Abstract
Fc Receptor-Like proteins are a family of cellular receptors homologous to FcγRI, and predominantly expressed by B cells. They function to co-stimulate or inhibit B cell receptor signaling through consensus ITAMs and ITIMs, however the extracellular ligands of these receptors remain unknown or controversial. In this study, we tested the ability of human FcRL proteins to bind immunoglobulins and found FcRL4 and FcRL5 to be bona fide Fc receptors. In cellular binding assays, FcRL4 bound efficiently to IgA and FcRL5 binds all IgG isotypes with varied efficiency. In addition, we generated monoclonal antibodies capable of specifically blocking these interactions. Given their expression on activated B cells and potential for inhibitory signaling, FcRL4 and FcRL5 are likely to be important for immune complex-dependent human B cell regulation, and represent novel therapeutic targets for receptor blockade therapies.
Introduction
Receptors for immunoglobulin (Ig) are essential mediators of immune responses. They bridge innate and adaptive immune responses by mediating phagocytosis of opsonized pathogens, antibody-dependent cell-mediated cytotoxicity (ADCC), trans-epithelial Ig transport, and immune complex (IC) retention by follicular dendritic cells, as well as enhancing antigen presentation and regulating leukocyte activation (1, 2).
Human Fc Receptor-Like (FcRL) proteins are a family of receptors homologous to FcγRI in one or more of their Ig superfamily (IgSF) domains. Most are predominantly expressed by B cells (3-13), while FcRL3 can be additionally found on NK cells (14) and Tregs (15), and FcRL6 is found mainly on NK cells and effector cytotoxic T cells (7, 8). Subset analyses have refined our knowledge of the expression of each of the receptors. For example, FcRL5 appears to be broadly expressed by B cell subsets (3-5, 14), whereas FcRL4 expression is limited to a unique subset of tissue memory B cells, concentrated in the sub-epithelial and marginal zones of mucosal lymphoid tissues (16-18).
FcRL1-6 contain consensus immunoreceptor tyrosine-based activation motifs (ITAMs) and/or immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic tails. In addition to the six cell-surface FcRL proteins are two intracellular receptors, FcRLA and FcRLB. These receptors lack transmembrane domains, but contain a flexible C-terminal proline-rich stalk followed by a coiled-coiled region (9-13, 19). Among the cell surface FcRLs, only FcRL1 has been shown to exhibit costimulatory activity with the B cell receptor (20). Of the others, FcRL2-5 have all been shown to recruit SHP-1 and inhibit BCR signaling (16, 21-23), while functional outcome of tyrosine phosphorylation in FcRL6 remains elusive (7, 8).
Beyond their potential signaling capabilities, the biological roles of these receptors have remained unknown, largely due to the absence of knowledge about their extracellular ligands. Recently, FcRL6 has been reported to bind HLA-DR (24), and we and others have shown that intracellular FcRLA associates with multiple Ig isotypes within the lumen of the endoplasmic reticulum (25, 26). Given the homology of FcRL3-5 with FcγRI and the association between FcRLA and Igs, we decided to examine whether any of the FcRL proteins could bind Ig. In fact, we found that FcRL4 is an IgA receptor and FcRL5 is an IgG receptor. The ability of these receptors to bind Ig, along with their demonstrated ability to regulate B cell antigen receptor signaling, suggests a role for FcRL4 and FcRL5 in the regulation of B cell responses by antibodies.
Materials and Methods
Immunoglobulin Binding Assays
In order to test for Ig binding by FcRL family members, cDNA encoding mouse FcRH3/FcRL5, human CD200R, FcRL1, FcRL3, FcRL4, FcRL5 (Open Biosystems), and FcRL6(7) were ligated into pFLAG-CMV-3 (Sigma, St. Louis, MO). cDNA encoding human CD32 was ligated into pEF6 (Invitrogen). Proteins were expressed in 293 cells by transient transfection using Lipofectamine 2000. Transiently transfected 293 cells were used for antibody binding assays 36-42 hours after transfection. Purified human Igs were obtained from Sigma (St. Louis, MO) in the following formats: IgG and IgM from serum; IgA from colostrum; IgG1, IgG2, IgG3, and IgG4 (all kappa light chain) from myeloma plasma. Purified human IgA from serum was from Bethyl Laboratories (Montgomery, TX). For the heat aggregation assay, Igs were aggregated by heating to 60°C for 30 minutes. Igs were then diluted to 100μg/ml in PBS/1% BSA. 293 cells were incubated for 30 minutes on ice with the Igs and washed four times, followed by incubation with biotin-conjugated goat F(ab’)2 anti-human kappa light chain antibody (for IgG1, IgG2, IgG3 and IgG4), or a combination of biotinylated goat F(ab’)2 anti-human kappa and goat F(ab’)2 anti-human lambda antibody (for total IgG and IgA) (Southern Biotech) for 20 minutes on ice. Cells were washed three times and incubated with a combination of APC-conjugated streptavidin (Invitrogen) and FITC-conjugated anti-Flag antibody (M2; Sigma) for 20 minutes on ice. To detect CD32-expressing cells, a PE-conjugated anti-human CD32 antibody (Beckman-Coulter) was added to CD32-transfected samples. Cells were washed twice and analyzed by flow cytometry on a FACSCalibur (BD Biosciences) for antibody binding. Dead cells were excluded by propidium iodide staining.
As an alternative to heat aggregation, IgG1-λ was pre-incubated with equimolar quantities of biotinylated F(ab’)2 fragments specific for human λ light chains in order to simulate immune complexes (ICs). The binding assay was performed as described. Secondary detection of bound Ig was achieved using streptavidin-PE.
Generation of monoclonal antibodies
Mouse P815 cells stably expressing surface FcRL4 or FcRL5 were used for immunization. Balb/c mice were immunized three times at two week intervals with paraformaldehyde-fixed FcRL4 or FcRL5 stable transfectants, first in CFA, then twice in IFA + CpG 1826. Three days prior to fusion with SP2/0 cells, the final immunization was performed by IP injection of irradiated cells in PBS. Hybridoma clones were screened by FACS for the ability to stain FcRL4 or FcRL5 expressing P815 cells, but not the P815 parent cell line. Blocking antibodies for FcRL4 were identified by FACS using a 1:1 mixture of FcRL4-expressing and non-expressing P815 cells pre-incubated with anti-FcRL4 antibody. CFSE-labelled colostral IgA was heat aggregated and incubated with cells as described above. Blocking antibodies for FcRL5 were identified in the primary screen for FcRL5-specific antibodies. A 1:1 mixture of FcRL5 expressing and non-expressing P815 cells were first incubated with supernatants from hybridoma clones. Goat-F(ab’)2 anti-mouse IgG-PE and anti-mouse IgM-FITC were used for secondary detection of FcRL5-specific Mabs. Blocking antibodies were simultaneously identified among FcRL5-specific clones by the ability of FcRL5-specific Mabs to block the non-specific association of Goat anti-rabbit IgG-Alexafluor647 with FcRL5 expressing P815 cells. Animal procedures have been reviewed and approved by the Washington University Animal Care and Use Committee.
For epitope mapping of anti-FcRL5 antibodies, FcRL5 cDNA encoding IgSF domains 1-3, 4-6, or 7-9 were amplified by PCR and ligated into the expression vector pDisplay (Clontech). 293 cells transiently transfected with these constructs using Lipofectamine 2000 (Invitrogen) were assayed by flow cytometry for anti-FcRL5 antibody staining. Selected antibodies were additionally tested for cross-reactivity against other FcRL proteins. Anti-FcRL5 (509F6) and Anti-FcRL4 (413D12) are not cross-reactive against FcRL4 and FcRL5 respectively.
FACS staining of tonsillar B cells with anti-FcRL4 and anti-FCRL5
Tonsils were obtained from pediatric patients undergoing elective tonsillectomy (Children’s Hospital, Washington University School of Medicine, St. Louis, MO). Approval was obtained from the Washington University School of Medicine institutional review board. Lymphocyte suspensions were prepared from tonsils by dissociation of the tissue, followed by Ficoll density gradient centrifugation. Tonsil lymphocytes were stained with APC-conjugated anti-CD19 and FITC-conjugated anti-CD27 (mouse IgG1, BD Biosciences) in the presence of FcR blocking reagent (Miltenyi Biotec), and either with isotype-matched control antibodies or with anti-FCRL4 (413D12 – IgG2b) and anti-FcRL5 (509F6 – IgG2a). Secondary staining was performed with FITC- or PE-conjugated antibodies to mouse IgG2a or IgG2b (Southern Biotech).
Results
FcRL4 and FcRL5 are immunoglobulin receptors
Fc receptor-like proteins are diverse in their expression patterns and functional roles among human B cell subsets. Despite the growing body of literature surrounding the expression patterns of these receptors and their associations with disease, little is known about the extracellular interactions on the cell surface. Since some of the FcRL family members are highly homologous to FcγRI, and anecdotal reports in the literature suggest Ig binding potential (4, 14), the ability of FcRL family members to bind Igs was assayed. In a preliminary test, FcRL5 was able to bind heat-aggregated IgG1. Weak binding of IgG1 to FcRL3 and FcRL4 was also observed, however these interactions were not consistently reproducible. No detectable binding to IgG1 was observed by control transfected or untransfected cells, or by cells transfected with FcRL1 or FcRL6 (not shown). In order to analyze binding specificity of FcRL proteins, 293 cells transiently expressing FcRL proteins or control CD200R or CD32 were incubated with heat aggregated IgG, IgA, IgE, or IgM. No binding to IgE or IgM was detected for any of the FcRL family members (not shown), whereas IgG bound FcRL5 and CD32, and human serum IgA was found to bind FcRL4 (Figure 1).
Figure 1. FcRL4 and FcRL5 bind human immunoglobulins.
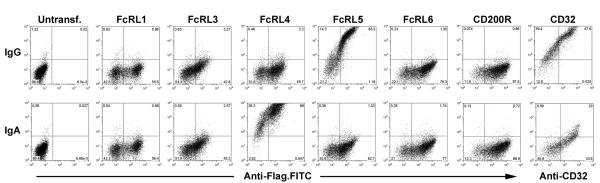
HEK293T cells were transiently transfected with human cDNA for FcRL1, 3, 4, 5, 6 and CD200R expressed in pFLAG-CMV-3, or CD32 in pEF6. Binding of heat-aggregated human serum IgG and IgA (Y-axis) to cells expressing human FcRL4 and FcRL5 was easily observable by FACS. Cell surface expression of each protein was confirmed using FITC-conjugated anti-FLAG M2 monoclonal antibody (X-axis), or by staining with anti-CD32 antibody. Binding to CD200R and CD32 are included as negative and positive controls respectively. No binding of human IgM to any of the FcRL proteins was observed (not shown).
Since CD32 is known to have differential affinity for the individual IgG subtypes (27), we examined the ability of FcRL5 to bind human IgG isotypes. Human IgG1-κ, IgG2-κ, IgG3-κ, and IgG4-κ were heat aggregated and incubated with 293 cells transiently transfected with FcRL4, FcRL5, CD32b, or CD200R. While no association was consistently found between FcRL4 and IgG1 or IgG2, binding of IgG3 and IgG4 by FcRL4 was weak but detectable. FcRL5 was able to bind all IgG isotypes, showing consistently stronger binding to IgG1 and IgG2 over IgG3, while associating only weakly with IgG4. The binding of CD32 in this assay to IgGs showed the qualitative preferences of IgG3>IgG1>IgG4>IgG2 (Figure 2A). This pattern precisely matches previously identified binding specificities for CD32b (27), providing validation for the observed binding specificity of FcRL4 and FcRL5.
Figure 2. Specificity of FcRL4 and FcRL5 for immunoglobulins.
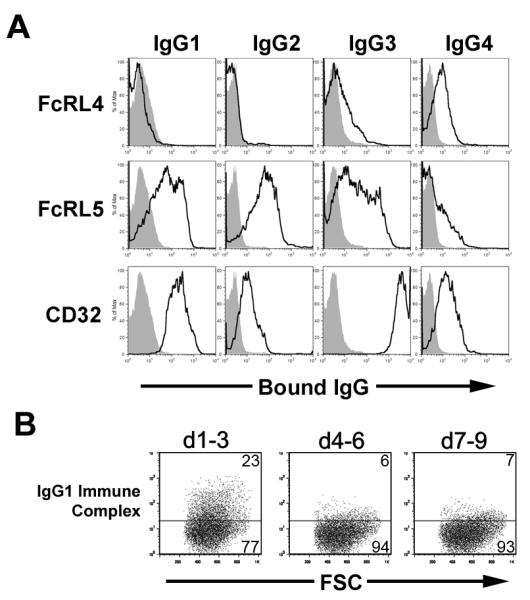
A) Human IgG isotypes were assayed by FACS for binding to FcRL4, FcRL5, and CD32. Dark lines indicate aggregated IgG bound to the indicated receptor-transfected 293 cells. Gray shading indicates binding of Igs to negative control CD200R-transfected cells. Histograms show populations gated on receptor-expressing cells by simultaneous staining with anti-Flag (FcRL4, FcRL5 and CD200R) or anti-CD32 antibodies B) Portions of the ectodomain of FcRL5 comprising Ig domains 1-3, 4-6, or 7-9 were expressed on the surface of 293 cells. Binding of IgG1 mock ICs to FcRL5 d1-3 was measured by FACS.
The N-terminal domains of FcRL5 are necessary and sufficient for IC binding
FcRL5 contains nine IgSF domains, with the N-terminal three domains sharing homology with those of FcγRI. We predicted that these three domains would be sufficient for Ig binding. In order to test this hypothesis, cDNA encoding d1-3, d4-6, or d7-9 of FcRL5 were expressed transiently in 293 cells using the vector pDisplay, and analyzed for the ability to bind Ig. While surface-expressed forms of FcRL5 D4-6 or D7-9 were unable to bind IgG1, cells expressing FcRL5 D1-3 readily bound IgG1 mock ICs (Figure 2B). These data indicate that the first three domains of FcRL5 are necessary and sufficient for Ig binding.
Development of blocking antibodies for FcRL4 and FcRL5
In order to further study the expression and function of FcRL4 and FcRL5, a panel of monoclonal antibodies specific for these receptors was generated. Hybridoma clones were screened for reactivity to either FcRL4 or FcRL5 transfected P815 cells, as well as the ability to block the binding of antibody to these receptors. Since FcRL5 contains nine Ig domains, anti-FcRL5 Mabs were further characterized for epitope location. Using 293 cells transiently transfected with FcRL5 d1-3, d4-6, or d7-9, we determined that Mab 509F6 and Mab 501H9 (both IgG2a) bind FcRL5 d1-3 and d4-6 respectively (Figure 3A). By pre-incubating these antibodies with FcRL5-transfected 293 cells, we determined that Mab 509F6 can specifically block the association of FcRL5 with mock ICs, whereas 501H9 is a non-blocking antibody. (Figure 3B) Similarly, anti-FcRL4 Mab 413D12 is able to block the binding of FcRL4-transfected P815 cells with heat aggregated IgA, while isotype-matched anti-FcRL4 Mab 418C8 (IgG2b) has no blocking potential. (Figure 3C) These data confirm the importance of the FcR-homologous domains of FcRL5 in antibody binding, and further support a role for FcRL4 and FcRL5 as Ig receptors.
Figure 3. Specific blockade of Ig binding to FcRL4 and FcRL5 by monoclonal antibodies.
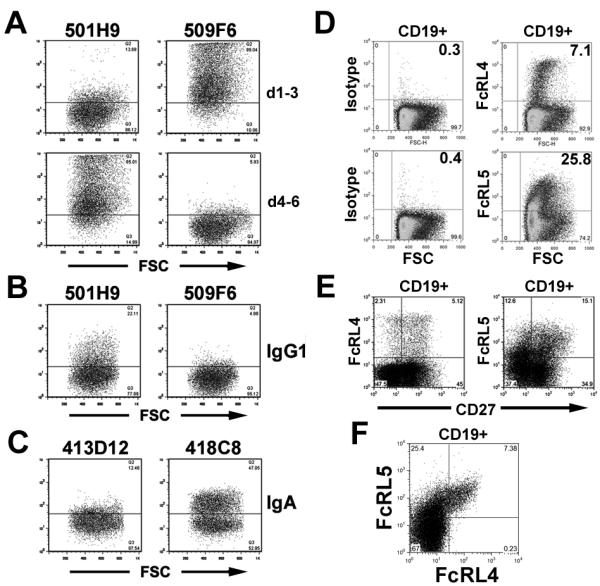
A) FACS staining of FcRL5 d1-3 or d4-6 with Mab 501H9 and 509F6. B) Pre-incubation of antibody 509F6 with FcRL5-expressing 293 cells prevents binding of FcRL5-transfected 293 cells to mock ICs. C) Blockade of IgA binding to FcRL4 by anti-FcRL4 clone 413D12 (IgG2b) but not isotype matched anti-FcRL4 clone 418C8. D) Staining of tonsillar B cells with anti-FcRL4 (413D12) and anti-FcRL5 (509F6) monoclonal antibodies or isotype controls. (Plots show cells following gating on live CD19+ cells.) E) Anti-FcRL4 and anti-FcRL5 identify mixed populations of CD27+ and CD27− tonsillar B cells. F) All FcRL4+ tonsillar B cells co-express FcRL5. E,F) Plots are representative of results obtained with tonsil samples from five different donors.
FcRL4 expression has been described as limited to a memory B cell population concentrated within mucosal sites, and with variable expression of the memory B cell marker CD27 (4, 14, 17, 18). Staining human tonsil sections and cell suspensions with anti-FcRL4 (413D12) was largely consistent with the published expression patterns. FcRL4+ cells were found to be abundant in the tonsillar sub-mucosa and marginal zones (Supplementary Figure 1). In addition, the proportion of FcRL4+ cells which were also CD27+ showed substantial variation among individuals, ranging from 50-70% in 5 tonsillar preparations, while FcRL5 expression was found among CD27−, CD27+, and CD27high cells (Figure 3E and data not shown). Interestingly, all FcRL4+ cells also expressed FcRL5 at a high level (Figure 3F). The combined expression of these two inhibitory Fc receptors may help explain the reported resistance of FcRL4+ memory cells to stimulation through the BCR (18).
Discussion
Human FcRL proteins are an important family of immunomodulatory receptors. Earlier studies have indicated an inhibitory function for both FcRL4 and FcRL5 through the recruitment of SHP-1 (16, 21), and our own unpublished observations have confirmed these findings. Anecdotal reports have previously suggested that FcRL4 and FcRL5 might be Fc receptors (4, 14). However, these observations were not initially reproducible by all groups studying these receptors (28), and so there has been no consensus ligand for these receptors. Our data clearly show that FcRL4 can bind IgA and FcRL5 can bind IgG.
Non-specific binding of soluble monomeric mouse and goat IgG by FcRL5-transfected P815 cells was observed while screening anti-FcRL5 hybridoma clones, leading initially to the conclusion that FcRL5 might be a high affinity receptor. In contrast, binding of soluble monomeric IgG to FcRL4 or FcRL5-transfected 293 cells was not readily observable in FACS-based assays, indicating that these receptors are likely to be low-medium affinity Fc receptors. Since P815 cells express classical IgG Fc receptors, these observations may instead indicate that FcRL5 and classical Fc receptors can bind IgG cooperatively on the cell surface. Structural studies of the interactions between antibodies and the classical FcγRs have shown a small conformational change in the Ig Fc region upon receptor binding, ensuring a 1:1 IgG:FcR stoichiometry (29). Though the FcRL proteins are homologous to FcγRI in their three N-terminal IGSF domains, they lack sequence homology with classical receptors at the putative Ig-binding interface (28), leading to the assumption that FcRL proteins would not bind antibodies. A model that includes cooperative IgG binding on B cells by FcγRIIb and FcRL5 explains observed binding phenomena and is consistent with the presence of structural similarity between the two receptors, but differences in the specific contact residues.
The finding that human B cells contain additional inhibitory Fc receptors is critically important for understanding the regulation of human B cell responses. Importantly, FcRL4 is the only known inhibitory receptor for IgA. Given the abundance of FcRL4 on mucosal memory B cells, IgA responses to mucosal antigens may be substantially regulated by FcRL4. Although it is possible that FcRL5 is somewhat redundant with FcγRIIb in humans, the potential for simultaneous recruitment of SHIP-1 by FcγRIIb and SHP-1 to FcRL5 provides a substantial barrier to recurrent activation of B cells. Together, FcRL4 and FcRL5 may limit B cell activation against chronic pathogens, self antigen, or commensal flora.
Mouse studies have clearly shown the importance of FcγRIIb in B cell regulation and autoimmune disease (1). Of the mouse cell surface FcRL proteins, only FcRH3/FcRL5 has a structure similar to FcRL4 or FcRL5. In our assays, mouse FcRH3/FcRL5 failed to bind heat aggregated mouse IgG (data not shown), and so FcγRIIb remains the sole inhibitory Fcγ receptor on mouse B cells. As a result of this functional difference, the regulatory function of human FcRL5 is unlikely to be determined from the study of FcRH3/FcRL5 knockout mice. Instead, FcRL4 or FcRL5 transgenes, expressed under appropriate promoters, would be more relevant for in vivo studies.
With extracellular ligands known and blocking antibodies available, FcRL4 and FcRL5 are attractive as therapeutic targets. First, FcRL4 and FcRL5 are both expressed by previously activated B cells. By blocking their ability to bind existing antibody, the antibody titer and repertoire generated by pathogen or tumor vaccines may be augmented. Secondly, down-regulation of FcRL4 in “exhausted” memory B cells from HIV patients was shown to enhance their responsiveness to HIV (30). Blocking FcRL4 might lead to renewed generation of neutralizing antibodies for HIV and other chronic viral infections. Lastly, the expression of FcRL5 by antigen experienced B cells makes it an attractive target for antibody-mediated cellular depletion. Autoimmune disorders which are dependent on auto-antibodies for their pathology might be successfully managed by depleting FcRL5+ cells. Additionally, the prevalence of FcRL5 on lymphomas with translocations at 1q21 (4) provides a specific target for depletion of tumors of this type. In conclusion, further study of the fundamental biology of FcRL proteins in general, and of FcRL4 and FcRL5 in particular, offer exciting possibilities for understanding human B cell biology and mucosal immunity.
Supplementary Material
Acknowledgments
The authors would like to thank Chris Nelson, Daved Fremont, Sergei Radaev and Peter Sun for advice and assistance on biochemical assays.
Footnotes
TJ Wilson was sponsored by a pre-doctoral training grant in Tumor Immunology from the Cancer Research Institute, and a post-doctoral National Research Service Award (T32) from the National Institutes of Health
This work was funded in part by the Center for HIV/AIDS Vaccine Immunology, AI067854-02.
References
- 1.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 2.Raghavan M, Bjorkman PJ. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol. 1996;12:181–220. doi: 10.1146/annurev.cellbio.12.1.181. [DOI] [PubMed] [Google Scholar]
- 3.Davis RS, Wang YH, Kubagawa H, Cooper MD. Identification of a family of Fc receptor homologs with preferential B cell expression. Proc Natl Acad Sci U S A. 2001;98:9772–9777. doi: 10.1073/pnas.171308498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatzivassiliou G, Miller I, Takizawa J, Palanisamy N, Rao PH, Iida S, Tagawa S, Taniwaki M, Russo J, Neri A, Cattoretti G, Clynes R, Mendelsohn C, Chaganti RS, Dalla-Favera R. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity. 2001;14:277–289. doi: 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama Y, Weissman SM, Bothwell AL. BXMAS1 identifies a cluster of homologous genes differentially expressed in B cells. Biochem Biophys Res Commun. 2001;285:830–837. doi: 10.1006/bbrc.2001.5231. [DOI] [PubMed] [Google Scholar]
- 6.Miller I, Hatzivassiliou G, Cattoretti G, Mendelsohn C, Dalla-Favera R. IRTAs: a new family of immunoglobulinlike receptors differentially expressed in B cells. Blood. 2002;99:2662–2669. doi: 10.1182/blood.v99.8.2662. [DOI] [PubMed] [Google Scholar]
- 7.Wilson TJ, Presti RM, Tassi I, Overton ET, Cella M, Colonna M. FcRL6, a new ITIM-bearing receptor on cytolytic cells, is broadly expressed by lymphocytes following HIV-1 infection. Blood. 2007;109:3786–3793. doi: 10.1182/blood-2006-06-030023. [DOI] [PubMed] [Google Scholar]
- 8.Schreeder DM, Pan J, Li FJ, Vivier E, Davis RS. FCRL6 distinguishes mature cytotoxic lymphocytes and is upregulated in patients with B-cell chronic lymphocytic leukemia. Eur J Immunol. 2008;38:3159–3166. doi: 10.1002/eji.200838516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis RS, Li H, Chen CC, Wang YH, Cooper MD, Burrows PD. Definition of an Fc receptor-related gene (FcRX) expressed in human and mouse B cells. Int Immunol. 2002;14:1075–1083. doi: 10.1093/intimm/dxf074. [DOI] [PubMed] [Google Scholar]
- 10.Facchetti F, Cella M, Festa S, Fremont DH, Colonna M. An unusual Fc receptor-related protein expressed in human centroblasts. Proc Natl Acad Sci U S A. 2002;99:3776–3781. doi: 10.1073/pnas.022042699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mechetina LV, Najakshin AM, Volkova OY, Guselnikov SV, Faizulin RZ, Alabyev BY, Chikaev NA, Vinogradova MS, Taranin AV. FCRL, a novel member of the leukocyte Fc receptor family possesses unique structural features. Eur J Immunol. 2002;32:87–96. doi: 10.1002/1521-4141(200201)32:1<87::AID-IMMU87>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Chikaev NA, Bykova EA, Najakshin AM, Mechetina LV, Volkova OY, Peklo MM, Shevelev AY, Vlasik TN, Roesch A, Vogt T, Taranin AV. Cloning and characterization of the human FCRL2 gene. Genomics. 2005;85:264–272. doi: 10.1016/j.ygeno.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Masuda K, Davis RS, Maruyama T, Zhang J, He T, Cooper MD, O. W. J, Burrows PD. FcRY, an Fc receptor related gene differentially expressed during B lymphocyte development and activation. Gene. 2005;363:32–40. doi: 10.1016/j.gene.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Polson AG, Zheng B, Elkins K, Chang W, Du C, Dowd P, Yen L, Tan C, Hongo JA, Koeppen H, Ebens A. Expression pattern of the human FcRH/IRTA receptors in normal tissue and in B-chronic lymphocytic leukemia. Int Immunol. 2006;18:1363–1373. doi: 10.1093/intimm/dxl069. [DOI] [PubMed] [Google Scholar]
- 15.Nagata S, Ise T, Pastan I. Fc receptor-like 3 protein expressed on IL-2 nonresponsive subset of human regulatory T cells. J Immunol. 2009;182:7518–7526. doi: 10.4049/jimmunol.0802230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrhardt GR, Davis RS, Hsu JT, Leu CM, Ehrhardt A, Cooper MD. The inhibitory potential of Fc receptor homolog 4 on memory B cells. Proc Natl Acad Sci U S A. 2003;100:13489–13494. doi: 10.1073/pnas.1935944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falini B, Tiacci E, Pucciarini A, Bigerna B, Kurth J, Hatzivassiliou G, Droetto S, Galletti BV, Gambacorta M, Orazi A, Pasqualucci L, Miller I, Kuppers R, Dalla-Favera R, Cattoretti G. Expression of the IRTA1 receptor identifies intraepithelial and subepithelial marginal zone B cells of the mucosa-associated lymphoid tissue (MALT) Blood. 2003;102:3684–3692. doi: 10.1182/blood-2003-03-0750. [DOI] [PubMed] [Google Scholar]
- 18.Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, Cooper MD. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202:783–791. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson TJ, Colonna M. A new Fc receptor homolog, FREB2, found in germinal center B cells. Genes Immun. 2005;6:341–346. doi: 10.1038/sj.gene.6364185. [DOI] [PubMed] [Google Scholar]
- 20.Leu CM, Davis RS, Gartland LA, Fine WD, Cooper MD. FcRH1: an activation coreceptor on human B cells. Blood. 2005;105:1121–1126. doi: 10.1182/blood-2004-06-2344. [DOI] [PubMed] [Google Scholar]
- 21.Haga CL, Ehrhardt GR, Boohaker RJ, Davis RS, Cooper MD. Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc Natl Acad Sci U S A. 2007;104:9770–9775. doi: 10.1073/pnas.0703354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochi Y, Myouzen K, Yamada R, Suzuki A, Kurosaki T, Nakamura Y, Yamamoto K. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J Immunol. 2009;183:5502–5510. doi: 10.4049/jimmunol.0901982. [DOI] [PubMed] [Google Scholar]
- 23.Jackson TA, Haga CL, Ehrhardt GR, Davis RS, Cooper MD. FcR-like 2 Inhibition of B cell receptor-mediated activation of B cells. J Immunol. 2010;185:7405–7412. doi: 10.4049/jimmunol.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreeder DM, Cannon JP, Wu J, Li R, Shakhmatov MA, Davis RS. Cutting edge: FcR-like 6 is an MHC class II receptor. J Immunol. 2010;185:23–27. doi: 10.4049/jimmunol.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson TJ, Gilfillan S, Colonna M. Fc receptor-like A associates with intracellular IgG and IgM but is dispensable for antigen-specific immune responses. J Immunol. 2010;185:2960–2967. doi: 10.4049/jimmunol.1001428. [DOI] [PubMed] [Google Scholar]
- 26.Santiago T, Kulemzin SV, Reshetnikova ES, Chikaev NA, Volkova OY, Mechetina LV, Zhao M, Davis RS, Taranin AV, Najakshin AM, Hendershot LM, Burrows PD. FCRLA is a resident endoplasmic reticulum protein that associates with intracellular Igs, IgM, IgG and IgA. Int Immunol. 2011;23:43–53. doi: 10.1093/intimm/dxq456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warmerdam PA, van den Herik-Oudijk IE, Parren PW, Westerdaal NA, van de Winkel JG, Capel PJ. Interaction of a human Fc gamma RIIb1 (CD32) isoform with murine and human IgG subclasses. Int Immunol. 1993;5:239–247. doi: 10.1093/intimm/5.3.239. [DOI] [PubMed] [Google Scholar]
- 28.Davis RS, Dennis G, Jr., Odom MR, Gibson AW, Kimberly RP, Burrows PD, Cooper MD. Fc receptor homologs: newest members of a remarkably diverse Fc receptor gene family. Immunol Rev. 2002;190:123–136. doi: 10.1034/j.1600-065x.2002.19009.x. [DOI] [PubMed] [Google Scholar]
- 29.Woof JM, Burton DR. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol. 2004;4:89–99. doi: 10.1038/nri1266. [DOI] [PubMed] [Google Scholar]
- 30.Kardava L, Moir S, Wang W, Ho J, Buckner CM, Posada JG, O’Shea MA, Roby G, Chen J, Sohn HW, Chun TW, Pierce SK, Fauci AS. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest. 2011;121 doi: 10.1172/JCI45685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


