Abstract
The meninges produce essential signaling molecules and major protein components of the pial basement membrane during normal brain development. Disruptions in the pial basement membrane underlie neural ectopia seen in those congenital muscular dystrophies (CMDs) caused by mutations in genes involved in O-mannosyl glycosylation. In mammals, biosynthesis of O-mannosyl glycans is initiated by a complex of mutually indispensable protein O-mannosyltransferases 1 and 2 (POMT1 and 2). To study the roles of O-mannosylation in brain development we generated a conditional allele of POMT2. POMT2 nulllizygosity resulted in embryonic lethality because of a defective Reichert's membrane. Brain-specific deletion of POMT2 resulted in hypoglycosylation of α-dystroglycan (DG) and abolished laminin binding activity. The effect of POMT2 deletion on brain development was dependent on timing, as earlier deletion resulted in more severe phenotypes. Multiple brain malformations including overmigration of neocortical neurons and migration failure of granule cells in the cerebellum were observed. Immunofluorescence staining and transmission electron microscopy revealed that these migration defects were closely associated with disruptions in the pial basement membrane. Interestingly, POMT2 deletion in the meninges (and blood vessels) did not disrupt the development of the neocortex. Thus, normal brain development requires protein O-mannosylation activity in neural tissue but not the meninges. These results suggest that gene therapy should be directed to the neural tissue instead of the meninges.
Keywords: congenital muscular dystrophy, neocortex, brain development, neural migration, basement membrane, dystroglycan, protein glycosylation
Congenital muscular dystrophies (CMDs) that are associated with central nervous system (CNS) malformations, Walker–Warburg syndrome (WWS), muscle–eye–brain disease (MEB), Fukuyama congenital muscular dystrophy (FCMD), and congenital muscular dystrophy 1D (MDC1D), can be caused by mutations in genes encoding glycosyltransferases (and presumed glycosyltransferases) including POMT1, POMT2, POMGnT1, FKTN, FKRP, and LARGE (Kobayashi et al., 1998; Brockington et al., 2001a,b; Yoshida et al., 2001; Beltran-Valero de et al., 2002; de Bernabe et al., 2003; Longman et al., 2003; van Reeuwijk et al., 2005; Currier et al., 2005). At least some of these genes are involved in the synthesis of O-linked mannosyl glycans such as Siaα2,3Galβ1,4GlcNAcβ1,2-Man-Ser/Thr and Galβ1,4(Fucα1,3)GlcNAcβ1,2Man-Ser/Thr (Chiba et al., 1997; Sasaki et al., 1998; Smalheiser et al., 1998). POMT1 and 2 (protein O-mannosyltransferases 1 and 2) are an enzyme complex that transfers mannose to serine or threonine residues; this is the first step in biosynthesis of this glycosylation (Manya et al., 2004; Akasaka-Manya et al., 2006). POMGnT1 (protein O-mannose N-acetylglucosaminyltransferase 1) then transfers N-acetylglucosamine to O-linked mannose (Yoshida et al., 2001; Zhang et al., 2002). The functions of the protein product of FKTN and LARGE, fukutin and LARGE, are not yet fully elucidated. Recent data indicate that LARGE is involved in extension of an unidentified phosphoryl glycosylation branch on O-linked mannose (Yoshida-Moriguchi et al., 2010) and complex N- and mucin O-glycosylations of α-dystroglycan (DG) (Patnaik and Stanley, 2005; Aguilan et al., 2009).
O-linked mannosyl glycans account for 1/3 of O-linked glycans in the brain (Finne et al., 1979; Krusius et al., 1986; Chai et al., 1999; Kogelberg et al., 2001). The bestknown O-mannosylated glycoprotein is α-DG, a cell surface glycoprotein that binds to the transmembrane β-DG (Ervasti and Campbell, 1991; Ibraghimov-Beskrovnaya et al., 1992). Both DG subunits are widely expressed and function as a transmembrane linker between the extracellular matrix and cytoskeleton (Ervasti and Campbell, 1991; Winder, 2001). α-DG binds with high affinity to laminin as well as several other extracellular matrix components including agrin, perlecan, neurexin, and pikachurin (Ervasti and Campbell, 1993; Gee et al., 1993; Yamada et al., 1994; Smalheiser and Kim, 1995; Montanaro et al., 1999; Sato et al., 2008). Mutations in POMT1, POMT2, and POMGnT1, as well as mutations in LARGE and fukutin, lead to hypoglycosylation of α-DG with markedly reduced laminin binding activity (Grewal et al., 2001; Michele et al., 2002; Kano et al., 2002; Takeda et al., 2003; Kim et al., 2004; Liu et al., 2006). Mouse knockout of dystroglycan recapitulates CMD phenotypes (Satz et al., 2008), supporting that defective glycosylation of α-DG is the key molecular cause of these CMDs.
CMDs with CNS manifestations are characterized by congenital muscular dystrophy, retinal atrophy, and type II lissencephaly in the brain. Overmigration of some neurons beyond the limits of the cerebral cortical boundary (Dobyns et al., 1985; Parano et al., 1995; Haltia et al., 1997; van der Knaap et al., 1997; Ross and Walsh, 2001; Jimenez-Mallebrera et al., 2005; Lian and Sheen, 2006) is a hallmark of these brain malformations. Analysis of animal models has provided insights into the mechanisms of type II lissencephaly (Holzfeind et al., 2002; Chiyonobu et al., 2005; Liu et al., 2006). Disruptions in the pial basement membrane are the key initial events leading to overmigration of neurons during development (Hu et al., 2007).
Major components of the pial basement membrane are produced by the meninges, a tissue of mesenchymal origin essential for brain development (Sievers et al., 1994). Destruction of meninges in the cerebellum of newborn rodents by 6-hydroxydopamine causes disruptions of the pial basement membrane with ectopia of granule cells, suggesting continued production of the extracellular matrix proteins by the meninges is required to maintain integrity of the pial basement membrane (Sievers et al., 1983, 1994). Interestingly, a meninges-specific deletion of focal adhesion kinase (FAK) disrupts the pial basement membrane and leads to overmigration of neurons through the disruptions. Thus, FAK is required in the meninges to maintain the integrity of the pial basement membrane, suggesting that the meninges function as more than just a source of extracellular matrix molecules (Beggs et al., 2003). The meninges have also been shown to be a source of retinoic acid that regulates proliferation of neural progenitor cells in the forebrain (Siegenthaler et al., 2009).
Analysis of POMGnT1 knockout mice shows a requirement for O-mannosyl glycosylation in brain development. However, knockout of POMGnT1 does not affect the first step of O-mannosyl glycosylation, which apparently provides residual laminin binding activity to α-DG (Kanagawa et al., 2009). In contrast, POMT1 nulllizygosity causes embryonic lethality and precludes the analysis of fetal development (Willer et al., 2004). Thus, a detailed examination of O-mannosyl glycosylation in brain development has not been performed nor is it known if O-mannosyl glycosylation is required in the meninges for normal brain development. In this study we generated POMT2-floxed allele to study the effect of deleting O-mannosyl glycosylation in developing brain in a tissue-specific manner. POMT2-floxed mice were crossed with GFAP-Cre (Zhuo et al., 2001), Emx1-Cre (Gorski et al., 2002), and Wnt1-Cre (Danielian et al., 1998) to specifically knockout POMT2 in the forebrain and the forebrain meninges. Our results indicated that O-mannosyl glycosylation is required in the brain for normal neocortical development in a temporal-specific manner. Surprisingly, POMT2 deletion in the meninges does not affect forebrain development.
MATERIALS AND METHODS
Animals
Protocols for animal use were approved by the Institutional Animal Care and Use Committee of SUNY Upstate Medical University and were in accordance with the National Institutes of Health guidelines.
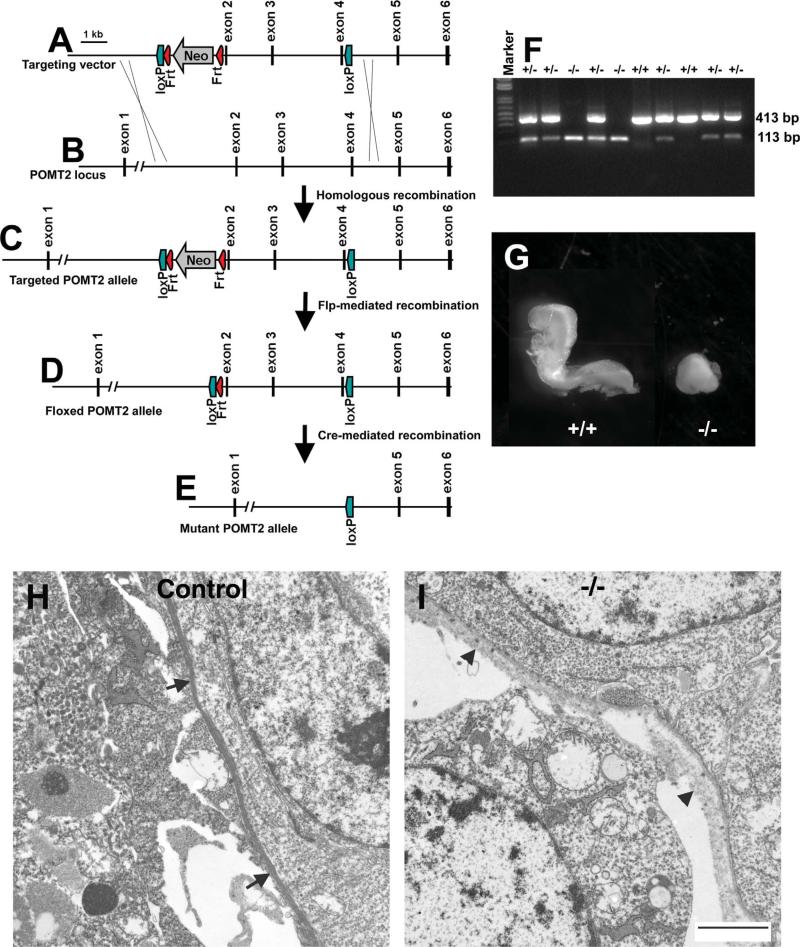
To generate mice with floxed POMT2 allele for conditional knockout, a targeting vector (Fig. 1A) was constructed by recombineering (Liu et al., 2003b) from a BAC clone containing the POMT2 locus obtained from the Children's Hospital Oakland Research Institute (CHORI) (Cat. no. RP23-212M4). The targeting vector was electroporated into D2 ES cell line derived from blastocysts of F1 hybrid of 129SvEvTac and C57BL/6J mice. Nested polymerase chain reaction (PCR) was used for screening of targeted event. ES cell clones with targeted POMT2 allele (Fig. 1C) were cocultured with CD-1 morulas to form chimeric blastocysts. The chimeric blastocysts were then implanted into pseudopregnant CD-1 females. Chimeras were crossed with female Rosa26-Flp transgenic mice (Farley et al., 2000) (Stock no. 003946; Jackson Laboratories, Bar Harbor, ME) to remove the Neo cassette and thus generated heterozygous mice with a floxed POMT2 allele (Fig. 1D). To generate mice with null POMT2 alleles, heterozygous floxed mice were crossed with a deleter line (Cre knockin at the hprt locus; Stock no. 004302, Jackson Laboratories) (Tang et al., 2002). Cre-mediated recombination deleted exons 2, 3, and 4, which resulted in a mutant allele encoding a truncated POMT2 mRNA species (Fig. 1E) with a frameshift mutation resulting in a premature stop of translation. Truncated POMT2 is expected to be nonfunctional because the conserved region of the wildtype protein (amino acid residues 350–712) that includes the mannosyltransferase domain is deleted (Girrbach et al., 2000; Willer et al., 2002).
Figure 1.
Targeting construct and the diagram for generation of floxed POMT2 allele. A: Targeting construct: a cassette containing loxP-Frt-Neo-Frt was inserted into intron 1. A second loxP site was inserted into intron 4. Thus, the targeting vector is designed to have exons 2, 3, and 4 flanked by two loxP sequences. Flanking the two loxP sites are homologous sequences of the POMT2 locus. After homologous recombination in ES cells, one of the two POMT2 alleles will become the targeted allele (shown in C). B: Wildtype POMT2 allele. (C) Targeted POMT2 allele. This allele is present in ES cells that underwent homologous recombination with the targeting vector after electroporation of the targeting vector. D: Floxed POMT2 allele. This allele is obtained by crossing chimeric mice with ROSA26-Flp transgenic mice to remove neomycin resistance cassette. E: Mutant POMT2 allele. This allele is obtained after Cre-mediated conditional removal of the floxed sequences (exons 2–4) of the floxed POMT2 allele. F: RT-PCR showed that the knockout embryos expressed only truncated POMT2 mRNA. The RT-PCR product of truncated POMT2 mRNA is 113 bp. G: E8.5 POMT2 null embryos were runted. H,I: Transmission EM analysis of the Reichert's membrane of control and POMT2 null embryos revealed that the POMT2 null Reichert's membrane was less dense with multiple breaks at E6.5 (arrowheads in I). Scale bar = 2 μm in I (applies to H). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Genotyping of POMT2 null (deletion of exons 2-4) was carried out by PCR of DNA extract from tissue pieces of embryos or microdissected materials from tissue sections. The primers for POMT2 null allele were TTGT CATGTTTGGAGGCTGA and CCCACCTAAATTGGTCTGGA with an expected 217 basepair (bp) amplicon. The primers for POMT2 wildtype were CCTCAGATGCTGATCGGTCT and TCATTCCCATGTAGCTGTGG with an expected 106 bp amplicon.
Genotyping of POMT2 floxed animals was carried out by PCR of tail DNA extract with specific primers (forward: TGCCCAGAAGTAGCTGTGAA and reverse: CCCACCTAAATTGGTCTGGA). The wildtype allele produces a 171 bp amplicon while the floxed allele produces a 262 bp amplicon.
Heterozygous Hprt-Cre knockin mice (Tang et al., 2002), hemizygous GFAP-Cre transgenic mice (Zhuo et al., 2001), homozygous EMX1-Cre knock-in mice (Gorski et al., 2002), hemizygous Wnt1-cre transgenic mice (Danielian et al., 1998) from the Jackson Laboratories were crossed with POMT2-floxed mice to obtain POMT2 null allele and mice with tissue-specific knockout of POMT2 in the brain and in the forebrain meninges. Genotyping of GFAP-Cre, EMX1-Cre, and Wnt1-Cre mice were determined by PCR with specific primers recommended by the Jackson Laboratories. For timed pregnancy, noon on the date of plug observation was considered embryonic day (E) 0.5. Date of birth was considered postnatal day (P) 0.
The double reporter mice, (ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo transgenic mice (Muzumdar et al., 2007), were obtained from Jackson Laboratories and crossed with Wnt1-Cre transgenic mice to evaluate meningeal expression of Cre recombinase.
Genotyping and POMT2 mRNA reverse transcription (RT)-PCR analysis of embryos
Segments of uterus containing E6.5 or 7.5 embryos were frozen in OCT medium, cryosectioned, and collected onto Leica FrameSlides with PET-Membrane (1.4 μm). The embryonic tissue was microdissected with a Nikon SMZ-2T microdissection microscope and collected into 35 μL of Buffer RLT (Qiagen, Chatsworth, CA) with 10 μL/mL β-mercaptoethanol. DNA and RNA were then extracted using the Qiagen AllPrep DNA/RNA micro kit. Extracted DNA was used for genotyping as above. RT-PCR of POMT2 was carried out with a Qiagen OneStep RT-PCR kit with a pair of primers spanning the floxed exons (forward: GTGACGCTGCTGTCCTTTG and reverse: AACATCAGGATGGGGTCAAG). The predicted product size was 413 bp for wildtype POMT2 mRNA and 113 bp for the knockout mRNA.
Antibodies
Please see Table 1 for a list of all antibodies used.
TABLE 1.
List of Antibodies Used
| Antibodies | Immunogen | Manufacturer | Dilution |
|---|---|---|---|
| IIH6C4 | Purified α-DG from rabbit skeletal muscle membrane | Millipore (Billerica, MA), Mouse monoclonal IgM, #05-593 | 1:300 |
| β-DG | Synthetic C-Terminal peptide (15 aa) | Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA) | 1:500 |
| Mouse monoclonal IgG, # MANADAG2, clone 7D11 | |||
| RC-2 | Rat fetal brain | Developmental Studies Hybridoma Bank Mouse monoclonal IgM | 1:10 |
| Ctip2 | Synthetic peptide, aa396-445 of human origin | Abcam (Cambridge, MA). Rabbit polyclonal, #ab91044 | 1:200 |
| Cux1 | Synthetic peptide, aa143-192 of human origin | Sigma-Aldrich (St. Louis, MO), rabbit polyclonal, #SAB2105137 | 1:200 |
| ER-TR7 | Mouse thymic reticulum | Abcam, Rat monoclonal, #ab51824 | 1:200 |
| Laminin-1 | Laminin-1 isolated from EHS sarcoma | Sigma-Aldrich, rabbit polyclonal, #L9393 | 1:1000 |
| GFAP | GFAP from pig spinal cord | Sigma-Aldrich, mouse monoclonal, #G3893 | 1:1000 |
| GABARA6 | Synthetic peptide, aa 20-37 of rat GABA(A) α6 | Sigma-Aldrich, rabbit polyclonal, #G5544 | 1:500 |
| CR-50 | Fetal mouse brain lysate | Drs. K. Nakajima and Ogawa Mouse monoclonal IgG. | 1:300 |
Antibody characterization
α-DG antibody IIH6C4 recognized carbohydrate epitopes of α-DG because chemical deglycosylation by trifluoromethanesulfonic acid abolished immunoreactivity (Ervasti and Campbell, 1993). The antibody recognized a 125-kDa band in glycoproteins isolated from the mouse brain with wheat germ agglutinin (WGA)-agarose (Michele et al., 2002). Staining was diminished in various CMD models because α-DG is hypoglycosylated (Kano et al., 2002; Michele et al., 2002; Takeda et al., 2003; Kim et al., 2004). We also observed a band at 125 kDa on western blot of glycoproteins isolated from the mouse brain and cultured neural progenitor cell. Immunoreactivity was diminished in POMGnT1 knockout mice (Liu et al., 2006) and eliminated in cultured dystroglycan knockout neural progenitor cells.
β-DG antibody (MANDAG2-7D11) recognized a single 43 kDa of expected size on western blot of total mouse brain lysate or brain glycoproteins isolated with WGA-agarose (Liu et al., 2010). Immunoreactivity at the 43-kDa band was completely eliminated in our cultured dystroglycan knockout neural progenitor cells.
RC-2 antibody recognized a 295-kDa band in the lysate of neural progenitor cells on western blot which corresponds to the expected molecular weight of nestin (Park et al., 2009). Nestin null mutation abolished RC2 immunoreactivity in neural progenitor cells on western blot and immunofluorescence staining (Park et al., 2009). In immunofluorescence staining it recognized cells with morphologies of radial glial cells (Misson et al., 1988). The antibody stained cells with morphology of radial glial cells when we stained fetal and newborn mouse brain sections as well.
Ctip2 antibody (25B6) labeled subsets of neurons in layer V–VI (Hevner, 2007). It recognized a specific band at 88 kDa on western blot of newborn brain lysate according to the manufacturer's datasheet. In our evaluation with immunofluorescence staining, it strongly labeled a subset of neurons within the cortical plate of newborn brain as expected.
ER-TR7 specifically recognized cells with fibroblast morphologies and distributions in tissues that included intestine, stomach, pancreas, liver, skin, and tendon (Van Vliet et al., 1986). The nature of the antigen is not known. In our evaluation of antibody specificity by immunofluorescence staining of primary cell cultures isolated from the neocortical wall, it only recognized fibronectin-positive cells with morphologies typical of fibroblasts. In the mouse brain it recognized cells located in the meninges and in some blood vessels where fibroblasts are present.
Laminin-1 antibody specifically recognized basement membranes in immunofluorescence staining according to the manufacturer's datasheet. It did not recognize fibronectin, vitronectin, collagen IV, or chondroitin sulfate proteoglycans. When we evaluated this antibody by western blot analysis it recognized a band at ≈350 kDa in glycoproteins isolated from the brain, skeletal muscle, and cultured neural stem cells by WGA-agarose, corresponding to expected molecular weight of laminin a1 chain. When we carried out immunofluorescence staining of brain sections it recognized blood vessels and meningeal covering, two locations where basement membranes are located.
Glial fibrillary acidic protein (GFAP) antibody recognized a single band of 50 kDa in mouse brain lysate on western blot according to the manufacturer's datasheet. When we evaluated the antibody by immunofluorescence staining, it specifically labeled astrocytes but not any other cell types in the mouse brain.
According to the manufacturer, GABA A receptor, α6 subunit (GABARA6) antibody specifically recognized a 55-kDa band in rat brain membrane extract on western blot consistent with the expected molecular weight of GABARA6 receptor. In our evaluation of the antibody, it recognized a 55-kDa band from cerebellar lysate of adult mouse. When we carried out immunofluorescence staining of cerebellar sections, the antibody specifically recognized granule cells but not other cell types in the cerebellum (Li et al., 2008).
Cux1 antibody specifically recognized a band at 42 kDa and an additional band at ≈80 kDa on western blot of fetal spleen lysate according to the manufacturer's datasheet. Cux1-positive neurons were mainly located in layer II–IV of adult neocortex (Nieto et al., 2004). When we stained the neocortex of newborn mice it recognized a subset of neurons in the neocortical wall, as expected.
CR-50 antibody specifically recognized Cajal-Retzius cells and its immunoreactivity was lost in the reeler mice deficient in Reelin (Ogawa et al., 1995). It recognized a band of ≈400 kDa in conditioned medium of Reelin expressing COS cells and in cerebellar cell lysate on western blot (D'Arcangelo et al., 1997). This antibody was a kind gift from Drs. K. Nakajima and M. Ogawa. When we stained neocortical sections with this antibody it recognized a subset of cells in the marginal zone where Cajal-Retzius cells were located.
Immunostaining
Mice were sacrificed by an overdose of pentobarbital and the brains were removed from the skull, embedded in OCT compound, frozen, and cryostat-sectioned in the coronal plane at 10 μm and mounted on Superfrost plus slides (Fisher Scientific, Pittsburgh, PA). The sections were fixed with 4% paraformaldehyde for 30 minutes. Primary and secondary antibody incubation were performed as described previously (Hu, 2000; Hu et al., 2007) and counterstained for 10 minutes with 0.10% DAPI (Sigma-Aldrich, St. Louis, MO) or 0.01% propidium iodide. All antibodies were used at dilutions recommended by the manufacturers. Fluorescence was visualized with a Zeiss Axioskop upright fluorescence microscope equipped with a digital camera (Carl Zeiss Microimaging, Thornwood, NY).
Neural progenitor cell cultures
Primary neural progenitor cells were isolated from E13.5 neocortex. The neocortical wall was dissected out, trypsinized, and triturated. The dissociated cells were cultured as neural spheres in neural basal medium (Invitrogen, Carlsbad, CA) supplemented with B27 (minus vitamin A), FGF-2 (20 ng/mL), EGF (20 ng/mL), and heparin (2 ng/mL).
To obtain clones of neural progenitor cells, the neural spheres were trypsinized and triturated into individual cells. Ten mL of diluted cells (at 6 cells/mL concentration) were seeded in a 100-mm fibronectin-coated tissue culture dish. Fresh FGF-2, EGF, and heparin were added once every 3 days. The individual colonies were picked up with a pipettor and subcultured in a 12-well plate with 1 mL culture medium for 1 week. The cloned neural progenitor cells were expanded as neural spheres. The genotypes of the POMT2 locus in cloned neural progenitor cells were identified as above.
Western blot and laminin overlay experiments
Neocortex was dissected out from the brain, meninges peeled off, and homogenized in a Dounce homogenizer in cold lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, pH 7.4) supplemented with protein inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), and centrifuged at 16,100g for 20 minutes at 4°C. The supernatants were collected. For 2 mg of proteins of the total lysate, 50 μL of WGA-agarose (EY Laboratories, San Mateo, CA) was added. After binding for 4 hours the WGA-agarose was washed 3 times with the lysis buffer. Bound glycoproteins were eluted by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading dye and separated on SDS-PAGE and electrotransferred onto polyvinylidene fluoride (PVDF) membranes.
For the laminin-overlay assay, the PVDF membrane was incubated with Tris-buffered saline (50 mM Tris, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2) containing 3% bovine serum albumin (BSA) for an hour to block nonspecific binding. The membrane was then incubated with 1.25 μg/mL laminin-1 (Invitrogen) in Tris-buffered saline (50 mM Tris, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2) overnight at 4°C. After washing with the same buffer, the membrane was incubated with a rabbit antibody against mouse laminin-1 (1:2,000) for 2 hours. After washing, the membrane was incubated with goat antirabbit IgG conjugated with horseradish peroxidase (1:3,000) for 45 minutes. After washing the signal was visualized with SuperSignal west pico chemiluminescent substrate (Thermo Scientific, Rockford, IL).
For detection of α-DG with IIH6C-4 and β-DG with monoclonal antibody MANDAG2-7D11, standard immunoblotting procedures were carried out.
Transmission electron microscopy (EM)
Transmission EM analysis was carried out essentially as described previously (Liu et al., 2003a). E13.5 fetuses were fixed in 3.7% glutaraldehyde in phosphate-buffered saline (PBS) (pH 7.4) overnight. The neocortical wall was dissected out from vibratome sections of the forebrain, postfixed in 1% osmium tetroxide, and stained en bloc with 1.0% uranyl acetate. The tissues were then dehydrated by an ethanol series, embedded in Poly/Bed 812 resin (Polysciences, Warrington, PA), and cut into 1-μm sections with a glass knife. The region of interest was then cut into 500-nm ultrathin sections, stained with 2.0% uranyl acetate, and Reynold's lead citrate (Polysciences). The samples were observed and photographs were taken with a Tecnai BT12 transmission electron microscope (FEI, Salem, MA).
Preparation of figures
All figures were prepared with Photoshop 5.0 (Adobe Systems, San Jose, CA). Contrast and/or brightness of micrographs in some figure panels were adjusted for clarity.
RESULTS
Fetal lethality of POMT2 null caused by defective Reichert's membrane
To create a conditional allele of POMT2, exons 2–4 were engineered with two flanking LoxP sequences. Deletion of exons 2–4 by the Cre recombinase creates a truncated POMT2 mRNA with a frameshift mutation at the junction between exons 1 and 5. In contrast to the 820 amino acid wildtype POMT2 polypeptide, the truncated POMT2 mRNA theoretically translates into a 152 amino acid polypeptide because of premature termination of translation.
To generate POMT2 null allele, POMT2 floxed mice were crossed with Hprt-Cre knockin mice. To confirm expression of the truncated POMT2 allele we carried out RT-PCR on total RNA isolated from E6.5 embryos with a pair of primers located in exon 1 and 5 of POMT2. The truncated POMT2 mRNA is expected to yield a 113 bp amplicon. The wildtype POMT2 mRNA is expected to yield a 413 bp fragment. Indeed, the wildtype embryos gave only a 413 bp fragment (Fig. 1F). The heterozygous embryos produced both 413 and 113 bp fragments. POMT2 knockout embryos produced only 113 bp fragment. These results indicate that the knockout embryos expressed only truncated POMT2 mRNA.
Intercross of animals heterozygous for POMT2 null mutation (+/–) did not produce any live births of homozygous POMT2 knockout pups (–/–). Table 2 lists genotyping results for the offspring from heterozygous intercrosses at various developing stages. No homozygous null (–/–) fetuses were found beyond E9.5. However, resorption sites were often observed in the uterus at E9.5 and E8.5, suggesting that the homozygous null fetuses died before E9.5. Indeed, runted POMT2 null embryos were found at E8.5 (Fig. 1G). Thus, as expected, POMT2 null embryos died at a similar stage as POMT1 null fetuses (Willer et al., 2004).
TABLE 2.
Genotypes of POMT2 Knockout at Various Ages
| Age | Wildtype (+/+) | Heterozygous (+/–) | Knockout (–/–) |
|---|---|---|---|
| E6.5 | 3 | 10 | 2 |
| E7.5 | 1 | 5 | 4 |
| E8.5 | 3 | 15 | 4 |
| E9.5 | 8 | 17 | 0 |
| E10.5 | 2 | 5 | 0 |
| Postnatal | 42 | 76 | 0 |
Both dystroglycan null and POMT1 null embryos die because of defective Reichert's membrane (Williamson et al., 1997; Willer et al., 2004), the extraembryonic basement membrane. To determine the integrity of the Reichert's membrane in POMT2 null fetuses, we carried out transmission EM at E6.5. In control embryos the Reichert's membrane was continuous without breaks (arrows in Fig. 1H). By contrast, the Reichert's membrane in POMT2 null embryos was less dense with frequent breaks (arrowheads in Fig. 1I). Thus, embryonic lethality of POMT2 null embryos is likely caused by defective Reichert's membrane as well.
POMT2 deficiency caused hypoglycosylation of α-DG and abolished its laminin binding activity
To determine the effect of POMT2 deficiency on brain development, POMT2 floxed mice were crossed with GFAP-Cre and Emx1-Cre transgenic mice to generate brain-specific knockouts of POMT2. We first evaluated the functional effect of POMT2 knockout by analyzing α-DG glycosylation in the neocortices of the knockout mice.
Immunoblots probed with anti-α-DG antibody IIH6C4 (specific for the glycosylated form of α-DG) or laminin overlay experiments were carried out on glycoproteins isolated from neocortices of adult POMT2f/f;GFAP-Cre+, POMT2f/f;Emx1-Cre+, and control (Cre-negative) animals (Fig. 2A). IIH6C4 immunoreactivity was markedly reduced in both GFAP-Cre and Emx1-Cre mediated knockouts. Similarly, laminin binding activity was also markedly reduced in the knockouts. As a control, β-DG immunoreactivity was not changed in the knockouts. Some residual IIH6C4 immunore-activity and laminin binding activity was detected in both knockouts, likely due to the presence of blood vessels and perhaps incomplete Cre recombination in all neural cells.
Figure 2.
Hypoglycosylation of α-DG in POMT2-deficient cells. Glycoproteins from the neocortex (A) and cloned wildtype and POMT2 knockout neural progenitor cells (B) were used for western blot with an antibody specific for glycosylated form of α-DG (IIH6C4) and laminin overlay experiments. Note dramatically reduced laminin binding and reduced IIH6C4 immunoreactivity in POMT2 conditional knockout brain and undetectable IIH6C4 immunoreactivity in POMT2 knockout neural progenitor cells. β-DG, β-dystroglycan; f/f, floxed/floxed.
To evaluate the effect of complete POMT2 deletion on α-DG glycosylation, we cultured neural progenitor cells from E13.5 neocortex. When primary cultures of POMT2f/f;GFAP-Cre+ neural progenitor cells were tested for α-DG glycosylation and laminin binding activity, residual glycosylation and lamination was detectable (data not shown). We reasoned that the residual activity was likely due to the lack of Cre transgene expression in some neural progenitor cells. Thus, POMT2f/f;GFAP-Cre+ neural progenitor cells were isolated for clonal expansion and 7 of 16 clones were found to be complete POMT2 nulls. Immunoblots with IIH6C4 were then carried out on glycoprotein isolated from lysates of cloned POMT2 null neural progenitor cells. As shown in Figure 2B, IIH6C4 immunoreactivity was undetectable in POMT2 null neural progenitor cells. By contrast, the wildtype showed strong IIH6C4 immunoreactivity. These results indicate that the truncated POMT2 is not functional.
Malformation of the forebrain in POMT2 knockout mediated by Emx1-Cre knockin mice
Migration failure of some granule cells in the cerebellum was observed in POMT2f/f;GFAP-Cre+ mice (data not shown). Unexpectedly, no apparent abnormalities in tissue architecture were observed in the forebrain of these mice. Breaches in pial basement membrane and the glia limitans were found only in limited areas at the midline between the cerebral hemispheres in the adult (data not shown). GFAP-Cre transgenic mice express Cre recombinase starting at E13.5 (Zhuo et al., 2001). We suspected functional deletion of POMT2 protein in POMT2f/f;GFAP-Cre+ mice may have occurred after the time when intact pial basement membrane was crucial for normal migration of neurons. Therefore, POMT2-floxed mice were crossed with Emx1-Cre knockin mice that express Cre recombinase before E10.5 in the neocortical wall. Adult POMT2f/f;Emx1-Cre+ mice (n = 13) exhibited clear lamination defects in the forebrain, as layer I was indistinct, giving the appearance of excessive cellular infiltration into layer I (Fig. 3B). Similarly, layer I could not be identified between cerebral hemispheres because two cerebral hemispheres of POMT2f/f;Emx1-Cre+ mice were fused (Fig. 3D and compare with control in Fig. 3C) obscuring layer I. Indistinct layer I of POMT2f/f;Emx1-Cre+ brain was similar to that in the POMGnT1 knockout (Liu et al., 2006), Largemyd mice (Grewal et al., 2001), chimeric fukutin knockout (Takeda et al., 2003), and brain-specific knockout of DG (Moore et al., 2002). Furthermore, layers II/III, IV, V, and VI could not be readily identified in Nissl-stained sections of the knockout cortex, suggesting that additional lamination defects were present. Indeed, when newborn sections were stained with an antibody against Ctip2, an antibody that labels subsets of layer V and VI neurons, severe lamination defects were observed (Fig. 3G,H). In control littermates there were two populations of Ctip2-labeled neurons, strongly labeled and weakly labeled. Strongly labeled Ctip2-positive neurons were located as a layer within the cortical plate of P0 animals (Fig. 3F). Weakly labeled Ctip2-positive neurons were widely distributed within the cortical plate. In the knockout, by contrast, strongly labeled and weakly labeled Ctip2-positive neurons were intermingled and widely distributed above the intermediate zone (IZ) in a diffuse manner throughout the developing cortex (Fig. 3H). To quantify this effect, we divided the cortical plate together with the marginal zone into three equal bins (the pial, middle, and the inner parts) and counted strongly labeled Ctip2-positive nuclei in each bin from images of three wildtype and three knockout animals, four images per animal (Table 3). In Cre-negative animals, most strongly labeled Ctip2-positive nuclei were located in the middle bin, with only a few in the pial and inner bins. In the POMT2f/f;Emx1-Cre+ animals, there were significant increases of Ctip2-positive neurons in the pial bin and the inner bin with a reduction in the middle bin (P < 0.0001, chi-square analysis).
Figure 3.
Lamination defects in the neocortex and hippocampus of POMT2f/f;Emx1-Cre+ mice. Frozen sections were stained with cresyl violet (A–E,G,K–N) or immunostained with antibodies against Ctip2 (F,H) and Cux1 (I,J). A,C,E,F,I,K,M: Cre-negative controls. B,D,G,H,J,L,N: POMT2f/f;GFAP-Cre+. A–D,K–N: Adult. E–J: P0. The knockout forebrain exhibited multiple defects. Cortical layering could not be identified in the knockout (B). Two cerebral hemispheres were fused in the knockout (D). The knockout hippocampus showed abnormal dentate gyrus morphology (arrow in L) and laminination defects of CA neurons. Also note that location of Ctip2- and Cux1-positive neurons was disrupted in newborn knockout (compare H with F and J with I). KO, knockout. Scale bars = 200 μm in H (applies to A–D,K–L); 100 μm for E–H; 25 μm for M–N; 50 μm in J (applies to I).
TABLE 3.
Numbers and Percentages of Ctip2-Positive Neurons in the Pial, Middle, and Inner Third Portions from the Pial Surface to the Inner Edge of the Cortical Plate of P0 Mice
| Bin | Pial | Middle | Inner | Total |
|---|---|---|---|---|
| Control | 13 | 1465 | 31 | 1509 |
| Percent | 0.86 | 97.08 | 2.05 | |
| Knockout | 544 | 698 | 101 | 1343 |
| Percent | 40.51 | 51.97 | 7.52 |
Chi-square analysis, χ2 = 808.39, P < 0.0001.
We also examined distribution of Cux1-positive neurons in newborn animals. Cux1 mainly labels subsets of neurons in layers II–IV of adult animals. In the Cre-negative newborns, Cux1-positive neurons were distributed throughout the cortical plate with a few in the marginal zone (Fig. 3I). In POMT2f/f;Emx1-Cre+ animals, the organization of Cux1-positive nuclei was apparently disorganized (Fig. 3J). To quantify this effect, we divided the cortical plate together with the marginal zone into 10 equal bins and counted Cux1-positive nuclei in each bin from five randomly selected images of two wildtype and two knockout animals (Table 4). There were apparent increases in Cux1-positive labeled neurons in the superficial bin (bin #1) and inner bins (bins #5–10) but decreases in bins #2–4 (P < 0.0001, chi-square analysis). These results indicate that the knockout exhibited severe lamination defects that involve multiple cortical layers.
TABLE 4.
Numbers and Percentages of Cux 1-Positive Neurons in 10 Equal Bins from the Pial Surface to the Inner Edge of the Cortical Plate in P0 Mice
| Bin | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 319 | 677 | 755 | 651 | 561 | 553 | 552 | 608 | 592 | 464 | 5732 |
| Percent | 5.57 | 11.81 | 13.17 | 11.36 | 9.79 | 9.65 | 9.63 | 10.61 | 10.33 | 8.09 | |
| Knockout | 454 | 487 | 580 | 604 | 639 | 637 | 637 | 625 | 623 | 566 | 5852 |
| Percent | 7.76 | 8.32 | 9.91 | 10.32 | 10.92 | 10.89 | 10.89 | 10.68 | 10.65 | 9.67 |
Chi-square analysis, χ2 = 106.26, P < 0.0001.
Other defects in the forebrain of POMT2f/f;Emx1-Cre+ mice included lamination defects in the hippocampus (Fig. 3K–N). The inferior blade of the dentate gyrus in the knockout showed a wavy morphology (arrow in Fig. 3L; 12 out of 13 animals). Ten out of 13 animals also showed dispersion of CA3 of the Ammon's horn with CA3 cells dispersed (asterisk in Fig. 3L). Two animals showed lamination defects of pyramidal neurons in all CA fields throughout the Ammon's horn with some pyramidal neurons displaced (# sign in Fig. 3L and arrow in Fig. 3N).
O-mannosylation is not required in the meninges for normal brain development
Deletion of FAK in the meninges resulted in disruption of the pial basement membrane and overmigration of neurons (Beggs et al., 2003). We therefore sought to determine whether POMT2 is also required in the meninges. Wnt1 is highly expressed by the neural crest cells during development. Thus, Wnt1-Cre transgenic mice may express Cre recombinase in meningeal covering of the forebrain because forebrain meninges are derived from the neural crest. Wnt1-Cre transgenic mice were crossed with a double reporter mouse line, (ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo transgenic mice (Muzumdar et al., 2007). Strong green fluorescence was observed in the meninges and blood vessels (green) but not in the neural tissue of the forebrain (magenta) at E11.5 (Fig. 4A) indicating Wnt1 driven Cre expression started before E11.5 in nonneural tissues. Continued meningeal and blood vessel expression of green fluorescent protein was also observed at E13.5 and P3 neocortical wall (Fig. 4B,C). These results indicate that Wnt1-Cre transgenic mice exhibit specific Cre-mediated recombination in the meninges (and blood vessels) of the forebrain but, importantly, no Wnt1-Cre-mediated recombination is observed in forebrain neural tissue. It was not possible to discern the exact cell type of Cre-expression in the blood vessels from the images.
Figure 4.
Wnt1-Cre mediated deletion of POMT2 in the meninges does not affect forebrain development. A–C: Wnt1-Cre positive (ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo transgenic mice revealed specificity of Cre-recombination event in the forebrain meninges. D: RT-PCR of POMT2 using total RNA isolated from the meningeal cells of POMT2f/f;Wnt1-Cre- (ff/Cre-) and POMT2f/f;Wnt1-Cre+ (ff/Cre+) mice. Wildtype POMT2 mRNA was barely detectable in the meninges of the knockout by RT-PCR (413 bp), while mutant mRNA was detected (113 bp). E,F: Cresyl violet stain of Cre-negative control and POMT2f/f;Wnt1-Cre+ mice, respectively, showing normal histology in the knockout. G,H: Distribution of Ctip2-positive neurons in POMT2f/f;Wnt1-Cre+ animals was indistinguishable from the controls at P0. KO, knockout. Scale bars = 100 μm in C (applies to A,B); 200 μm in F (applies to E); 100 μm in H (applies to G).
We therefore crossed POMT2-floxed mice with Wnt1-Cre transgenic mice. RT-PCR analysis revealed that the meninges in POMT2f/f;Wnt1-Cre+ mice expressed truncated POMT2 mRNA (Fig. 4D), indicating efficient recombination at the floxed POMT2 locus in the meningeal cells. As shown in Figure 4F, deletion of POMT2 in the meninges did not affect the tissue architecture of the neocortex. Immunofluorescence staining with Ctip2 antibody showed identical pattern of strongly (and weakly) labeled neurons between POMT2f/f;Wnt1-Cre+ (Fig. 4H) and control mice (Fig. 4G). Similarly, no obvious difference was observed with Cux1 immunostaining (not shown). Further, there was no observable defect in the hippocampus and the pial basement membrane of POMT2f/f;Wnt1-Cre+ mice (not shown). These results indicated that POMT2 is not required in the meninges for proper brain development including integrity of the pial basement membrane.
Disruptions in the pial basement membrane and the glia limitans in POMT2f/f;Emx1-Cre+ mice
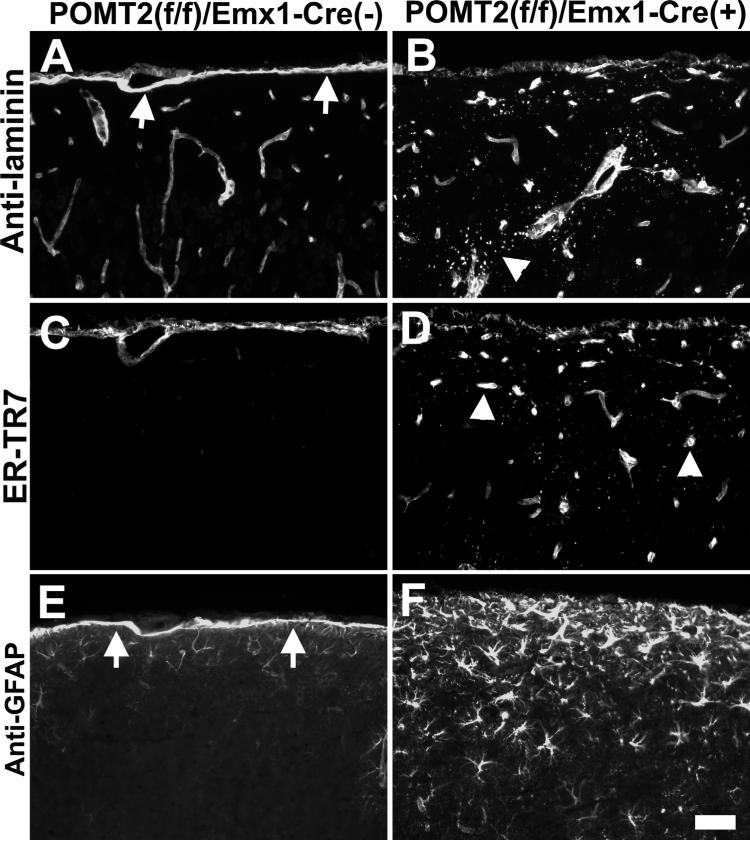
To examine the integrity of the pial basement membrane in the neocortex of POMT2f/f;Emx1-Cre+ mice, we carried out immunofluorescence staining with basement membrane marker laminin-1. The pial basement membrane in the control showed a continuous pial basement membrane (arrows in Fig. 5A). POMT2f/f;Wnt1-Cre+ mice showed similar pattern of staining as the controls (data not shown). By contrast, POMT2f/f;Emx1-Cre+ neocortical surface was devoid of such an uninterrupted continuous line of laminin immunofluorescence (Fig. 5B), indicating the absence of a pial basement membrane in most regions of the neocortical surface. However, punctate staining patterns of laminin were found at the surface and the upper half of the neocortex, indicating ectopic deposits of laminin (arrowhead in Fig. 5B).
Figure 5.
Disruptions of the pial basement membrane covering the neocortex of POMT2f/f;Emx1-Cre+ mice. Frozen sections of adult forebrain were immunofluorescence stained with anti-laminin (A,B), ER-TR7 (C,D), and anti-GFAP (E,F). A,C,E: Cre-negative control. B,D,,F: POMT2f/f;Emx1-Cre+. Note absence of the pial basement membrane (B) and glia limitans (F) at the neocortical surface in POMT2f/f;Emx1-Cre+ animals. Ectopic meningeal fibroblasts were observed in the cortex (arrowheads in D). Excess GFAP-positive astrocytes were observed in the upper half of the knockout neocortex (F). Scale bar = 50 μm.
POMGnT1 knockout exhibits ectopic fibroblasts in the upper half of the neocortex (Yang et al., 2007). Therefore, immunostaining with ER-TR7 (a fibroblast marker) was carried out. The upper half of the POMT2f/f;Emx1-Cre+ neocortex exhibited ectopic meningeal fibroblasts as well (arrowheads in Fig. 5D). The ectopic fibroblasts were likely associated with blood vessels as POMGnT1 knockout (Yang et al., 2007) because the staining pattern resembled that of blood vessels.
In addition, GFAP immunofluorescence staining showed an undisrupted line of GFAP fluorescence at the neocortical surface of control mice, indicative of a glia limitans at the neocortical surface (arrows in Fig. 5E). By contrast, POMT2f/f;Emx1-Cre+ neocortical surface did not show a line of continuous GFAP fluorescence staining, indicating the absence of a glia limitans at the neocortical surface (Fig. 5F). However, overall GFAP fluorescence is increased, with many more GFAP-positive cells in the upper half of the neocortex (Fig. 5F). These results indicated that, similar to POMGnT1 knockout, the upper half of the neocortex in POMT2f/f;Emx1-Cre+ mice exhibits reactive astrogliosis.
Disruptions in the pial basement membrane during neocortical development of POMT2f/f;Emx1-Cre+ mice
To determine whether the pial basement membrane and the glia limitans were disrupted in the developing brain of POMT2f/f;Emx1-Cre+ fetuses, we carried out transmission EM analysis at E13.5. While the pial basement membrane of neocortical wall was continuous in the control fetuses (arrows in Fig. 6A), the knockout exhibited broken pial basement membrane often with protrusion of radial glia endfeet into the pia-arachnoid space of the neocortical wall (arrowheads in Fig. 6B). Some breaks in pial basement membrane were found to be without protrusion of radial glial endfeet (arrowhead in Fig. 6D), suggesting that breaks in the pial basement membrane precedes ectopia of neural cells into the pia-arachnoid space. These breaks were more frequently found in the dorsal aspect than the lateral aspect of the neocortical wall. Neurons could be observed migrating through the disruptions ($ symbol in Fig. 6C). Some had already migrated into the pia-arachnoid space (not shown). These results indicate that disruptions in the pial basement membrane underlie overmigration of neurons.
Figure 6.
Disruptions of the pial basement membrane during development underlie the lamination defects in POMT2f/f;Emx1-Cre+ mice. Transmission EM (A–D) and immunofluorescence staining for laminin-1 (E,F) were carried out to evaluate the pial basement membrane at E13.5 and 15.5, respectively. A: Control. B–D: POMT2f/f;Emx1-Cre+. E: Control. F: POMT2f/f;Emx1-Cre+. Note intact pial basement membrane in the control (arrows in A) and disrupted pial basement membrane in the knockout (arrowheads in B–D). $ symbol in C indicates the leading process of a cell (its nucleus indicated by # symbol) that is migrating through the disruption of the pial basement membrane. Asterisks in F indicate location of the disrupted pial basement membrane and remnants of the pial basement membrane can clearly be identified at lateral aspect of this section (arrowheads). CP, cortical plate; fib, fibroblast; IZ, intermediate zone; KO, knockout; SVZ, subventricular zone; VZ, ventricular zone. Scale bars = 500 nm in A–D; 100 μm in E (applies to F).
By E15.5, many more neurons have migrated past the disrupted pial basement membrane in POMT2f/f;Emx1-Cre+ fetuses. In the POMT2f/f;Emx1-Cre- mice, the developing pia-arachnoid and the pial basement membrane constitute a thick band of immunofluorescence above the developing neocortical wall when stained with an antibody against laminin (arrows in Fig. 6E). This pattern of laminin immunofluorescence was absent in the knockout (Fig. 6F). Instead, the developing pia-arachnoid was disrupted and apparently scattered by neural cells that bypassed the pia-arachnoid. The overmigrated neurons formed a diffuse cell zone (DCZ) above the disrupted pial basement membrane (location indicated by asterisks in Fig. 6F). A thin line of laminin immunofluorescence could often be identified between the DCZ and the cortical plate (CP), especially in the lateral aspect of the neocortical wall (arrowheads in Fig. 6F). This line of staining was also immunoreactive to collagen IV, nidogen, and perlecan, all four major components of the basement membrane, indicating that the line of immunoreactivity constituted the remnants of the original pial basement membrane (not shown). The original pial basement membrane was absent in the adult mutant brain, indicating a postnatal loss during further development of the cortex. These results indicate that the overmigrated neurons in the pia-arachnoid space formed a DCZ above the original pial basement membrane similar to POMGnT1 knockout (Hu et al., 2007). The knockout neocortex was thus formed by the cells in the DCZ and the cells that remained in the cortical plate.
Disruptions of radial glia and Cajal-Retzius cells during neocortical development of POMT2f/f;Emx1-Cre+ mice
To determine whether the radial glia are affected in the developing POMT2f/f;Emx1-Cre+ neocortical wall, E15.5 sections were immunostained with RC-2 antibody. In controls, all radial glial processes (green fluorescence) terminated at the neocortical surface (asterisks in Fig. 7A). In POMT2f/f;Emx1-Cre+ fetuses, however, many radial glial processes protruded out through the neocortical surface (asterisks in Fig. 7B) into the DCZ (see arrowheads for examples), indicating that radial glia extended their processes beyond the disruptions of the pial basement membrane.
Figure 7.
Abnormal radial glial morphology and displacement of Cajal-Retzius cells are associated with neuronal overmigration in POMT2f/f;Emx1-Cre+ mice. E15.5 frozen sections were immunostained with RC2 antibody (green fluorescence) (A,B) and double stained with anti-laminin (red fluorescence) and CR-50 (green fluorescence) (C,D). Propidium iodide (magenta) was used as a nuclear counterstain in A and B while DAPI (blue) was used as a nuclear counterstain stain in C and D. A,C: Cre-negative controls. B,D: POMT2f/f;Emx1-Cre+ mice. Note protrusion of radial glia fibers into the DCZ and displacement of Cajal-Retzius cells in the DCZ (arrowheads in B,D, respectively). Scale bar = 50 μm.
Overmigration of neurons is expected to affect the Cajal-Retzius cells of the marginal zone. Cajal-Retzius cells secrete reelin, a protein that is an essential cue for correct neuronal positioning in the developing neocortex. To evaluate the distribution of Cajal-Retzius cells, E15.5 sections were double-immunostained with CR-50 and laminin-1 antibodies. CR-50-positive Cajal-Retzius cells (green fluorescence) were found under the pial basement membrane (red fluorescence indicated by arrow) in the marginal zone of control fetuses (Fig. 7C). By contrast, Cajal-Retzius cells in the knockout were located between the DCZ and the cortical plate (Fig. 7D); with an absence of or broken pial basement membrane. Some Cajal-Retzius cells were located in the DCZ (arrowheads in Fig. 7D). In some regions, Cajal-Retzius cells were absent, likely due to displacement of the Cajal-Retzius cells from their original location into the DCZ (not shown). These results indicate that the location of Cajal-Retzius cells is abnormal in the knockout.
DISCUSSION
To overcome the early embryonic lethality of POMT2 deficiency, we generated a conditional allele of POMT2 and crossed this line of mice to three different Cre-expressing transgenic lines to study the effects of tissue-specific deletion of O-mannosylation on development of the brain. GFAP-Cre-mediated deletion of POMT2 did not cause neuronal lamination defect in the neocortex. Disruptions of the pial basement membrane were found in only limited areas in these animals. In contrast, when Emx1-Cre knockin line was used to delete the floxed POMT2, extensive lamination defects were observed in both the neocortex and the hippocampus. The lamination defect in the neocortex was caused by migration of neurons past the disrupted pial basement membrane. The different phenotypes in the neocortex between GFAP-Cre- and Emx1-Cre-mediated POMT2 knockouts were likely due to differences in timing of Cre expression in the developing neocortical wall, since GFAP-Cre expression occurs at E13.5, while Emx1-Cre expression is detected at E10.5. In the cerebellum, POMT2f/f;GFAP-Cre+ mice exhibited migration arrest of many granule cells secondary to disruptions of the pial basement membrane. However, the cerebellum of POMT2f/f;Emx1-Cre+ mice exhibited normal histology because Emx1-Cre knockin does not drive Cre expression in the cerebellum. We next examined the role of POMT2-mediated glycosylation in the meninges by crossing POMT2-floxed mice with Wnt1-Cre transgenic mice. Deletion of POMT2 in the meninges covering the forebrain did not affect the development of the neocortex. These results indicate that POMT2-mediated glycosylation is essential in the brain but dispensable in the meninges for maintaining the integrity of the pial basement membrane and normal migration of neurons.
Deficiencies of DG and enzymes (or presumed enzymes) involved in α-DG functional glycosylation result in lamination defects in the neocortex due to disruptions of the pial basement membrane. In POMGnT1 knockout (Hu et al., 2007) and POMT2 brain-specific knockout (this study), disruptions in the pial basement membrane are followed by exodus of migrating neurons out of the neural boundary into the pia-arachnoid space to form a DCZ. The disrupted pial basement membrane eventually disappears during postnatal maturation of the brain. The knockout neocortex is thus formed by the cells that overmigrated (DCZ) and the cells that did not overmigrate (remaining cortical plate). It is likely that the formation of the neocortex in DG knockout and Largemyd mice occur in a similar fashion because the pial basement membrane defect is observed in both animal models at adulthood.
Meninges are a source of retinoic acid that regulate proliferation of neural stem cells (Siegenthaler et al., 2009). It also produces major protein components of the pial basement membrane, which is sandwiched between the meninges and the glia limitans formed by the radial glia endfeet (Sievers et al., 1994). Ultrastructural analyses showed a close apposition of the pial basement membrane with the glia limitans but no apparent association with the meningeal cells. This suggests that the glia limitans but not the meninges may organize the assembly of the pial basement membrane. However, microinjection of a Cre-expressing adenovirus into the pia-arachnoid space was carried out to selectively delete FAK in the meninges. This local deletion of FAK resulted in disruption of the pial basement membrane and overmigration of neurons into the pia-arachnoid space through the disruptions (Beggs et al., 2003). This finding outlines a contrasting model; meninges may also participate in organizing formation of the pial basement membrane or provide additional structural support as an ensheathing tissue.
FAK is a key molecule in mediating integrin signaling. Interestingly, breaches in pial basement membrane are also found in knockouts of α6 and β1 integrins and another integrin signaling mediator integrin-linked kinase (Georges-Labouesse et al., 1998; Graus-Porta et al., 2001; Beggs et al., 2003; Niewmierzycka et al., 2005). Our results show that POMT2 is dispensable in the meninges but essential in the brain for maintaining the integrity of the pial basement membrane. These results are consistent with a model of differential requirement of FAK and POMT2 for maintaining pial basement membrane, FAK is required by the meninges but POMT2 is not. Since POMT2 likely exerts its effect through glycosylation of DG, there may be differential requirement of DG and FAK signaling mechanisms in the meninges for maintaining integrity of the pial basement membrane. We propose that integrin signaling in the meninges is critical for maintaining of the pial basement membrane but dystroglycan signaling is not. It is known that dystroglycan knockout in the brain causes disruptions of the pial basement membrane (Michele et al., 2002; Moore et al., 2002). Further studies are needed to determine whether there is differential requirement in the meninges for integrins (such as α6 and β1 integrin) and dystroglycan and the underlying mechanisms.
It is of some interest that GFAP-Cre mediated deletion of POMT2 resulted in only minor disruptions in the pial basement membrane in only limited regions, with no noticeable lamination defects in the neocortex. In contrast, deletion of DG mediated by the same GFAP-Cre transgenic mice resulted in a more widespread disruptions in the pial basement membrane, with profound neuronal migration defects in the neocortex and reactive gliosis (Moore et al., 2002), similar to POMGnT1 knockout mice (Yang et al., 2007) and POMT2f/f;Emx1-Cre+ mice (this study). These results suggest that GFAP-Cre mediated knockout of POMT2 had a less severe phenotype than GFAP-Cre mediated knockout of DG. The phenotypic differences between GFAP-Cre-mediated knockout of DG and POMT2 suggest that although POMT2-mediated glycosylation is essential for ECM binding activities of α-DG, it is not completely required for all DG functions. Alternatively, POMT1 still keeps some POMT residual activity that is enough to maintain some dystroglycan function.
Why does the pial basement membrane break during development? In POMGnT1 knockout mice the pial basement membrane is normal before E11.5 but disrupted at E13.5 (Hu et al., 2007). In POMT2f/f;Emx1-Cre+ mice the pial basement membrane is assumed to be normal before the start of the Cre expression but also breaks by E13.5. Thus, in both cases disruptions in pial basement membrane occur at a time when the neocortex is undergoing rapid expansion. As the brain size increases, new basement membrane must be assembled to accommodate the expanding surface area. We speculate that reduced binding of the extracellular matrix molecules causes a reduction of the rate of basement membrane assembly, which in turn results in a physically weakened basement membrane that ultimately ruptures due to ongoing brain expansion.
While Emx1-Cre knockin mice express Cre recombinase at E10.5, GFAP-Cre transgenic mice start Cre expression at E13.5 (Zhuo et al., 2001; Gorski et al., 2002). Functional deletion of POMT2 protein likely occurs at a later time than the start of Cre expression. The differential effect of POMT2 deletion on development of the neocortex mediated by GFAP-Cre and Emx1-Cre lines suggest that there is a critical period for O-mannosylation requirement in maintaining the integrity of the pial basement membrane. This critical period is likely the period of rapid neocortical expansion during development. The consequence of POMT2 deficiency on the pial basement membrane is relatively minimal after this critical period. such that migration and lamination of neurons are not affected. Thus, for future gene therapy it may be unnecessary to have a sustained expression in the developing brain to alleviate the neuronal migration defect.
ACKNOWLEDGMENTS
We thank Drs. Eric Olson and Mary Lou Vallano for critical reading of the article; Drs. K. Nakajima and M. Ogawa for supplying CR-50 antibody; the Developmental Studies Hybridoma Bank at the University of Iowa for antibodies.
Grant sponsor: National Institutes of Health; Grant numbers: HD060458 and NS066582 (to H.H).
LITERATURE CITED
- Aguilan JT, Sundaram S, Nieves E, Stanley P. Mutational and functional analysis of Large in a novel CHO glycosylation mutant. Glycobiology. 2009;19:971–986. doi: 10.1093/glycob/cwp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasaka-Manya K, Manya H, Nakajima A, Kawakita M, Endo T. Physical and functional association of human protein O-mannosyltransferases 1 and 2. J Biol Chem. 2006;281:19339–193345. doi: 10.1074/jbc.M601091200. [DOI] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Valero de BD, Currier S, Steinbrecher A, Celli J, van BE, van der ZB, Kayserili H, Merlini L, Chitayat D, Dobyns WB, Cormand B, Lehesjoki AE, Cruces J, Voit T, Walsh CA, van BH, Brunner HG. Mutations in the O-mannosyl-transferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 2002;71:1033–1043. doi: 10.1086/342975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington M, Blake DJ, Prandini P, Brown SC, Torelli S, Benson MA, Ponting CP, Estournet B, Romero NB, Mercuri E, Voit T, Sewry CA, Guicheney P, Muntoni F. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet. 2001a;69:1198–1209. doi: 10.1086/324412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington M, Yuva Y, Prandini P, Brown SC, Torelli S, Benson MA, Herrmann R, Anderson LV, Bashir R, Burgunder JM, Fallet S, Romero N, Fardeau M, Straub V, Storey G, Pollitt C, Richard I, Sewry CA, Bushby K, Voit T, Blake DJ, Muntoni F. Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet. 2001b;10:2851–2859. doi: 10.1093/hmg/10.25.2851. [DOI] [PubMed] [Google Scholar]
- Chai W, Yuen CT, Kogelberg H, Carruthers RA, Margolis RU, Feizi T, Lawson AM. High prevalence of 2-mono- and 2,6-di-substituted manol-terminating sequences among O-glycans released from brain glycopeptides by reductive alkaline hydrolysis. Eur J Biochem. 1999;263:879–888. doi: 10.1046/j.1432-1327.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J Biol Chem. 1997;272:2156–2162. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- Chiyonobu T, Sasaki J, Nagai Y, Takeda S, Funakoshi H, Nakamura T, Sugimoto T, Toda T. Effects of fukutin deficiency in the developing mouse brain. Neuromuscul Disord. 2005;15:416–426. doi: 10.1016/j.nmd.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Currier SC, Lee CK, Chang BS, Bodell AL, Pai GS, Job L, Lagae LG, Al-Gazali LI, Eyaid WM, Enns G, Dobyns WB, Walsh CA. Mutations in POMT1 are found in a minority of patients with Walker-Warburg syndrome. Am J Med Genet A. 2005;133:53–57. doi: 10.1002/ajmg.a.30487. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- de Bernabe DB, van BH, van BE, Van den AW, Kant S, Dobyns WB, Cormand B, Currier S, Hamel B, Talim B, Topaloglu H, Brunner HG. A homozygous nonsense mutation in the fukutin gene causes a Walker-Warburg syndrome phenotype. J Med Genet. 2003;40:845–848. doi: 10.1136/jmg.40.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobyns WB, Kirkpatrick JB, Hittner HM, Roberts RM, Kretzer FL. Syndromes with lissencephaly. II: Walker-Warburg and cerebro-oculo-muscular syndromes and a new syndrome with type II lissencephaly. Am J Med Genet. 1985;22:157–195. doi: 10.1002/ajmg.1320220118. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Wide-spread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Finne J, Krusius T, Margolis RK, Margolis RU. Novel mannitol-containing oligosaccharides obtained by mild alkaline borohydride treatment of a chondroitin sulfate proteoglycan from brain. J Biol Chem. 1979;254:10295–10300. [PubMed] [Google Scholar]
- Gee SH, Blacher RW, Douville PJ, Provost PR, Yurchenco PD, Carbonetto S. Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J Biol Chem. 1993;268:14972–14980. [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Girrbach V, Zeller T, Priesmeier M, Strahl-Bolsinger S. Structure-function analysis of the dolichyl phosphate-mannose: protein O-mannosyltransferase ScPmt1p. J Biol Chem. 2000;275:19288–19296. doi: 10.1074/jbc.M001771200. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Grewal PK, Holzfeind PJ, Bittner RE, Hewitt JE. Mutant glycosyltransferase and altered glycosylation of alpha-dystroglycan in the myodystrophy mouse. Nat Genet. 2001;28:151–154. doi: 10.1038/88865. [DOI] [PubMed] [Google Scholar]
- Haltia M, Leivo I, Somer H, Pihko H, Paetau A, Kivela T, Tarkkanen A, Tome F, Engvall E, Santavuori P. Muscle-eye-brain disease: a neuropathological study. Ann Neurol. 1997;41:173–180. doi: 10.1002/ana.410410208. [DOI] [PubMed] [Google Scholar]
- Hevner RF. Layer-specific markers as probes for neuron type identity in human neocortex and malformations of cortical development. J Neuropathol Exp Neurol. 2007;66:101–109. doi: 10.1097/nen.0b013e3180301c06. [DOI] [PubMed] [Google Scholar]
- Holzfeind PJ, Grewal PK, Reitsamer HA, Kechvar J, Lassmann H, Hoeger H, Hewitt JE, Bittner RE. Skeletal, cardiac and tongue muscle pathology, defective retinal transmission, and neuronal migration defects in the Large(myd) mouse defines a natural model for glycosylation-deficient muscle–eye–brain disorders. Hum Mol Genet. 2002;11:2673–2687. doi: 10.1093/hmg/11.21.2673. [DOI] [PubMed] [Google Scholar]
- Hu H. Polysialic acid regulates chain formation by migrating olfactory interneuron precursors. J Neurosci Res. 2000;61:480–492. doi: 10.1002/1097-4547(20000901)61:5<480::AID-JNR2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hu H, Yang Y, Eade A, Xiong Y, Qi Y. Breaches of the pial basement membrane and disappearance of the glia limitans during development underlie the cortical lamination defect in the mouse model of muscle-eye-brain disease. J Comp Neurol. 2007;501:168–183. doi: 10.1002/cne.21238. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mallebrera C, Brown SC, Sewry CA, Muntoni F. Congenital muscular dystrophy: molecular and cellular aspects. Cell Mol Life Sci. 2005;62:809–823. doi: 10.1007/s00018-004-4510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa M, Nishimoto A, Chiyonobu T, Takeda S, Miyagoe-Suzuki Y, Wang F, Fujikake N, Taniguchi M, Lu Z, Tachikawa M, Nagai Y, Tashiro F, Miyazaki J, Tajima Y, Takeda S, Endo T, Kobayashi K, Campbell KP, Toda T. Residual laminin-binding activity and enhanced dystroglycan glycosylation by LARGE in novel model mice to dystroglycanopathy. Hum Mol Genet. 2009;18:621–631. doi: 10.1093/hmg/ddn387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano H, Kobayashi K, Herrmann R, Tachikawa M, Manya H, Nishino I, Nonaka I, Straub V, Talim B, Voit T, Topaloglu H, Endo T, Yoshikawa H, Toda T. Deficiency of alpha-dystroglycan in muscle-eye-brain disease. Biochem Biophys Res Commun. 2002;291:1283–1286. doi: 10.1006/bbrc.2002.6608. [DOI] [PubMed] [Google Scholar]
- Kim DS, Hayashi YK, Matsumoto H, Ogawa M, Noguchi S, Murakami N, Sakuta R, Mochizuki M, Michele DE, Campbell KP, Nonaka I, Nishino I. POMT1 mutation results in defective glycosylation and loss of laminin-binding activity in alpha-DG. Neurology. 2004;62:1009–1011. doi: 10.1212/01.wnl.0000115386.28769.65. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, Segawa M, Yoshioka M, Saito K, Osawa M, Hamano K, Sakakihara Y, Nonaka I, Nakagome Y, Kanazawa I, Nakamura Y, Tokunaga K, Toda T. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–392. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- Kogelberg H, Chai W, Feizi T, Lawson AM. NMR studies of mannitol-terminating oligosaccharides derived by reductive alkaline hydrolysis from brain glycoproteins. Carbohydr Res. 2001;331:393–401. doi: 10.1016/s0008-6215(01)00051-9. [DOI] [PubMed] [Google Scholar]
- Krusius T, Finne J, Margolis RK, Margolis RU. Identification of an O-glycosidic mannose-linked sialylated tetrasaccharide and keratan sulfate oligosaccharides in the chondroitin sulfate proteoglycan of brain. J Biol Chem. 1986;261:8237–8242. [PubMed] [Google Scholar]
- Li X, Zhang P, Yang Y, Xiong Y, Qi Y, Hu H. Differentiation and developmental origin of cerebellar granule neuron ectopia in POMGnT1 knockout mice. Neuroscience. 2008;152:391–406. doi: 10.1016/j.neuroscience.2007.06.041. [DOI] [PubMed] [Google Scholar]
- Lian G, Sheen V. Cerebral developmental disorders. Curr Opin Pediatr. 2006;18:614–620. doi: 10.1097/MOP.0b013e328010542d. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang L, Wang D, Shen H, Jiang M, Mei P, Hayden PS, Sedor JR, Hu H. Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice. Mech Dev. 2003a;120:1059–1070. doi: 10.1016/s0925-4773(03)00161-8. [DOI] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003b;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ball SL, Yang Y, Mei P, Zhang L, Shi H, Kaminski HJ, Lemmon VP, Hu H. A genetic model for muscle-eye-brain disease in mice lacking protein O-mannose beta1,2-N-acetylglucosaminyltransferase (POMGnT1). Mech Dev. 2006;123:228–240. doi: 10.1016/j.mod.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang Y, Li X, Zhang P, Qi Y, Hu H. Cellular and molecular characterization of abnormal brain development in protein o-mannose N-acetylglucosaminyltransferase 1 knockout mice. Methods Enzymol. 2010;479:353–366. doi: 10.1016/S0076-6879(10)79020-0. [DOI] [PubMed] [Google Scholar]
- Longman C, Brockington M, Torelli S, Jimenez-Mallebrera C, Kennedy C, Khalil N, Feng L, Saran RK, Voit T, Merlini L, Sewry CA, Brown SC, Muntoni F. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet. 2003;12:2853–2861. doi: 10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci U S A. 2004;101:500–505. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Misson JP, Edwards MA, Yamamoto M, Caviness VS., Jr Identification of radial glial cells within the developing murine central nervous system: studies based upon a new immunohistochemical marker. Brain Res Dev Brain Res. 1988;44:95–108. doi: 10.1016/0165-3806(88)90121-6. [DOI] [PubMed] [Google Scholar]
- Montanaro F, Lindenbaum M, Carbonetto S. alpha-Dystroglycan is a laminin receptor involved in extracellular matrix assembly on myotubes and muscle cell viability. J Cell Biol. 1999;145:1325–1340. doi: 10.1083/jcb.145.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- Niewmierzycka A, Mills J, St-Arnaud R, Dedhar S, Reichardt LF. Integrin-linked kinase deletion from mouse cortex results in cortical lamination defects resembling cobblestone lissencephaly. J Neurosci. 2005;25:7022–7031. doi: 10.1523/JNEUROSCI.1695-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Parano E, Pavone L, Fiumara A, Falsaperla R, Trifiletti RR, Dobyns WB. Congenital muscular dystrophies: clinical review and proposed classification. Pediatr Neurol. 1995;13:97–103. doi: 10.1016/0887-8994(95)00148-9. [DOI] [PubMed] [Google Scholar]
- Park D, Xiang AP, Zhang L, Mao FF, Walton NM, Choi SS, Lahn BT. The radial glia antibody RC2 recognizes a protein encoded by Nestin. Biochem Biophys Res Commun. 2009;382:588–592. doi: 10.1016/j.bbrc.2009.03.074. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Stanley P. Mouse large can modify complex N- and mucin O-glycans on alpha-dystroglycan to induce laminin binding. J Biol Chem. 2005;280:20851–20859. doi: 10.1074/jbc.M500069200. [DOI] [PubMed] [Google Scholar]
- Ross ME, Walsh CA. Human brain malformations and their lessons for neuronal migration. Annu Rev Neurosci. 2001;24:1041–1070. doi: 10.1146/annurev.neuro.24.1.1041. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Yamada H, Matsumura K, Shimizu T, Kobata A, Endo T. Detection of O-mannosyl glycans in rabbit skeletal muscle alpha-dystroglycan. Biochim Biophys Acta. 1998;1425:599–606. doi: 10.1016/s0304-4165(98)00114-7. [DOI] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado T, Furukawa T. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- Satz JS, Barresi R, Durbeej M, Willer T, Turner A, Moore SA, Campbell KP. Brain and eye malformations resembling Walker-Warburg syndrome are recapitulated in mice by dystroglycan deletion in the epiblast. J Neurosci. 2008;28:10567–10575. doi: 10.1523/JNEUROSCI.2457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, Napoli JL, Peterson AS, Pleasure SJ. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers J, Mangold U, Berry M. 6-OHDA-induced ectopia of external granule cells in the subarachnoid space covering the cerebellum. Genesis and topography. Cell Tissue Res. 1983;230:309–336. doi: 10.1007/BF00213807. [DOI] [PubMed] [Google Scholar]
- Sievers J, Pehlemann FW, Gude S, Berry M. Meningeal cells organize the superficial glia limitans of the cerebellum and produce components of both the interstitial matrix and the basement membrane. J Neurocytol. 1994;23:135–149. doi: 10.1007/BF01183867. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Kim E. Purification of cranin, a laminin binding membrane protein. Identity with dystroglycan and reassessment of its carbohydrate moieties. J Biol Chem. 1995;270:15425–15433. doi: 10.1074/jbc.270.25.15425. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Haslam SM, Sutton-Smith M, Morris HR, Dell A. Structural analysis of sequences O-linked to mannose reveals a novel Lewis X structure in cranin (dystroglycan) purified from sheep brain. J Biol Chem. 1998;273:23698–236703. doi: 10.1074/jbc.273.37.23698. [DOI] [PubMed] [Google Scholar]
- Takeda S, Kondo M, Sasaki J, Kurahashi H, Kano H, Arai K, Misaki K, Fukui T, Kobayashi K, Tachikawa M, Imamura M, Nakamura Y, Shimizu T, Murakami T, Sunada Y, Fujikado T, Matsumura K, Terashima T, Toda T. Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum Mol Genet. 2003;12:1449–1459. doi: 10.1093/hmg/ddg153. [DOI] [PubMed] [Google Scholar]
- Tang SH, Silva FJ, Tsark WM, Mann JR. A Cre/loxP-deleter transgenic line in mouse strain 129S1/SvImJ. Genesis. 2002;32:199–202. doi: 10.1002/gene.10030. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Smit LM, Barth PG, Catsman-Berrevoets CE, Brouwer OF, Begeer JH, de C I, Valk J. Magnetic resonance imaging in classification of congenital muscular dystrophies with brain abnormalities. Ann Neurol. 1997;42:50–59. doi: 10.1002/ana.410420110. [DOI] [PubMed] [Google Scholar]
- van Reeuwijk J, Janssen M, van den Elzen C, Beltran-Valero de BD, Sabatelli P, Merlini L, Boon M, Scheffer H, Brockington M, Muntoni F, Huynen MA, Verrips A, Walsh CA, Barth PG, Brunner HG, van Bokhoven H. POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J Med Genet. 2005;42:907–912. doi: 10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet E, Melis M, Foidart JM, Van Ewijk W. Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J Histochem Cytochem. 1986;34:883–890. doi: 10.1177/34.7.3519751. [DOI] [PubMed] [Google Scholar]
- Willer T, Amselgruber W, Deutzmann R, Strahl S. Characterization of POMT2, a novel member of the PMT protein O-mannosyltransferase family specifically localized to the acrosome of mammalian spermatids. Glycobiology. 2002;12:771–783. doi: 10.1093/glycob/cwf086. [DOI] [PubMed] [Google Scholar]
- Willer T, Prados B, Falcon-Perez JM, Renner-Muller I, Przemeck GK, Lommel M, Coloma A, Valero MC, de Angelis MH, Tanner W, Wolf E, Strahl S, Cruces J. Targeted disruption of the Walker-Warburg syndrome gene Pomt1 in mouse results in embryonic lethality. Proc Natl Acad Sci U S A. 2004;101:14126–14131. doi: 10.1073/pnas.0405899101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- Winder SJ. The complexities of dystroglycan. Trends Biochem Sci. 2001;26:118–124. doi: 10.1016/s0968-0004(00)01731-x. [DOI] [PubMed] [Google Scholar]
- Yamada H, Shimizu T, Tanaka T, Campbell KP, Matsumura K. Dystroglycan is a binding protein of laminin and merosin in peripheral nerve. FEBS Lett. 1994;352:49–53. doi: 10.1016/0014-5793(94)00917-1. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhang P, Xiong Y, Li X, Qi Y, Hu H. Ectopia of meningeal fibroblasts and reactive gliosis in the cerebral cortex of the mouse model of muscle-eye-brain disease. J Comp Neurol. 2007;505:459–477. doi: 10.1002/cne.21474. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, Herrmann R, Straub V, Talim B, Voit T, Topaloglu H, Toda T, Endo T. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science. 2010;327:88–92. doi: 10.1126/science.1180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Betel D, Schachter H. Cloning and expression of a novel UDP-GlcNAc:alpha-D-mannoside beta1,2-N-acetylglucosaminyltransferase homologous to UDP-GlcNAc:alpha-3-D-mannoside beta1,2-N-acetylglucosaminyltransferase I. Biochem J. 2002;361(Pt 1):153–162. doi: 10.1042/0264-6021:3610153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, varez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]