Previews
Cancer cells reprogram their metabolism to support a high proliferative rate. A new study shows that, upon serine starvation, the tumor suppressor p53 activates p21 to shift metabolic flux from purine biosynthesis to glutathione production, which enhances cellular proliferation and viability by combating ROS (Maddocks et al., 2012).
Cancer cells have designated hallmarks differentiating them from non-neoplastic cells: genomic instability, resistance to cell death, uncontrolled proliferation, and metabolic reprogramming, to name a few (Hanahan and Weinberg, 2011). The latter has been gaining increased attention. Cancer cells can reprogram their metabolism by shifting from oxidative phosphorylation to aerobic glycolysis, which defines the Warburg effect (Ward and Thompson, 2012). Although aerobic glycolysis produces far less ATP, an increase in macromolecule production along with an avoidance of heightened ROS accumulation from oxidative phosphorylation have been postulated for this metabolic reprograming (Vander Heiden et al., 2009). Recent advances have shown that several features of altered metabolism can be dictated by specific oncogenes or tumor suppressors (Ward and Thompson, 2012). Classically known for inhibiting malignant transformation by regulating DNA repair, cell cycle arrest, and apoptosis, the tumor suppressor p53 also upregulates metabolic targets to inhibit tumorigenesis (Li et al., 2012). Further, recent evidence suggests that p53 may also regulate glycolysis and oxidative phosphorylation in a cell- and context-specific manner (Gottlieb and Vousden, 2010). In an elegant study conducted by Karen Vousden’s group, Maddocks et al. demonstrate that serine starvation activates p53 to reprogram metabolism and increase cancer cell survival (Maddocks et al., 2012).
Reprograming metabolic flux makes cancer cells increasingly dependent on specific metabolites; de novo serine synthesis has been recently shown to be crucial for cancer cell proliferation and survival (Chaneton et al., 2012; Locasale et al., 2011). Under serine starvation conditions, Maddocks et al. initially observed a decrease in cell proliferation and survival that was more pronounced in p53-deficient colon cancer cells compared to isogenic p53 wild-type cells. When these tumor cells were xenografted in nude mice fed a diet lacking serine, p53-deficient tumors exhibited a stronger decrease in tumor volume than wild-type tumors (Maddocks et al., 2012). It has recently been shown that serine deprivation forces cancer cells into a ‘fuel-efficient mode’ where the glycolytic intermediate 3-phosphoglycerate (3-PG) shuttles into the Serine Synthesis Pathway (SSP), while pyruvate enters the TCA cycle, thereby increasing oxidative phosphorylation and limiting the production of lactate (Chaneton et al., 2012). Maddocks et al. found that SSP enzymes were similarly activated upon serine starvation, independent of p53 status, ruling out a defective SSP and subsequent improper de novo serine synthesis as a potential candidate for the observed proliferative failure in p53−/− cells (Maddocks et al., 2012). Using oxygen consumption as a read-out for oxidative phosphorylation, the authors showed that serine deprivation increased oxygen consumption in p53−\− colon cancer cells while p53+\+ cells had decreased respiration. Although the glycolytic flux channeled to the TCA cycle was initially indistinguishable between the two genotypes, TCA intermediates returned to basal levels in wild-type cells, while p53 deficient-cells sustained cycling after serine starvation, therefore increasing oxygen consumption (Maddocks et al., 2012). In the absence of serine, 3-PG was re-routed towards serine synthesis, which limited the amount of pyruvate available for ATP production. The authors then tested the hypothesis that increasing TCA cycle flux by adding exogenous pyruvate would restore ATP levels and improve proliferation after serine starvation: indeed, a partial rescue of cellular proliferation was observed, caused specifically by an increase in TCA flux and not from the de novo conversion of 3-PG into serine.
Serine is converted through a glycine intermediate to produce either inosine monophosphate (IMP) to generate purines (GMP and AMP) or glutathione (GSH), a known anti-oxidant (Figure 1). Maddocks et al. found that serine starvation decreased GMP and AMP levels in both p53+/+ and p53−/− colon cancer cells (Maddocks et al., 2012). Depleted GMP has been shown to induce a p53/p21-dependent cell cycle arrest (Linke et al., 1996). Consistent with these findings, serine starvation increased p53 expression levels, which markedly induced p21 levels to trigger a transient cell cycle arrest, not observed in either p53−\− or p21−\− isogenic cancer cells (Maddocks et al., 2012). Moreover, cell survival in p21−\− cancer cells was similar to isogenic p53−\− cancer cells, underscoring the importance of p21 activation after serine removal. Lastly, the authors found that p53 expression levels influenced serine utilization and showed that p53+\+ cancer cells failed to convert serine into IMP while both p53−\− and p21−\− isogenic cancer cells retained this ability. Conversely, although GSH levels decreased after serine starvation, p53+\+ cancer cells enhanced GSH flux unlike the p53−\− or p21−\− isogenic cells. To test the importance of the antioxidant activity of GSH, exogenous GSH was added to p53−\− cells starved of serine. Cellular proliferation was only partially rescued with the addition of GSH, while it was fully restored when GSH was combined with exogenous pyruvate, (Maddocks et al., 2012).
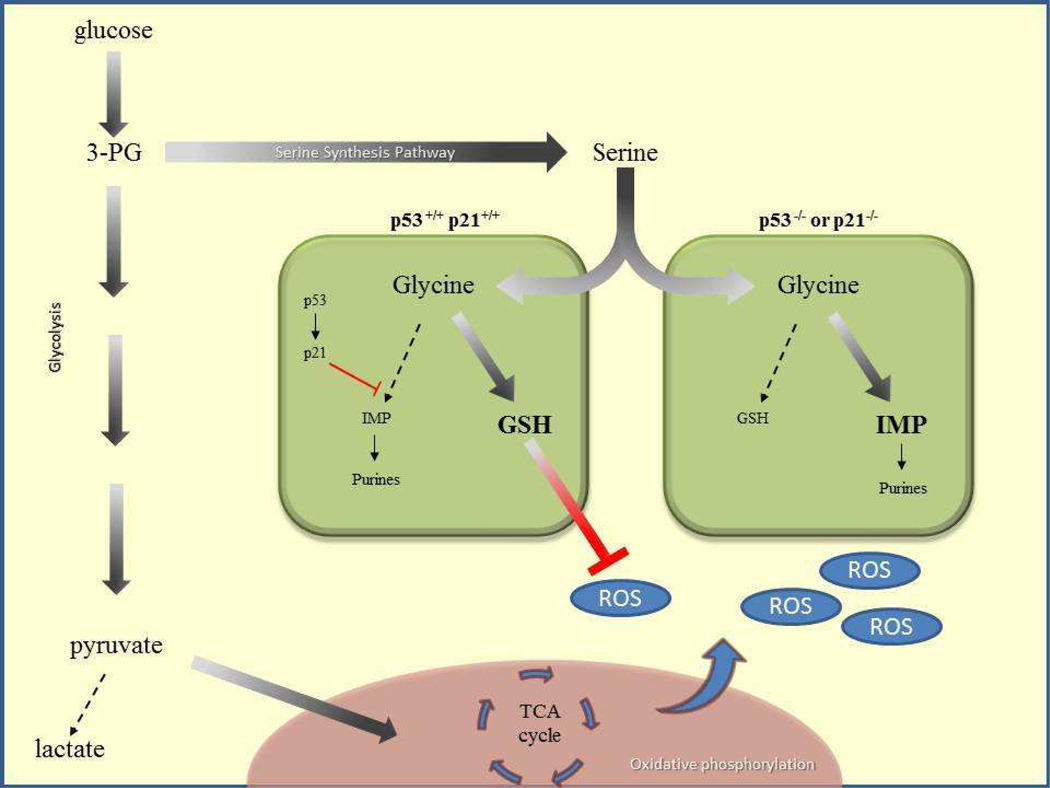
Figure. Schematic of p53 controlling metabolic fate upon serine starvation.
Through glycolysis, glucose is converted into 3-PG, 3-phosphoglycerate, and shuttled into the serine synthesis pathway, as the demand for serine is great. Serine is then converted into glycine, where, p53 is elevated and activates p21 to promote cell cycle arrest and replenish GSH, glutathione. Conversion to inosine monophosphate (IMP) and resultant purine biosynthesis is suppressed. GSH pools suppress reactive oxygen species (ROS) generated from the TCA cycle. Cells lacking p53 or p21 generate IMP instead of replenishing GMP pools and succumb to excessive ROS.
Taken together, this study reveals that the proliferative defects seen in p53−\− cells after serine starvation result from decreased pyruvate caused by the shuttling of glycolytic intermediates into serine biosynthesis, as well as the lack of GSH production to combat increased ROS generated from the TCA cycle. Furthermore, as serine starvation decreases GMP levels, p53 is activated and induces a p21 transient cell cycle arrest, allowing for a recovery in GSH pools (Figure 1). The study by Maddocks et al. raises a number of interesting questions. How does p53 senses metabolic distress under serine starvation or other metabolic stimuli? Additionally, since p21 is not critical for p53-mediated tumor suppression (Li et al., 2012), are additional downstream partners of p53 regulating this pathway? Considering the specific regulation of the M2 isoform of pyruvate kinase (PKM2) in cancer, as well as the suppression of PKM2 activity by serine deprivation (Chaneton et al., 2012) and the described roles for p53, it is tempting to speculate that p53 may regulate PKM2 levels, or vice versa.
The study by Maddocks et al. adds to the complexity of the roles of p53 in tumor suppression. Targeting metabolic enzymes involved in the Serine Synthesis Pathway or in the production of glutathione that can either mimic or work in concert with serine starvation may help in the design of future therapies for cancers harboring mutated or complete loss of p53.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Chaneton B, Hillmann P, Zheng L, Martin AC, Maddocks OD, Chokkathukalam A, Coyle JE, Jankevics A, Holding FP, Vousden KH, Frezza C, O'Reilly M, Gottlieb E. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E, Vousden KH. p53 regulation of metabolic pathways. Cold Spring Harb Perspect Biol. 2010;2:a001040. doi: 10.1101/cshperspect.a001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W. Tumor Suppression in the Absence of p53-Mediated Cell-Cycle Arrest, Apoptosis, and Senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke SP, Clarkin KC, Di Leonardo A, Tsou A, Wahl GM. A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev. 1996;10:934–947. doi: 10.1101/gad.10.8.934. [DOI] [PubMed] [Google Scholar]
- Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, Sasaki AT, Anastasiou D, Mullarky E, Vokes NI, Sasaki M, Beroukhim R, Stephanopoulos G, Ligon AH, Meyerson M, Richardson AL, Chin L, Wagner G, Asara JM, Brugge JS, Cantley LC, Vander Heiden MG. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks OD, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, Vousden KH. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2012 doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]