Abstract
The goal of this mini-review is to address the long standing argument that the pathogenesis of disc disease is due to the loss and/or the replacement of the notochordal cells by other cell types. We contend that although cells of different size and morphology exist, there is no strong evidence to support the view that the nucleus pulposus contains cells of distinct lineages. Based on lineage mapping studies and studies of other notochordal markers, we hypothesize that in all animals including human, nucleus pulposus retains notochordal cells throughout life. Moreover, all cells including chondrocyte-like cells are derived from notochordal precursors and that variations in morphology and size are representative of different stages of maturation, and or, function. Thus, the most critical choice for a suitable animal model should relate more to the anatomical, and mechanical characteristics of the motion segment than concerns of cell loss and replacement by non-notochordal cells.
Keywords: notochord, nucleus pulposus, intervertebral disc, axial skeleton, brachyury, sonic hedgehog, disc degeneration
INTRODUCTION
The incidence of low back pain, which is often linked to degenerative changes in the intervertebral disc, is extraordinary high. As many as 80% of adults will experience at least one episode of pain during their lifetime (Shvartzman et al., 1992), and 5% will experience chronic spinal disease (Deyo and Tsui-Wu, 1987). The annual total cost of back pain to the US health care industry is almost 200 billion dollars (Katz, 2006). Noteworthy, degeneration of the intervertebral disc is also believed to contribute to spinal arthritis, myelopathy and radiculopathy. In most cases, the pain caused by spine disease is incapacitating, and may become chronic. Although there are numerous surgical approaches for dealing with damaged or traumatized discs, the commonest strategies are aimed at providing symptomatic relief. None of the current therapies can completely restore the function of the degenerative intervertebral disc and thereby prevent further deterioration of the health of the compromised spine.
The intervertebral disc is a complex tissue that permits a range of motions between vertebrae and accommodates applied biomechanical forces to the spine. At the disc periphery, annulus fibrosus forms a ligamentous structure, composed of tightly packed parallel collagen type I fibrils; these sharpey fibers are inserted into the contiguous superior and inferior vertebral bodies. The inner surface of the annulus fibrosus is comprised of a poorly organized fibrocartilage containing collagen type II fibrils and proteoglycans. The annulus and the cartilagenous endplates enclose the nucleus pulposus, an aggrecan-rich gel-like tissue sparsely populated by cells (Fig. 1). During development, the nucleus pulposus is highly cellular with relatively little extracellular proteoglycan. In contrast, in the mature nucleus pulposus, the proportion of cells to matrix is low.
Figure 1.
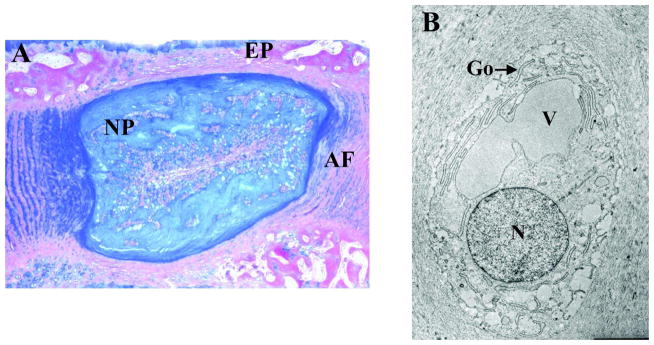
A) A saggital section through a rat intervertebral disc showing the nucleus pulposus (NP), annulus fibrosus (AF) and endplate cartilage (EP). B) TEM image of a rat nucleus pulposus cell showing characteristic cytoplasmic vacuoles (V). N: nucleus; Golgi: Go. Mag. 10,000
A defining characteristic of the disc is a proteoglycan-rich extracellular matrix with a high osmotic pressure, and low vascularity. Since, vascularity of the tissue is limited, cells tune their metabolism to the available oxygen supply. In this case, nucleus pulposus cells evidence almost complete reliance on the glycolytic pathway to generate metabolic energy (Agrawal et al., 2007). Indeed, the disc cells have very few mitochondria, extensive ER and a large number of vacuoles filled with an osmotically active material. Cells generate lactic acid as an end point of metabolism. If the tissue oxygen tension was altered, then aberrant cell function would be expected, and normal biochemical activities would be subverted. Thus, perturbation in oxygen tension within the disc, due to herniation or sclerosis of the cartilage endplate would influence the energy status of the nucleus pulposus cells. Since levels of ATP, NADH and reduced thiols impact on almost every biochemical activity, this type of alteration would be expected to profoundly impact metabolism and normal anabolic activity and decrease cell survival. Moreover, although progenitor cells are present in the tissue, it is likely that their number or activity is too low to mount a robust repair response (Risbud et al., 2007).
Discussions concerning the origin of cells of the nucleus pulposus have been ongoing since the latter half of the nineteenth century. Gegenbauer and Hertig considered that the cells of the nucleus pulposus were derived from the notochord; counter arguments by luminaries such as Virchove, Heildberg and Weiss opined that the nucleus pulposus was derived from peri- or extra-notochordal tissues. Surprisingly, these arguments have continued to the present day, impacting our understanding of the fate of cells of the nucleus pulposus in the adult, especially in relationship to degenerative disc disease (Hunter et al., 2003). As the disc matures, the composition of the nucleus pulposus changes: the large vacuolated cells that have been assumed to be of notochordal origin decrease in number, whereas smaller chondrocyte-like cells, assumed to be of a different origin, increase. The focus of the current debate is whether the pathogenesis of disc disease in humans is due to the loss and/or the replacement of the original notochordal cells by other cell types.
This dispute even impacts investigational strategies where the choice of an animal model is governed by considerations of whether notochordal cells are present in the disc at maturity or have been replaced by chondrocytic cells that are non-notochordal. Accordingly, the use of animal models including rodents, rabbits and pigs in which the nucleus pulposus is populated by notochordal cells well into maturity is discouraged on the basis that disc cells would be different from those of the human and hence they would not mimic the pathognomonic changes that characterize human degenerative disc disease.
The goal of this mini-review is to address these long standing arguments in the light of recent studies, including fate mapping of notochordal cells in the disc and profiling of notochord-specific genes. In addition, we consider the utility of reports on the possible replacement of nucleus pulposus cells by cells from surrounding tissues within the intervertebral disc. We contend that as a result of these current investigations, the source of cells of the nucleus pulposus can now be determined with some finality, while rationale decisions can be made concerning the choice of animal models for studies of the normal and degenerative disc.
DEVELOPMENT OF THE NOTOCHORD AND THE SURROUNDING TISSUES
The notochord is a rod-like midline structure of mesodermal origin that forms during gastrulation in chordate embryos and represents a primitive axial skeleton (Adams et al., 1990; Hogan et al., 1994; Stempe DL, 2005). With respect to axial skeleton formation during embryogenesis, osmotic swelling of notochordal cells resisted by the peri-notochordal sheath, results in an elevation in internal tissue pressure and elongation and straightening of the notochord (Adams et al., 1990). Signals from the notochord induce sclerotome cell migration, condensation, and differentiation to generate a peri-notochordal sheath. The sheath exhibits a metameric pattern of more condensed (chordascheidenstrang) and less condensed (chordasegment) zones that give rise to the outer and inner annulus and the vertebrae (Schaffer, 1910). Enclosed within the primitive annulus fibrosus the notochord condenses to form the nucleus pulposus (Bell, 1996; Horwitz; 1977). However, there remains some uncertainty whether the notochordal cells, located in the developing vertebral bodies, disintegrate or migrate to the intervertebral regions and contribute to the formation of the nucleus pulposus (Aszódi et al.; 1998; Rufai et al., 1995). Possibly a few dormant notochordal cells persist in the adult vertebral body and give rise to chordomas (Choi et al., 2008). Thus, in chordate embryos, the nucleus pulposus is the only tissue that is completely derived from the notochord (Choi et al., 2008). Based on these earlier investigations, considerable information has accrued concerning the relationship between the notochord and the nucleus pulposus during development. In contrast, the fate of the notochordal cells as well as their involvement in functional maintenance of the intervertebral disc in post-natal life is less well understood. Whether the notochordal nature of the nucleus pulposus is preserved in the adult remains controversial and is the main focus of this mini-review.
KEY MOLECULES REGULATING NOTOCHORD AND DISC DEVELOPMENT
During embryogenesis, the development of notochord is specified by the activity of a number of gene products (Cunliffe and Ingham, 1999). Foxa2/HNF-3β is a key transcription factor required for the development of the node, notochord, and floor plate of the neural tube (Ang and Rossant, 1994; Weinstein et al., 1994). Foxa2 mutants do not generate a node and lack all notochordal cells. Another of the important genes that has been linked to the notochord development is brachyury, a T-box transcription factor which determines cell differentiation and survival (Herrmann and Kispert, 1994). Nadine Dobrovolskaïa-Zavadskaïa first described a mutation in this gene which was associated with developmental defects in tail and vertebrae (Dobrovolskaïa-Zavadskaïa, 1927). She reported that mutations in brachyury in homozygotes were lethal at embryonic day 10 due to defects in mesoderm formation and notochord differentiation. Since then, brachyury has been shown to be required for notochord development in all chordates. During early vertebrate development, brachyury is first expressed in the mesoderm, and only later, after separation of axial and paraxial lineages, is its expression restricted to the notochord and tailbud (Wilkinson et al 1990).
Noteworthy, notochord development can also be indirectly influenced by the abnormal formation of vertebrae. Thus, mouse mutants that lack collagen type II (Aszódi et al., 1998; Li et al., 1995), the homeobox transcription factors Bapx1 (Tribioli and Lufkin, 1999; Lettice et al., 1999) and the paired box transcription factors Pax1 and Pax9 (Wallin et al., 1994; Peters et al., 1999) that are expressed in the sclerotome, display abnormal development of the vertebral bodies and notochord. In these mutants, the notochord forms normally but it is not removed from the vertebral bodies, thereby compromising the formation of the nucleus pulposus. In addition, two other genes, Sox5 and Sox6 encode highly homologous transcription factors, L-Sox5 and Sox6, respectively, which are expressed in sclerotome-derived cells and in the notochord (Lefebvre, 2002). These factors are required for peri-notochordal sheath formation, cell survival and development of the nucleus pulposus (Smits et al., 2003). Not surprisingly, Sox5−/−/Sox6−/− embryos exhibit extreme defects in notochord development and formation of the axial skeleton (Smits et al., 2003).
Retinoids are also involved in development of vertebrae and intervertebral disc. Imbalance of retinoic acid levels leads to abnormal development of the axial skeleton, disrupted notochord segmentation and formation of oversized vertebrae and vertebral fusion (Lohnes et al. 1994, Kessel and Gruss 1991). It is thus likely that retinoids may have a role in the establishment of somites (Sakai et al., 2001), if so, this will explain the presence of vertebral fusions and misshaped/sized vertebrae. Retinoids are also known to regulate expression of Hox genes, which are important in specifying the anterior posterior axis and the formation of vertebral elements (Wellik D 2009).
Another important gene, Sonic Hedgehog (Shh) is involved in notochord development and along with noggin mediates inductive actions of the notochord (Chiang et al., 1996; Teillet et al., 1998, McMahon et al., 1998). However, little is know about Shh expression and function in the post-natal nucleus pulposus. Not surprisingly, Shh has been used for lineage studies of the notochord, while brachyury has been utilized as both an indicator of the notochord as well as a molecular marker of the cells of the nucleus pulposus. We propose to use this information to delineate the ontology of the cells that contribute to and are retained in the mature nucleus pulposus.
IS THE NUCLEUS PULPOSUS POPULATED BY CELLS OF DIFFERENT LINEAGES?
The disposition and importance of notochordal cells in the post-embryonic state is not clear. Hunter and his colleagues evaluated cells of the nucleus pulposus and showed the presence of chondrocyte-like and “actin-filled’ notochordal cells (Hunter et al., 2003b; Hunter et al., 2004). The large notochordal cells were 25–85 μm in diameter, contained glycogen stores and were highly vacuolated (probably due to unsecreted proteoglycan). The cells exhibited a well demarcated golgi, possessed “immature” mitochondria associated with a generous endoplasmic reticulum, and formed fasiculi with contiguous cells. In addition to the notochordal cells, a second population of smaller cells “chondrocyte-like” cells was noted. Subsequently, two groups of cells were isolated from the nucleus pulposus by flow cytometric analysis, based on autofluorescence and cell size, this finding lent support to the argument that the nucleus contained small chondrocyte-like cells and large notochordal cells (Chen et al., 2006). Chen et al. analyzed gene expression profile of these two populations and showed what appeared to be differences in levels of expression of select phenotypic markers. Moreover, cell mixing studies indicated that notochordal cells stimulated glycosaminoglycan and aggrecan core protein synthesis by nucleus pulposus cells (Aguiar et al., 1999). It is therefore possible that a sub-population of notochordal cells may serve as “signaling centers” and/or progenitor cells influencing the activities of surrounding cells and their homeostasis, thus maintaining tissue integrity (Henriksson et al., 2009; Risbud et al., 2010).
It has been argued that the number of notochordal cells in the nucleus pulposus declines after birth due to their slow transformation and/or replacement by chondrocyte-like cells (Chen et al., 2006; Buckwalter, 1995; Butler, 1989; Walmsley, 1953; Trout et al.; 1982). Some authorities contend that a “centripetal sequential replacement mechanism” serves to replace notochordal remnants with chondrocytes (Kim et al., 2003). This migration of the endplate chondrocytes is thought to be facilitated by chemotactic signals generated by notochordal cells (Kim et al., 2009). It is argued that the cell composition of the nucleus pulposus is species specific (Gan et al., 2003; Poiraudeau et al., 1999; Guilak et al., 1999). It is known that certain breeds of dogs are more susceptible to disc degeneration than others and this has been linked to decline in notochordal-like cell content of the nucleus pulposus (Hunter et al., 2003 a,b, 2004; Aguiar et al., 1999). In mature rabbits, notochordal cells are retained in the disc (Poiraudeau et al., 1999) whereas, in rodents, it is thought that notochordal cells are the dominant cell population in the immature tissue but few notochordal cells are present after one year (Rufai et al., 1995; Stevens et al., 2000). Likewise, it has been surmised that notochordal cells are rarely present after adolescence in humans (Horwitz, 1977; Trout et al., 1982b; Pazzaglia et al., 1989); this prediction has fueled the debate that the disappearance of the notochordal cells and their replacement by chondrocytic cells may initiate or contribute to degenerative disc disease. Supporting this argument, histological studies using needle punctured mouse discs show sequential transformation of large notochordal cells first into chondrocyte-like cells and then into cells with a fibrocartilage phenotype (Yang et al., 2009). That two cell types existed in the bovine as well as human discs was supported by recent studies of cytokeratins, vimentin and brachyury expression in the nucleus pulposus (Gilson et al., 2010; Minogue et al., 2010). Urban’s group indicated that a small cohort of cells in the adult bovine nucleus pulposus expressed the notochordal marker cytokeratin 8, suggesting the presence of both notochordal and non-notochordal cell types (Gilson et al., 2010). Surprisingly, these cytokeratin 8 positive cells were of similar size to the chondrocyte-like cells of the nucleus pulposus. To explain this finding the authors proposed that this may be due to cell contraction and loss of vacuoles in culture. In contrast, Hoyland and colleagues could culture the large (notochordal) and small (chondroytic) cells from the bovine discs (Minogue et al., 2010).
While the studies mentioned above were of great interest, build logically on each other and generated what appeared to be a rational explanation for the observed variations in cell size within the nucleus pulposus, there are several problems with the conclusions generated by these investigations. First, within any tissue, cell size differences are the norm, not the exception. Rather than indicating differences in ontology, these differences frequently signal variations in RNA/DNA ratios, metabolic/secretive activity and cell cycle status. Indeed, it would be very unusual if differences in cell size were not apparent in this tissue. Directly relevant to this point, Zavala and Vazquez-Nin have reported the presence of two cell types with distinct morphologies in the late stages of development of the notochord: large vacuolated cells with numerous cisternae as well as much smaller non-vacuolated spindle shaped cell containing ribosomes unattached to membranes and mitochondria (Zavala and Vázquez-Nin, 2003). Thus, the notochord, the presumptive tissue of origin of the nucleus pulposus, contains cells of differing size that are similar to those seen in the nucleus itself. Relevant to the discussion of cell size, noteworthy, even in a related tissue, the growth plate, during maturation, chondrocyte volumes can vary by as much as eight to ten fold (Hunziker et al., 1987). Second, it would not be unreasonable to assume that within a single population, cells of differing size express different gene profiles. This phenomenon is seen in liver cells, where cells closest to the lobular artery express a profile of genes that differ from those removed from the arteriole (Gebhardt, 1992). In the study mentioned above the two cell types in the nucleus pulposus, evidenced substantive differences in expression levels of integrins, collagens, biglycan and MMP’s (Chen et al., 2006). Again, in comparison with the maturing chondrocyte, substantive differences in transcript expression would be expected; there is even, the expression of new transcript (collagen type X) by the large mature hypertrophic cell (Reginato et al., 1988). Lastly, while differences in transcript levels are a useful guide to phenotype, measurement of protein expression is a requirement. Interestingly, even within sorted notochordal and small chondrocyte-like nucleus pulposus populations, non-homogeneity in terms of integrin subunit expression was noted (Chen et al., 2006).
Based on the limitations discussed above and the lack of a highly specific marker that is homogenously expressed within a specific population, it is difficult to accept the view that cells with different lineages (notochord and mesenchymal cells) are present within the intervertebral disc. Moreover, without unequivocal lineage information the argument that notochordal cells are lost and replaced by cells of a different lineage is far from convincing.
EVIDENCE IN SUPPORT OF THE ARGUMENT THAT CELLS OF THE NUCLEUS PULPOSUS ARE ENTIRELY OF NOTOCHORDAL LINEAGE
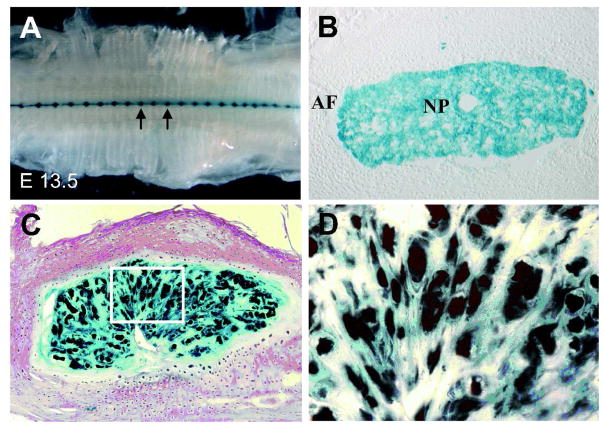
In contrast to the studies mentioned above, there are a number of investigations in which definitive markers of the notochord have been used to delineate the ontology of cells of the intervertebral disc. Use has been made of the T-box gene, brachyury which is required for notochordal cell differentiation and survival (Herrmann and Kispert, 1994), and Shh a secreted protein that is necessary for normal disc development; (Chiang et al., 1996; Teillet et al., 1998). Choi et al. created fate maps of the disc cells by knocking into the Shh gene the recombinase gene cre which is inducible by tamoxifen (ShhCreERT2) (Choi et al., 2008). These workers had previously shown that Shh-Cre is highly expressed in the notochord (Tian et al., 2005). When animals containing ShhCreERT were mated with R26R Cre reporter mice, all cells in the nucleus pulposus were labeled (Fig. 2A, B). Based on these observations, these workers concluded that the entire nucleus pulposus is descended from the notochord. While this finding confirms many earlier studies of notochordal development, it said little about the adult disc. To address this issue, these workers then evaluated the expression of the reporter in an old mouse (19 months). They showed that in the skeletally mature animals, all of the cells of the nucleus pulposus were labeled (Fig. 2C, D). This data lent considerable strength to the notion that cells in the adult disc were descended from “a homogeneous population of Shhcre” expressing notochordal cells. Noteworthy, the annulus fibrosus and the cartilaginous endplates, were devoid of Shhcre descendant cells. It was concluded that even the chondrocyte-like cells of the adult nucleus pulposus are derived from Shh-expressing notochord cells, and not from cells of the surrounding Shh-negative mesenchyme.
Figure 2.
Fate mapping studies of Sonic hedgehog (shh) expressing cells in the intervertebral disc. A) Ventral whole mount of the developing vertebral column of ShhcreERT2;R26R mouse at E13.5 that has been pulse labeled with tamoxifen at E 8.0. Sections were stained with β-galacosidase to identify the Shh expressing cells. Arrows show the forming nucleus pulposus of the intervertebral discs. B) A transverse section of the intervertebral disc of a new born mice. Note, that all the cells in the nucleus pulposus (NP) are positively labeled, while the developing annulus fibrosus (AF) is negative. C) 19 month old Shhcre;R26R mouse showing that all the cells of the nucleus pulposus are labeled. D) High magnification image of the insert area shown in panel C (40X). Courtesy of Dr. Brian Harfe (Choi et al., 2008)
Aside from Shh, considerable attention has been directed at the product of brachyury in both the notochord and the nucleus pulposus. As was discussed earlier, we now know that brachyury is required for differentiation of axial midline mesoderm into notochord (Chesley 1935; Halpern et al., 1993; Schulte-Merker et al., 1992) and that misexpression of brachyury can give rise to a fully differentiated ectopic notochord (Takahashi et al., 1999). In a recent study, Yang et al. has reported that duplication of the brachyury gene resulted in a tumor (familial chordoma) that is derived from notochordal remnants (Yang et al., 2009). Thus from all perspectives, the expression of brachyury should serve as a useful guide to the presence of notochordal cells in the adult nucleus pulposus. Genome wide microarray analysis indicates that adult bovine, as well as human discs, expressed brachyury and different cytokeratins (CK8, 18 and 19) (Gilson et al., 2010; Minogue et al., 2010); the latter genes are usually expressed by epithelial derived tissues as well as the notochord (Salisbury and Isaacson, 1985; Salisbury et al., 1993). If it is assumed that in these species the notochordal cells are lost from the disc early in life, then these results were unexpected. Moreover, Minogue et al. showed that both the large notochordal and small chondrocyte-like nucleus pulposus cells have substantially overlapping expression patterns; for some marker genes, the level of notochordal expression is even higher (Minogue et al., 2010). Since, the differences in expression of marker genes between the two cells types were never greater than ~10-fold it was suggested that both cell types may have been derived from a common lineage. This inference is in accord with a recent observation that the rabbit notochordal cells can differentiate into cells of different morphologies in vitro (Kim et al., 2009). Importantly, these studies question the much debated notion that notochordal cells are lost during the post-natal life of bovine and human discs (Gilson et al., 2010; Minogue et al., 2010; Stosiek et al., 1988; Weiler et al., 2007).
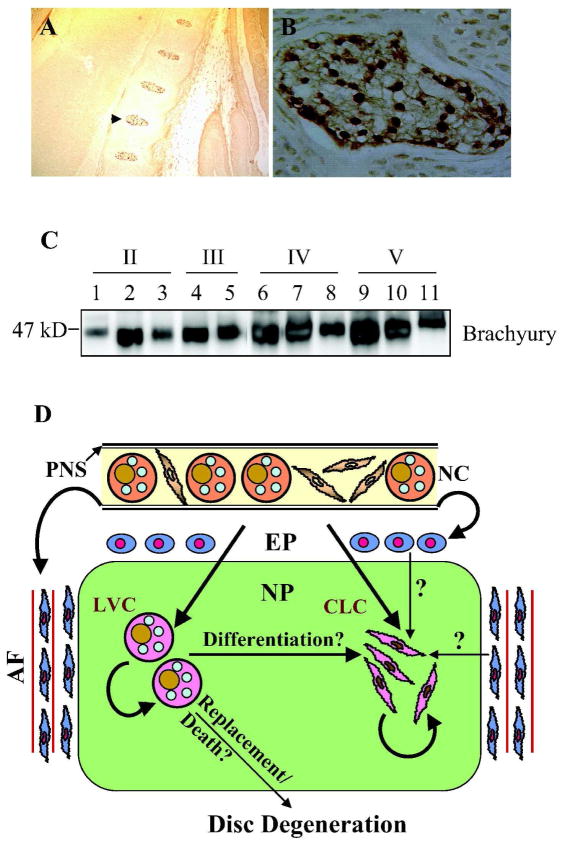
We have measured brachyury protein expression in embryonic mouse as well as in adult human nucleus pulposus tissue samples (Fig. 3A–C). Our data shows that there is a pronounced expression of brachyury in the nucleus pulposus, even during degeneration (Fig. 3C). Interestingly, expression of brachyury, an unequivocal marker of notochord is also reported in bovine as well as human nucleus pulposus (Minogue et al., 2010). Hoyland and her colleagues showed that unlike cytokeratins 8 and 18, mRNA expression of brachyury remained unchanged with degeneration in the human nucleus pulposus (Minogue et al., 2010). Moreover, expression of brachyury was also pronounced in both notochordal and chondrocyte-like nucleus pulposus cells of the adult bovine disc (Minogue et al., 2010).
Figure 3.
Brachyury expression in cells of the nucleus pulposus. A) Low magnification saggital section of the E15.5 mouse embryo showing brachyury staining in the developing nucleus pulposus. Note that the annulus fibrosus and endplate cartilage is negative (10X). B) High magnification image the developing nucleus pulposus that is shown by an arrow in panel A (20X). All cells show intense nuclear staining of brachyury. C) Western blot analysis of brachyury expression in nucleus pulposus tissue isolated from progressively degenerate human discs (Thompson Grade II–V). A robust expression of brachyury was seen in all the nucleus pulposus samples. D) Schematic indicating current theories concerning the origin of cells of the NP. Although, it is well established that the notochord (NC) gives rise to large NP cells during embryogenesis, there is discord concerning the origin of small chondrocytic cells in the adult. As the NC contains both small spindle shaped cells as well as large vacuolated cells, we hypothesize that both the large vacuolated cells (LVC) and chondrocyte-like cells (CLC) of the NP are derived from the notochord. These cells then undergo self renewal to maintain cellular homeostasis of the NP tissue. It is possible that LVC may differentiate into CLC. It is known that the perinotochordal sheath (PNS) gives rise to both endplate (EP) chondrocytes and annulus fibrosus (AF) cells. Some workers are of the opinion that in the adult, endplate chondrocytes and inner annulus fibrosus cells give rise to CLC at the same time replacing LVC. There is debate that loss or replacement of LVC in the nucleus pulposus initiates disc degeneration.
While there is considerable agreement on the expression of brachyury, Vujovic et al. were unable to show that this gene was expressed by the adult nucleus pulposus and indeed concluded that this tissue was not derived from the notochord (Vujovic et al., 2006). Casting some doubt on this conclusion, the authors also failed to detect cytokeratins, proteins that are known to be expressed by the notochord as well as nucleus pulposus cells (Gilson et al., 2010; Minogue et al., 2010; Stosiek et al., 1988; Weiler et al., 2007; Lee et al., 2007; Sakai et al., 2009). Based on our own data, studies of other notochordal markers by several workers and the lineage mapping studies, we forward the hypothesis that in all of the animal species mentioned earlier, including human, nucleus pulposus tissue retains notochordal cells throughout life. Although it is still possible that development of disc disease is linked to loss of a sub-population of notochordal cells i.e. “large cells” either by death or by de-differentiation to a modified phenotype (Yang et al., 2009), more definitive studies using molecular genetic approaches are required. We propose that all nucleus pulposus cells including chondrocyte-like cells are derived from notochordal precursors and that variations in morphology and size are representative of different stages of maturation, and or, function (see Fig. 3D). Finally, while an overall decrease in cell number and a reduction in their functional activity may contribute to development of degenerative disc disease, the existing notion that degeneration is due to a selective loss of only notochordal cells in untenable.
Perspective
Although cells of different size and shape exist, and species-specific differences have been reported, there is no strong evidence to support the view that the nucleus pulposus contains mixed populations of cells of distinct lineages or that the nucleus recruits cells from the endplate or annulus fibrosus. Based on the published literature, it is quite clear that during embryogenesis, cells of the nucleus pulposus are derived from the notochord. There is compelling evidence that in the rodent, the cells maintain their notochordal characteristics into maturity. Moreover, using a very well established notochordal marker brachyury, it was shown that even human degenerate disc cells express this protein, implying that these cells are notochordal. If these contentions are valid, then the conclusions impact current disc research endeavors that require use of animal models. While large animal models are important for testing surgical procedures and devices, the value of small animal models such as rodents, for genetic, biochemical and molecular studies can not be underestimated. Thus, the authors contend that the most critical choice for a suitable animal model should relate more to the anatomical, spatial and mechanical characteristics of the spinal motion segment than concerns of cell loss and replacement by non-notochordal cells of the nucleus pulposus.
Acknowledgments
Supported by grants from the National Institutes of Health R01-AR050087, R01-AR055655 and T35RR07065. We thank Dr. Brian Harfe for providing images of the Sonic hedgehog reporter mice. The authors are grateful to Dr. Norman Dollahon for TEM images of the disc and Kathleen Rhodes for brachyury staining of the nucleus pulposus.
References
- Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–30. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud MV. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–31. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:12. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–74. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- Aszódi A, Chan D, Hunziker E, Bateman JF, Fässler R. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol. 1998;143:1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GR. Anatomy of the lumbar spine: developmental to normal adult anatomy. In: Wiesel SW, Weinstein JN, Herkowitz HN, editors. The Lumbar Spine. Philadelphia: Saunders; 1996. pp. 43–52. [Google Scholar]
- Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- Butler WF. Comparative anatomy and development of the mammalian disc. In: Gosh P, editor. The Biology of the Intervertebral Disc. Boca Raton: CRC Press; 1989. pp. 84–108. [Google Scholar]
- Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15(Suppl 3):S303–11. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesley P. Development of the short-tailed mutant in the house mouse. J Exp Zool. 1935;70:429–459. [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–8. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe VT, Ingham PW. Switching on the notochord. Genes Dev. 1999;13:1643–6. doi: 10.1101/gad.13.13.1643. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine. 1987;12:264–8. doi: 10.1097/00007632-198704000-00013. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaïa-Zavadskaïa N. Sur la mortification spontanee de la chez la souris nouveau-nee et sur l’existence d’un caractere (facteur) hereditaire, non-viable. Crit Rev Soc Biol. 1927;97:114–116. [Google Scholar]
- Gan JC, Ducheyne P, Vresilovic EJ, Shapiro IM. Intervertebral disc tissue engineering II: cultures of nucleus pulposus cells. Clin Orthop. 2003:315–324. doi: 10.1097/01.blo.0000063797.98363.d3. [DOI] [PubMed] [Google Scholar]
- Gebhardt R. Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther. 1992;53:275–354. doi: 10.1016/0163-7258(92)90055-5. [DOI] [PubMed] [Google Scholar]
- Gilson A, Dreger M, Urban JP. Differential expression levels of cytokeratin 8 in cells of the bovine nucleus pulposus complicates the search for specific intervertebral disc cell markers. Arthritis Res Ther. 2010;12:R24. doi: 10.1186/ar2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Ting-Beall HP, Baer AE, Trickey WR, Erickson GR, Setton LA. Viscoelastic properties of intervertebral disc cells: Identification of two biomechanically distinct cell populations. Spine. 1999;24:2475–2483. doi: 10.1097/00007632-199912010-00009. [DOI] [PubMed] [Google Scholar]
- Halpern ME, Ho RK, Walker C, Kimmel CB. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell. 1993;75:99–111. [PubMed] [Google Scholar]
- Henriksson H, Thornemo M, Karlsson C, Hägg O, Junevik K, Lindahl A, Brisby H. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine. 2009;34:2278–87. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- Herrmann BG, Kispert A. The T genes in embryogenesis. Trends Genet. 1994;10:280–6. doi: 10.1016/0168-9525(90)90011-t. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy F. Manipulating the Mouse Embryo. A Laboratory Manual. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. Early mouse development; pp. 63–81. [Google Scholar]
- Horwitz T. The Human Notochord: A Study of Its Development and Regression, Variations, and Pathologic Derivative. Chordoma; Indianapolis: 1977. [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003a;9:667–77. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. The three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat. 2003b;202:279–91. doi: 10.1046/j.1469-7580.2003.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–62. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker EB, Schenk RK, Cruz-Orive LM. Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J Bone Joint Surg Am. 1987;69:162–73. [PubMed] [Google Scholar]
- Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88:21–4. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kim JH, Deasy BM, Seo HY, Studer RK, Vo NV, Georgescu HI, Sowa GA, Kang JD. Differentiation of intervertebral notochordal cells through live automated cell imaging system in vitro. Spine. 2009;34:2486–9. doi: 10.1097/BRS.0b013e3181b26ed1. [DOI] [PubMed] [Google Scholar]
- Kim KW, Ha KY, Lee JS, Nam SW, Woo YK, Lim TH, An HS. Notochordal cells stimulate migration of cartilage end plate chondrocytes of the intervertebral disc in in vitro cell migration assays. Spine. 2009;9:323–9. doi: 10.1016/j.spinee.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28:982–90. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, Grad S. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174–85. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V. Toward understanding the functions of the two highly related Sox5 and Sox6 genes. J Bone Miner Metab. 2002;20:121–130. doi: 10.1007/s007740200017. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Purdie LA, Carlson GJ, Kilanowski F, Dorin J, Hill RE. The mouse bagpipe gene controls development of axial skeleton, skull, and spleen. Proc Natl Acad Sci U S A. 1999;96:9695–700. doi: 10.1073/pnas.96.17.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Prockop DJ, Helminen H, Fassler R, Lapvetelainen T, Kiraly K, Peltarri A, Arokoski J, Lui H, Arita M, Khillan JS. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995;9:2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–48. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required fro growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12:R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazzaglia UE, Salisbury JR, Byers PD. Development and involution of the notochord in the human spine. J R Soc Med. 1989;82:413–5. doi: 10.1177/014107688908200714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H, Wilm B, Sakai N, Imai K, Maas R, Balling R. Pax1 and Pax9 synergistically regulate vertebral column development. Development. 1999;126:5399–408. doi: 10.1242/dev.126.23.5399. [DOI] [PubMed] [Google Scholar]
- Poiraudeau S, Monteiro I, Anract P, Blanchard O, Revel M, Corvol MT. Phenotypic characteristics of rabbit intervertebral disc cells. Comparison with cartilage cells from the same animals. Spine. 1999;24:837–844. doi: 10.1097/00007632-199905010-00002. [DOI] [PubMed] [Google Scholar]
- Reginato AM, Shapiro IM, Lash JW, Jimenez SA. Type X collagen alterations in rachitic chick epiphyseal growth cartilage. J Biol Chem. 1988;263:9938–9945. [PubMed] [Google Scholar]
- Risbud MV, Guttapalli A, Tsai TT, Lee JY, Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D, Shapiro IM. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine. 2007;32:2537–44. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Schipani E, Shapiro IM. Hypoxic regulation of nucleus pulposus cell survival: from niche to notch. Am J Pathol. 2010;176:1577–83. doi: 10.2353/ajpath.2010.090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufai A, Benjamin M, Ralphs JR. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol. 1995;192:5242–46. doi: 10.1007/BF00186991. [DOI] [PubMed] [Google Scholar]
- Sakai D, Nakai T, Mochida J, Alini M, Grad S. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. Spine. 2009;34:1448–5. doi: 10.1097/BRS.0b013e3181a55705. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, Rossant J, Hamada H. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 2001;15:213–25. doi: 10.1101/gad.851501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury JR, Deverell MH, Cookson MJ, Whimster WF. Three-dimensional reconstruction of human embryonic notochords: clue to the pathogenesis of chordoma. J Pathol. 1993;171:59–62. doi: 10.1002/path.1711710112. [DOI] [PubMed] [Google Scholar]
- Salisbury JR, Isaacson PG. Demonstration of cytokeratins and an epithelial membrane antigen in chordomas and human fetal notochord. Am J Surg Pathol. 1985;9:791. doi: 10.1097/00000478-198511000-00002. [DOI] [PubMed] [Google Scholar]
- Schaffer . In von Mollendorff’s “Handbuch der Mikroskopischen Anatomie des Menschen. Vol. 2. Berlin: Springer; 1910. pp. 31–44. [Google Scholar]
- Schulte-Merker S, Ho RK, Herrmann BG, Nüsslein-Volhard C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development. 1992;116:1021–32. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- Shvartzman L, Weingarten E, Sherry H, Levin S, Persaud A. Cost-effectiveness analysis of extended conservative therapy versus surgical intervention in the management of herniated lumbar intervertebral disc. Spine. 1992;17:176–82. doi: 10.1097/00007632-199202000-00010. [DOI] [PubMed] [Google Scholar]
- Smits P, Lefebvre V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development. 2003;130:1135–48. doi: 10.1242/dev.00331. [DOI] [PubMed] [Google Scholar]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–12. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- Stevens JW, Kurriger GL, Carter AS, Maynard JA. CD44 expression in the developing and growing rat intervertebral disc. Dev Dyn. 2000;219:381–90. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1060>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Stosiek P, Kasper M, Karsten U. Expression of cytokeratin and vimentin in nucleus pulposus cells. Differentiation. 1988;39:78–81. doi: 10.1111/j.1432-0436.1988.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Hotta K, Erives A, Di Gregorio A, Zeller RW, Levine M, Satoh N. Brachyury downstream notochord differentiation in the ascidian embryo. Genes Dev. 1999;13:1519–23. doi: 10.1101/gad.13.12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teillet M, Watanabe Y, Jeffs P, Duprez D, Lapointe F, Le Douarin NM. Sonic hedgehog is required for survival of both myogenic and chondrogenic somitic lineages. Development. 1998;125:2019–30. doi: 10.1242/dev.125.11.2019. [DOI] [PubMed] [Google Scholar]
- Tian H, Jeong J, Harfe BD, Tabin CJ, McMahon AP. Mouse Disp1 is required in sonic hedgehog-expressing cells for paracrine activity of the cholesterol-modified ligand. Development. 2005;132:133–42. doi: 10.1242/dev.01563. [DOI] [PubMed] [Google Scholar]
- Tribioli C, Lufkin T. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development. 1999;126:5699–711. doi: 10.1242/dev.126.24.5699. [DOI] [PubMed] [Google Scholar]
- Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982a;14:359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral disc. II. Cells of the nucleus pulposus. Anat Rec. 1982b;204:307–314. doi: 10.1002/ar.1092040403. [DOI] [PubMed] [Google Scholar]
- Vujovic S, Henderson S, Presneau N, Odell E, Jacques TS, Tirabosco R, Boshoff C, Flanagan AM. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209:157–65. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. The role of Pax-1 in axial skeleton development. Development. 1994;120:1109–21. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- Walmsley R. The development and growth of the intervertebral disc. Edinburgh Med J. 1953;60:341–363. [PMC free article] [PubMed] [Google Scholar]
- Weiler C, Schaaf R, Nerlich A, Boss N. Immunohistochemical identification of notochordal cell phenotype in the ageing human lumbar intere-vertebral disc. In Pathol Res Pract. 2007;203:233–234. [Google Scholar]
- Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE., Jr The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–88. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Wellik DM. Hox genes and vertebrate axial pattern. Curr Top Dev Biol. 2009;88:257–78. doi: 10.1016/S0070-2153(09)88009-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–9. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Yang F, Leung VY, Luk KD, Chan D, Cheung KM. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol. 2009;218:113–21. doi: 10.1002/path.2519. [DOI] [PubMed] [Google Scholar]
- Yang XR, Ng D, Alcorta DA, Liebsch NJ, Sheridan E, Li S, Goldstein AM, Parry DM, Kelley MJT. Brachyury gene duplication confers major susceptibility to familial chordoma. Nat Genet. 2009;41:1176–8. doi: 10.1038/ng.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala G, Vázquez-Nin GH. Analysis of nuclear ribonucleoproteic structures during notochordal cell differentiation and maturation in chick embryos. Anat Rec. 2000;259:113–23. doi: 10.1002/(SICI)1097-0185(20000601)259:2<113::AID-AR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]