Abstract
Background/Objectives
Association of insulin-induced gene 2 (INSIG2) variants with obesity has been confirmed in several but not all follow-up studies. Differences in environmental factors across populations may mask some genetic associations and therefore gene-environment interactions should be explored. We hypothesized that the association between dietary patterns and components of the metabolic syndrome could be modified by INSIG2 variants.
Subjects/Methods
We conducted a longitudinal study of adiposity and cardiovascular disease risk among 427 and 290 adults from Samoa and American Samoa (1990–95). Principal component analysis on food items from a validated FFQ was used to identify neo-traditional and modern dietary patterns. We explored gene-dietary pattern interactions with the INSIG2 variants rs9308762 and rs7566605.
Results
Results for American Samoans were mostly non-significant. In Samoa, the neo-traditional dietary pattern was associated with lower triglycerides, BMI, waist circumference, systolic and diastolic blood pressure, and fasting glucose (all p-for-trend<0.05). The modern pattern was significantly associated with higher triglycerides, BMI, waist circumference, and lower HDL cholesterol (all p-for-trend<0.05). A significant interaction for triglycerides was found between the modern pattern and the rs9308762 polymorphism (p=0.04). Those from Samoa consuming the modern pattern have higher triglycerides if they are homozygous for the rs9308762 C allele.
Conclusions
The common INSIG2 rs9308762 variant was associated with poorer metabolic control and a greater sensitivity of trigylcerides to a modern dietary pattern. Environmental factors need to be taken into account when assessing genetic associations across and within populations.
Keywords: INSIG2, dietary patterns, gene-diet interactions, metabolic risk, Samoa
Introduction
Insig2 is an endoplasmic reticulum protein that blocks the processing of sterol regulatory element-binding proteins (SREBPs) (1, 2). Dysregulation of SREBP-1c affects lipid metabolism and may lead to insulin resistance and obesity (3). Results from genome-wide association studies (GWAS) have shown that a variant in the INSIG2 gene (rs7566605) on chromosome 2q14.2 is associated with obesity (4). However, this association has only been confirmed in several (5–11) but not all replication studies conducted in other populations (12–31), including our prior report in Samoans. The lack of replication may be due to variation in linkage disequilibrium among different populations if the variant is not causal (32). On the other hand, most obesity genes are unlikely to cause obesity in the absence of an obesogenic environment (33). Therefore, it is possible that differences in environmental factors across and within populations may mask some genetic associations. In particular, some genetic associations may not be evident in the absence of obesogenic dietary patterns. Therefore, it is important to study these associations in populations that still retain traditional dietary patterns.
The population of the Samoan Islands is characterized by high prevalence of obesity, metabolic syndrome, and diabetes (34–38). Rates of obesity have been increasing dramatically in the last decades as the population experienced rapid modernization (39). As a consequence of this rapid modernization, Samoans have undergone a nutrition transition, largely abandoning their neo-traditional diets and acquiring a modern dietary pattern and sedentary lifestyle (39, 40). In a study conducted in 2002–03 among 723 American Samoans and 785 Samoans, we have shown previously that a modern dietary pattern was significantly associated with metabolic syndrome in Samoa and with increased triglyceride levels in both polities (41). In addition, a neo-traditional dietary pattern was associated with higher HDL-cholesterol in American Samoa and lower waist circumference in both polities (41). In another study conducted in the Samoan islands from 1990 to 1995 with the study sample used here, we have shown also that a variant in the INSIG2 gene (rs9308762) was associated with BMI and waist circumference in the combined Samoa and American Samoa sample and with BMI in the sample from Samoa. However, rs7566605 was not associated with BMI or waist circumference in the combined sample nor in either polity (32).
The goal of this study was to use principal component analysis to derive dietary patterns in the Samoan Islands population and evaluate their association with metabolic health in order to reproduce the associations previously seen in the 2002–03 population, and assess for the first time if the INSIG2 variants, rs9308762 and rs7566605, modify these associations. Out of the 4 INSIG2 variants that were previously genotyped in this study population, we chose to focus upon rs9308762 due to our earlier study (32) which showed association with BMI and waist circumference, and on rs7566605 due to the many prior studies of this single nucleotide polymorphism (SNP) in other populations (5–31).
Materials and Methods
Study Population
The study population consists of adults 25 to 55 years old with all four grandparents of Samoan ancestry. A detailed description of this longitudinal study has been reported previously (42, 43). Briefly, participants were recruited from 46 villages and worksites in American Samoa in 1990 and nine villages in (then Western) Samoa in 1991, and followed up four years later in 1994 and 1995, respectively. All participants were free of self-reported history of heart disease, hypertension, or diabetes at baseline. At baseline, 563 and 694 individuals were recruited from American Samoa in 1990, and 1991 in Samoa, respectively. In 1994 and 1995 when outcome data were collected, 361 and 560 individuals were available for the four-year follow-up in American Samoa and Samoa, respectively.
Informed consent was obtained from all participants. All study protocols were approved by the human subjects committees of the Miriam Hospital of Providence, RI, The Department of Health, American Samoa and the Ministry of Health, Samoa.
Data collection
Dietary intake was measured at baseline with a validated 42-item food frequency questionnaire (FFQ) specifically designed for this population (41). Information on socio-demographic and lifestyle variables was obtained through a questionnaire, including number of hours farming (used as a proxy of physical activity (34)), education, and occupation. The outcome variables at the four-year follow-up included BMI, waist, leptin, triglycerides, HDL-cholesterol, LDL-cholesterol, fasting glucose, insulin, systolic blood pressure, and diastolic blood pressure. Anthropometric measures were taken with participants wearing light tropical clothing without shoes. Height was measured using a portable GPM anthropometer (Pfister Imports, New York, NY) and they were weighed with a calibrated spring-balance scale. Waist circumference was measured twice at the umbilicus level with a metal tape and the average of the two measurements was used in the analysis. Blood pressure was measured three times using a mercury sphygmomanometer with appropriately sized upper arm cuffs after the participants were seated and resting for 5 minutes. The average of the three measurements was used in the analysis. Blood was collected in the morning after a minimum of 10 hours fasting. Serum glucose was analyzed with a Berkman CX4 automatic analyzer. Total cholesterol and triglycerides were measured by enzymatic assays on a Gilford Impact 400 computer directed analyzer. HDL cholesterol was measured after precipitation of VLDL and LDL with heparin-Mn2+ reagent. Leptin was measured by radioimmunoassay (RIA) using a kit from ALPCO (Windham, NH, USA). Insulin was measured using standard RIA kits from Diagnostic Products Inc.
Genotyping
Genotyping data from four tagging SNPs spanning a region of 28kb in INSIG2 including rs7566605 were already available for this study population (32). Details about the genotyping can be found in Deka et al. (32).
Of the four previously genotyped and analyzed SNPs (rs756605, rs1352083, rs2161829, rs9308762), only rs9308762, located in intron 3 of INSIG2, was associated with BMI and waist circumference in the Samoan study population (32). Therefore, we focused our main analysis of gene-dietary pattern interactions on this SNP. However, we also explored potential gene-dietary pattern interactions with rs7566605, i.e., the SNP found to be associated to obesity in GWAS of other populations, under the assumption that differences in environmental exposures may mask genetic associations. r2 and D′ for rs9308762 and rs7566605 were 0.37 and 0.95 respectively, as reported previously (32). Genotype frequencies were in Hardy-Weinberg equilibrium.
Data Analysis
There are no genetic differences between Samoans and American Samoans and genetically they can be considered a unique homogenous single population without evidence of population substructure as shown by our previous studies (32, 44, 45). However, there are substantial economic disparities between the two countries. Because these economic disparities are reflected in different stages of the nutrition transition, we decided to explore our main aims stratifying by country.
In order to maximize power, only participants with missing information in age, sex, or the FFQ, or pregnant women were deleted from the analysis. The final sample size at baseline was 427 for Samoa and 290 for American Samoa (Table 1). However, due to missing values in some of the four-year outcome variables, potential confounders, and genotype information, the sample size varied from 290 to 390 in Samoa, and from 200 to 278 in American Samoa, in the multivariable regression models. Participants with missing values in the outcome variables or in the FFQ did not differ in socio-demographic baseline variables except for education in Samoa. They reported more education (0.6 y more on average).
Table 1.
Characteristics of the study population at baseline (1990–1991) by polity and sex1
| Samoa 2 | American Samoa 3 | |||
|---|---|---|---|---|
|
| ||||
| Women (N=216) | Men (N=211) | Women (N=175) | Men (N=115) | |
| Age (y) | 38.5 (8.3) | 39.3 (8.8) | 39.3 (9.8) | 40.3 (10.3) |
| Farm work (h/w) | 4.9 (6.3) | 13.5 (11.3) | 2.5 (4.2) | 5.9 (8.7) |
| Education (y) | 10.1 (2.2) | 9.1 (2.5) | 12.6 (2.3) | 12.8 (3) |
| Waist circumference (cm) | 97.8 (12.7) | 93.0 (13.0) | 109.3 (15.9) | 106.7 (14.5) |
| BMI (Kg/m2) | 31.1 (5.1) | 29.2 (5.0) | 35.9 (7.1) | 33.9 (6.0) |
| Systolic blood pressure (mmHg) | 120.1 (13.2) | 124.6 (13.1) | 124.1 (17.2) | 125.2 (13.3) |
| Diastolic blood pressure (mmHg) | 76.1 (9.6) | 80.3 (11.2) | 80.9 (10.9) | 84.1 (10.9) |
| Fasting blood glucose (mg/dL) | 86.3 (14.6) | 87.9 (20.0) | 96.6 (17.4) | 101.7 (29.9) |
| Insulin (μU/mL) | 12.5 (10.7) | 10.7 (9.0) | 22.3 (20.2) | 23.7 (32.5) |
| Leptin (ng/mL) | 17 (9.3) | 4.3 (3.7) | 25 (11.9) | 10.3 (23.1) |
| rs9308762 genotype frequencies (%) | ||||
| CC | 16 | 16 | 20 | 16 |
| CT | 52 | 51 | 47 | 50 |
| TT | 32 | 33 | 33 | 35 |
| rs9308762 C allele frequency (%) | 42 | 41 | 44 | 41 |
| rs7566605 genotype frequencies (%) | ||||
| CC | 15 | 10 | 19 | 13 |
| CG | 41 | 47 | 44 | 43 |
| GG | 44 | 43 | 37 | 44 |
| rs7566605 C allele frequency (%) | 35 | 34 | 41 | 35 |
Values are means (SD) for continuous variables and percentages for categorical values
We used principal component analysis (PCA) to derive dietary patterns. The factors obtained were rotated by an orthogonal transformation to achieve a simpler structure that assists interpretability. We considered eigenvalues >1, the Scree test, and the general interpretability of the factors to determine the number of factors to retain (46). The standardized frequencies of intake for each food group were multiplied by the factor score coefficients and the sum of these products was the score for each derived factor. Adjusted least square means by quintiles of dietary pattern were estimated from linear regression models for each outcome variable. We used robust estimates of the variance (47) to overcome potential deviations from normality in the error distributions for linear regression models. To test for trends across quintiles of dietary patterns, the median intake of each quintile was assigned to each subject in the same quintile and treated as a continuous variable in regression analyses. These models were adjusted for age, sex, years of education, and number of hours farming at baseline. Adjustment for other potential confounders, like type of job, did not change the results. Age- and sex-adjusted least square means by genotype were estimated from linear regression models for each outcome variable and p-values from genetic additive models were reported. Analyzing the data under a co-dominant model did not change the significance of the models and conclusions. Genotypes were coded as follows: CC=0, CT=1, TT=2 for rs9308762, and GG=0, CG=1, CC=2 for rs756660. Standard regression diagnostics, such as Cook’s d, leverage, and change in beta estimates were used to evaluate potential influential points. Points that were considered influential based on conventional cutoffs for the mentioned diagnostics were removed. Effect modification by genotype was assessed using an F test for the interaction term. P-values of 0.05 were considered significant for the metabolic-diet associations and we used the false discovery rate method to adjust the p-values of the interaction analysis. All analyses were conducted using Statistical Analyses System software version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Descriptive characteristics of the population at baseline stratified by sex and country are shown in Table 1. Subjects from Samoa had lower waist circumference, BMI, systolic and diastolic blood pressure, fasting blood glucose, insulin, and leptin compared to American Samoans. They also reported higher number of hours of farm work and less years of education. There were not major differences in the frequency of the rs9308762 and rs7566605 genotypes by sex and polity.
Using principal component analysis, three factors were retained in each polity (Supplementary Table 1). Based on the loadings on neo-traditional or modern foods, factors retained in Samoa were named as “modern”, “neo-traditional 1”, and “neo-traditional 2”. Factors retained in American Samoa were named as “modern”, “transitional”, and “neo-traditional”, respectively. The three factors retained in each polity explained 32% and 29% of the variability in diet in Samoa and American Samoa respectively. The neo-traditional dietary patterns were characterized by high intake of local foods, including coconut products, taro, tropical fruits and low intake of processed foods. The modern patterns were characterized by high intake of processed foods, non-traditional foods like milk, cheese, and eggs, and low intake of local foods. The transitional pattern in American Samoa is characterized by both local and non-local foods (Supplementary Table 1).
Adherence to the modern pattern in Samoa was consistently associated with a worse metabolic profile, including higher BMI, larger waist, higher leptin, higher triglycerides, lower HDL-cholesterol, higher fasting glucose, and higher insulin (Table 2). Absolute differences between extreme quintiles of adherence to the modern pattern were 3 Kg/m2 for BMI, 6 cm for waist, 4.1 ng/mL for leptin, 43 mg/dL for triglycerides, 3 mg/dL for HDL cholesterol, 6 mg/dL for fasting glucose, and 5 mU/mL for insulin. On the other hand, adherence to the neo-traditional 2 pattern was consistently associated with a healthier metabolic profile, including lower BMI, lower waist, lower leptin, lower triglycerides, higher HDL-cholesterol, lower insulin, and lower systolic and diastolic blood pressure (Table 2). Absolute differences between extreme quintiles of adherence to the traditional 2 pattern were 2.1 Kg/m2 for BMI, 5 cm for waist, 2.8 ng/mL for leptin, 22 mg/dL for triglycerides, 4 mg/dL for HDL cholesterol, 3.9 mU/mL for insulin, 7 mmHg for systolic blood pressure, and 5 mmHg for diastolic blood pressure. The neo-traditional 1 pattern was only associated with lower HDL-cholesterol with 6 mg/dL difference between extreme quintiles (Table 2). None of the dietary patterns were associated with LDL-cholesterol.
Table 2.
Adjusted least square means for metabolic parameters in Samoa at follow-up (1995) by quintiles of dietary patterns1,2
| N | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | p for trend | |
|---|---|---|---|---|---|---|---|
| BMI (Kg/m2) | |||||||
| Modern | 372 | 28.5 (27.6, 29.5) | 30.4 (29.2, 31.6) | 29.7 (28.5, 30.9) | 31.3 (30.2, 32.5) | 31.5 (30.1, 33) | 0.001 |
| Neotraditional 1 | 372 | 28.9 (27.8, 30) | 30 (28.6, 31.4) | 30.6 (29.3, 31.8) | 31.5 (30.4, 32.7) | 30.2 (29.1, 31.3) | 0.25 |
| Neotraditional 2 | 372 | 31.2 (30, 32.3) | 30.9 (30, 31.8) | 30.5 (29.1, 31.9) | 29.8 (28.5, 31) | 29.1 (27.8, 30.3) | 0.006 |
| Waist (cm) | |||||||
| Modern | 377 | 91 (89, 94) | 96 (93, 99) | 94 (91, 97) | 99 (96, 102) | 97 (94, 100) | 0.006 |
| Neotraditional 1 | 377 | 91 (88, 94) | 96 (93, 100) | 96 (93, 99) | 99 (96, 102) | 95 (93, 98) | 0.20 |
| Neotraditional 2 | 377 | 98 (95, 101) | 97 (95, 100) | 95 (92, 99) | 94 (91, 97) | 93 (90, 96) | 0.005 |
| Leptin (ng/mL) | |||||||
| Modern | 354 | 8.4 (7.2, 9.7) | 10 (8.3, 11.8) | 10.3 (8.7, 12) | 12.9 (11, 14.8) | 12.5 (10.5, 14.5) | 0.0005 |
| Neotraditional 1 | 354 | 8.8 (7.2, 10.5) | 10.1 (8.3, 11.9) | 10.8 (9.4, 12.3) | 13.1 (11.3, 14.9) | 10.6 (8.9, 12.4) | 0.18 |
| Neotraditional 2 | 354 | 11.7 (10.1, 13.3) | 11 (9.6, 12.4) | 11.1 (9, 13.1) | 11.2 (9.4, 13) | 8.9 (7.2, 10.6) | 0.03 |
| Triglycerides (mg/dL)3 | |||||||
| Modern | 363 | 72 (63, 81) | 76 (67, 85) | 78 (67, 90) | 93 (80, 106) | 115 (96, 134) | <0.0001 |
| Neotraditional 1 | 363 | 89 (75, 104) | 74 (65, 83) | 79 (69, 89) | 99 (82, 117) | 87 (75, 100) | 0.36 |
| Neotraditional 2 | 363 | 94 (83, 105) | 98 (79, 118) | 93 (80, 105) | 74 (66, 83) | 72 (63, 81) | 0.0002 |
| HDL (mg/dL) | |||||||
| Modern | 366 | 44 (42, 47) | 44 (42, 46) | 45 (42, 47) | 42 (39, 44) | 41 (38, 44) | 0.045 |
| Neotraditional 1 | 366 | 46 (43, 49) | 43 (41, 46) | 44 (41, 46) | 43 (40, 46) | 40 (38, 42) | 0.001 |
| Neotraditional 2 | 366 | 41 (39, 43) | 43 (40, 46) | 44 (41, 46) | 44 (41, 46) | 45 (42, 47) | 0.045 |
| LDL (mg/dL) | |||||||
| Modern | 366 | 130 (125, 135) | 138 (132, 145) | 136 (130, 142) | 144 (136, 151) | 137 (128, 146) | 0.23 |
| Neotraditional 1 | 366 | 138 (131, 145) | 139 (131, 146) | 134 (127, 140) | 137 (130, 144) | 137 (130, 144) | 0.96 |
| Neotraditional 2 | 366 | 137 (131, 143) | 136 (129, 143) | 144 (136, 151) | 132 (126, 139) | 135 (129, 142) | 0.45 |
| Fasting glucose (mg/dL)3 | |||||||
| Modern | 376 | 86 (83, 90) | 85 (82, 88) | 85 (82, 88) | 89 (82, 95) | 92 (86, 98) | 0.04 |
| Neotraditional 1 | 376 | 89 (84, 93) | 86 (82, 90) | 87 (83, 91) | 86 (81, 92) | 88 (84, 92) | 0.97 |
| Neotraditional 2 | 376 | 90 (85, 95) | 84 (81, 87) | 87 (83, 91) | 86 (82, 90) | 89 (84, 94) | 0.95 |
| Insulin (mU/mL)3 | |||||||
| Modern | 373 | 8 (5.8, 10.1) | 7.9 (6.5, 9.3) | 10.2 (7.7, 12.6) | 10.6 (8.1, 13) | 13 (9.4, 16.5) | 0.007 |
| Neotraditional 1 | 373 | 9.2 (7.2, 11.3) | 8.4 (5.8, 11) | 9.4 (7, 11.8) | 12.1 (9.3, 15) | 9.5 (7.2, 11.8) | 0.53 |
| Neotraditional 2 | 373 | 10.9 (8.4, 13.4) | 12.1 (8.9, 15.2) | 10.1 (7.5, 12.8) | 9.1 (7, 11.2) | 7 (5.6, 8.4) | 0.002 |
| Systolic blood pressure (mmHg) | |||||||
| Modern | 390 | 120 (117, 124) | 123 (120, 126) | 123 (119, 126) | 124 (121, 127) | 123 (120, 126) | 0.24 |
| Neotraditional 1 | 390 | 122 (118, 125) | 123 (119, 126) | 123 (120, 126) | 125 (122, 128) | 122 (118, 125) | 0.94 |
| Neotraditional 2 | 390 | 128 (124, 131) | 125 (122, 128) | 121 (118, 123) | 119 (116, 122) | 121 (118, 124) | 0.0004 |
| Diastolic blood pressure (mmHg) | |||||||
| Modern | 390 | 77 (74, 79) | 78 (76, 80) | 78 (76, 81) | 77 (75, 79) | 79 (76, 81) | 0.45 |
| Neotraditional 1 | 390 | 77 (75, 80) | 78 (75, 80) | 77 (74, 79) | 80 (78, 82) | 77 (75, 80) | 0.8 |
| Neotraditional 2 | 390 | 80 (77, 83) | 80 (78, 82) | 77 (75, 79) | 77 (75, 80) | 75 (73, 77) | 0.006 |
Values are adjusted least square means and 95% confidence intervals in brackets from linear regression models with robust variances.
Models are adjusted for age, sex, years of education, and number of hours farming.
In American Samoa, none of the dietary patterns were significantly associated with any of the outcome variables analyzed (data not shown), except for the transitional pattern that was associated with glucose, insulin, and systolic blood pressure. Higher adherence to the transitional pattern was associated with higher glucose, higher insulin and higher systolic blood pressure (all p value <0.05).
The rs9308762 C allele was associated with higher BMI, and higher waist circumference, as reported previously, and higher leptin, and higher triglycerides, among people from Samoa. Absolute differences comparing homozygous for the variant with homozygous for the wild type were 2.9 Kg/m2 for BMI, 6 cm for waist, 4 ng/mL for leptin, and 23 mg/dL for triglycerides. Interestingly, there were no significant associations between the rs9308762 genotype and metabolic variables among American Samoans (Table 3). Genotypes at rs7566605 were not associated with metabolic traits in this population as reported previously (data not shown).
Table 3.
Adjusted least square means for metabolic parameters in Samoa and American Samoa at follow-up (1994–5) by rs9308762 genotype 1,2
| N | rs9308762 genotype | p value from additive model | |||
|---|---|---|---|---|---|
| CC | CT | TT | |||
| BMI (Kg/m2) | |||||
| Samoa | 317 | 32.3 (30.5, 34.1) | 30.1 (29.4, 30.9) | 29.4 (28.3, 30.4) | 0.009 |
| American Samoa | 261 | 35 (32.6, 37.3) | 35.8 (34.7, 36.9) | 35.8 (34.6, 36.9) | 0.61 |
| Waist (cm) | |||||
| Samoa | 321 | 100 (96, 104) | 95 (93, 97) | 94 (91, 96) | 0.02 |
| American Samoa | 271 | 111 (106, 116) | 111 (109, 114) | 111 (109, 114) | 0.92 |
| Leptin (ng/mL) | |||||
| Samoa | 301 | 13.6 (11.5, 15.7) | 10.3 (9.2, 11.4) | 9.6 (8.4, 10.9) | 0.004 |
| American Samoa | 212 | 17.7 (14.6, 20.7) | 19.4 (17.3, 21.5) | 17.7 (16.1, 19.3) | 0.75 |
| Triglycerides (mg/dL)3 | |||||
| Samoa | 315 | 100 (83, 118) | 89 (80, 98) | 77 (71, 84) | 0.007 |
| American Samoa | 232 | 138 (118, 159) | 129 (112, 147) | 125 (112, 137) | 0.27 |
| HDL (mg/dL) | |||||
| Samoa | 315 | 40 (38, 43) | 44 (42, 45) | 44 (41, 46) | 0.14 |
| American Samoa | 224 | 34 (31, 36) | 35 (34, 37) | 34 (32, 35) | 0.77 |
| LDL (mg/dL) | |||||
| Samoa | 315 | 135 (126, 144) | 137 (133, 142) | 131 (126, 137) | 0.29 |
| American Samoa | 224 | 137 (126, 148) | 135 (128, 142) | 143 (136, 149) | 0.24 |
| Fasting glucose (mg/dL)3 | |||||
| Samoa | 324 | 85 (81, 89) | 88 (85, 91) | 85 (82, 87) | 0.58 |
| American Samoa | 235 | 104 (92, 116) | 103 (96, 110) | 100 (93, 107) | 0.52 |
| Insulin (mIU/mL)3 | |||||
| Samoa | 323 | 12.1 (8.5, 15.7) | 9.3 (7.6, 11) | 9.3 (7.7, 11) | 0.22 |
| American Samoa | 234 | 20.7 (14.6, 26.8) | 19.3 (15.7, 22.9) | 17.7 (15, 20.4) | 0.33 |
| Systolic blood pressure (mmHg) | |||||
| Samoa | 338 | 124 (121, 127) | 123 (120, 125) | 122 (119, 124) | 0.28 |
| American Samoa | 278 | 131 (127, 136) | 130 (127, 133) | 131 (129, 134) | 0.87 |
| Diastolic blood pressure (mmHg) | |||||
| Samoa | 338 | 81 (78, 83) | 77 (75, 78) | 77 (75, 79) | 0.07 |
| American Samoa | 278 | 86 (83, 90) | 85 (83, 87) | 85 (83, 88) | 0.65 |
Values are adjusted least square means and 95% confidence intervals in brackets from linear regression models with robust variances. P values correspond to additive models
Models are adjusted for age and sex.
Influential points removed in models for leptin (1 in American Samoa), triglycerides (3 in Samoa), fasting glucose (1 in Samoa), and insulin (1 in Samoa and 1 in American Samoa)
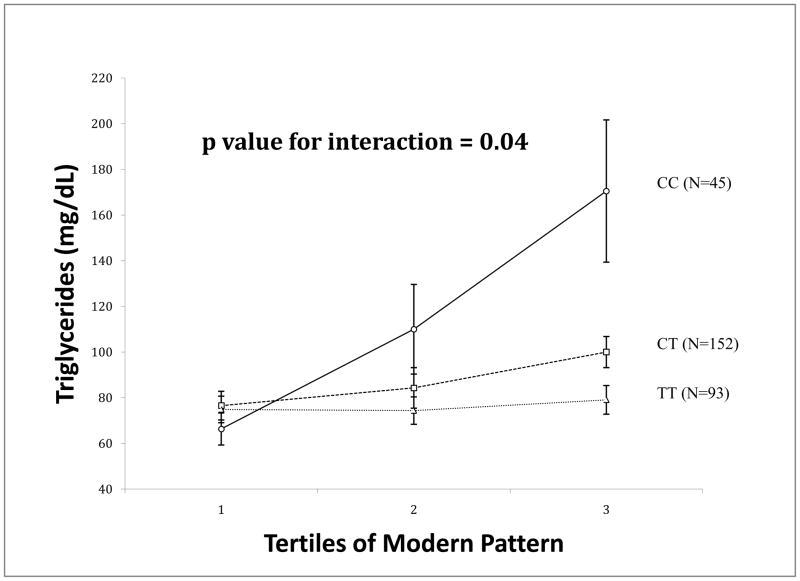
We next explored interactions between dietary patterns and the rs9308762 genotype among participants from Samoa for BMI, waist circumference, and triglycerides (i.e., for those outcomes that had significant main effects). A significant interaction was detected for triglycerides and the modern pattern (Figure 1). The association between triglycerides and the modern pattern was most pronounced among people homozygous for the C allele and almost nonexistent among those homozygous for the T allele (p value for interaction = 0.04 after adjusting for multiple comparisons).
Figure 1.
Association between triglycerides and tertiles of the modern dietary pattern by rs9308762 genotype among participants from Samoa (N=293). Triangles, squares, and circles are least square means adjusted by age, sex, and hours of farming, and bars are standard errors. CC are represented by circles, CT by squares, and TT by triangles.
Finally, we explored if similar interactions with triglycerides could be found among those from Samoa for rs7566605, i.e., the SNP found to be associated with obesity in previous GWAS, even though rs7566605 was not associated as a main effect with metabolic traits in this population. We saw a potential interaction between rs7566605 and the neo-traditional 2 pattern on triglyceride concentrations that did not reach statistical significance after adjusting for multiple comparisons (data not shown).
Discussion
Out of the three dietary patterns identified in Samoa in 1991, the modern pattern was associated with a poorer metabolic profile four-years later while the neo-traditional 2 pattern was associated with a better metabolic profile. The rs9308762 C allele was associated with higher BMI, higher waist circumference, higher leptin, and higher triglycerides, among people from Samoa. Samoans consuming the modern pattern have higher triglycerides if they are homozygous for the rs9308762 C allele. Results from American Samoans were mostly non-significant.
Our results on dietary patterns and metabolic syndrome components are consistent with our previous published work of an independent study conducted in the Samoan Islands in 2002–2003 (41). In that study we used a different method to extract dietary patterns (partial least square regression) but findings were similar. The modern dietary pattern was significantly associated with metabolic syndrome in Samoa and with increased triglyceride concentrations in both polities. In addition, a neo-traditional dietary pattern was associated with higher HDL-cholesterol in American Samoa and lower waist circumference in both polities (41). Our results are reassuring that the previously seen associations are not due to chance but to a potential real biological mechanism.
As mentioned before, INSIG2 is involved in the regulation of SREBPs that play an important role in lipid metabolism. Through SREBPs inhibition, upregulation of insig2 decreases fatty acid and triglyceride synthesis (48). Consequently, the INSIG2 polymorphism rs9308762 has been associated with adiposity measures in the Samoan population and in a population of 1,495 Hispanics (11). Although most studies have focused on the association of INSIG2 variants with adiposity measures, others have also explored associations with related metabolic traits such as plasma lipids and glucose homeostasis (11, 17, 19, 21, 26, 28–30). Results from these studies are not consistent either. For example, similar to our findings, Wang et al. found the INSIG2 variant rs7566605 to be associated with triglycerides among obese Chinese children (23). However, this association was not confirmed in other studies (26, 28, 29). Lack of replication may be due to gene-gene or gene-environment interactions. For example, the association between the INSIG1 variant rs2721 and triglycerides was strengthened by the INSIG2 rs7566605 variant in a U.S. family study of 1,560 Northern European-ancestry individuals (49). Likewise, our findings showing an interaction between dietary patterns and the INSIG2 variant rs9308762 on triglyceride concentrations serve as a potential explanation of why results may not be homogenous across populations. It is noteworthy that we found a potential interaction, although not significant after correcting for multiple testing, between the neo-traditional 2 pattern and the rs7566605 variant on triglyceride concentrations among subjects from Samoa, regardless of not finding a significant genetic main effect for rs7566605. Therefore, our results offer an alternative explanation for the lack of consistent association across populations. Interactions between the INSIG2 variant rs7566605 and other environmental factors have been reported in previous studies. In a population-based study of 6,514 Danish subjects, BMI was higher for rs7566605 C-allele carriers compared with G-allele carriers among physically inactive people (20). However, this interaction was not replicated in another study carried out in 2,003 European children (22).
Our mostly non-significant results in the American Samoa population offer some interesting insights. The lack of significant results could be attributed to lack of power, since the sample size in America Samoa was much lower. However, there were not even noticeable non-significant trends in our results. It is also possible that dietary patterns are not as clearly defined as in the Samoa study sample. On the other hand, the genetic results were also non-significant among American Samoans. Compared to most populations in the world, overweight and obesity prevalence is much higher among people from the Samoan Islands (34, 50, 51), but there are still important differences between the two polities (Table 1). The American Samoa population has even a higher prevalence of obesity than the Samoa population. Therefore, it is possible that when BMI reaches very high values at the population level, and if there are not enough thin people as a reference, it becomes hard to find associations with environmental or even genetic factors. Since American Samoans and Samoans come from the same genetic pool, our study became a natural experiment that allowed us to evaluate the impact of dietary pattern variation on genetic associations. Our results emphasize that comparison of genetic associations across populations need to take into account environmental heterogeneity. The lack of main effects in American Samoa between dietary patterns and metabolic and cardiovascular phenotypes, and between allelic variants and those phenotypes precluded our exploration of possible gene by dietary pattern interactions.
Strengths of our study include its longitudinal design, the variability of dietary exposures in the population, and the use of dietary patterns that better capture the diet as a whole. Our study has some limitations. First, we had a relatively small sample size. Low power may have been responsible of lack of significant associations among American Samoans, and reaching significance for some of the potential interactions. Second, we excluded people with missing values, which may result in bias. Third, although we used number of hours farming to adjust for physical activity, we cannot totally rule out residual confounding. However, we have found an inverse association between participation in farming and adult BMI and percent body fat in both Samoa and American Samoa in another independent study (34). Furthermore, it could be argued that rather than confounding for physical activity, our dietary patterns may also reflect lifestyle patterns that include physical activity as one more component. As expected, number of hours farming was positively correlated with the neo-traditional and transitional patterns and negatively correlated with the modern pattern in both Samoa and American Samoa. Finally, we acknowledge that our results need replication before reaching any definitive conclusion.
For some of the studied metabolic outcomes, we found important differences between quintiles of adherence to the modern and neo-traditional 2 patterns and between genotypes. For example, differences in BMI between extreme quintiles of the modern and neotraditional 2 pattern translate into an increase in 9% and a decrease in 12% of the obesity prevalence, respectively. Similarly, differences in triglycerides between extreme quintiles of the modern and neotraditional 2 pattern translate into an increase in 17% and a decrease in 8% of the prevalence of high triglycerides, respectively. These differences in metabolic outcomes clearly have important public health implications and highlights that departure from traditional lifestyles is affecting the cardiometabolic risk of Samoans. Also, based on our interaction analysis, genetic background can increase or attenuate the effect of environmental factors. Although our interaction results need to be replicated in other populations and we are still far from performing genetic testing at the population level, it will be important to keep this heterogeneity in mind when considering general public health interventions.
In conclusion, we found that the modern dietary pattern in Samoa was associated with a poorer metabolic profile compared to the neo-traditional pattern. Homozygotes for the rs9308762 C allele were more susceptible to the hypertriglyceridemic effect of the modern pattern. We did not see any significant association between dietary patterns and SNPs with metabolic factors among American Samoans. Our findings present an interesting example of the complex interplay between genes and the environment on the risk of metabolic traits and suggest that results from GWAS may be attenuated or masked if gene-environment interactions are not taken into account.
Supplementary Material
Acknowledgments
This study was supported by NIH grants AG09375, HL52611, DK55406, and DK59642 and from the Dean’s fund of the University of Cincinnati College of Medicine
Footnotes
Conflict of interest: None
References
- 1.Yabe D, Brown MS, Goldstein JL. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc Natl Acad Sci U S A. 2002;99(20):12753–8. doi: 10.1073/pnas.162488899. Epub 2002/09/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–31. doi: 10.1172/JCI15593. Epub 2002/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghow R, Yellaturu C, Deng X, Park EA, Elam MB. SREBPs: the crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol Metab. 2008;19(2):65–73. doi: 10.1016/j.tem.2007.10.009. Epub 2008/02/23. [DOI] [PubMed] [Google Scholar]
- 4.Herbert A, Gerry NP, McQueen MB, Heid IM, Pfeufer A, Illig T, et al. A common genetic variant is associated with adult and childhood obesity. Science. 2006;312(5771):279–83. doi: 10.1126/science.1124779. Epub 2006/04/15. [DOI] [PubMed] [Google Scholar]
- 5.Lyon HN, Emilsson V, Hinney A, Heid IM, Lasky-Su J, Zhu X, et al. The association of a SNP upstream of INSIG2 with body mass index is reproduced in several but not all cohorts. PLoS Genet. 2007;3(4):e61. doi: 10.1371/journal.pgen.0030061. Epub 2007/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Wu Y, Li H, Yu Z, Li X, Liu Y, et al. Potential association of INSIG2 rs7566605 polymorphism with body weight in a Chinese subpopulation. Eur J Hum Genet. 2008;16(6):759–61. doi: 10.1038/ejhg.2008.8. Epub 2008/02/14. [DOI] [PubMed] [Google Scholar]
- 7.Liu YJ, Liu XG, Wang L, Dina C, Yan H, Liu JF, et al. Genome-wide association scans identified CTNNBL1 as a novel gene for obesity. Hum Mol Genet. 2008;17(12):1803–13. doi: 10.1093/hmg/ddn072. Epub 2008/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Lin R, Wang F, Lu M, Lin RY, Wang SZ, et al. A common polymorphism is associated with body mass index in Uyghur population. Diabetes Res Clin Pract. 2008;81(2):e11–3. doi: 10.1016/j.diabres.2008.03.022. Epub 2008/06/03. [DOI] [PubMed] [Google Scholar]
- 9.Chu X, Erdman R, Susek M, Gerst H, Derr K, Al-Agha M, et al. Association of morbid obesity with FTO and INSIG2 allelic variants. Arch Surg. 2008;143(3):235–40. doi: 10.1001/archsurg.2007.77. discussion 41. Epub 2008/03/19. [DOI] [PubMed] [Google Scholar]
- 10.Hotta K, Nakamura M, Nakata Y, Matsuo T, Kamohara S, Kotani K, et al. INSIG2 gene rs7566605 polymorphism is associated with severe obesity in Japanese. J Hum Genet. 2008;53(9):857–62. doi: 10.1007/s10038-008-0317-8. Epub 2008/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbert ME, Langefeld CD, Ziegler JT, Haffner SM, Norris JM, Bowden DW. INSIG2 SNPs associated with obesity and glucose homeostasis traits in Hispanics: the IRAS Family Study. Obesity (Silver Spring) 2009;17(8):1554–62. doi: 10.1038/oby.2009.94. Epub 2009/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall DH, Rahman T, Avery PJ, Keavney B. INSIG-2 promoter polymorphism and obesity related phenotypes: association study in 1428 members of 248 families. BMC Med Genet. 2006;7:83. doi: 10.1186/1471-2350-7-83. Epub 2006/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AJ, Cooper JA, Li LK, Humphries SE. INSIG2 gene polymorphism is not associated with obesity in Caucasian, Afro-Caribbean and Indian subjects. Int J Obes (Lond) 2007;31(11):1753–5. doi: 10.1038/sj.ijo.0803645. Epub 2007/05/02. [DOI] [PubMed] [Google Scholar]
- 14.Kumar J, Sunkishala RR, Karthikeyan G, Sengupta S. The common genetic variant upstream of INSIG2 gene is not associated with obesity in Indian population. Clin Genet. 2007;71(5):415–8. doi: 10.1111/j.1399-0004.2007.00795.x. Epub 2007/05/11. [DOI] [PubMed] [Google Scholar]
- 15.Kuzuya M, Ando F, Iguchi A, Shimokata H. No association between rs7566605 variant and being overweight in Japanese. Obesity (Silver Spring) 2007;15(11):2531–4. doi: 10.1038/oby.2007.301. Epub 2007/12/12. [DOI] [PubMed] [Google Scholar]
- 16.Tabara Y, Kawamoto R, Osawa H, Nakura J, Makino H, Miki T, et al. No association between INSIG2 Gene rs7566605 polymorphism and being overweight in Japanese population. Obesity (Silver Spring) 2008;16(1):211–5. doi: 10.1038/oby.2007.25. Epub 2008/01/29. [DOI] [PubMed] [Google Scholar]
- 17.Boes E, Kollerits B, Heid IM, Hunt SC, Pichler M, Paulweber B, et al. INSIG2 polymorphism is neither associated with BMI nor with phenotypes of lipoprotein metabolism. Obesity (Silver Spring) 2008;16(4):827–33. doi: 10.1038/oby.2007.132. Epub 2008/02/02. [DOI] [PubMed] [Google Scholar]
- 18.Marvelle AF, Lange LA, Qin L, Adair LS, Mohlke KL. Association of FTO with obesity-related traits in the Cebu Longitudinal Health and Nutrition Survey (CLHNS) Cohort. Diabetes. 2008;57(7):1987–91. doi: 10.2337/db07-1700. Epub 2008/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oki K, Yamane K, Kamei N, Asao T, Awaya T, Kohno N. The single nucleotide polymorphism upstream of insulin-induced gene 2 (INSIG2) is associated with the prevalence of hypercholesterolaemia, but not with obesity, in Japanese American women. Br J Nutr. 2009;101(3):322–7. doi: 10.1017/S0007114508006557. Epub 2008/06/24. [DOI] [PubMed] [Google Scholar]
- 20.Andreasen CH, Mogensen MS, Borch-Johnsen K, Sandbaek A, Lauritzen T, Sorensen TI, et al. Non-replication of genome-wide based associations between common variants in INSIG2 and PFKP and obesity in studies of 18,014 Danes. PLoS One. 2008;3(8):e2872. doi: 10.1371/journal.pone.0002872. Epub 2008/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiedmann S, Neureuther K, Stark K, Reinhard W, Kallmunzer B, Baessler A, et al. Lack of association between a common polymorphism near the INSIG2 gene and BMI, myocardial infarction, and cardiovascular risk factors. Obesity (Silver Spring) 2009;17(7):1390–5. doi: 10.1038/oby.2008.669. Epub 2009/02/07. [DOI] [PubMed] [Google Scholar]
- 22.Vimaleswaran KS, Franks PW, Brage S, Sardinha LB, Andersen LB, Wareham NJ, et al. Absence of association between the INSIG2 gene polymorphism (rs7566605) and obesity in the European Youth Heart Study (EYHS) Obesity (Silver Spring) 2009;17(7):1453–7. doi: 10.1038/oby.2008.650. Epub 2009/02/07. [DOI] [PubMed] [Google Scholar]
- 23.Wang HJ, Zhang H, Zhang SW, Pan YP, Ma J. Association of the common genetic variant upstream of INSIG2 gene with obesity related phenotypes in Chinese children and adolescents. Biomed Environ Sci. 2008;21(6):528–36. doi: 10.1016/S0895-3988(09)60013-1. Epub 2009/03/07. [DOI] [PubMed] [Google Scholar]
- 24.Peeters A, Beckers S, Verrijken A, Mertens I, Van Gaal L, Van Hul W. Possible role for ENPP1 polymorphism in obesity but not for INSIG2 and PLIN variants. Endocrine. 2009;36(1):103–9. doi: 10.1007/s12020-009-9194-y. Epub 2009/04/29. [DOI] [PubMed] [Google Scholar]
- 25.Bressler J, Fornage M, Hanis CL, Kao WH, Lewis CE, McPherson R, et al. The INSIG2 rs7566605 genetic variant does not play a major role in obesity in a sample of 24,722 individuals from four cohorts. BMC Med Genet. 2009;10:56. doi: 10.1186/1471-2350-10-56. Epub 2009/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha S, Koo I, Choi SM, Park BL, Kim KS, Kim JR, et al. Association analyses of the INSIG2 polymorphism in the obesity and cholesterol levels of Korean populations. BMC Med Genet. 2009;10:96. doi: 10.1186/1471-2350-10-96. Epub 2009/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heid IM, Huth C, Loos RJ, Kronenberg F, Adamkova V, Anand SS, et al. Meta-analysis of the INSIG2 association with obesity including 74,345 individuals: does heterogeneity of estimates relate to study design? PLoS Genet. 2009;5(10):e1000694. doi: 10.1371/journal.pgen.1000694. Epub 2009/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fornage M, Papanicolaou G, Lewis CE, Boerwinkle E, Siscovick DS. Common INSIG2 polymorphisms are associated with age-related changes in body size and high-density lipoprotein cholesterol from young adulthood to middle age. Metabolism. 2010;59(8):1084–91. doi: 10.1016/j.metabol.2009.11.005. Epub 2010/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubacek JA, Kuthanova L, Bohuslavova R, Adamkova V, Lanska V, Meitinger T, et al. INSIG2 promoter variant, obesity markers and lipid parameters - No association in a large Slavonic Caucasian population sample. Folia Biol (Praha) 2010;56(3):131–4. Epub 2010/07/27. [PubMed] [Google Scholar]
- 30.Do R, Bailey SD, Pare G, Montpetit A, Desbiens K, Hudson TJ, et al. Fine mapping of the insulin-induced gene 2 identifies a variant associated with LDL cholesterol and total apolipoprotein B levels. Circ Cardiovasc Genet. 2010;3(5):454–61. doi: 10.1161/CIRCGENETICS.109.917039. Epub 2010/09/23. [DOI] [PubMed] [Google Scholar]
- 31.Angeli CB, Kimura L, Auricchio MT, Vicente JP, Mattevi VS, Zembrzuski VM, et al. Multilocus Analyses of Seven Candidate Genes Suggest Interacting Pathways for Obesity-Related Traits in Brazilian Populations. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2010.325. Epub 2011/01/15. [DOI] [PubMed] [Google Scholar]
- 32.Deka R, Xu L, Pal P, Toelupe PT, Laumoli TS, Xi H, et al. A tagging SNP in INSIG2 is associated with obesity-related phenotypes among Samoans. BMC Med Genet. 2009;10:143. doi: 10.1186/1471-2350-10-143. Epub 2009/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreasen CH, Andersen G. Gene-environment interactions and obesity--further aspects of genomewide association studies. Nutrition. 2009;25(10):998–1003. doi: 10.1016/j.nut.2009.06.001. Epub 2009/07/15. [DOI] [PubMed] [Google Scholar]
- 34.Keighley ED, McGarvey ST, Turituri P, Viali S. Farming and adiposity in Samoan adults. Am J Hum Biol. 2006;18(1):112–22. doi: 10.1002/ajhb.20469. Epub 2005/12/27. [DOI] [PubMed] [Google Scholar]
- 35.McGarvey ST. Cardiovascular disease (CVD) risk factors in Samoa and American Samoa, 1990–95. Pac Health Dialog. 2001;8(1):157–62. Epub 2002/05/23. [PubMed] [Google Scholar]
- 36.Galanis DJ, Sobal J, McGarvey ST, Pelletier DL, Bausserman L. Ten-year changes in the obesity, abdominal adiposity, and serum lipoprotein cholesterol measures of Western Samoan men. J Clin Epidemiol. 1995;48(12):1485–93. doi: 10.1016/0895-4356(95)00060-7. Epub 1995/12/01. [DOI] [PubMed] [Google Scholar]
- 37.Galanis DJ, McGarvey ST, Sobal J, Bausserman L, Levinson PD. Relations of body fat and fat distribution to the serum lipid, apolipoprotein and insulin concentrations of Samoan men and women. Int J Obes Relat Metab Disord. 1995;19(10):731–8. Epub 1995/10/01. [PubMed] [Google Scholar]
- 38.Tsai HJ, Sun G, Weeks DE, Kaushal R, Wolujewicz M, McGarvey ST, et al. Type 2 diabetes and three calpain-10 gene polymorphisms in Samoans: no evidence of association. Am J Hum Genet. 2001;69(6):1236–44. doi: 10.1086/324646. Epub 2001/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keighley ED, Mc Garvey ST, Quested C, McCuddin C, Viali S, Maga U. Nutrition and Health in modernizing Samoans: temporal trends and adaptive perspectives. In: Ohtsuka R, Ulijaszek SJ, editors. Health change in the Asia-Pacific: biocultural and epidemiological approaches. Cambridge (UK): Cambridge University Press; 2007. pp. 147–91. [Google Scholar]
- 40.Seiden A, Hawley NL, Schulz D, Raifman S, McGarvey ST. Long-term trends in food availability, food prices, and obesity in samoa. Am J Hum Biol. 2012 doi: 10.1002/ajhb.22237. Epub 2012/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiBello JR, McGarvey ST, Kraft P, Goldberg R, Campos H, Quested C, et al. Dietary patterns are associated with metabolic syndrome in adult Samoans. J Nutr. 2009;139(10):1933–43. doi: 10.3945/jn.109.107888. Epub 2009/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galanis DJ, McGarvey ST, Quested C, Sio B, Afele-Fa’amuli SA. Dietary intake of modernizing Samoans: implications for risk of cardiovascular disease. J Am Diet Assoc. 1999;99(2):184–90. doi: 10.1016/s0002-8223(99)00044-9. Epub 1999/02/11. [DOI] [PubMed] [Google Scholar]
- 43.McGarvey ST, Levinson PD, Bausserman L, Galanis DJ, Hornick C. Population change in adult obesity and blood lipid in American Samoa from 1976–78 to 1990. Am J Hum Biol. 1993;5:17–30. doi: 10.1002/ajhb.1310050106. [DOI] [PubMed] [Google Scholar]
- 44.Deka R, Mc Garvey ST, Ferrell RE, Kamboh MI, Yu LM, Aston CE, et al. Genetic characterization of American and Western Samoans. Hum Biol. 1994;66(5):805–22. Epub 1994/10/01. [PubMed] [Google Scholar]
- 45.Tsai HJ, Sun G, Smelser D, Viali S, Tufa J, Jin L, et al. Distribution of genome-wide linkage disequilibrium based on microsatellite loci in the Samoan population. Hum Genomics. 2004;1(5):327–34. doi: 10.1186/1479-7364-1-5-327. Epub 2004/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–9. doi: 10.1093/ajcn/69.2.243. Epub 1999/02/16. [DOI] [PubMed] [Google Scholar]
- 47.White HA. Heteroscedasticity-consistent covariance matrix estimator and a direct test for heterscedasticity. Econometrica. 1980;48:817–38. [Google Scholar]
- 48.Takaishi K, Duplomb L, Wang MY, Li J, Unger RH. Hepatic insig-1 or -2 overexpression reduces lipogenesis in obese Zucker diabetic fatty rats and in fasted/refed normal rats. Proc Natl Acad Sci U S A. 2004;101(18):7106–11. doi: 10.1073/pnas.0401715101. Epub 2004/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith EM, Zhang Y, Baye TM, Gawrieh S, Cole R, Blangero J, et al. INSIG1 influences obesity-related hypertriglyceridemia in humans. J Lipid Res. 2010;51(4):701–8. doi: 10.1194/jlr.M001404. Epub 2009/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aberg K, Dai F, Sun G, Keighley ED, Indugula SR, Roberts ST, et al. Susceptibility loci for adiposity phenotypes on 8p, 9p, and 16q in American Samoa and Samoa. Obesity (Silver Spring) 2009;17(3):518–24. doi: 10.1038/oby.2008.558. Epub 2009/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGarvey ST. Obesity in Samoans and a perspective on its etiology in Polynesians. Am J Clin Nutr. 1991;53(6 Suppl):1586S–94S. doi: 10.1093/ajcn/53.6.1586S. Epub 1991/06/01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.