Abstract
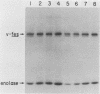
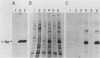
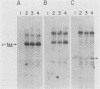
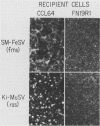
A contact-inhibited revertant of mink cells transformed by the Gardner-Arnstein strain of feline sarcoma virus was isolated by fluorescence-activated sorting of cells stained with the mitochondria-specific dye rhodamine 123. The revertant cell line exhibited a decrease in its proliferative rate and saturation density and a complete loss of its capacity for anchorage-independent growth, but it remained tumorigenic when inoculated into nude mice. The revertant cells retained a rescuable Gardner-Arnstein feline sarcoma provirus, expressed high levels of the v-fes oncogene product and its associated tyrosine kinase activity, manifested elevated levels of phosphotyrosine-containing cellular proteins similar to those observed in v-fes-transformed cells, and were refractory to retransformation by retroviruses containing the v-fes, v-fms, and v-ras oncogenes. Fusion of the revertant and parental cells generated somatic cell hybrids which formed colonies in semisolid medium, indicating that the block in transformation was recessive. These data together with the observation that the revertant phenotype is unstable in continuous culture suggest that the loss of transformation is due to the presence of limiting quantities of a gene product which functions downstream of the v-fes-coded kinase in the mitogenic pathway.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernal S. D., Lampidis T. J., McIsaac R. M., Chen L. B. Anticarcinoma activity in vivo of rhodamine 123, a mitochondrial-specific dye. Science. 1983 Oct 14;222(4620):169–172. doi: 10.1126/science.6623064. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Oliff A. I., Linemeyer D. L., Lander M. R., Lowy D. R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981 Sep;39(3):777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H. The proto-oncogene c-ets is preferentially expressed in lymphoid cells. Mol Cell Biol. 1985 Nov;5(11):2993–3000. doi: 10.1128/mcb.5.11.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. W., Sager R. Suppression of tumorigenicity in hybrids of normal and oncogene-transformed CHEF cells. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2062–2066. doi: 10.1073/pnas.82.7.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Rettenmier C. W., Sherr C. J. Ligand-induced tyrosine kinase activity of the colony-stimulating factor 1 receptor in a murine macrophage cell line. Mol Cell Biol. 1988 Apr;8(4):1795–1799. doi: 10.1128/mcb.8.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Even J., Anderson S. J., Hampe A., Galibert F., Lowy D., Khoury G., Sherr C. J. Mutant feline sarcoma proviruses containing the viral oncogene (v-fes) and either feline or murine control elements. J Virol. 1983 Mar;45(3):1004–1016. doi: 10.1128/jvi.45.3.1004-1016.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele L. A., Even J., Garon C. F., Donner L., Sherr C. J. Recombinant bacteriophages containing the integrated transforming provirus of Gardner--Arnstein feline sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4036–4040. doi: 10.1073/pnas.78.7.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Gilbert J. H., Porzig K. J., Scolnick E. M., Aaronson S. A. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. doi: 10.1128/jvi.30.3.821-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman W. L., Rettenmier C. W., Chen J. H., Roussel M. F., Quinn C. O., Sherr C. J. Antibodies to distal carboxyl terminal epitopes in the v-fms-coded glycoprotein do not cross-react with the c-fms gene product. Virology. 1986 Jul 30;152(2):432–445. doi: 10.1016/0042-6822(86)90145-5. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Adhya S., Das A. Transcription antitermination by bacteriophage lambda N gene product. J Mol Biol. 1980 Jun 15;140(1):57–75. doi: 10.1016/0022-2836(80)90356-3. [DOI] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Inoue H., Yutsudo M., Hakura A. Rat mutant cells showing temperature sensitivity for transformation by wild-type Moloney murine sarcoma virus. Virology. 1983 Feb;125(1):242–245. doi: 10.1016/0042-6822(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Summerhayes I. C., Chen L. B. Decreased uptake and retention of rhodamine 123 by mitochondria in feline sarcoma virus-transformed mink cells. Cell. 1982 Jan;28(1):7–14. doi: 10.1016/0092-8674(82)90369-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampidis T. J., Bernal S. D., Summerhayes I. C., Chen L. B. Selective toxicity of rhodamine 123 in carcinoma cells in vitro. Cancer Res. 1983 Feb;43(2):716–720. [PubMed] [Google Scholar]
- Lee C. Y., Huang Y. S., Hu P. C., Gomel V., Menge A. C. Analysis of sperm antigens by sodium dodecyl sulfate gel/protein blot radioimmunobinding method. Anal Biochem. 1982 Jun;123(1):14–22. doi: 10.1016/0003-2697(82)90617-0. [DOI] [PubMed] [Google Scholar]
- Morris A., Clegg C., Jones J., Rodgers B., Avery R. J. The isolation and characterization of a clonally related series of murine retrovirus-infected mouse cells. J Gen Virol. 1980 Jul;49(1):105–113. doi: 10.1099/0022-1317-49-1-105. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. D., Cook F., Roberts P. C., Clewley J. P., Avery R. J. Expression of Kirsten murine sarcoma virus in transformed nonproducer and revertant NIH/3T3 cells: evidence for cell-mediated resistance to a viral oncogene in phenotypic reversion. J Virol. 1984 May;50(2):439–444. doi: 10.1128/jvi.50.2.439-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka M., Ihara S., Ogawa R., Watanabe T., Watanabe Y. Preparation and characterization of antibodies to O-phosphotyrosine and their use for identification of phosphotyrosine-containing proteins. Int J Cancer. 1984 Dec 15;34(6):855–861. doi: 10.1002/ijc.2910340618. [DOI] [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A. Purification of the catalytically active phosphorylated form of insulin receptor kinase by affinity chromatography with O-phosphotyrosyl-binding antibodies. Arch Biochem Biophys. 1985 Oct;242(1):176–186. doi: 10.1016/0003-9861(85)90491-6. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Aaronson S. A. Complete nucleotide sequence and organization of the Moloney murine sarcoma virus genome. Science. 1981 Oct 23;214(4519):445–450. doi: 10.1126/science.6170110. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Chen J. H., Roussel M. F., Sherr C. J. The product of the c-fms proto-oncogene: a glycoprotein with associated tyrosine kinase activity. Science. 1985 Apr 19;228(4697):320–322. doi: 10.1126/science.2580348. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Turek L. P., Sherr C. J. Three independent isolates of feline sarcoma virus code for three distinct gag-x polyproteins. J Virol. 1980 Jul;35(1):259–264. doi: 10.1128/jvi.35.1.259-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. W., Christensen J. B., Imperiale M. J., Brockman W. W. Isolation of a simian virus 40 T-antigen-positive, transformation-resistant cell line by indirect selection. Mol Cell Biol. 1985 Dec;5(12):3577–3582. doi: 10.1128/mcb.5.12.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samid D., Flessate D. M., Friedman R. M. Interferon-induced revertants of ras-transformed cells: resistance to transformation by specific oncogenes and retransformation by 5-azacytidine. Mol Cell Biol. 1987 Jun;7(6):2196–2200. doi: 10.1128/mcb.7.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Donner L., Turek L. P. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: localization of sarcoma-specific sequences. J Virol. 1979 Dec;32(3):860–875. doi: 10.1128/jvi.32.3.860-875.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stephenson J. R., Reynolds R. K., Aaronson S. A. Characterization of morphologic revertants of murine and avian sarcoma virus-transformed cells. J Virol. 1973 Feb;11(2):218–222. doi: 10.1128/jvi.11.2.218-222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerhayes I. C., Lampidis T. J., Bernal S. D., Nadakavukaren J. J., Nadakavukaren K. K., Shepherd E. L., Chen L. B. Unusual retention of rhodamine 123 by mitochondria in muscle and carcinoma cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5292–5296. doi: 10.1073/pnas.79.17.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Pollack R. Methods for obtaining revertants of transformed cells. Methods Cell Biol. 1974;8(0):75–92. doi: 10.1016/s0091-679x(08)60444-6. [DOI] [PubMed] [Google Scholar]
- Wang J. Y. Isolation of antibodies for phosphotyrosine by immunization with a v-abl oncogene-encoded protein. Mol Cell Biol. 1985 Dec;5(12):3640–3643. doi: 10.1128/mcb.5.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbl H., Latreille J., Jolicoeur P. Revertants of v-fos-transformed fibroblasts have mutations in cellular genes essential for transformation by other oncogenes. Cell. 1987 Nov 6;51(3):357–369. doi: 10.1016/0092-8674(87)90632-5. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., Graham F. L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65(1):826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]