Abstract
Lipoprotein lipase (LPL) is synthesized primarily in muscle and adipose tissue and by hydrolyzing triglycerides in chylomicrons and very low density lipoprotein allows uptake of the resultant free fatty acids by these tissues. This report describes the cloning of the mouse LPL gene from which probes were derived to study the regulation of LPL synthesis in the 3T3-L1 adipocyte cell culture system. Preconfluent 3T3-L1 preadipocytes had very small amounts of LPL mRNA (less than 1 pg/micrograms of RNA). At confluency, LPL mRNA levels increased to 5 to 15 pg/micrograms of RNA. After insulin and dexamethasone were added, LPL activity and mRNA levels rose in parallel. Peak mRNA levels were reached within 4 to 10 days, achieving LPL mRNA concentrations of 150 to 500 pg/micrograms of RNA. This represents a 15- to 50-fold increase over confluent cells. Two cytokines known to diminish adipose tissue LPL activity were studied to see how their effects were regulated. Recombinant human cachectin/tumor necrosis factor diminished both LPL activity and LPL mRNA levels. The effect on LPL activity compared with mRNA levels was quicker, at a lower dose, and more complete (95 versus 75% maximum effect). The effect of recombinant human cachectin tumor necrosis factor on LPL mRNA levels was shown by nuclear run-on experiments to be exerted transcriptionally. It was also independent of new protein synthesis. Recombinant human interleukin-1 alpha diminished only LPL activity but not mRNA levels. This study suggests that during times of stress, cytokines secreted by activated macrophages can alter energy balance by affecting transcriptional and posttranscriptional processes in adipocytes.
Full text
PDF


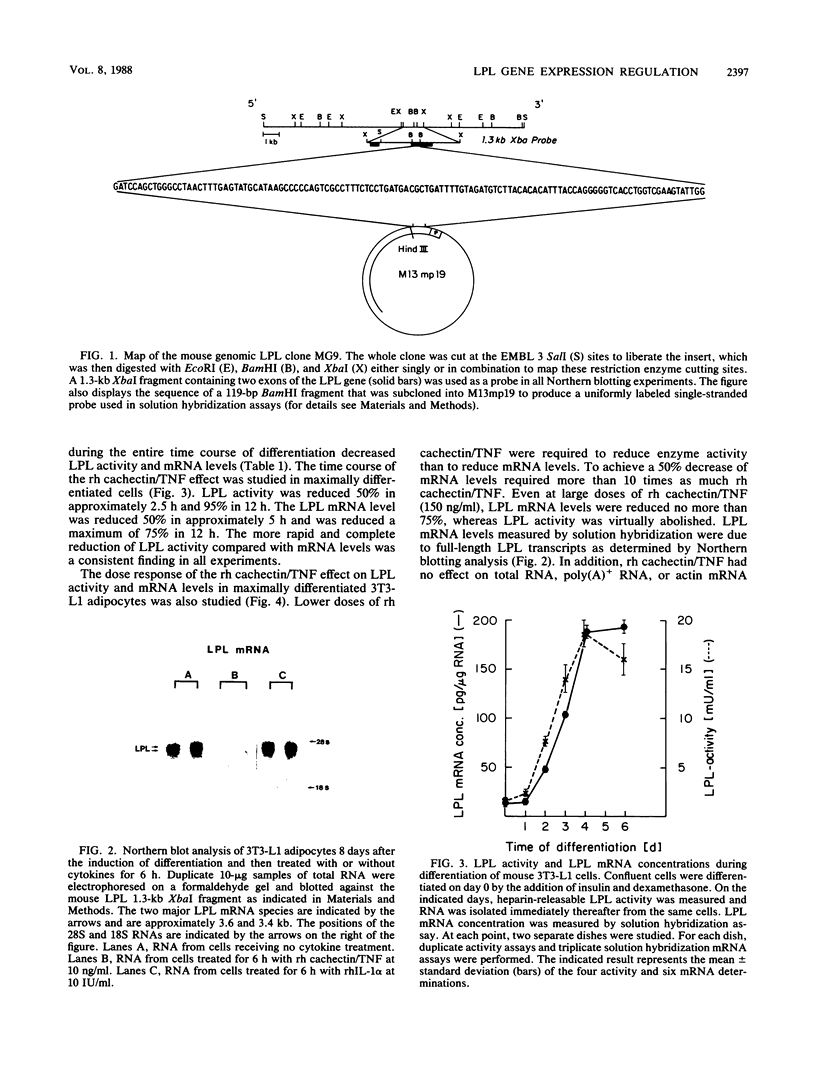
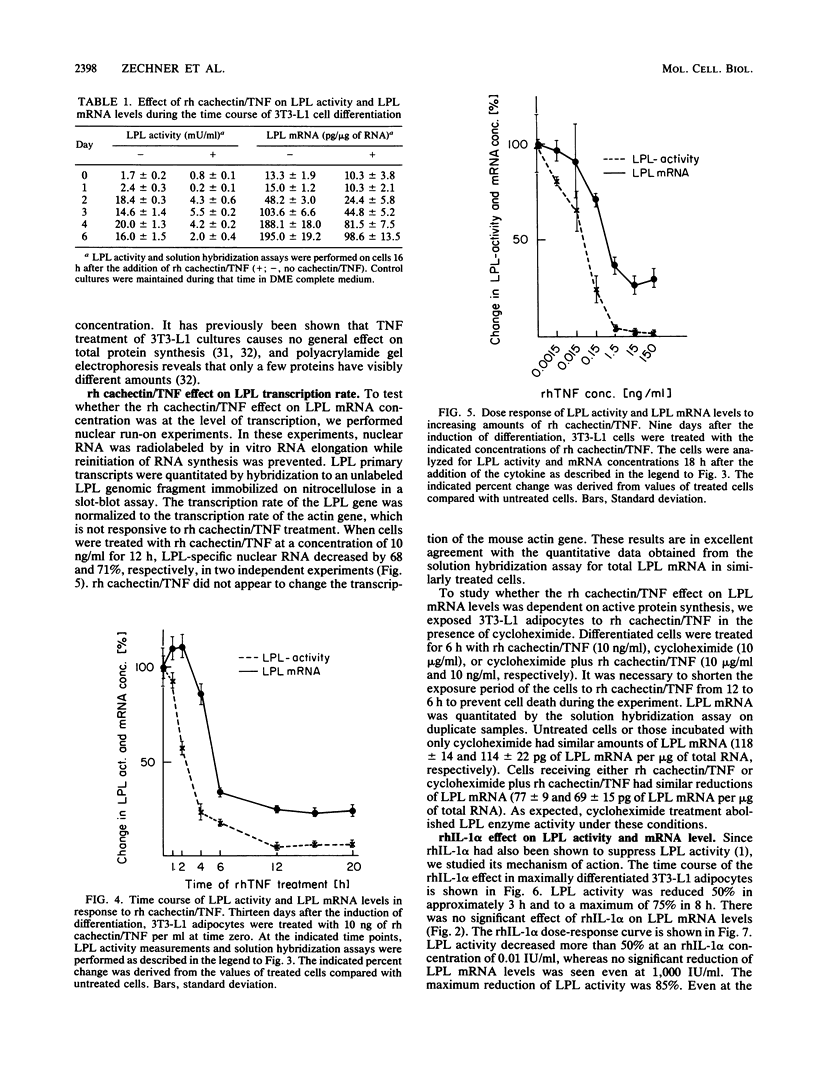
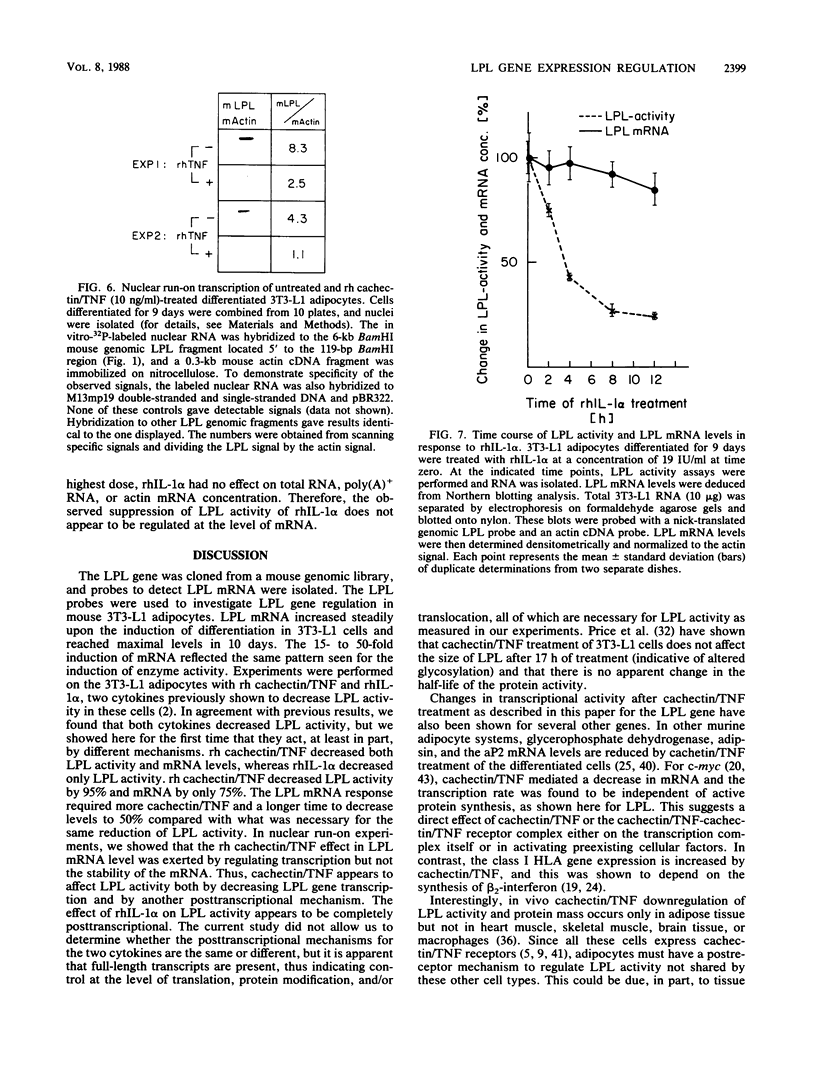

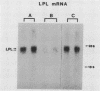
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami A., Ikeda Y., Le Trang N., Hotez P. J., Beutler B. Weight loss associated with an endotoxin-induced mediator from peritoneal macrophages: the role of cachectin (tumor necrosis factor). Immunol Lett. 1985;11(3-4):173–177. doi: 10.1016/0165-2478(85)90165-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Lai E., Darnell J. E., Jr Transcriptional control of the mouse prealbumin (transthyretin) gene: both promoter sequences and a distinct enhancer are cell specific. Mol Cell Biol. 1986 Dec;6(12):4697–4708. doi: 10.1128/mcb.6.12.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasey A. A., Yamamoto R., Vitt C. R. A high molecular weight component of the human tumor necrosis factor receptor is associated with cytotoxicity. Proc Natl Acad Sci U S A. 1987 May;84(10):3293–3297. doi: 10.1073/pnas.84.10.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer A. Tissue lipoprotein lipase activity and its action in lipoprotein metabolism. Int J Biochem. 1981;13(5):525–541. doi: 10.1016/0020-711x(81)90177-4. [DOI] [PubMed] [Google Scholar]
- Dobson D. E., Groves D. L., Spiegelman B. M. Nucleotide sequence and hormonal regulation of mouse glycerophosphate dehydrogenase mRNA during adipocyte and muscle cell differentiation. J Biol Chem. 1987 Feb 5;262(4):1804–1809. [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature. 1985 Feb 14;313(6003):597–600. doi: 10.1038/313597a0. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Hybridization of oligo(dT) to RNA on nitrocellulose. Gene Anal Tech. 1987 Mar-Apr;4(2):17–22. doi: 10.1016/0735-0651(87)90013-6. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med. 1981 Sep 1;154(3):631–639. doi: 10.1084/jem.154.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner T. G., Svenson K. L., Lusis A. J., Schotz M. C. The sequence of cDNA encoding lipoprotein lipase. A member of a lipase gene family. J Biol Chem. 1987 Jun 25;262(18):8463–8466. [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Krönke M., Schlüter C., Pfizenmaier K. Tumor necrosis factor inhibits MYC expression in HL-60 cells at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1987 Jan;84(2):469–473. doi: 10.1073/pnas.84.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff T., Elkaim R., Goding C. R., Jalinot P., Sassone-Corsi P., Perricaudet M., Kédinger C., Chambon P. Individual products of the adenovirus 12S and 13S EIa mRNAs stimulate viral EIIa and EIII expression at the transcriptional level. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4381–4385. doi: 10.1073/pnas.81.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Min H. Y., Spiegelman B. M. Adipsin, the adipocyte serine protease: gene structure and control of expression by tumor necrosis factor. Nucleic Acids Res. 1986 Nov 25;14(22):8879–8892. doi: 10.1093/nar/14.22.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T. C., Dawson P. A., Rudel L. L., Williams D. L. Quantitation of apolipoprotein E mRNA in the liver and peripheral tissues of nonhuman primates. J Biol Chem. 1985 Feb 25;260(4):2452–2457. [PubMed] [Google Scholar]
- Nilsson-Ehle P., Garfinkel A. S., Schotz M. C. Lipolytic enzymes and plasma lipoprotein metabolism. Annu Rev Biochem. 1980;49:667–693. doi: 10.1146/annurev.bi.49.070180.003315. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Schotz M. C. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976 Sep;17(5):536–541. [PubMed] [Google Scholar]
- Patton J. S., Shepard H. M., Wilking H., Lewis G., Aggarwal B. B., Eessalu T. E., Gavin L. A., Grunfeld C. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8313–8317. doi: 10.1073/pnas.83.21.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala P. H., Kawakami M., Angus C. W., Lane M. D., Cerami A. Selective inhibition of synthesis of enzymes for de novo fatty acid biosynthesis by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2743–2747. doi: 10.1073/pnas.80.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. R., Olivecrona T., Pekala P. H. Regulation of lipoprotein lipase synthesis by recombinant tumor necrosis factor--the primary regulatory role of the hormone in 3T3-L1 adipocytes. Arch Biochem Biophys. 1986 Dec;251(2):738–746. doi: 10.1016/0003-9861(86)90384-x. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey P. G., Whittaker P. A., Southern E. M. Removal of repeated sequences from hybridisation probes. Nucleic Acids Res. 1985 Mar 25;13(6):1905–1922. doi: 10.1093/nar/13.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semb H., Peterson J., Tavernier J., Olivecrona T. Multiple effects of tumor necrosis factor on lipoprotein lipase in vivo. J Biol Chem. 1987 Jun 15;262(17):8390–8394. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Student A. K., Hsu R. Y., Lane M. D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980 May 25;255(10):4745–4750. [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Yip Y. K., Vilcek J. Tumor necrosis factor: specific binding and internalization in sensitive and resistant cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7626–7630. doi: 10.1073/pnas.82.22.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Newman T. C., Shelness G. S., Gordon D. A. Measurement of apolipoprotein mRNA by DNA-excess solution hybridization with single-stranded probes. Methods Enzymol. 1986;128:671–689. doi: 10.1016/0076-6879(86)28099-4. [DOI] [PubMed] [Google Scholar]
- Yarden A., Kimchi A. Tumor necrosis factor reduces c-myc expression and cooperates with interferon-gamma in HeLa cells. Science. 1986 Dec 12;234(4782):1419–1421. doi: 10.1126/science.3097823. [DOI] [PubMed] [Google Scholar]