Abstract
Aims
Although cardiac resynchronization therapy (CRT) reduces morbidity and mortality in patients with heart failure, a significant minority of patients do not respond adequately to this therapy. The objective of this study was to examine the impact of a ‘multidisciplinary care’ (MC) approach on the clinical outcome in CRT patients.
Methods and results
The clinical outcome in patients prospectively receiving MC (n = 254) was compared with a control group of patients who received conventional care (CC, n = 173). The MC group was followed prospectively in an integrated clinic setting by a team of subspecialists from the heart failure, electrophysiology, and echocardiography service at 1-, 3-, and 6-months post-implant. All patients had echocardiographic-guided optimization at their 1-month visit. The proportional hazards model (adjusting for all covariates) and Kaplan–Meier time to first event curves were compared between the two groups, over a 2-year follow-up. The long-term outcome was measured as a combined endpoint of heart failure hospitalization, cardiac transplantation, or all-cause mortality. The clinical characteristics between the MC and CC groups at baseline were comparable (age, 68 ± 13 vs. 69 ± 12; NYHA III, 90 vs. 82%; ischaemic cardiomyopathy 55 vs. 64%, P = NS, respectively). The event-free survival was significantly higher in the multidisciplinary vs. the CC group (P = 0.0015). A significant reduction in clinical events was noted in the MC group vs. the CC group (hazard ratio: 0.62, 95% CI: 0.46–0.83, P = 0.001).
Conclusion
Integrated MC may improve 2-year event-free survival in patients receiving cardiac resynchronization therapy. Prospective randomized studies are needed to validate our findings.
Keywords: Cardiac resynchronization therapy, Heart failure, Integrated clinic, Multidisciplinary care
Introduction
Several prospective randomized studies have shown that cardiac resynchronization therapy (CRT) is associated with a significant reduction in hospitalization rates for heart failure (HF) and improved long-term survival.1–3 Consequently, CRT has gained widespread acceptance as a safe and efficacious therapeutic strategy for patients with advanced HF and evidence of systolic dysfunction (ejection fraction ≤35%), intraventricular conduction delay (QRS duration >120 ms), and HF symptoms refractory to maximal medical therapy (NYHA class III and IV). Recent publications have also demonstrated benefit in even mildly symptomatic (class I and II NYHA) HF patients.4–6
Although CRT has been shown to favourably alter the natural course of HF, significant proportions of patients remain non-responsive and have recurrent hospitalization for HF.1–6 Although previous reports have suggested that multidisciplinary strategies for congestive HF are associated with a reduction in HF hospitalizations, this has not been examined in the CRT patient population. Heart failure patients with implanted CRT devices are a frail group of patients often requiring care from multiple cardiovascular subspecialties inclusive of the HF, electrophysiology (EP), and echocardiography (ECHO)/imaging services. In most instances, the care delivered is fragmented with limited cross talk within the subspecialties. There has been a recent emphasis on disease management initiatives to integrate the care delivered to the CRT patient between the different subspecialties. The aim of this study was to examine the impact of this ‘multidisciplinary care’ (MC) approach on the clinical outcome in CRT patients.
Methods
The multidisciplinary clinic
The Massachusetts General Hospital (MGH) CRT clinic was established in November 2005 to provide MC to HF patients receiving CRT devices. The clinic works in conjunction with referring physicians consisting of primary cardiologists, advanced HF cardiologists, and electrophysiologists both inside and outside of the MGH system who provide care to patients with refractory HF. Patients are referred at various stages throughout the process of HF management and device implantation, although, in most cases, patients first visit the CRT clinic just before or just after CRT implantation. The primary objective is to provide MC during individual patient visits.
The clinic consists of a team of physicians, nurse practitioners, technologists, and support staff with expertise in HF, EP/arrhythmia/device management, and ECHO. Figure 1 outlines the post-CRT device implant MC clinic protocol. The protocol consists of three visits to the MC clinic over a 6-month period, after which patients who respond well to CRT typically return to conventional care (CC). The first MC CRT clinic visit typically occurs 1 month after implant. At this visit, patients undergo a 6-minute walk test, quality of life assessment (using the Minnesota Living with Heart Failure Questionnaire), and device interrogation. In addition, an ECHO-guided atrio-ventricular and inter-ventricular device optimization by a physician echocardiographer is performed. The patient is evaluated by both an EP and HF specialist in order to adjust medications, refer for relevant diagnostic tests, and make any necessary device adjustments.
Figure 1.
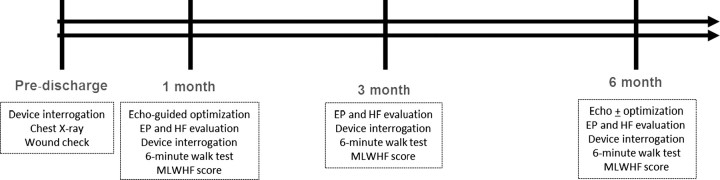
Schematic representation of multidisciplinary care. The figure outlines the components of the integrated care delivered at 1-, 3-, and 6-months post-CRT implant. EP, electrophysiology; HF, heart failure; MLWHFQ, Minnesota living with heart failure questionnaire.
Second visit occurs at 3 months, where the patient once again undergoes a 6-minute walk test, quality of life assessment, device interrogation, and assessment by an EP and HF specialist. There is careful evaluation of device diagnostics including assessment of heart rate variability, activity monitors, arrhythmia burden, frequency of premature ventricular contractions (PVCs) and per cent biventricular pacing with a particular focus to identify and correct problems for those patients who may have little or no symptomatic improvement at this early stage after CRT.
The third visit occurs at 6 months. At this visit, the patient once again undergoes a 6-minute walk test, quality of life assessment, device interrogation, and assessment by an EP and HF physician. An echocardiogram is performed to assess for left ventricular remodelling. Patients subsequently ‘graduate’ from the clinic and continue to the follow-up in a CC setting. Those patients showing continued evidence of lack of improvement in the form of HF hospitalization or refractory symptoms after CRT are re-evaluated and may undergo repeat echo-guided device optimization as well as comprehensive assessment for causes of non-response.
Data were collected prospectively on each patient seen in the CRT clinic. Patients who underwent de novo CRT device implant or upgrade from a pacemaker or defibrillator between September 2005 and February 2010, and were seen, or scheduled to be seen, in the MC clinic were the patients that were included in the study and grouped into the MC cohort. Prospectively obtained baseline characteristics and clinical outcomes including death, cardiac transplant, and HF hospitalization were reconfirmed with review of the electronic medical record and comparison with the social security death index (SSDI). The current project and proposed analysis was approved by the MGH Institutional Review Board and Ethics Committee.
Conventional care
In the CC setting, patients were seen as needed by each subspecialist and in EP device clinic in separate visits at varying intervals. Echocardiogram-guided optimizations were dictated by physician discretion and not performed routinely. Patients who underwent CRT device implantation and were followed conventionally at MGH between March 2003 and November 2009 (i.e. were never seen in the MC) were included as part of the CC cohort. Either due to physician or patient preference a small number patients (n = 25) did not to participate in the MC approach. Medical records were retrospectively reviewed for baseline characteristics using pre-specified search parameters. The clinical outcome was obtained from the medical records and by a search of the SSDI where appropriate. Hospitalizations for HF were adjudicated by a blinded reviewer.
Follow-up
All patients were followed up for hard clinical endpoints, i.e. all-cause mortality, HF hospitalizations, left ventricular-assist device implantation and cardiac transplant. For both cohorts, HF hospitalization was defined as inpatient admission with signs and/or symptoms of HF, including shortness of breath, peripheral oedema, and/or congestion on the chest radiograph and improvement of these signs and/or symptoms with medical therapy. Left ventricular lead location was adjudicated using venous angiograms, PA, and lateral chest radiographs.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation and were compared using the unpaired two-tailed Student's t-test. Categorical variables are expressed as a percentage and were compared using Fisher's exact probability test. Survival curves were constructed using the Kaplan–Meier method and compared between the two groups using the log-rank test. Patients were censored after their first event. In a secondary analysis, a blanking period of 1 month was used to rule out the confounding influence of early procedure-related events (prior to the first post-implant MC clinic visit). For both analyses, the follow-up was truncated at 2 years. Unadjusted and adjusted Cox proportional hazards models controlling for all variables for which the two cohorts showed differences with a P-value <0.2 at baseline were used to compare the risk for an event between the groups. We determined whether interaction occurred between pre-selected covariates and the two groups in unadjusted Cox regression models and provided separated hazard rate ratio regarding death/transplant/HF hospitalization between the MC and CC group for the clinically defined subgroups. The proportional hazards assumption was tested for all variables within the main 2-year outcome Cox regression model using cumulative sums of martingale residuals (Kolmogorov–Smirnov Supremum test),7 with no violations observed. Paired t-test was used to test whether the change between baseline and follow-up of echo parameters (LV ejection fraction and end-diastolic/systolic diameter) differed significantly from zero. Further, unpaired t-test was used to assess differences in these measures between the two groups. All performed tests were two-sided and a P-value of <0.05 was considered as statistically significant. All analyses were performed with SAS (version 9.2, SAS Institute, Inc., Cary, NC, USA).
Results
Patient population
There were 254 patients included in the MC cohort, inclusive of three patients who died prior to their first scheduled visit in the clinic. There were 173 patients included in the CC cohort including 7 patients who were initially followed conventionally for >6 months but were subsequently referred to the MC after an HF decompensation clinical event. Most baseline characteristics of patients in the MC group were comparable with those in the CC group (Table 1). However, there were a higher proportion of CC patients with NYHA functional class IV symptoms, prior revascularization, and who were taking loop diuretics and digoxin. The baseline left ventricular ejection fraction (LVEF) was also slightly lower in the CC group.
Table 1.
Baseline characteristics
| Variable | Multidisciplinary care (n = 254) | Conventional care (n = 173) | P-value |
|---|---|---|---|
| Age (SD) | 68 ± 13 | 69 ± 12 | 0.41 |
| Male (%) | 205 (81) | 142 (82) | 0.80 |
| QRS (ms)a | 160.4 ± 29 | 159.4 ± 28 | 0.73 |
| NYHA class IVa (%) | 23 (10) | 16 (18) | 0.05 |
| HTN (%) | 186 (73) | 130 (75) | 0.73 |
| DM (%) | 102 (40) | 70 (40) | 1.00 |
| Atrial fibrillation (%) | 149 (59) | 110 (64) | 0.32 |
| Ischaemic cardiomyopathy (%) | 140 (55) | 111 (64) | 0.07 |
| CAD (%) | 162 (64) | 127 (73) | 0.045 |
| Post-CABG (%) | 124 (49) | 107 (62) | 0.01 |
| Valve surgery (%) | 40 (16) | 26 (15) | 0.89 |
| Creatinine >2 prior to implant (%) | 41 (18) | 33 (22) | 0.29 |
| Loop diuretics (%) | 217 (85) | 161 (93) | 0.02 |
| Aldosterone antagonist (%) | 90 (35) | 57 (33) | 0.68 |
| Digoxin (%) | 92 (36) | 100 (58) | <0.0001 |
| Beta-blockers (%) | 228 (90) | 147 (85) | 0.17 |
| ACE inhibitors/AR blockers (%) | 209 (82) | 136 (79) | 0.38 |
| New implant (%) | 149 (59) | 102 (59) | 1.00 |
| Transvenous (%) | 237 (93) | 160 (93) | 1.00 |
| Apical lead locationa (%) | 55 (22) | 25 (16) | 0.12 |
| Non-lateral lead locationa (%) | 36 (15) | 42 (26) | 0.004 |
| Baseline ejection fraction (%)a | 24.2 ± 6.8 | 22.5 ± 7.2 | 0.02 |
| Baseline end-systolic diametera | 54.9 ± 9.1 | 54.1 ± 9.1 | 0.47 |
| Baseline end-diastolic diametera | 62.9 ± 8.9 | 62.0 ± 8.5 | 0.32 |
aAmong patients with available data.
Clinical outcome
In total, 177 patients had at least one event within 2 years. There were 87 deaths, 14 transplants, and 137 hospitalizations because of HF. In the MC cohort (n = 254), 88 patients had at least one event (46 deaths, 7 transplants, 70 hospitalizations because of HF). In the CC cohort (n = 173), 89 patients had at least one event (41 deaths, 7 transplants, 67 hospitalizations because of HF). Among the entire cohort of the study (n = 315), 74% either completed 2 years of follow-up or died prior to this. The median follow-up time was 24 months with an inter-quartile range of 13–24 months. Kaplan–Meier estimates for survival free of cardiac transplant or HF at 2 years were performed (Figure 2A).
Figure 2.
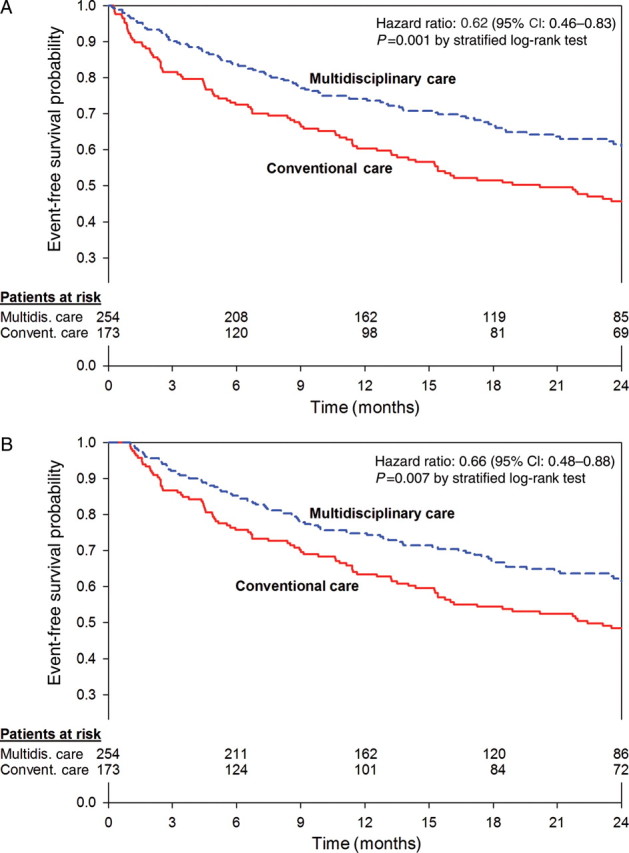
Kaplan–Meier estimates of the probability of survival free of heart failure or death. (A) The Kaplan–Meier curve of survival free of heart failure hospitalization or death at 2 years for the multidisciplinary care and the conventional care cohorts. (B) The Kaplan–Meier analysis for these two groups in which events occurring within the first 30 days of implant are censored to eliminate the influence of procedure-related complications.
After 1 and 2 years, 89 and 77% of patients in the MC cohort survived, whereas only 78 and 65% survived within the CC cohort, respectively (log-rank test, P = 0.01). Subjects receiving MC had a lower risk for death over a 2-year follow-up in comparison to the CC cohort (HR: 0.58, 95% CI: 0.38–0.88, P = 0.01).
After 1 and 2 years, patients in the MC cohort remained event-free at a rate of 73 and 61%, while only 61 and 46% were event free within the CC cohort, respectively (log-rank test, P = 0.001). Accordingly, the risk for an event within 2 years was reduced in the MC cohort when compared with CC (HR: 0.62, 95% CI: 0.46–0.83, P = 0.001). This result remained significant when the analysis included a 30-day blanking period in which 17 early events were not included in order to account for any difference in procedurally related complications between the two groups (HR: 0.66, 95% CI: 0.49–0.89, P = 0.007; Figure 2B).
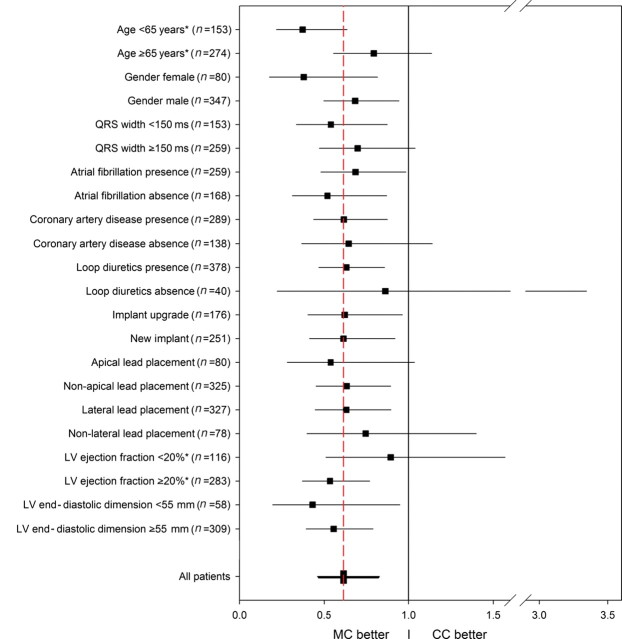
A Cox proportional hazards model was constructed with all variables for which the two groups differed at baseline (P ≤ 0.20) which included NYHA functional class, aetiology of cardiomyopathy, history of coronary artery disease, prior coronary artery revascularization, LVEF, LV lead location, and use of digoxin, loop diuretics, and beta-blocker at baseline. After adjustment, patients receiving MC continued to show a significant reduction in death, transplant, or HF hospitalization (HR: 0.69, 95% CI: 0.48–0.98; P = 0.04). After adjustment the difference between the two groups there was only a trend towards improved overall mortality for patients in the MC group (HR: 0.70, 95% CI: 0.42–1.18; P = 0.18). Patients in the CC group consisted primarily of a historical cohort who received CRT prior to the existence of our multidisciplinary clinic. However, there were patients in the analysis who were implanted while the multidisciplinary clinic was on-going, but received conventional follow-up due to patient or physician preference (n = 25). Interestingly, when only these patients in the CC group were compared with the MC group, event-free survival remained significantly improved among those receiving MC (HR: 0.23, 95% CI: 0.14–0.39, P < 0.0001). When these 25 patients were excluded altogether from the analysis in order to assess whether there was bias introduced by non-referral to our clinic, there continued to be improved event-free survival in those receiving MC (HR: 0.72, 95% CI: 0.53–0.99, P = 0.04). The effects of the impact of MC in 12 subgroups are presented in Figure 3.
Figure 3.
Risk of death or heart failure, according to type of care received. The hazard ratios for death or non-fatal heart failure hospitalization (whichever came first) are shown for various subgroups among patients who received multidisciplinary care. The red dashed vertical line represents the results for the entire analysis for multidisciplinary care (hazard ratio, 0.62), and the horizontal lines indicate 95% confidence intervals. Subgroup treatment interaction was identified for the left ventricular ejection fraction and age (P = 0.01 and 0.04, respectively), marked with *. LVEF, left ventricular ejection fraction; MC, multidisciplinary care; CC, conventional care.
Interaction effects between the subgroup and MC were identified in two subsets: MC was associated with a significantly better outcome in patients with an LVEF ≥20% (n = 283; HR: 0.54, 95% CI: 0.37–0.77) when compared with those with an LVEF <20% (n = 116; HR: 0.90, 95% CI: 0.51–1.57; P = 0.01 for the interaction between the groups and LVEF) and in patients with age <65 years (n = 153; HR: 0.37, 95% CI: 0.22–0.64) when compared with those with age ≥65 years (n = 274; HR: 0.80, 95% CI: 0.56–1.14; P = 0.04 for the interaction between the groups and age). These subgroup interactions, however, should be interpreted with caution, in view of the multiple testing involved.
A secondary subset analysis examining the impact of the multidisciplinary clinic on echocardiographic reverse remodelling was performed. For the whole cohort, there was an absolute increase in an LVEF by 8 ± 11% (P < 0.001), a relative decrease in the LV end-diastolic diameter (LVEDD) of 6 ± 10% (P < 0.001), and a relative decrease in the LV end-systolic diameter (LVESD) of 8 ± 13% (P < 0.001). Patients receiving MC had a significantly greater improvement in EF compared with those receiving CC (9.3 ± 10 vs. 3.2 ± 10%, P < 0.001), but no significant differences in change in the LVEDD or the LVESD (P = 0.97 and P = 0.67, respectively).
Discussion
This study shows that a multidisciplinary approach is associated with a better clinical outcome in the CRT patients and reduced HF hospitalization and all-cause mortality. There was a 38% relative risk reduction for HF hospitalization, transplant, and/or mortality over a 2-year follow-up in the group receiving MC vs. clinical care. These differences remained significant after adjusting for all clinical covariates and accounting for procedural adverse events. Our findings, drawn from a ‘real-world’ cohort of heterogeneous and often morbid HF patients receiving CRT, complement the data originally obtained from controlled trials enrolling highly selected patients and extend applicability to a wider cohort of CRT patients.
Clinical outcome
Heart failure, a final common pathway for most cardiovascular illnesses, affects nearly 5.8 million Americans, with ∼660 000 new patients being added yearly.8,9 Heart failure remains a leading cause of hospital readmissions, and despite therapeutic innovations, the long-term mortality from either pump failure or arrhythmic death remains high. Heart failure management programmes have been shown to be successful in improving quality of life and patient satisfaction, while reducing hospital admissions and hospital stay. Many such models involving home-care and specialized nursing follow-up of patients with HF have also been shown to improve the clinical outcome with a 27% reduction in hospitalization rates and a 43% reduction in HF hospitalizations.10 Previously reported multidisciplinary approaches for HF management have neither primarily involved HF patients with implanted devices nor have they involved care delivered across different subspecialties.
The high prevalence of HF and the expanding patient population eligible for device therapy has created a new genre of ambulatory HF patients with implanted devices. The complex nature of these patients often requires participation of an electrophysiologist, HF specialist, with support from a cardiovascular imaging specialist. Current post-device implant care is lacking on many fronts, namely: attention to device diagnostic information, evaluating and optimizing device programming in patients, and early identification and treatment of non-responders. The present study showed the positive impact of these collective interventions in a patient population through a multidisciplinary clinic.
Despite enhanced patient selection strategies and technological advances in devices and left ventricular lead systems, however, nearly a third of patients continue to remain non-responsive to CRT. In the current cost-conscious healthcare environment, worsening HF requiring emergent care and recurrent hospitalizations substantially diminishes the cost-effectiveness profile of this therapeutic modality. The integrated clinic approach provides a means to identify early on patients that may be potential non-responders, thereby enabling pre-emptive intervention.
The total mortality in our integrated-clinic cohort was comparable with the recent data reported from the ALTITUDE survival study11 and lower than that reported in the Medicare registry.12 Notably, Bilchik et al.12 reported that the observed rates of mortality in the CRT patients in a real-world setting was much higher than that seen in major clinical trials, with the Medicare registry reporting a 3-year mortality of 31.8%. In contrast to the ALTITUDE study and Medicare registry, our study also examined HF hospitalization, which was significantly lower in the MC cohort. Compared with the clinical trial population, our patients in the multidisciplinary group reflect a sicker cohort, and included all patients receiving CRT, irrespective of their renal function, presence of atrial fibrillation, and other co-morbidities.
Importantly, the additional benefit of a multidisciplinary approach was similar in magnitude to that which has been reported in clinical trials evaluating new drugs13–16 and device therapy for HF patients.1–6 Furthermore, the benefit seen in this trial could be considered as ‘additive’ to conventional therapies. This incremental improvement in the clinical outcome is impressive particularly within the context that most multicentre trials have had a carefully selected patient population with limited co-morbidities. Interestingly the beneficial effect of MC was evident across the different subgroups of patients, stratified by age, gender, aetiology of cardiomyopathy, lead location, and presence of atrial fibrillation and renal dysfunction.
Clinical components
Given the integrated delivery of care, it is difficult to disaggregate the relative contribution from different components of care delivered through the multidisciplinary clinic. A protocol-based multidisciplinary post-implant follow-up strategy not only ensures increased accountability but also guarantees good communication between care-givers, appropriate use of the HF and arrhythmia-specific data obtained from the device interrogation, along with individualized echo-guided programming of the atrio-ventricular and inter-ventricular timings in the device. The patients are more engaged and consequently there is earlier detection and intervention in non-responders.
Experience from the ADHERE registry suggests medical management of HF has significantly improved over the past two decades. Despite clear guidelines for managing HF, however, there remains substantial variation in treatment practices among physicians treating patients with HF.17 In the multidisciplinary approach having the HF specialist closely involved in the post-implant care of the CRT patient, may have by itself had a significant impact on the outcome, via intensified titration of the medications along with education component pertinent to salt and water intake and self-monitoring of HF symptoms.
A controversial component to this treatment model is the role of AV and VV optimization. Although prior clinical work showed that AV optimization may be useful,18 the recent SMART-AV study has questioned its utility in the CRT population.19 Although the SMART-AV study was a well-conducted randomized study, it was underpowered, the population was less sick and the endpoint of anatomical remodelling examined was different from the harder clinical endpoints evaluated in our study. Notably in the secondary analysis, we did find a greater improvement in the ejection fraction in the MC group, as opposed to those in the CC arm.
Of note, Mullens et al.,20 in their work with CRT non-responders, showed that suboptimal AV timing is one of the commonest and easily correctable causes for non-responsiveness. Our effort to optimize all patients at their first-month visit was to ensure uniformity in our approach, and programme the intervals to the best baseline haemodynamic settings. We used echocardiographic-guided optimization of the AV and VV interval, per a fixed protocol ensuring reproducibility within our clinic. Importantly, no changes were made to the VV intervals unless ≥15% increase in the stroke volume was observed. This was based on the inter-observer variability for these measurements in our ECHO laboratory.
Cardiac resynchronization therapy devices can record and provide detailed information pertinent to patient activity, heart rate, autonomic activity, and transthoracic impedance and in the near future they may also provide real-time haemodynamic data.21 Much of these data can be used for risk stratifying and prognosticating22 and adjusting their medical regimen.23 A multidisciplinary clinic provides an ideal structure for a collaborative approach to treat this sick patient population, enabling the different specialties to allow these data to be used more efficiently in the care of these patients. Device diagnostic data were evaluated at each visit and information derived from that was used to help facilitate patient care. For example, the physical activity log was routinely evaluated to (i) get an objective assessment of the patient's day-to-day activity and (ii) to encourage the patient to exercise and stay active. The mean and nocturnal heart rate data were used in conjunction with other clinical data to up-titrate beta-blockers. Transthoracic impedance when available was used in the context of the clinical situation to guide therapy.
Limitations
These data must be interpreted in the context of the study design. This is a single-centre non-randomized study. Importantly, only patients who received their entire care at MGH were included, thereby ensuring complete follow-up data. Data collection for patient seen in the CRT clinic was prospective, whereas that for the historical group was retrospective. Prospective randomized studies to validate our findings are needed. The improved outcomes in the integrated clinic approach in contrast to the historical control group could be related to advances in device technology and operator experience. The higher incidence of lateral wall lead placements in the multidisciplinary programme may be reflective of this. Of note, recent work has shown the absence of the impact of lateral free wall placement on the clinical outcome.24,25 There were no differences within the two groups in the location of the left ventricular lead in the apical segment, which has been recently shown to be associated with an adverse clinical outcome.24,26 Importantly, in a subgroup analysis, the MC improved outcomes across both apical and non-apical lead locations. Notably, at baseline, patients in both groups were noted to be on similar pharmacological therapy for HF. Uptitration of neurohormonal blockers after CRT therapy in the MC group was usual, but our study did not systematically track changes in medications in the CC group. However, univariate analysis showed the beneficial effect of MC across most subgroups, including those on loop diuretics, aldosterone antagonists, beta-blockers, and digoxin. Although attempts were made to control for differences between the MC and CC patient cohorts, it is possible that differences between these groups may have impacted the study results. Also, the number covariates used in the full-adjusted model may be un-proportional to the number of events using only death as an outcome and may explain the widening of the confidence intervals. Finally, although a multidisciplinary programme as shown in this study may be successful in improving the long-term outcome, it still remains to be assessed if the front-loaded expenditure is cost-effective in the long run.
Clinical implications
Importantly, HF is a progressive disease and our data suggest that a multidisciplinary effort may set the patient on an appropriate trajectory, thereby reducing cardiovascular events and improving the long-term outcome. As the HF population eligible for device therapy rapidly expands, the need to implement hospital protocols, which simultaneously amplify the accountability of practitioners, yield superior cost-effectiveness, and improve clinical outcomes is becoming increasingly imperative. To ensure the early detection of non-response to CRT, and trigger remedial actions through modifying drug therapy or device settings, the communication lines between the electrophysiologist, echocardiographer, and HF specialist need to be open and fluid. An integrated MC model may make this process achievable, and could improve the 2-year event-free survival in patients receiving CRT.
Funding
This study was supported in part by a research grant from St Jude Medical.
Conflict of interest: E.K.H. reports receiving research grants from St Jude Medical, Biotronik, Sorin Group and Boston Scientific, lecture fees from Boston Scientific Corp and St Jude Medical and consulting fees from Boston Scientific, St Jude Medical, Biotronik and Sorin group. C.D.B. reports receiving research grants from St Jude Medical and Medtronic and consulting fees from St Jude Medical. Q.A.T. reports receiving research grant support from NIH (grant K23HL098370). K.A.P. reports receiving lecture fees from Biotronik and Sorin Group and has served as a consultant for Thoratec, Inc. J.P.S. reports receiving research grants from St Jude Medical, Medtronic Inc., Boston Scientific Corp. and Biotronik, consulting from Boston Scientific Corp., Medtronic Inc, Sorin Group, St Jude Medical, Biosense Webster, Thoratec Inc. and Respicardia Inc. and receiving lecture fees from Boston Scientific Corp., St Jude Medical and Sorin Group.
References
- 1.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. doi:10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. doi:10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. doi:10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 4.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. doi: 10.1016/j.jacc.2008.08.027. doi:10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G, Szili-Torok T, Linde C. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the reverse (resynchronization reverses remodeling in systolic left ventricular dysfunction) trial. J Am Coll Cardiol. 2009;54:1837–1846. doi: 10.1016/j.jacc.2009.08.011. doi:10.1016/j.jacc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, III, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. doi:10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 7.Lin DY, Wei LJ, Ying Z. Checking the cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. doi:10.1093/biomet/80.3.557. [Google Scholar]
- 8.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American heart association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. doi:10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 9.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the acc/aha 2005 guidelines for the diagnosis and management of heart failure in adults a report of the American college of cardiology foundation/American heart association task force on practice guidelines developed in collaboration with the international society for heart and lung transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. doi:10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 10.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 11.Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, Meyer TE, Jones PW, Boehmer JP. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the altitude survival study. Circulation. 2010;122:2359–2367. doi: 10.1161/CIRCULATIONAHA.110.960633. doi:10.1161/CIRCULATIONAHA.110.960633. [DOI] [PubMed] [Google Scholar]
- 12.Bilchick KC, Kamath S, DiMarco JP, Stukenborg GJ. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in medicare patients. Circulation. 2010;122:2022–2030. doi: 10.1161/CIRCULATIONAHA.110.956011. doi:10.1161/CIRCULATIONAHA.110.956011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rutherford J, Wertheimer JH, Hawkins CM, on behalf of the SAVE investigators Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. doi:10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 14.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The solvd investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. doi:10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 15.Waagstein F, Bristow MR, Swedberg K, Camerini F, Fowler MB, Silver MA, Gilbert EM, Johnson MR, Goss FG, Hjalmarson A. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in dilated cardiomyopathy (MDC) trial study group. Lancet. 1993;342:1441–1446. doi: 10.1016/0140-6736(93)92930-r. doi:10.1016/0140-6736(93)92930-R. [DOI] [PubMed] [Google Scholar]
- 16.A randomized trial of beta-blockade in heart failure. The cardiac insufficiency bisoprolol study (CIBIS). CIBIS investigators and committees. Circulation. 1994;90:1765–1773. doi: 10.1161/01.cir.90.4.1765. [DOI] [PubMed] [Google Scholar]
- 17.Fonarow GC, Yancy CW, Heywood JT. Adherence to heart failure quality-of-care indicators in us hospitals: analysis of the adhere registry. Arch Intern Med. 2005;165:1469–1477. doi: 10.1001/archinte.165.13.1469. doi:10.1001/archinte.165.13.1469. [DOI] [PubMed] [Google Scholar]
- 18.Auricchio A, Ding J, Spinelli JC, Kramer AP, Salo RW, Hoersch W, KenKnight BH, Klein HU. Cardiac resynchronization therapy restores optimal atrioventricular mechanical timing in heart failure patients with ventricular conduction delay. J Am Coll Cardiol. 2002;39:1163–1169. doi: 10.1016/s0735-1097(02)01727-8. doi:10.1016/S0735-1097(02)01727-8. [DOI] [PubMed] [Google Scholar]
- 19.Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, Lemke B, Singh JP, Spinale FG, Van Eyk JE, Whitehill J, Weiner S, Bedi M, Rapkin J, Stein KM. Primary results from the smart delay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (smart-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660–2668. doi: 10.1161/CIRCULATIONAHA.110.992552. doi:10.1161/CIRCULATIONAHA.110.992552. [DOI] [PubMed] [Google Scholar]
- 20.Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, Tang WH. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765–773. doi: 10.1016/j.jacc.2008.11.024. doi:10.1016/j.jacc.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Ritzema J, Troughton R, Melton I, Crozier I, Doughty R, Krum H, Walton A, Adamson P, Kar S, Shah PK, Richards M, Eigler NL, Whiting JS, Haas GJ, Heywood JT, Frampton CM, Abraham WT. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation. 2010;121:1086–1095. doi: 10.1161/CIRCULATIONAHA.108.800490. doi:10.1161/CIRCULATIONAHA.108.800490. [DOI] [PubMed] [Google Scholar]
- 22.Singh JP, Rosenthal LS, Hranitzky PM, Berg KC, Mullin CM, Thackeray L, Kaplan A. Device diagnostics and long-term clinical outcome in patients receiving cardiac resynchronization therapy. Europace. 2009;11:1647–1653. doi: 10.1093/europace/eup250. doi:10.1093/europace/eup250. [DOI] [PubMed] [Google Scholar]
- 23.Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar S, Porter CB, Pavri BB, O'Connor CM. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from partners HF (program to access and review trending information and evaluate correlation to symptoms in patients with heart failure) study. J Am Coll Cardiol. 2010;55:1803–1810. doi: 10.1016/j.jacc.2009.11.089. doi:10.1016/j.jacc.2009.11.089. [DOI] [PubMed] [Google Scholar]
- 24.Singh JP, Klein HU, Huang DT, Reek S, Kuniss M, Quesada A, Barsheshet A, Cannom D, Goldenberg I, McNitt S, Daubert JP, Zareba W, Moss AJ. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation. 2011;123:1159–1166. doi: 10.1161/CIRCULATIONAHA.110.000646. doi:10.1161/CIRCULATIONAHA.110.000646. [DOI] [PubMed] [Google Scholar]
- 25.Saxon LA, Olshansky B, Volosin K, Steinberg JS, Lee BK, Tomassoni G, Guarnieri T, Rao A, Yong P, Galle E, Leigh J, Ecklund F, Bristow MR. Influence of left ventricular lead location on outcomes in the companion study. J Cardiovasc Electrophysiol. 2009;20:764–768. doi: 10.1111/j.1540-8167.2009.01444.x. doi:10.1111/j.1540-8167.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- 26.Merchant FM, Heist EK, McCarty D, Kumar P, Das S, Blendea D, Ellinor PT, Mela T, Picard MH, Ruskin JN, Singh JP. Impact of segmental left ventricle lead position on cardiac resynchronization therapy outcomes. Heart Rhythm. 2010;7:639–644. doi: 10.1016/j.hrthm.2010.01.035. doi:10.1016/j.hrthm.2010.01.035. [DOI] [PubMed] [Google Scholar]