an alternative diagnosis. In the remaining 20, presumptive diagnosis of IgA nephropathy (IgA) was made in 10 cases, focal segmental glomerulosclerosis (FSGS) in 5, membranoproliferative GN (MPGN) in 3, lupus nephritis in 1 and Henoch-Schonlein Purpura in 1 case. In the historical GN cohort maintained on steroids, the percentage of patients who did have a native kidney biopsy was 58%. Serologic confirmation, however, was available for all biopsied and nonbiopsied cases of lupus, Wegener’s granulomatosis, and antiglomerular basement membrane disease.
Of the 216 recipients transplanted for GN under RDS, 123 recipients (57%) underwent an allograft biopsy for an unexplained rise in creatinine or proteinuria. Of these, 96 (77%) did not have recurrence and 27 (23%) did; FSGS was seen in 14; IgA, 6; membranous nephropathy (MN), 3; MPGN, 3; and Wegener’s granulomatosis, 1. All recipients with MPGN diagnosis were C4d negative. All, but one recipient with recurrent disease had a native kidney biopsy that revealed the same histologic diagnosis. Overall, the mean time from transplant to biopsy-proven recurrence was 19.5±24.5 months. For IgA nephropathy, it was 44.4±31.9 months; FSGS, 9.2±14.2 months; MPGN, 30.6±30 months; MN, 12.2±5.5 months; and Wegener’s granulomatosis: 0.8 months. Of note, 7 of 14 recipients (50%) with FSGS had recurrence within 1 month posttransplant.
The baseline characteristics and graft outcomes for GN recipients under RDS with and without recurrence were comparable. Most were of primary transplants; the proportions of deceased donor transplants and history of previous graft loss due to recurrence were comparable. Serum creatinine and estimated glomerular filtration rate (eGFR) were similar at last follow-up but the urine protein to creatinine ratio was higher in those with recurrence (2.0±1.7 g/day vs. 0.68±1.72 g/day; P=0.0004).
In historic GN group (group 3), 118 recipients (45%) underwent at least one allograft biopsy and 15 (12.7%) had histologic recurrence: 6 FSGS, 4 IgA, 4 MPGN, and 1 with MN. Eleven of these 15 had concordant pretransplant biopsy diagnoses. Mean time to recurrence was 46.5±42 months and as follows for individual diseases: IgA, 59.4±49.7 months; FSGS, 38.4±44.3 months; MPGN, 32±29.4 months; and MN, 100.8±32.1 months. In contrast to those transplanted more recently for FSGS, only one of the six with recurrent FSGS recipients occurred within 1 month of transplantation.
Recurrence rates for the two GN groups are outlined in Table 2. Overall, recurrence rates for those transplanted under RDS were 6.7%, 13.7%, and 19.2% at 1, 5, and 7 years posttransplant when compared with 2.4%, 3.8%, and 5.3%, respectively, in historic GN patients on maintenance steroids (P<0.0001). In total, 10 of 216 steroid-free GN patients and 6 of 260 of the historic GN recipients with recurrent disease lost their grafts to recurrence (P=0.16).
TABLE 2.
Rate of histologic recurrence (%)
| Group 1 (GN: no steroids) |
Group 3 (GN: steroids) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | 1 yr | 5 yr | 7 yr | n | 1 yr | 5 yr | 7 yr | P | |
| Focal segmental GN | 61 | 17 | 28.8 | 28.8 | 49 | 6.4 | 9.2 | 12.3 | 0.02 |
| Membranous nephropathy | 12 | 8 | 31 | 31 | 12 | 0 | 0 | 10a | 0.04 |
| Membranoproliferativea | 18 | 5.5 | 11.2 | 31 | 31 | 6.7 | 11 | 16 | 0.5 |
| IgA nephropathy | 65 | 1.6 | 8.8 | 22 | 66 | 1.6 | 3.3 | 5.2 | 0.02 |
| Chronic GN | 27 | 0 | 0 | 0 | 49 | 0 | 0 | 0 | |
| SLE | 15 | 0 | 0 | 0 | 22 | 0 | 0 | 0 | |
| Rapidly progressive GN | 8 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | |
| Anti-GBM | 3 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | |
| Henoch-Schonlein purpura | 4 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | |
| Wegener’s granulomatosis | 2 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | |
| Overall recurrence rate (%) | 6.7 | 13.7 | 19.2 | 2.4 | 3.8 | 5.3 | <0.0001 | ||
All MPGN types.
GN, glomerulonephritis; SLE, systemic lupus erythematosus; GBM, glomerular basement membrane.
Risk of Recurrence
In a model that used the entire cohort of patients transplanted for GN (groups 1 and 3, n=476), we found that RDS was associated with a higher risk of recurrence (hazard ratio [HR] 4.86; 95% confidence interval [CI] 2.34 –10.07; P<0.0001). This heightened risk was observed in all GN types (Table 3). Receiving a preemptive transplant was also associated with a higher risk. Sirolimus use, on the other hand, seemed to be associated with the lower risk (HR 0.27; 95% CI 0.08–0.89; P=0.036).
TABLE 3.
Multivariate analysis of the risk for recurrent GN according to steroid use (n=459)
| Predictor | Hazard ratio (95% CI) |
P |
|---|---|---|
| Living donor | 1.44 (0.70–2.94) | 0.31 |
| African American | 1.59 (0.54–4.69) | 0.39 |
| Female | 1.08 (0.56–2.12) | 0.79 |
| Preemptive transplant | 2.58 (1.00–6.65) | 0.04 |
| Sirolimus use | 0.27 (0.08–0.89) | 0.036 |
| Rapid discontinuation of steroidsa | 4.86 (2.34–10.07) | <0.0001 |
| IgA nephropathy vs. other | 11.02 (1.36–88.91) | 0.02 |
| FSGS vs. other | 34.72 (4.6–261.8) | 0.0006 |
| MPGN vs. other | 23.03 (2.82–188.04) | 0.003 |
| Membranous nephropathy vs. other | 25.35 (2.78–230.86) | 0.004 |
Reference group: recipients with GN on steroids and transplanted between 1994 and 1999.
GN, glomerulonephritis; FSGS, focal segmental glomerulosclerosis; MPGN, membranoproliferative glomerulonephritis; CI, confidence interval.
To more carefully study the relationship between sirolimus and recurrent disease, we first performed a simple chi-square test comparing recurrence in the RDS patients on sirolimus (n=53) and those who were not (n=163). In addition, RDS + sirolimus and RDS without sirolimus were entered in a separate multivariate model where the reference group was group 3 (i.e., GN + steroids). There were 24 of 163 (14.7%) recurrent cases in those not maintained on sirolimus versus 3 of 53 (5.7%) in those on sirolimus; P=0.012. The adjusted HR for recurrent disease in RDS GN patient maintained on sirolimus was 1.32 (95% CI 0.37– 4.8; P=0.66), whereas it was 4.89 (95% CI 2.36–10.15; P<0.0001) in those not receiving sirolimus.
Patient and Allograft Outcomes
For recipients of kidneys from deceased donors, 1-year eGFR was the highest in group 2 at 55 mL/min/1.73 m2; in groups 1, 3, and 4, was 50, 49, and 47 mL/min/1.73 m2, respectively, P less than 0.003. At later time points, eGFR was not statistically different between groups. In living donor transplants, the 1-year eGFR was significantly higher in patients not on steroids (regardless of ESKD cause) at 53 mL/min/1.73 m2 compared with 45 and 47 mL/min/1.73 m2 in groups 3 and 4 (P<0.001). Subsequently, it remained statistically different with the highest eGFR observed in group 2. Graft survival at 1, 3, 5, and 7 years were comparable between the two GN groups and both were superior to the non-GN groups (Table 4). Death-censored graft survival, however, was not different among the groups. In regard to patient survival, the historic non-GN group maintained on steroids had the lowest patient survival and patient survival was similar in groups 1, 2, and 3. When we analyzed by diabetic status, we found that these differences were attenuated if we only looked at nondiabetic individuals in all groups (Table 4). In fact, graft survival was not different among groups and patient survival remained inferior in the historic non-group but to a much lesser degree than what we observed when diabetic subjects were included.
TABLE 4.
Graft and patient survival (%) for entire population compared with nondiabetic population
| Time (yr) |
Group 1 (GN: no steroids) |
Group 2 (non-GN: no steroids) |
Group 3 (GN: steroids) |
Group 4 (non-GN: steroids) |
P | |
|---|---|---|---|---|---|---|
| Entire population | ||||||
| N | 216 | 978 | 260 | 950 | ||
| Graft survival | 1 | 98 | 94 | 92 | 89 | 0.0007a |
| 3 | 88 | 84 | 87 | 83 | ||
| 5 | 81 | 77 | 77 | 75 | ||
| 7 | 74 | 70 | 70 | 66 | ||
| Death-censored graft survival | 1 | 99 | 97 | 95 | 94 | 0.47 |
| 3 | 91 | 91 | 91 | 91 | ||
| 5 | 85 | 87 | 83 | 85 | ||
| 7 | 81 | 83 | 78 | 80 | ||
| Patient survival | 1 | 99 | 96 | 95 | 94 | <0.0001b |
| 3 | 96 | 90 | 92 | 89 | ||
| 5 | 94 | 86 | 89 | 83 | ||
| 7 | 87 | 80 | 84 | 76 | ||
| Non-diabetic population | ||||||
| N | 203 | 446 | 251 | 317 | ||
| Graft survival | 1 | 98 | 96 | 92 | 91 | 0.07 |
| 3 | 89 | 87 | 87 | 84 | ||
| 5 | 81 | 84 | 77 | 77 | ||
| 7 | 73 | 78 | 70 | 69 | ||
| Death-censored graft survival | 1 | 99 | 97 | 95 | 94 | 0.21 |
| 3 | 92 | 92 | 92 | 90 | ||
| 5 | 85 | 89 | 84 | 84 | ||
| 7 | 80 | 87 | 78 | 80 | ||
| Patient survival | 1 | 99 | 98 | 95 | 96 | 0.03c |
| 3 | 96 | 93 | 93 | 91 | ||
| 5 | 94 | 93 | 89 | 86 | ||
| 7 | 87 | 83 | 85 | 80 |
Group 3 vs. 4, P=0.02; group 1 vs. 4, P=0.004.
Group 3 vs. 4, P=0.0002; group 2 vs. 4, P=0.03; group 1 vs. 4, P<0.0001.
Group 2 vs. 4, P=0.05; group 1 vs. 4, P=0.03.
GN, glomerulonephritis.
DISCUSSION
Our results show that: (1) recurrence rates are higher among recipients with GN transplanted under RDS protocol compared with historic GN counterparts who remained on life long steroids; (2) the overall mean time to recurrence of FSGS was significantly shorter than what was observed historically; and (3) Despite higher recurrence rates, outcomes of recipients transplanted for GN under RDS protocols are not inferior to those observed in their historical GN and non-GN comparators.
Among those with recurrence, roughly one fourth lost their grafts because of that and the remaining recipients have significant proteinuria; a known poor prognostic factor for poorer patient and allograft outcomes (9, 10). Why this higher rate of recurrence did not translate into detrimental effect on patient and graft survival is unclear but most likely reflects the relatively short follow-up or perhaps some factors that could not be accounted for by this retrospective analysis.
The higher risk of histologic recurrence under RDS has also been observed by others. Dube et al. (7) compared 109 steroid-free GN recipients transplanted from 2004 to 2008 with 52 GN controls transplanted between 2001 and 2003 but were maintained on steroids. Histologic recurrence occurred in 16.5% in those not receiving steroids, but only 9.6% in the historic GN controls but this difference did not reach statistical significance. Singh et al. (11) has also recently reported a higher incidence of recurrent IgA nephropathy in recipients on a steroid-free regimen; 46.6% at 9.5 years versus 31.7% at 6.8 years in those maintained on steroids.
Sirolimus appeared to be associated with a lower risk of recurrent disease. This appears to be counterintuitive as this agent has been linked to the development of new proteinuria and worsening existent one in kidney transplant recipients (12–15). To our knowledge, sirolimus has not been incriminated in increasing GN recurrence in renal allografts and recent observations highlight the fact most of the sirolimus-induced proteinuria is actually tubular in origin (16). Our observation regarding sirolimus, however, might be related to the relatively small number of recipients on this agent and needs to be confirmed.
Chailimpamontree et al. (17) compared the cumulative incidence of any posttransplant GN (de novo and recurrent) across different eras of immunosuppression protocols: CsA, steroids, and AZA (era 1); CsA, MMF, and steroids (era 2); and basiliximab, tacrolimus, and MMF with steroid minimization (era 3). The risk of posttransplant GN was lowest in era 1 and was similar in more recent steroid-containing and steroid-free eras (2 and 3). Regardless, conclusions can be drawn that newer immunosuppressive protocols do not adequately address the high risk of recurrent disease, which remains a significant risk factor for graft failure (17, 18).
No consensus exists regarding whether adding back steroids modifies the course of recurrent GN. Although it is not a uniform practice in our center, most recipients with documented histologic FSGS and MPGN recurrence were restarted on steroids. Sutherland et al. (19) reports that recurrence of disease is considered a steroid-free immunosuppression failure in their center and is the second most common reason, after early or refractory acute rejection, for conversion to steroid-based regimen, at least for selected glomerulonephritides. The small number of recipients studied and the variable approach to the treatment by the different practitioners precludes us from making conclusions regarding the utility of adding steroids to these patients.
Whether the risk related to increased recurrence of disease may be offset by benefits gained from avoiding long-term steroids therapy by improving triglyceride levels and weight changes that may translate into an improvement in cardiovascular risk profile is yet to be seen (20). Importantly, acute rejection rates were not increased compared with those on life long prednisone but this analysis is underpowered to make this assertion as this was not the primary outcome of this analysis.
This retrospective, single-center analysis has shortcomings. The historic GN group was different on many characteristics including the proportion with biopsy-proven diagnosis which may reflect a lower risk group or it may simply be due to increasing recent tendency by nephrologists to perform kidney biopsies. The latter possibility has not been documented but reflects our general impression. We acknowledge, that the difference in number of recipients who underwent native kidney biopsy, and the lower percentage of those labeled “chronic GN” in RDS group (12% in group 1 vs. 19% in group 3), may influence the results and lead to underestimation or overestimation of recurrence rate depending on the original diagnosis. Although we attempted to adjust for factors that impact the outcomes of interest this adjustment cannot be complete given the retrospective design of this analysis. The lack of protocol biopsies, the relatively small number of patients and the relatively short follow-up are added shortcomings.
In all, these data suggest that the use of steroid-free immunosuppression in recipients with GN is associated with a higher risk of histologic recurrence. Randomized controlled trials designed to test different antirejection protocols should include the risk of recurrent disease as an important outcome.
MATERIALS AND METHODS
Population
We studied recipients who received kidney transplants under a RDS protocol between October 1, 1999, and July 31, 2008. Initially, only first living donor (LD) transplant recipients were enrolled. Following encouraging results, the RDS protocol was expanded to include all first and second LD and deceased donor transplant recipients except those who required steroids for other conditions (such as asthma or inflammatory bowel disease) or were on steroids at the time of transplant (e.g., because of previous transplants). Within this group, we identified a cohort that was transplanted for GN as a cause of ESKD (group 1, n=216). Three comparator groups were studied. Recipients who were transplanted concurrently under RDS but for causes other than GN (group 2, n=978), recipients who received their transplant for GN between 1994 and 1999 and were maintained on long-term steroids (historical comparators group 3, n=260) and another historical comparator group that consisted of those also transplanted between 1994 and 1999 but for non-GN causes of ESKD (n=950). A concurrent GN group maintained on steroids would have been more appropriate but because our program routinely uses RDS such a group was not feasible. These groups were further subdivided according to diabetic status. The number of diabetic recipients in the four groups were 13, 532, 9, and 633, respectively (Fig. 1). The glomerulonephritides included in this analysis are as follows: FSGS, MN, all types of MPGN, Wegener’s granulomatosis, lupus nephritis, pauci-immune GN, chronic GN, IgA, Henoch-Schonlein Purpura, and also rapidly progressive GN. All studies were approved by the University of Minnesota Institutional Review Board.
Statistical Analysis
Continuous data are presented as mean±standard deviation and categorical data as percentages. Student’s t test was used to compare continuous variables and chi-square or Fisher’s exact test, when appropriate, to compare categorical data. Kaplan-Meier estimates were used to analyze patient and allograft survival. Graft failure was defined by retransplant, return to dialysis, or death with a functioning graft. Recipients, who underwent a pancreas transplant after their kidney and, as part of their pancreas transplant had another course of induction therapy and a recycling of a steroid taper, were censored at the time of their pancreas transplant. Actuarial recurrence rates at 1, 5, and 7 years in group 1 were compared with GN historical controls who were maintained on steroids. Actuarial graft survival, death-censored graft survival, rejection-free graft survival, and patient survival were analyzed and compared between all cohorts using log-rank and Wilcoxon tests. Additionally, to account for the impact of diabetes and also era effect, patient and allograft survival were obtained for diabetic and nondiabetic recipients in both eras. Regression analysis was used to address factors associated with the risk of histologic recurrence. Donor source, recipient gender, recipient ethnicity, recipient age, preemptive transplantation, steroid-containing versus steroid-free immunosuppression, FSGS diagnosis, IgA diagnosis, MPGN and MN diagnosis, and sirolimus use were included in the model. Including sirolimus but not other immunosuppressive strategies in the model was done considering the link between sirolimus and worsening proteinuria. eGFR, estimated using the Modification of Diet in Renal Disease study equation, was compared between the three groups at yearly intervals (21). P values less than 0.05 were considered significant. Analysis and graphs were completed using SAS version 8.12 (SAS Institute Inc., Cary, NC).
Footnotes
A.K., R.S., M.W., Y.E.-S., A.J.M., and H.N.I. participated in research design, writing of the manuscript, and data analysis; E.C. participated in research design and data analysis; and K.G. participated in data analysis.
The authors declare no conflict of interest.
REFERENCES
- 1.U.S. Renal Data System. [Accessed April 1, 2009];USRDS 2008 Annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Available at: www.usrds.org.
- 2.Matas AJ, Kandaswamy R, Gillingham KJ, et al. Prednisone-free maintenance immunosuppression —A 5-year experience. Am J Transplant. 2005;5:2473. doi: 10.1111/j.1600-6143.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- 3.Braun N, Schmutzler F, Lange C, et al. Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev. 2008;3:CD003233. doi: 10.1002/14651858.CD003233.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres A, Dominguez-Gil B, Carreno A, et al. Conservative versus immunosuppressive treatment of patients with idiopathic membranous nephropathy. Kidney Int. 2002;61:219. doi: 10.1046/j.1523-1755.2002.00124.x. [DOI] [PubMed] [Google Scholar]
- 5.Manno C, Torres D, Rossini M, et al. Randomized controlled clinical trial of corticosteroid plus ACE-inhibitors with long term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant. 2009;24:3694. doi: 10.1093/ndt/gfp356. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim H, Rogers T, Casingal V, et al. Graft loss from recurrent glomerulonephritis is not increased with a rapid steroid discontinuation protocol. Transplantation. 2006;81:214. doi: 10.1097/01.tp.0000188656.44326.53. [DOI] [PubMed] [Google Scholar]
- 7.Dube G, Crew R, Ratner L, et al. Graft loss from recurrent glomerular disease is not increased with steroid free maintenance immunosuppression (Abs) Am J Transplant. 2010;10(S4):514. [Google Scholar]
- 8.Humar A, Gillingham K, Kandaswamy R, et al. Steroid avoidance regimens: A comparison of outcomes with maintenance steroids versus continued steroid avoidance in recipients having an acute rejection episode. Am J Transplant. 2007;7:1948. doi: 10.1111/j.1600-6143.2007.01883.x. [DOI] [PubMed] [Google Scholar]
- 9.Halimi JM, Laouad I, Buchler M, et al. Early low-grade proteinuria: Causes, short-term evolution and long-term consequences in renal transplantation. Am J Transplant. 2005;5:2281. doi: 10.1111/j.1600-6143.2005.01020.x. [DOI] [PubMed] [Google Scholar]
- 10.Roodnat JI, Mulder PG, Rischen-Vos J, et al. Proteinuria after renal transplantation affects not only graft survival but also patient survival. Transplantation. 2001;72:438. doi: 10.1097/00007890-200108150-00014. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Gunay Y, Samavedi S, et al. Recurrence of IgA nephropathy post kidney transplantation in steroid free vs. steroid dependent immunosuppression protocol. Am J Transplant. 2010;10:519. [Google Scholar]
- 12.Letavernier E, Legendre C. mTOR inhibitors induced proteinuria: Mechanisms, significance, and management. Transplant Rev (Orlando) 2008;22:125. doi: 10.1016/j.trre.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Straathof-Golema L, Wetzels JF, Dijkman HB, et al. Sirolimus-associated heavy proteinuria in a renal transplant recipient: Evidence for a tubular mechanism. Am J Transplant. 2006;6:429. doi: 10.1111/j.1600-6143.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 14.Diekmann F, Budde K, Oppenheimer F, et al. Predictors of success in conversion from calcineurin inhibitor to sirolimus in chronic allograft dysfunction. Am J Transplant. 2004;4:1869. doi: 10.1111/j.1600-6143.2004.00590.x. [DOI] [PubMed] [Google Scholar]
- 15.Fervenza FC, Fitzpatric PM, Mertz J, et al. Acute rapamycin nephrotoxicity in native kidneys of patients with chronic glomerulopathies. Nephrol Dial Transplant. 2004;19:1288. doi: 10.1093/ndt/gfh079. [DOI] [PubMed] [Google Scholar]
- 16.Franz S, Regeniter A, Hopfer H, et al. Tubular toxicity in sirolimus- and cyclosporine-based transplant immunosuppression strategies: An ancillary study from a randomized controlled trail. Am J Kidney Dis. 2010;55:335. doi: 10.1053/j.ajkd.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Chailimpamontree W, Dmitrienko S, Li G, et al. Probability, predictors, and prognosis of posttransplantation glomerulonephritis. J Am Soc Nephrol. 2009;20:843. doi: 10.1681/ASN.2008050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briganti EM, Russ GR, McNeil JJ, et al. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347:103. doi: 10.1056/NEJMoa013036. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland S, Li L, Concepcion W, et al. Steroid-free immunosuppression in pediatric renal transplantation: Rationale outcomes following conversion to steroid based therapy. Transplantation. 2009;87:1744. doi: 10.1097/TP.0b013e3181a5df60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodle ES, First MR, Pirsch J, et al. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;284:564. doi: 10.1097/SLA.0b013e318187d1da. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, et al. for the Modification of Diet in Renal Disease Study group. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]