Abstract
Objective
To (a) compare the size of the dorsal and ventral striatum(caudate and putamen) in a large sample of antipsychotic-naïve individuals with schizotypal personality disorder (SPD) and healthy control participants; (b) examine symptom correlates of striatal size in SPD.
Methods
The left and right caudate and putamen were hand-traced on structural MRI at five dorsal to ventral slice levels in 76 SPD and 148 healthy control participants. A Group × Region (caudate, putamen) × Slice (1–5: ventral, 2, 3, 4, dorsal) × Hemisphere (left, right) mixed-model MANOVA was conducted on size relative to whole brain.
Results
Primary results showed that compared with the controls, the SPD group showed (a) larger bilateral putamen size overall and this enlargement was more pronounced at the most ventral and dorsal levels; in contrast, there were no between-group differences in caudate volume; (b) larger bilateral size of the striatum ventrally, averaged across the caudate and putamen. Among the SPD group, larger striatal size ventrally, particularly in the left hemisphere was associated with less severe paranoid symptoms.
Conclusions
Striatal size is abnormal in SPD and resembles that of patients with schizophrenia who respond well to antipsychotic treatment. The results suggest that striatal size may be an important endophenotype to consider when developing new pharmacological treatments and when studying factors mitigating psychosis.
Keywords: Schizotypal personality disorder, Putamen, Caudate, Striatum, Schizophrenia, MRI, Striatal size
1. Introduction
The striatum, mainly composed of the caudate nucleus and putamen, receives afferent inputs from various brain cortical regions and via the thalamus sends neural projections back to the cortex (Alexander et al., 1986; Parent and Hazrati, 1995; Haber, 2003). Through these cortico–striato–thalamo neural loops, the striatum serves as a center for the integration and modulation of many high-level cognitive, motor, and limbic processes (Menon et al., 2001; Simpson et al., 2010; Bernacer et al., 2012) and its dysregulation contributes to the psychotic symptoms of schizophrenia-spectrum disorders (Howes et al., 2007). As reviewed by Bernacer et al. (2012), functional territories are segregated within the striatum. The major portion of the caudate nucleus, together with the precommissural putamen, is considered the associative part of the striatum. The dorsolateral rim of the caudate and the postcommissural putamen constitute its sensorimotor territory and the posteroventral putamen has limbic projections.
Numerous structural neuroimaging studies have reported enlargement of striatal size in patients with schizophrenia who were treated with antipsychotic medications (Breier et al., 1992; Hokama et al., 1995; McCarley et al., 1999). Since dopamine (DA) is a key neurotransmitter in the striatum and D2 DA receptors are a major target of typical antipsychotic medications, striatal enlargement in people exposed to these drugs may be in response to D2 blockade (Siever and Davis, 2004). Conversely, striatal size in patients with schizophrenia who were never exposed to typical antipsychotic medication is reported to be decreased (Ohnuma et al., 1997; Shihabuddin et al., 1998; McCarley et al., 1999). Even when antipsychotic exposure is taken into account, reports of striatal size alterations in schizophrenia have been inconsistent. For example, Gunduz et al. (2002) found no volumetric abnormalities in the striatum of never-medicated patients, while Corson et al. (1999) found decreased striatal size in patients receiving atypical antipsychotics.
Individuals with schizotypal personality disorder (SPD) exhibit social and cognitive deficits, although less marked than those observed in schizophrenia (Siever and Davis, 2004; Miller and Lenzenweger, 2012). Schizophrenia and SPD also share several structural and functional neuroanatomical features (Siever et al., 2002). In contrast to schizophrenia, few striatal neuroimaging studies have been carried out in SPD. Compared with healthy controls, SPD subjects showed smaller size (Levitt et al., 2002; Koo et al., 2006) and shape differences (Levitt et al., 2004) of their caudate nucleus. Among the SPD group, smaller caudate volume was associated with greater cognitive impairment (Levitt et al., 2002). A prior study by our group (Shihabuddin et al., 2001) examined volume of the caudate and putamen in healthy controls, individuals with SPD, and patients with schizophrenia. While caudate size was similar in all three groups, putamen volume was significantly smaller in SPD compared with the other two groups. Striatal DA release, induced either by amphetamine (Abi-Dargham et al., 2002; Siever et al., 2002) or physiological stressors (alpha 2-deoxyglucose infusion) (Mitropoulou et al., 2004), is significantly lower in SPD than in schizophrenia. Conversely, compared with healthy controls and patients with personality disorders other than SPD, the plasma and CSF concentration of homovanillic acid (HVA), a DA metabolite, were found to be significantly increased in the SPD group (Siever and Davis, 2004). Despite these seemingly contradictory findings, prior work in SPD suggests that, similar to schizophrenia (Tritsch and Sabatini, 2012), striatal abnormalities may reflect circuit dysfunction and plasticity alterations secondary to perturbations of DA signaling in this disorder.
The aim of the present study was to examine the size of the caudate and putamen in a large sample of antipsychotic-naïve individuals with SPD compared with healthy controls. Given prior dorsal-to-ventral striatum gradients in schizophrenia-spectrum neuroimaging work (e.g., Buchsbaum et al., 1992), and laterality effects in schizophrenia (Crow et al., 1989; Oertel-Knochel and Linden, 2011), we also investigated these factors as repeated measures. Unlike patients with schizophrenia, individuals with SPD are rarely treated with antipsychotic medications or exposed to multiple hospitalizations. Thus, this study examining the striatum (i.e. caudate and putamen) in SPD—a schizophrenia-spectrum disorder—may be particularly informative as these confounds are avoided. Given that prior work examining striatal size in SPD is limited and contradictory, our hypothesis is parsimonious and did not predict the direction of size differences. We hypothesized that compared with healthy controls, individuals with SPD would have striatal-size abnormalities. Additionally, we conducted exploratory correlations to examine clinical symptom severity correlates of striatal size.
2. Methods
2.1. Participants
We studied two age- and sex-matched groups: 76 individuals with SPD and 148 healthy controls. The two groups did not differ significantly in age or sex. Demographic and clinical data are presented in Table 1.
Table 1.
Demographic and clinical characteristics of SPD patients and healthy controls.
| Characteristic | SPD patients (n = 76) | Healthy controls (n = 148) | Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | t | df | p | |
| Age (years) | 38.0 | 10.2 | 19–62 | 35.5 | 13.0 | 17–65 | −1.44 | 222 | 0.15 |
| Relative striatal size | 1.04 | 0.13 | 0.78–1.31 | 1.02 | 0.12 | 0.75–1.36 | −1.42 | 222 | 0.16 |
| Symptom severity | 6.8 | 1.1 | 5–10 | ||||||
| Cognitive/perceptual | 1.5 | 0.9 | 0–4 | ||||||
| Interpersonal (negative) | 2.9 | 0.9 | 1–5 | ||||||
| Paranoid (positive) | 2.4 | 0.9 | 1–5 | ||||||
| n | % | n | % | ||||||
| Sex | χ2 | df | p | ||||||
| Male | 60 | 79 | 99 | 67 | 3.54 | 1 | 0.06 | ||
| Female | 16 | 21 | 49 | 33 | |||||
All participants received an interview with a psychologist using the Structured Clinical Interview for DSM-IV Axis I disorders (First et al., 1996) and the Structured Interview for DSM-IV Personality Disorders (Pfohl et al., 1997). Participants were excluded if they had met lifetime criteria for substance dependence or abuse in the previous six months, previously taken antipsychotic medication, or had a positive urine toxicology screen for drugs of abuse. All participants were screened by a physician for severe medical or neurological illness and head injury by comprehensive medical history and laboratory tests. Individuals with SPD met the DSM-IV criteria based on structured diagnostic interview and had no history of Axis I disorder. Healthy controls had no Axis I or II disorder and no history of a first-degree relative with an Axis I disorder.
The healthy controls and majority of the SPD group (90%) were recruited from the community through advertisements in local newspapers. The remaining SPD participants were recruited through referrals from outpatient psychiatry clinics at Mount Sinai and its affiliate, the James J. Peters VA Medical Center. Participants provided written informed consent in accordance with the Institutional Review Board guidelines. A subgroup of the healthy controls and SPD individuals was previously included in studies examining Brodmann areas using FDG-PET (Buchsbaum et al., 2002), cingulate volume (Haznedar et al., 2004),whole temporal lobe volume (Downhill et al., 2001), and cortical volume (Hazlett et al., 2008).
Based on prior work (Bergman et al., 1996; Mitropoulou et al., 2002; Goldstein et al., 2009), we grouped the nine DSM criteria for SPD into three composite scores to assess symptom severity: cognitive/perceptual, interpersonal, and paranoid. Each of the nine DSM symptoms was rated on a 4-point scale (0 = absent, 0.5 = somewhat present, 1.0 = definitely present/prototypic, 2.0 = severe, pervasive). Overall symptom severity was measured as the total of all nine symptom scores. According to the three-factor model of schizotypal symptoms (Bergman et al., 1996), the cognitive/perceptual factor includes magical thinking and illusions, the interpersonal factor includes negative-like symptoms such as social isolation, odd speech, and poor rapport, and the paranoid factor includes positive-like symptoms, such as ideas of reference, suspiciousness, and hypersensitivity.
2.2. Imaging methods
2.2.1. Image acquisition
All participants received T1-weighted axial MRI scans on a 1.5 T Signa 5× system (GE Medical Systems) with the same acquisition parameters: repetition time = 24 ms, echo time = 5 ms, flip angle = 40°, slice thickness = 1.2 mm, pixel matrix = 256 × 256, field of view = 23 cm, and total slices = 128. MRI scans were re-sectioned to standard Talairach–Tournoux position (Talairach and Tournoux, 1988).
2.2.2. Tracing methods
Each participant's caudate and putamen were traced on five ventral to dorsal slice levels in each hemisphere by a tracer blind to diagnosis using our previously published tracing methods (e.g., Shihabuddin et al., 2001; Brickman et al., 2003; Buchsbaum et al., 2003). See Fig. 1 for details on the tracing methods employed.
Fig. 1.
Hand-tracing of the caudate and putamen. (A) MRI showing an axial MRI slice with caudate and putamen visible, (B) using our edge contrast-enhancing technique (Sobel-gradient filter), gray/white boundaries of the striatal regions (caudate and putamen) are enhanced, (C) caudate and putamen are outlined, and (D) the anatomical regions-of-interest are shown. As described by Buchsbaumet al. (2003), the most ventral and dorsal axial slices for which both the caudate and putamen were present were determined for each participant. An automated boundary-finding method based on the Sobel-gradient filter provides a reproducible structure edge, with little operator variability. The caudate and putamen were outlined on the MRI by depositing points by mouse on the magnified and enhanced white structure edge using a semi-automated 3 × 3 local pixel maximum search. This placed the point at the center of the edge, enhancing inter-operator consistency. A spline curve was fit to the points and the ROI edge was stored. We determined the top of the caudate and putamen as the most dorsal axial slice showing a visible gray patch and the bottomas the slice in which the caudate and putamen entirely merged. This distance was divided by six to yield five equally spaced slices for the caudate and putamen separately for tracing (e.g., most dorsal slice = 20, most ventral slice = 38, difference = 18. 18/6 = 3, slices 23, 26, 29, 32, and 35 are traced). For each region of interest, area was calculated in voxels, divided by whole brain volume, and then multiplied by 1000 to correct for variation in intracranial volume. Whole brain volume was expressed as the sum of the absolute gray and white matter volumes of 39 Brodmann areas within 33 coronal brain slices (see Mitelman et al., 2005). Although there are multiple ways to account for variation in brain size (O'Brien et al., 2006), our primary dependent variables are striatal size relative to whole brain because relative values are more widely reported than absolute size. However, we additionally conducted parallel analyses on absolute size and report these findings. To establish inter-rater reliability, two tracers from our group independently traced striatal ROIs at various ventral/dorsal slice levels in each hemisphere for a subset of the individuals (n = 10). The intra-class correlation coefficient (ICC) for the putamen was 0.98 and for the caudate, ICC = 0.92 (Buchsbaum et al., 2003) which is in line with kappa scores from other groups examining the striatum, e.g., Levitt et al. (2002).
2.2.3. Statistical analysis
As in our previous studies examining striatal size in healthy controls and schizophrenia-spectrum samples (e.g., Shihabuddin et al., 2001; Brickman et al., 2003; Buchsbaum et al., 2003), a Group (Healthy control-vs.-SPD participants) × Structure (caudate, putamen) × Slice level (1 to 5; from ventral- to dorsal-slice level) × Hemisphere (left, right) MANOVA examined whether there were differences in relative size of the striatal regions-of-interest. Diagnostic group was a between-subjects factor and all remaining factors were repeated measures. Follow-up simple effects were examined to identify the strongest sources of between-group differences. In reporting repeated-measures MANOVA, means for the main effect of Diagnostic group and interactions with Diagnostic group are averaged across levels of the repeated measures (e.g., the Group × Structure interaction is averaged across the five dorsoventral slices and two hemispheres).
We report the multivariate (Wilks Lambda) F from Statistica (Statsoft, Inc., 2009) to adjust probabilities for repeated-measures effects with more than two levels (e.g., slice level). Fisher's Least Significant Difference (LSD) tests were used to follow up significant interaction effects with diagnostic group. Compared with conducting all possible comparisons, this multivariate approach provides tests of hypothesized group differences and helps minimize Type I statistical error involved with t-tests for each area, group contrast, and hemisphere. Additionally, we only examined significant main effects for diagnostic group and interactions between the diagnostic group and the repeated-measures factors. To examine symptom correlates of striatal size, we used Spearman's rank correlations. A p-value of 0.05 was used for all analyses.
3. Results
3.1. Striatal size: relative area
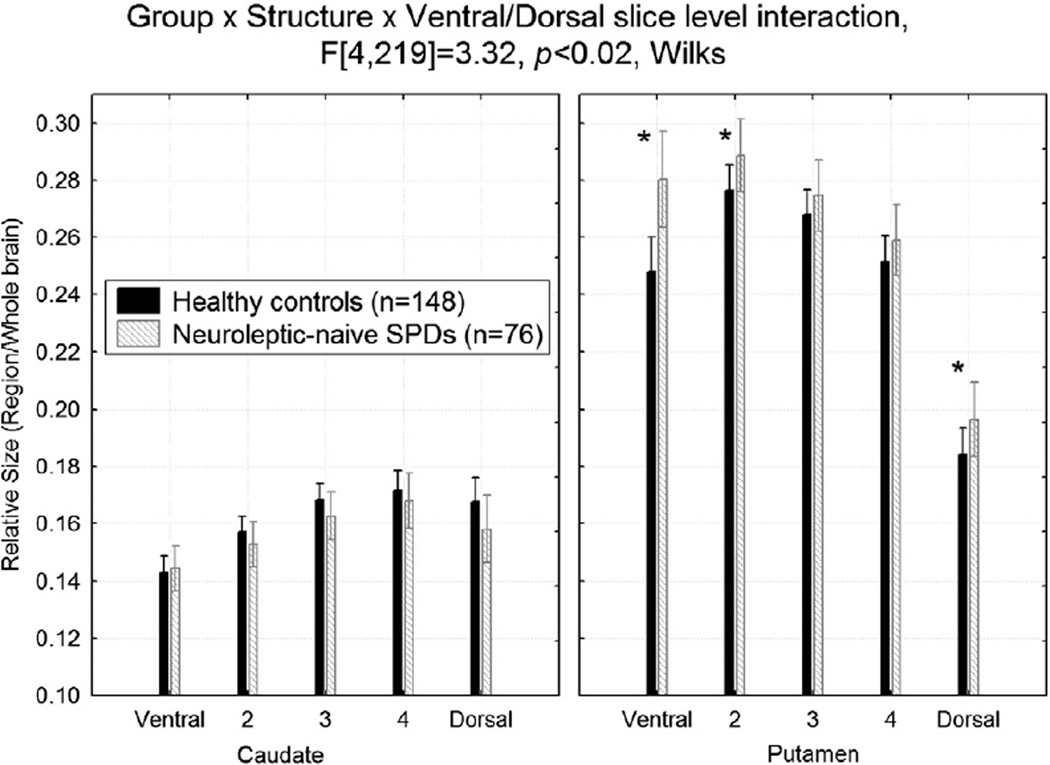
Compared with healthy controls, the SPD group had larger putamen size at the most ventral slice levels and the most dorsal slice level, but there were no differences in the caudate, Group × Structure (caudate, putamen) × Ventral/Dorsal slice (1 to 5) interaction, F[4,219] = 3.32, p<0.02 (Fig. 2). The follow-up Fisher's LSD tests were significant (p-values<0.04).
Fig. 2.
Compared with healthy controls, the schizotypal personality disorder (SPD) group had larger relative putamen size at the most ventral and dorsal slice levels, but there were no differences in the caudate nucleus. *Fisher's LSD tests, all p-values<0.04.
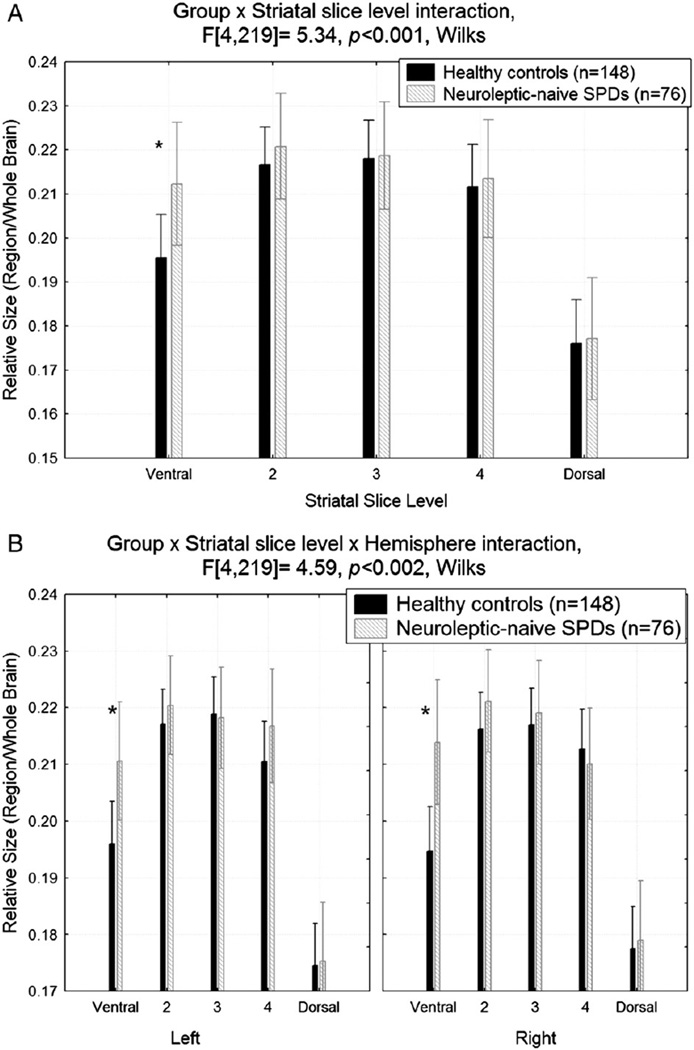
Overall, compared with healthy controls, the striatum, averaged across structure and hemisphere, was larger in the SPD group at the most ventral slice level (Group × Ventral/Dorsal slice, F[4,219] = 5.34, p<0.001; Fisher's LSD, p<0.0001; Fig. 3A). This effect was seen in both the left and right most ventral slice level (p-values<0.01, Group × Ventral/Dorsal slice × Hemisphere, F[4,219] = 4.59, p<0.002; Fig. 3B).
Fig. 3.
A. The striatum (averaged across structure and hemisphere) was larger at the most ventral slice level in schizotypal personality disorder (SPD) patients compared with healthy controls. *Fisher's LSD test, p<0.0001. B. The striatum (averaged across structure) was larger in schizotypal personality disorder (SPD) patients compared with healthy controls at the most ventral slice level in both hemispheres. *Fisher's LSD tests, both p-values<0.01.
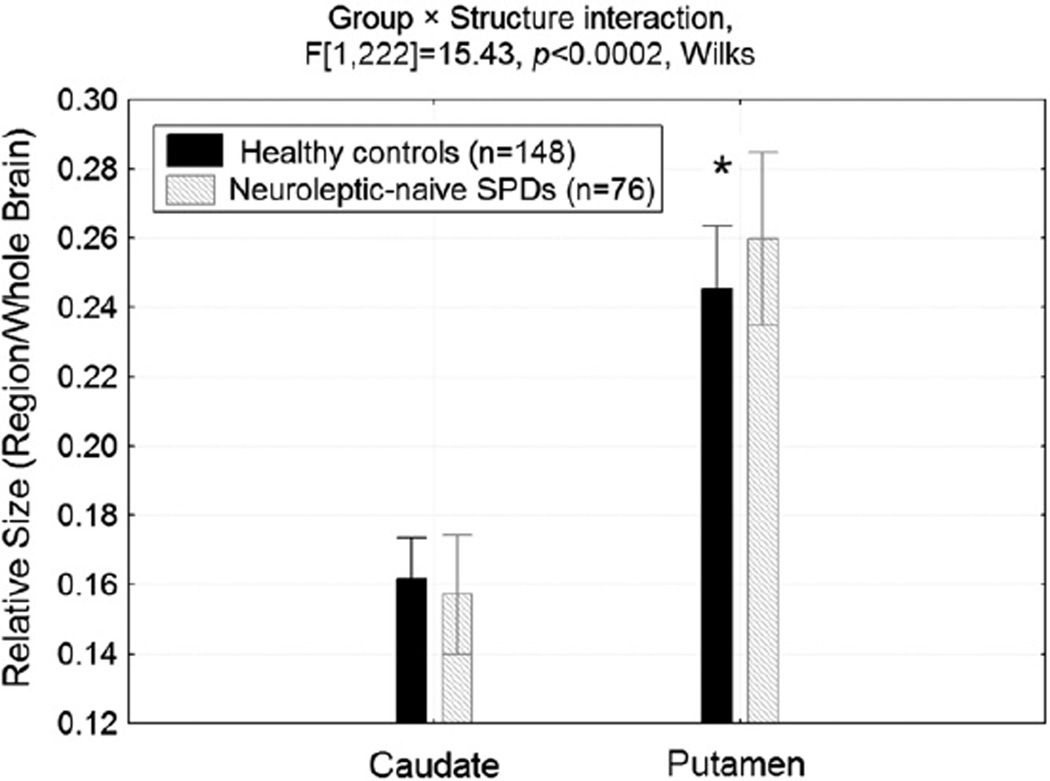
Mean putamen size, averaged across hemisphere and dorso-ventral slice level, was larger in the SPD group compared with the controls (p<0.001) and the caudate did not differ between groups (Group × Structure interaction, F[1,222] = 15.43, p<0.0002; Fig. 4).
Fig. 4.
Mean putamen size (averaged across hemisphere and slice) was larger in the SPD group compared with the healthy control group while the caudate did not differ between groups. *Fisher's LSD test, p<0.001.
3.2. Striatal size: absolute area
All four of the significant interactions reported above in Section 3.1 and the post-hoc follow-up tests remained significant for the absolute-size analyses. The interactions were all p<0.01 and the Fisher's LSD tests were p<0.05.
3.3. Symptom severity correlates of striatal size
Spearman's correlations were performed on only the relative striatal variables that showed significant between-group differences in the follow-up post-hoc tests from the MANOVA analysis. These included the ventral slices of the striatum averaged across hemisphere and for each hemisphere separately, overall mean putamen size, and mean putamen size for the two most ventral slices (i.e. slices = ventral, 2) and the most dorsal slice. Smaller overall relative area in the ventral striatal slices (averaged across structure and hemisphere) and smaller left relative area were associated with greater paranoid SPD symptom severity (r = −0.25 and r = −0.26, respectively, both p<0.05).
4. Discussion
The main findings of this study are that in SPD: (1) the putamen bilaterally is larger and this enlargement is more pronounced at the most ventral and dorsal levels, while there were no between-group differences in caudate volume; (2) ventrally, averaging across the caudate and putamen, the striatum is larger bilaterally; and (3) among the SPD group, larger striatal size ventrally, particularly in the left hemisphere is associated with less severe paranoid symptoms.
The finding of increased putamen size in SPD compared with healthy controls is consistent with longitudinal MRI work showing that good outcome schizophrenia patients have larger putamen volumes over time compared with poor outcome patients (Mitelman et al., 2009). Mitelman et al. (2009) proposed that, rather than being a consequence of prolonged neuroleptic exposure, putamen enlargement observed in several studies may be a marker of responsiveness to antipsychotic treatment with the caveat that its shrinkage may also be associated with poor clinical outcome. Other work indicates that compared with healthy controls, a significant increase in volume of the right putamen in first-episode schizophrenia patients following 6 weeks of antipsychotic treatment was associated with symptom improvement (Li et al., 2012). Taken together, these findings suggest that larger putamen size in SPD may be a protective factor, while it serves as a longitudinal marker of treatment responsiveness and outcome in schizophrenia. Several investigators (Raine and Lencz, 1995; Siever and Davis, 2004; Hazlett et al., 2008) have discussed the idea that individuals with SPD possess some biological characteristics that shield them from frank psychosis.
It is important to note that the present findings in 76 antipsychotic-naïve SPD patients are inconsistent with our earlier study (Shihabuddin et al., 2001), which is the only other study to examine putamen size in SPD. Shihabuddin et al. (2001) reported smaller-than-normal putamen volume in a much smaller sample of neuroleptic-naïve SPD individuals (n = 10). Possible explanations for this discrepancy include heterogeneity of the disorder, medication, and/or gender (94% male in Shihabuddin et al. and 79% in current study) differences. Prior work indicates that neuroleptic medication affects the morphology of the striatum (Benes et al., 1985; Chakos et al., 1994; Li et al., 2012), highlighting the need for future studies to examine medication and gender effects.
Although the SPD group showed smaller volume for all of the caudate slice levels, these between-group differences did not reach significance. In contrast to this finding, two prior studies of neuroleptic-naïve SPD individuals (n = 15 men: Levitt et al., 2002 and n = 32 women: Koo et al., 2006) reported smaller relative caudate volume compared with healthy controls. Differences in tracing methodology and/or sample characteristics (e.g., severity of the disorder) may explain these disparate results.
Our findings indicate that striatal abnormalities in SPD are most prominent at the more ventral slice levels, suggesting that this region is particularly important in schizophrenia-spectrum disorders. Consistent with this concept, Haber and Knutsen (2010) have shown that the ventral portion of the striatum has several unique characteristics: (1) the dopamine transporter (DAT) is relatively low in the ventral portion of the striatum relative to the dorsal striatum; (2) the ventral portion of the striatum contains many smaller and more densely packed neurons, while the dorsal striatum is more homogeneous; and (3) although both the dorsal and ventral regions of the striatum receive input from the cortex, thalamus, and brainstem, the ventral portion of the striatum also receives dense projections from the amygdala and hippocampus (Russchen et al., 1985; Friedman et al., 2002; Fudge and Haber, 2002). Thus, the ventral–dorsal striatal gradient warrants further examination across the schizophrenia spectrumin order to determine associations between dopamine neurotransmission and striatal size.
A limitation of the present study is that the horizontal division employed to divide ventral and dorsal levels of the striatum was based on the plane of the neuroimaging sectioning, and the extent to which this approximates the actual dorsal to ventral segregation of function within the striatum (Haber, 2003; Haber and Knutson, 2010) is not clear. Another limitation is the unknown significance of variations in striatal size. In schizophrenia, striatal volume has been repeatedly shown to increase with the administration of typical antipsychotics (Chakos et al., 1994; Keshavan et al., 1994). As reviewed by Chakos et al. (1998), in rat models this phenomenon is also associated with increased glucose utilization, synaptic markers, striatal neuronal and axon terminal volumes, and increased sprouting of axons in the substantia nigra. More recently, animal work indicates that dopamine D2 antagonists may promote cell genesis and survival in the adult brain (Kippin et al., 2005; Keilhoff et al., 2010). Along these lines, our finding of larger relative volume of the ventral portion of the striatumin SPD may reflect neuroplasticity (Li et al., 2012). Future studies should examine the striatumin age- and sex-matched groups of SPD and schizophrenia patients to determine whether between-group differences within the spectrum are consistent. Lastly, we did not trace the full volume of the caudate and putamen. Instead, these structures were traced using previously published and validated methods examining the size of each region-of-interest based upon five equally-spaced dorsal-to-ventral slices. However, for a subset of 15 subjects in this study, we traced the full volume for each region-of-interest and conducted correlations between these values and the mean areas calculated using the 5-slice methodology. These correlations were significant (range for r values: 0.93–0.96), suggesting that our 5-slice approach is both time effective and a good proxy for caudate and putamen volume.
Among the SPD sample, those with larger overall striatal size, particularly in the left hemisphere, exhibited less severe paranoid symptoms. Our finding is in line with two previous studies examining caudate size and symptomatology in SPD (Levitt et al., 2002; Koo et al., 2006). Similar to Levitt et al. (2002), who studied men with SPD, we did not find significant correlations with negative-like symptoms in our predominantly-male sample (79%). Conversely, Koo et al. (2006) reported that smaller caudate size was associated with negative-like symptoms in a sample of 15 women with SPD. Future work will be needed to examine gender differences in key regions, including the striatum, across the schizophrenia spectrum. Additionally, our SPD findings are consistent with observations in schizophrenia where increased volume in the striatum (caudate and putamen) (Taylor et al., 2005), putamen (Li et al., 2012) and caudate (Okugawa et al., 2007) is associated with fewer positive symptoms.
Strengths of our study include a large sample (n = 76) of antipsychotic-naïve individuals with SPD. Limitations include not assessing dopaminergic and/or metabolic function of the striatum. Despite our large SPD sample, we did not have enough women to examine gender differences, which prior work suggests is important (Dickey et al., 2003; Koo et al., 2006).
In conclusion, the present findings indicate that striatal size in SPD is abnormal and resembles that of schizophrenia patients who respond well to antipsychotic treatment. This suggests that striatal size may be an important endophenotype to consider when developing new pharmacological treatments and when studying factors mitigating psychosis.
Acknowledgment
The authors want to thank Dr. Monte Buchsbaum for sharing the MIPS software which was used for tracing the brain regions of interest.
Role of funding source
Funding for this study was provided by VA Merit Grants I01 CX000261 to Dr. Hazlett and 7609-028 to Dr. Siever. Partial support was also provided by the Mental Illness Research Education and Clinical Center, VISN3 Veterans Health Administration, and grant number #UL1TR000067 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The funding sources had no further role in study design, collection, analysis and interpretation of data, writing of the report, or in the decision to submit the paper for publication.
Footnotes
Contributors
Dr. Chemerinski wrote some sections of the manuscript, Drs. Byne, Haznedar, Novakovic, Siever, and Ms. Kolaitis provided feedback on the manuscript. Dr. Chu provided image-processing support. Ms. Glanton, Ms. Canfield, and Mr. Newmark were responsible for image-processing support and tracing of the regions of interest. Drs. Siever and Hazlett were responsible for recruitment and diagnosis. Dr. Hazlett was responsible for the idea of the study, supervised all aspects of the research, conducted all of the statistical analyses, made the figures, wrote sections of the manuscript, and provided feedback on others. All authors have approved the final manuscript.
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J. Neurosci. 2002;22(9):3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Benes FM, Paskevich PA, Davidson J, Domesick VB. The effects of haloperidol on synaptic patterns in the rat striatum. Brain Res. 1985;329(1–2):265–273. doi: 10.1016/0006-8993(85)90532-3. [DOI] [PubMed] [Google Scholar]
- Bergman AJ, Harvey PD, Mitropoulou V, Aronson A, Marder D, Silverman J, Trestman R, Siever LJ. The factor structure of schizotypal symptoms in a clinical population. Schizophr. Bull. 1996;22(3):501–509. doi: 10.1093/schbul/22.3.501. [DOI] [PubMed] [Google Scholar]
- Bernacer J, Prensa L, Gimenez-Amaya JM. Distribution of GABAergic interneurons and dopaminergic cells in the functional territories of the human striatum. PLoS One. 2012;7(1):e30504. doi: 10.1371/journal.pone.0030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia. Amagnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch. Gen. Psychiatry. 1992;49(12):921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MD, Shihabuddin L, Hazlett EA, Borod JC, Mohs RC. Striatal size, glucose metabolic rate, and verbal learning in normal aging. Brain Res. Cogn. Brain Res. 2003;17(1):106–116. doi: 10.1016/s0926-6410(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Potkin SG, Siegel BV, Jr, Lohr J, Katz M, Gottschalk LA, Gulasekaram B, Marshall JF, Lottenberg S, Teng CY, et al. Striatal metabolic rate and clinical response to neuroleptics in schizophrenia. Arch. Gen. Psychiatry. 1992;49(12):966–974. doi: 10.1001/archpsyc.1992.01820120054008. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Nenadic I, Hazlett EA, Spiegel-Cohen J, Fleischman MB, Akhavan A, Silverman JM, Siever LJ. Differential metabolic rates in prefrontal and temporal Brodmann areas in schizophrenia and schizotypal personality disorder. Schizophr. Res. 2002;54(1–2):141–150. doi: 10.1016/s0920-9964(01)00361-9. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Shihabuddin L, Brickman AM, Miozzo R, Prikryl R, Shaw R, Davis K. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophr. Res. 2003;64(1):53–62. doi: 10.1016/s0920-9964(02)00526-1. [DOI] [PubMed] [Google Scholar]
- Chakos M, Lieberman J, Bilder R. Increase in caudate nuclei volume of first episode schizophrenia patients taking antipsychotic drugs. Am. J. Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chakos M, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga C. Striatal enlargement in rats chronically treated with neuroleptic. Biol. Psychiatry. 1998;44:675–684. doi: 10.1016/s0006-3223(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Corson PW, Nopoulos P, Andreasen NC, Heckel D, Arndt S. Caudate size in first-episode neuroleptic-naive schizophrenic patients measured using an artificial neural network. Biol. Psychiatry. 1999;46(5):712–720. doi: 10.1016/s0006-3223(99)00079-7. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, Frith CD, Johnstone EC, Owens DG, Roberts GW. Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch. Gen. Psychiatry. 1989;46(12):1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM. An MRI study of superior temporal gyrus volume in women with schizotypal personality disorder. Am. J. Psychiatry. 2003;160:2198–2201. doi: 10.1176/appi.ajp.160.12.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downhill JE, Buchsbaum MS, Hazlett EA, Barth S, Lees Roitman S, Nunn M, Lekarev O, Wei T, Shihabuddin L, Mitropoulou V, Silverman J, Siever LJ. Temporal lobe volume determined by magnetic resonance imaging in schizotypal personality disorder and schizophrenia. Schizophr. Res. 2001;48(2–3):187–199. doi: 10.1016/s0920-9964(00)00131-6. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: New York State Psychiatric Institute; 1996. [Google Scholar]
- Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain. J. Comp. Neurol. 2002;450(4):345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. Defining the caudal ventral striatum in primates: cellular and histochemical features. J. Neurosci. 2002;22(23):10078–10082. doi: 10.1523/JNEUROSCI.22-23-10078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein KE, Hazlett EA, New AS, Haznedar MM, Newmark RE, Zelmanova Y, Passarelli V, Weinstein SR, Canfield EL, Meyerson DA, Tang CY, Buchsbaum MS, Siever LJ. Smaller superior temporal gyrus volume specificity in schizotypal personality disorder. Schizophr. Res. 2009;112(1–3):14–23. doi: 10.1016/j.schres.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz H, Wu H, Ashtari M, Bogerts B, Crandall D, Robinson DG, Alvir J, Lieberman J, Kane J, Bilder R. Basal ganglia volumes in first-episode schizophrenia and healthy comparison subjects. Biol. Psychiatry. 2002;51(10):801–808. doi: 10.1016/s0006-3223(01)01345-2. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Haznedar MM, Newmark R, Goldstein KE, Zelmanova Y, Glanton CF, Torosjan Y, New AS, Lo JN, Mitropoulou V, Siever LJ. Cortical gray and white matter volume in unmedicated schizotypal and schizophrenia patients. Schizophr. Res. 2008;101:111–123. doi: 10.1016/j.schres.2007.12.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, Shihabuddin L, New A, Siever LJ. Cingulate gyrus volume and metabolism in the schizophrenia spectrum. Schizophr. Res. 2004;71(2–3):249–262. doi: 10.1016/j.schres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Hokama H, Shenton M, Nestor P, Kikinis R, Levitt J, Metcalf D, Wible C, O'Donnell B, Jolesz F, McCarley R. Caudate, putamen and globus pallidus volume in schizophrenia: a quantitative MRI study. Psychiatry Res. Neuroimaging. 1995;61:209–229. doi: 10.1016/0925-4927(95)02729-h. [DOI] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br. J. Psychiatry. 2007;(Suppl. 51):s13–s18. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G, Grecksch G, Bernstein HG, Roskoden T, Becker A. Risperidone and haloperidol promote survival of stem cells in the rat hippocampus. Eur. Arch. Psychiatry Clin. Neurosci. 2010;260(2):151–162. doi: 10.1007/s00406-009-0033-1. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344(8934):1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J. Neurosci. 2005;25(24):5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo MS, Levitt JJ, McCarley RW, Seidman LJ, Dickey CC, Niznikiewicz MA, Voglmaier MM, Zamani P, Long KR, Kim SS, Shenton ME. Reduction of caudate nucleus volumes in neuroleptic-naive female subjects with schizotypal personality disorder. Biol. Psychiatry. 2006;60(1):40–48. doi: 10.1016/j.biopsych.2005.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JJ, McCarley RW, Dickey CC, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Ciszewski AA, Kikinis R, Jolesz FA, Shenton ME. MRI study of caudate nucleus volume and its cognitive correlates in neuroleptic-naive patients with schizotypal personality disorder. Am. J. Psychiatry. 2002;159(7):1190–1197. doi: 10.1176/appi.ajp.159.7.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JJ, Westin CF, Nestor PG, Estepar RS, Dickey CC, Voglmaier MM, Seidman LJ, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Shape of caudate nucleus and its cognitive correlates in neuroleptic-naive schizotypal personality disorder. Biol. Psychiatry. 2004;55(2):177–184. doi: 10.1016/j.biopsych.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen Z, Deng W, He Z, Wang Q, Jiang L, Ma X, Wang Y, Chua SE, Cheung C, McAlonan GM, Sham PC, Collier DA, Gong Q, Li T. Volume increases in putamen associated with positive symptom reduction in previously drug-naive schizophrenia after 6 weeks antipsychotic treatment. Psychol. Med. 2012;42(7):1475–1483. doi: 10.1017/S0033291711002157. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biol. Psychiatry. 1999;45(9):1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Anagnoson RT, Glover GH, Pfefferbaum A. Functional magnetic resonance imaging evidence for disrupted basal ganglia function in schizophrenia. Am. J. Psychiatry. 2001;158(4):646–649. doi: 10.1176/appi.ajp.158.4.646. [DOI] [PubMed] [Google Scholar]
- Miller AB, Lenzenweger MF. Schizotypy, social cognition, and interpersonal sensitivity. Personal. Disord. 2012;3(4):379–392. doi: 10.1037/a0027955. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Brickman AM, Shihabuddin L, Newmark R, Chu KW, Buchsbaum MS. Correlations between MRI-assessed volumes of the thalamus and cortical Brodmann's areas in schizophrenia. Schizophr. Res. 2005;75(2–3):265–281. doi: 10.1016/j.schres.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Canfield EL, Chu KW, Brickman AM, Shihabuddin L, Hazlett EA, Buchsbaum MS. Poor outcome in chronic schizophrenia is associated with progressive loss of volume of the putamen. Schizophr. Res. 2009;113(2–3):241–245. doi: 10.1016/j.schres.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Maldari LA, Moriarty PJ, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: evidence regarding diagnostic specificity. Biol. Psychiatry. 2002;52(12):1175–1182. doi: 10.1016/s0006-3223(02)01426-9. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Goodman M, Sevy S, Elman I, New AS, Iskander EG, Silverman JM, Breier A, Siever LJ. Effects of acute metabolic stress on the dopaminergic and pituitary-adrenal axis activity in patients with schizotypal personality disorder. Schizophr. Res. 2004;70(1):27–31. doi: 10.1016/j.schres.2003.10.008. [DOI] [PubMed] [Google Scholar]
- O'Brien LM, Ziegler DA, Deutsch CK, Kennedy DN, Goldstein JM, Seidman LJ, Hodge S, Makris N, Caviness V, Frazier JA, Herbert MR. Adjustment for whole brain and cranial size in volumetric brain studies: a review of common adjustment factors and statistical methods. Harv. Rev. Psychiatry. 2006;14(3):141–151. doi: 10.1080/10673220600784119. [DOI] [PubMed] [Google Scholar]
- Oertel-Knochel V, Linden DE. Cerebral asymmetry in schizophrenia. Neuroscientist. 2011;17(5):456–467. doi: 10.1177/1073858410386493. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Kimura M, Takahashi T, Iwamoto N, Arai H. A magnetic resonance imaging study in first-episode disorganized-type patients with schizophrenia. Psychiatry Clin. Neurosci. 1997;51(1):9–15. doi: 10.1111/j.1440-1819.1997.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Nobuhara K, Takase K, Saito Y, Yoshimura M, Kinoshita T. Olanzapine increases grey and white matter volumes in the caudate nucleus of patients with schizophrenia. Neuropsychobiology. 2007;55(1):43–46. doi: 10.1159/000103575. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. 1995;20(1):91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured Clinical Interview for DSM-IV Personality (SIDP-IV) Washington, D.C: American Psychiatric Press; 1997. [Google Scholar]
- Raine A, Lencz T. Conceptual and theoretical issues in schizotypal personality research. In: Raine A, Lencz T, Mednick SA, editors. Schizotypal Personality. New York: Cambridge University Press; 1995. pp. 3–15. [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985;329(1–2):241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Shihabuddin L, Buchsbaum M, Hazlett E, Haznedar M, Harvey P, Newman A, Schnur D, Spiegel-Cohen J, Wei T, Machac J, Knesaurek K, Vallabhojosula S, Biren M, Ciaravolo T, Luu-Hsia C. Dorsal striatal size, shape, and metabolic rate in neuroleptic-naive and previously medicated schizophrenic patients performing a verbal learning task. Arch. Gen. Psychiatry. 1998;55:235–243. doi: 10.1001/archpsyc.55.3.235. [DOI] [PubMed] [Google Scholar]
- Shihabuddin L, Buchsbaum M, Hazlett E, Silverman J, New A, Brickman A, Mitropoulou V, Nunn M, Fleischman M, Tang C, Siever L. Striatal size and relative glucosemetabolic rate in schizotypal personality disorder and schizophrenia. Arch. Gen. Psychiatry. 2001;58(9):877–884. doi: 10.1001/archpsyc.58.9.877. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am. J. Psychiatry. 2004;161(3):398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Koenigsberg HW, Harvey P, Mitropoulou V, Laruelle M, Abi-Dargham A, Goodman M, Buchsbaum M. Cognitive and brain function in schizotypal personality disorder. Schizophr. Res. 2002;54(1–2):157–167. doi: 10.1016/s0920-9964(01)00363-2. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65(5):585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statsoft, Inc. Statistica, 9.0 ed. Tulsa, OK: 2009. www.statsoft.com. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Taylor S, Christensen JD, Holcomb JM, Garver DL. Volume increases in striatum associated with positive symptom reduction in schizophrenia: a preliminary observation. Psychiatry Res. 2005;140(1):85–89. doi: 10.1016/j.pscychresns.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76(1):33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]