Abstract
This work presents the synthesis and use of surface-modified iron oxide nanoparticles for the covalent immobilization of Bacillus licheniformis γ-glutamyl transpeptidase (BlGGT). Magnetic nanoparticles were prepared by an alkaline solution of divalent and trivalent iron ions, and they were subsequently treated with 3-aminopropyltriethoxysilane (APES) to obtain the aminosilane-coated nanoparticles. The functional group on the particle surface and the amino group of BlGGT was then cross-linked using glutaraldehyde as the coupling reagent. The loading capacity of the prepared nanoparticles for BlGGT was 34.2 mg/g support, corresponding to 52.4% recovery of the initial activity. Monographs of transmission electron microscopy revealed that the synthesized nanoparticles had a mean diameter of 15.1 ± 3.7 nm, and the covalent cross-linking of the enzyme did not significantly change their particle size. Fourier transform infrared spectroscopy confirmed the immobilization of BlGGT on the magnetic nanoparticles. The chemical and kinetic behaviors of immobilized BlGGT are mostly consistent with those of the free enzyme. The immobilized enzyme could be recycled ten times with 36.2% retention of the initial activity and had a comparable stability respective to free enzyme during the storage period of 30 days. Collectively, the straightforward synthesis of aldehyde-functionalized nanoparticles and the efficiency of enzyme immobilization offer wide perspectives for the practical use of surface-bound BlGGT.
Keywords: Bacillus licheniformis, γ-glutamyl transpeptidase, magnetic nanoparticle, 3-aminopropyltriethoxysilane, covalent immobilization
1. Introduction
Enzymes are versatile biocatalysts that are useful for biotechnological applications, including food industries and pharmaceuticals [1,2]. The specific chemo-, regio- and stereo-selectivity of enzyme-mediated transformations remarkably render their superiority over chemical catalytic reactions [3–5]. For industrial applications, immobilized enzymes are currently the object of considerable interest, because of the advantage benefits over the soluble form of enzymes or alternative technologies [6]. The ability to retain or recover enzymes allows biocatalyst separation from the product, thereby permitting continuous processes, and prevents carry-through of protein or activity to subsequent process steps [7]. In some cases, the denaturation operation of immobilized enzymes can even be prevented [8–10]. Although the obvious advantages of enzyme immobilization have been reported [6,11], it is estimated that only 20% of the biological processes involve the utilization of immobilized enzymes [12]. However, a number of interesting findings indicate that enzyme immobilization has entered an exciting new phase [13].
The selected approach of immobilized enzymes should be able to stabilize the target biocatalysts and allow easier diffusion of substrates and products. Stabilization of enzymes against many different types of inactivation has been accomplished by a variety of immobilization strategies, including adsorption, polymer entrapment, microencapsulation, chemical aggregation, bioaffinity and covalent coupling [6,11,13]. Among the immobilization methods employed, covalent cross-linking of enzymes to inert supports is practically useful, since it can facilitate product separation and sometimes helps to improve the stability of biocatalysts [14–18]. Nanomaterials are currently attracting much attention as ideal supports for enzyme immobilization, since they could provide a very high-specific area surface for enzyme loading, lower the limitation of substrate and product diffusion and improve the catalytic efficiency [19]. To date, a variety of nanomaterials, including carbon nanotube, epoxy nanobeads and cellulose-bound magnetic nanoparticles, have been fabricated and used for the covalent immobilization of enzymes [20–22].
γ-Glutamyl transpeptidase (GGT) belongs to the N-terminal nucleophile (Ntn) hydrolase superfamily [23] and catalyzes the transfer of the γ-glutamyl moiety of glutathione to an amino acid, a short peptide or water molecule [24]. The gene encoding GGT is translated as a unique polypeptide that then undergoes an autocatalytic processing to form either a heterodimeric or heterotetrameric enzyme comprising of the typical large (L) and small (S) subunits [25–27]. The mature GGT enzyme plays a key role in the γ-glutamyl cycle, a pathway for the biosynthesis and degradation of glutathione, as well as xenobiotic detoxification [28–30]. Beyond its physiological function, GGT enzymes can be employed for the synthesis of γ-glutamyl derivatives, with great potential for pharmaceutical applications [31].
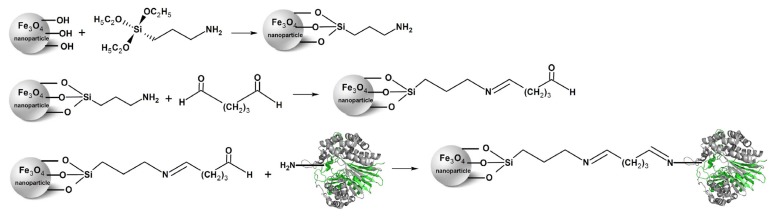
The putative B. licheniformis ggt gene can be translated into a 61.259 kDa precursor consisting of a signal peptide of 25 residues, a L-subunit of 374 residues and a S-subunit of 187 residues (Swiss-Prot Q65KZ6). Earlier, a recombinant B. licheniformis GGT (BlGGT) was functionally expressed in Escherichia coli M15 cells [32]. Deletion analyses of the recombinant enzyme have demonstrated that both N- and C-terminal sequences are crucial for the expression of its active form in host cells [33,34]. Given that nano-sized magnetic particles have recently received considerable attention for chemical cross-linking of enzymes [22,34–38], the use of surface-modified magnetic nanoparticles to covalently immobilize BlGGT should be a persuasive approach for obtaining stable and reusable biocatalyst preparations. In this work, we coated the prepared Fe3O4 nanoparticles with aminosilane and, subsequently, immobilized BlGGT on the nanomaterial through a glutaraldehyde coupling reaction (Figure 1). Moreover, the chemical properties and kinetic parameters of the immobilized BlGGT were determined and compared with its soluble counterpart.
Figure 1.
The strategy used to immobilize Bacillus licheniformis γ-glutamyl transpeptidase (BlGGT) on the magnetic nanoparticles.
2. Results and Discussion
2.1. Immobilization of the Recombinant Enzyme
In the present study, the purified BlGGT was covalently attached to the surface-modified magnetic nanoparticles. In order to obtain an immobilized biocatalyst with a high amount of enzyme loading and activity recovery, the effects of initial enzyme concentration and coupling time on the immobilization efficiency were investigated.
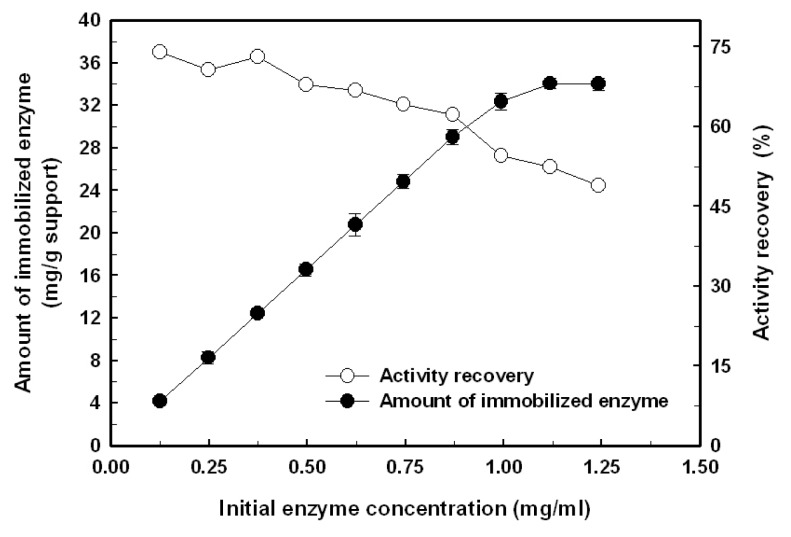
The effect of the initial BlGGT concentration on the amount of the biocatalyst covalently attached on the surface-modified magnetic nanoparticles and recovery of the enzymatic activity are shown in Figure 2. The amount of immobilized enzyme almost proportionally increased with the increment of initial enzyme concentration in the bulk solution from 0.125 to 0.875 mg/mL and then leveled off. Eventually, the maximum loading for BlGGT could reach 34.2 mg/g of support, which corresponds to an activity recovery of 52.4%. The activity recovery of BlGGT, defined as the ratio of the GGT activity of immobilized enzyme to the initial activity, was almost kept unchanged in the low range of initial enzyme concentration (0.125–0.375 mg/mL). However, a further increase in the initial enzyme concentration led to the reduction of activity recovery. The possible explanation for this phenomenon is that the multiple layers of enzyme molecules on the surface of magnetic nanoparticles are formed at high enzyme loading, which might block the active sites of the enzyme and cause the diffusion limitation of the substrate.
Figure 2.
Effect of initial BlGGT concentration on the amount of immobilized enzyme (closed circles) and activity recovery (open circles).
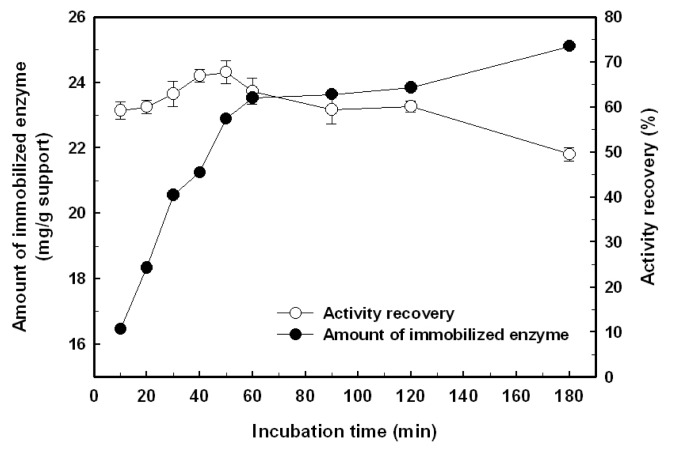
The effect of coupling time on the immobilization efficiency of BlGGT on the 3-aminopropyltriethoxysilane (APES)-modified magnetic nanoparticles is presented in Figure 3. The amount of immobilized enzyme was increased with the increment of coupling time, reaching a plateau after 1 h and the maximum amount of enzyme immobilization was determined to be 25.1 mg/g of support. The activity recovery of immobilized BlGGT maintained almost constant at about 57.7% for the first 50 min and then gradually decreased when the incubation time went further. A long immobilization time resulted in lower activity recovery, which could be the result of the creation of additional multiple covalent linkages between the nanoparticles and the enzyme, and, in turn, disturbs the structural conformation of the biocatalyst.
Figure 3.
Effect of the coupling time on the amount of immobilized BlGGT (closed circles) and activity recovery (open circles). In this experiment, the initial enzyme concentration was 0.92 mg/mL.
2.2. Properties of the Magnetic Nanoparticles
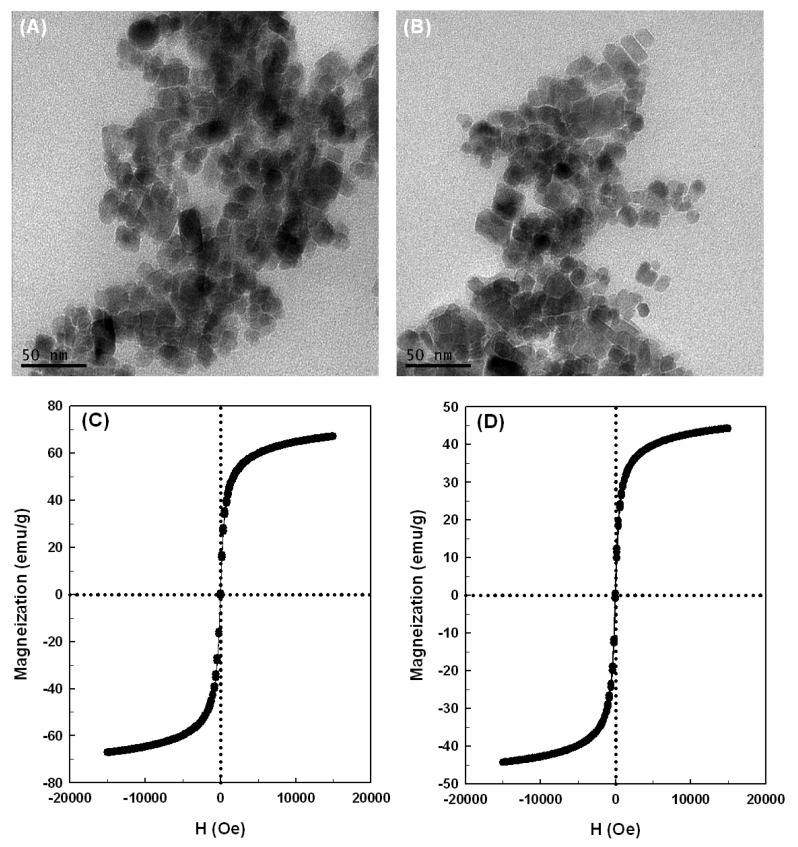
Typical transmission electron microscopy (TEM) micrographs for the magnetic nanoparticles without and with covalently cross-linked BlGGT are shown in Figure 4. The magnetic nanoparticles had a size distribution of 7.5–25.3 nm, and the statistical sampling of 103 particles in five TEM micrographs yielded a mean diameter of 15.1 ± 3.7 nm (Figure 4A). The particle diameter is believed to be an important feature for the immobilization matrix. Definitely, smaller particles have larger surface-to-volume ratios and larger capacity to bind more enzymes on their surface, and the substrate and product would give less restriction for diffusion [39,40]. In this case, the particles remained discrete after cross-linking to BlGGT and had a mean diameter of 17.2 ± 3.9 nm (Figure 4B), which is comparable to that of uncross-linked ones. These observations reveal that the immobilization step does not significantly change the average size of the magnetic nanoparticles.
Figure 4.
Transmission electron microscopy (TEM) micrographs (A) and (B) and magnetization curves (C) and (D) of Fe3O4 and Fe3O4/APES/BlGGT. Panels represent: (A) and (C), naked Fe3O4; (B) and (D), Fe3O4/3-aminopropyltriethoxysilane (APES)/BlGGT.
The magnetization curves of naked Fe3O4 and Fe3O4/APES/BlGGT measured at 300 K showed no detectable coercivity in the field sweep (Figure 4C,D), which suggests that the magnetic nanoparticles possess a characteristic of superparamagnetic materials [41], and the saturation magnetization of Fe3O4 and Fe3O4/APES/BlGGT was different with individual values of 67.2 and 42.1 emu/g, respectively. This indicates that the magnetic properties of Fe3O4/APES/BlGGT have a content of 62.6% with respect to the value of naked Fe3O4. Besides, the enzyme-conjugated magnetic nanoparticles in aqueous solution were a brown suspension, and they were easily recovered from the solution under an external magnetic field (data not shown). Once the external magnetic field was removed, they could be redispersed into a particle suspension with slight shaking. Based on the magnetization curves and the separation-redispersion process, it can be concluded that the prepared nanoparticles have the superparamagnetic properties.
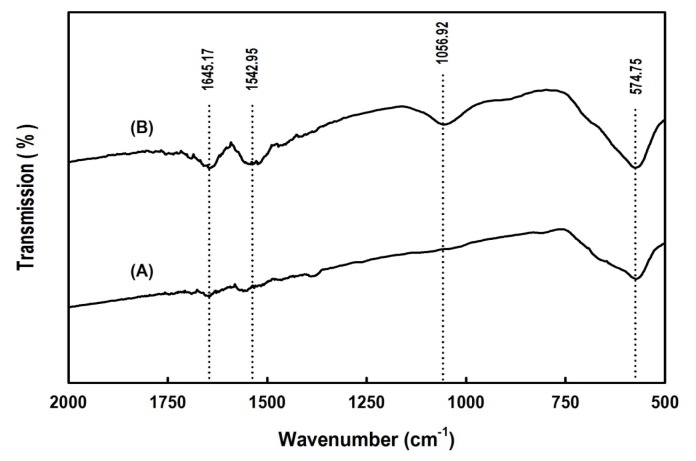
The cross-linking of BlGGT to the aldehyde-functionalized magnetic nanoparticles was also confirmed by FTIR analysis. Figure 5 shows the FTIR spectra of naked Fe3O4 and Fe3O4/APES/BlGGT. The peak at 569 cm−1 relates to the Fe–O group of Fe3O4. Compared with the spectrum of naked Fe3O4, the characteristic bands appear at 1542.95 and 1645.17 cm−1 in Fe3O4/APES/BlGGT and can be assigned to the NH and NH2 bending vibration, respectively. These results indicate that the BlGGT molecules are conjugated with the APES-modified magnetic nanoparticles successfully.
Figure 5.
Fourier transform infrared spectroscopy (FTIR) spectra of naked Fe3O4 and Fe3O4/APES/BlGGT. Lines represent: (A) naked Fe3O4; (B) Fe3O4/APES/BlGGT.
2.3. Characterization of Free and Immobilized Enzymes
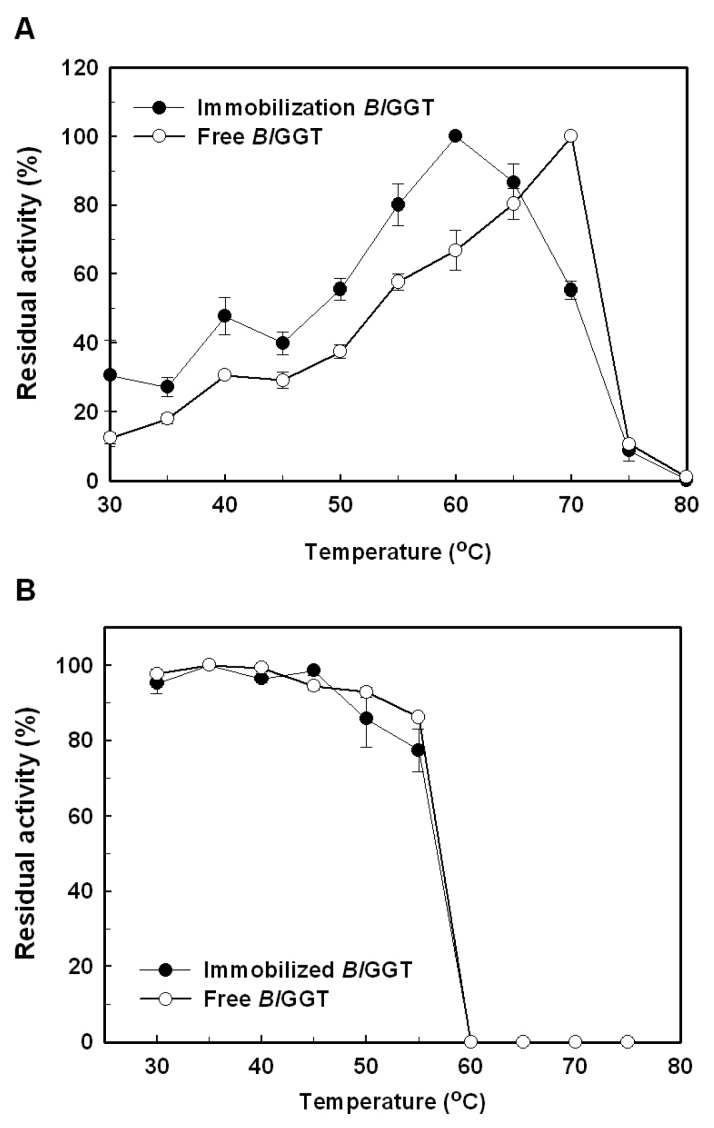
The optimal temperature for free BlGGT was seen at 70 °C, but the maximal activity of immobilized enzyme was shifted to 60 °C (Figure 6A). The thermal stability of free and immobilized BlGGTs was further determined by incubating the enzyme samples in a temperature range of 30–80 °C and, thereafter, measuring their residual activity. As shown in Figure 6B, both free and immobilized enzymes shared a similar profile of thermal stability. Surface immobilization of an enzyme on magnetic particles has been shown to improve the thermal stability of the biocatalyst, probably due to the increase of its molecular rigidity [42]. Thermal stabilization by immobilization could also be observed in a number of enzymes conjugated with magnetic nanoparticles [43–45]. However, the reasons for a downshift of the optimal temperature and no significant thermostabilization of BlGGT by the immobilization remain to be elucidated.
Figure 6.
Effect of temperature on activity (A) and stability (B) of free and immobilized enzymes. Results were reported as means of three independent experiments, and the standard deviations were lower than ±4.3%.
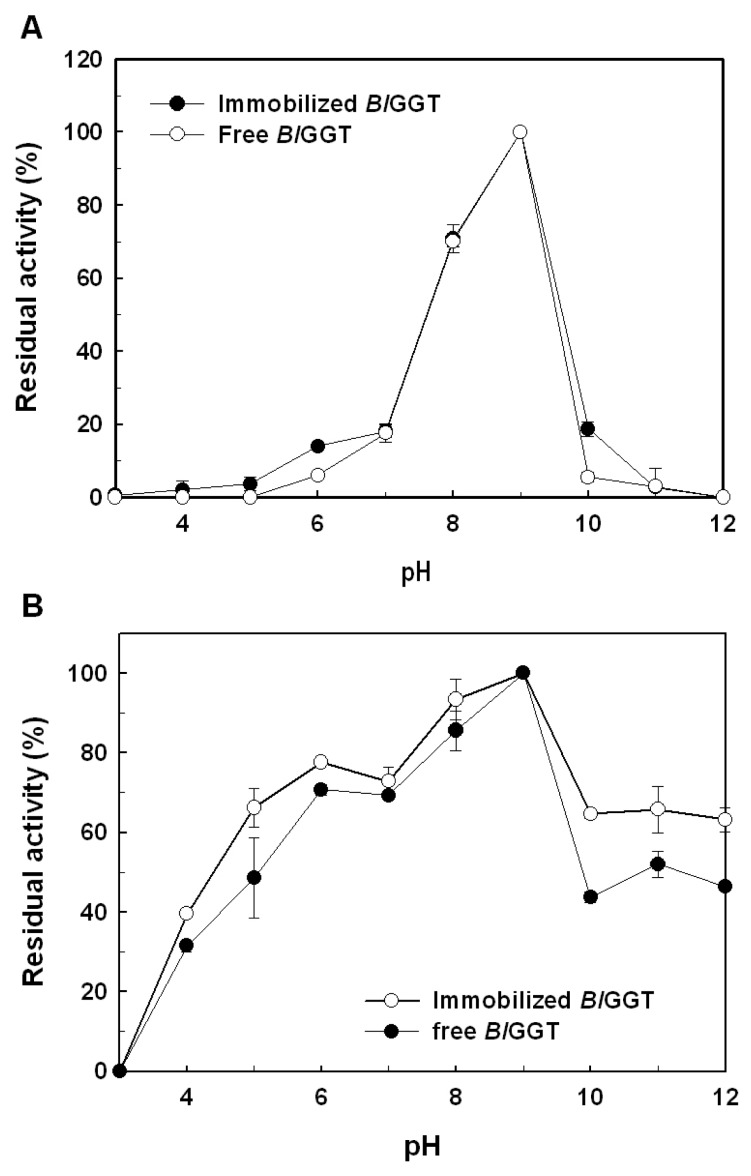
The effect of pH on the enzymatic activity of immobilized BlGGT is shown in Figure 7. The optimal pH of immobilized BlGGT was observed at 8.0, which is almost the same with respect to free enzyme (Figure 7A). A higher stability for the immobilized BlGGT was kept in the pH range of 6–9, and the free enzyme was also in the same pH range (Figure 7B). Practically, it would be ideal if the immobilized enzyme could be functional over a wide range of pH values.
Figure 7.
Effect of pH on activity (A) and stability (B) of free and immobilized enzymes. Results were reported as means of three independent experiments, and the standard deviations were lower than ±3.7%.
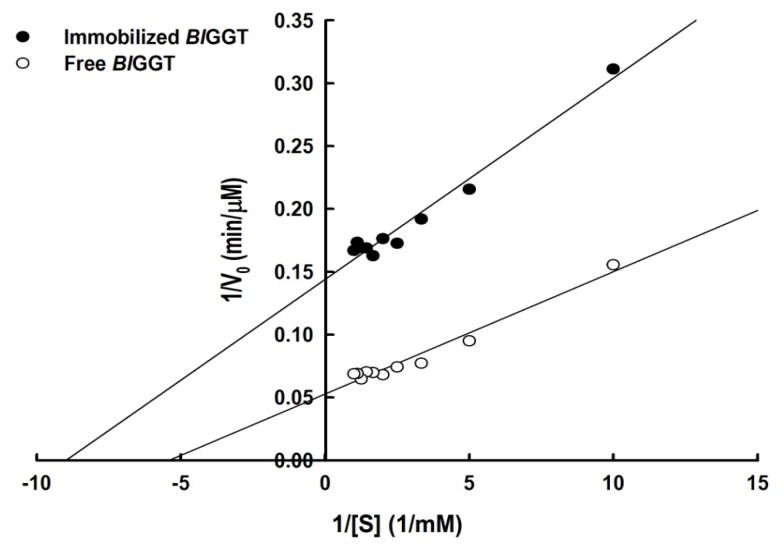
Lineweaver-Burk plots for free and immobilized enzymes on the transpeptidation reaction of l-γ-Glu-p-NA were used to obtain kinetic parameters (Figure 8). The Michaelis–Menten constants, KM and Vmax, for free enzyme were 0.18 mM and 18.94 μmol/min/mg, respectively. The immobilized BlGGT had an apparent KM of 0.11 mM and a Vmax value of 7.01 μmol/min/mg, leading to a reduction of the catalytic efficiency (Vmax/KM) from 10.51 to 3.05 s−1. These observations indicate that the immobilization process significantly reduces the substrate affinity of BlGGT and may also hinder the accessibility of reactants to the active site. These results are consistent with the kinetic observations of other enzymes immobilized on magnetic nanomaterials [37,43,46–48].
Figure 8.
Lineweaver–Burk plot for free and immobilized enzymes.
2.4. Reusability
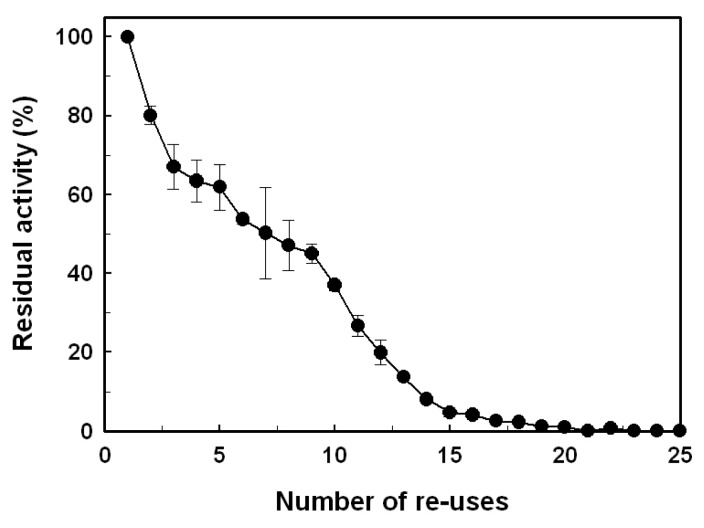
The duration of a biocatalyst is an important aspect to consider its potential for industry applications. In this regard, the operational stability of immobilized BlGGT was evaluated in a repeated batch process. At each of the repetitive cycles, the immobilized enzyme was recovered by magnetic separation and recycled for the transpeptidation reaction of l-γ-Glu-p-NA. As shown in Figure 9, the GGT activity of immobilized enzyme did not decrease significantly during the first cycle. The immobilized enzyme retained approximately 36.2% of the initial activity, even after 10 cycles of usage. Given that BlGGT was stable for 24 h at 40 °C (data not shown), the gradual loss of the GGT activity may be attributed to the conformational changes of immobilized enzyme during reuse or a distributive stability of each enzyme due to differences in the number of interactions between enzyme molecules and the support matrix [49–52].
Figure 9.
Operational stability of the immobilized enzymes. Results were reported as the means of three independent experiments, and the standard deviations were lower than ±5.2%.
2.5. Storage Stability of Free and Immobilized Enzymes
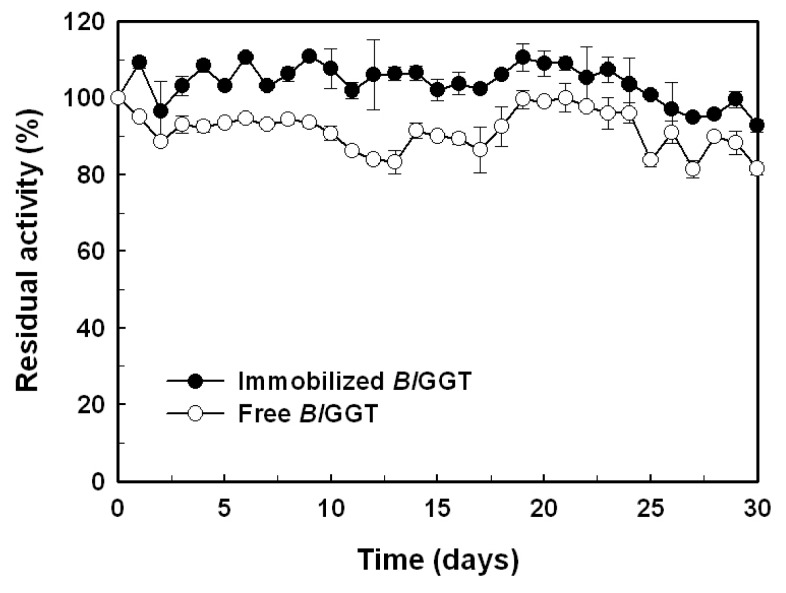
The storage stability of an enzyme is important criteria for biocatalyst-mediated processes, due to the economics of industrial bioprocesses, which are closely tied to the production cost of enzymes. In this regard, we also evaluated the storage stability of free and immobilized enzymes (Figure 10). After an incubation period of 30 days, the residual activity of free and immobilized enzymes was 90.2% and 82.3%, respectively. The experimental results suggest that BlGGT is quite stable after being conjugated to the surface-modified magnetic nanoparticles.
Figure 10.
Storage stability of free and immobilized enzymes. Results were reported as means of three independent experiments, and the standard deviations were lower than ±4.1%.
3. Materials and Methods
3.1. Chemicals
Ferric chlorides 6-hydrate, ferrous chloride tetrahydrate, glutaraldehyde and 3-aminopropyltriethoxysilane (APES) were acquired from Wako Pure Chemicals Industries, Ltd. (Osaka, Japan). Nickel-nitrilotriacetate (Ni2+-NTA) resin was purchased from Qiagen Inc. (Valencia, CA, USA). Chemical compounds for enzyme assay, including l-γ-glutamyl-p-nitroanilide (l-γ-Glu-p-NA), Gly-Gly and p-nitroaniline (p-NA) were brought from Sigma-Aldrich Fine Chemicals (St. Louis, MO, USA). All other chemicals were commercial products of analytical or molecular biological grade.
3.2. Expression and Purification of the Recombinant GGT
To purify BlGGT from the crude extract of E. coli M15 (pQE-BlGGT) [32], the recombinant bacterium was grown at 37 °C in 100 mL of Luria-Bertani (LB) medium containing 100 μg ampicillin mL−1 and 25 μg kanamycin mL−1 to an absorbance at 600 nm of 1.0. Then, isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM, and the cultivation of bacteria continued at 20 °C for 12 h. Bacterial cells were harvested by centrifugation at 9000 × g for 10 min at 4 °C, resuspended in 30 mL of 20 mM Tris-HCl buffer (pH 8.0) after decanting the supernatant and disrupted by sonication (Sonicator XL-2000; Misonix, Inc., Farmingdale, NY, USA). The extract was clarified by centrifugation at 12,000× g for 20 min at 4 °C, and the resulting supernatant was mixed with Ni2+-NTA resin pre-equilibrated with the binding buffer (5 mM imidazole, 0.5 M NaCl and 20 mM Tris-HCl; pH 7.9). The adherent BlGGT was eluted from the column by an elution buffer consisting of 0.5 M imidazole, 0.5 M NaCl and 20 mM Tris-HCl (pH 7.9).
3.3. Determination of GGT Activity
GGT activity was assayed at 50 °C according to the method of Orlowski and Meister [53], and the formation of p-NA was recorded by monitoring the absorbance changes at 410 nm. The reaction mixture contained 1.25 mM l-γ-Glu-p-NA, 30 mM Gly-Gly, 1 mM MgCl2, 20 mM Tris-HCl buffer (pH 8.0) and 20 μL of enzyme solution at a suitable dilution and enough distilled water to bring the final volume to 1 mL. One unit of GGT activity is defined as the amount of enzyme required to produce 1 μmol of p-NA per min under the assay conditions.
Kinetic parameters of free and immobilized enzymes were estimated by measuring p-NA production in 1 mL of 20 mM Tris-HCl buffer (pH 8.0) containing various concentrations of l-γ-Glu-p-NA (0.1–2.0 KM), 30 mM Gly-Gly, 1 mM MgCl2 and an appropriate amount of free and immobilized enzymes. The KM and Vmax values were calculated from the slope and the y-axis intercept, respectively, on the Lineweaver-Burke plot.
3.4. Preparation of Aldehyde-Functionalized Magnetic Nanoparticles and Enzyme Immobilization
Magnetic nanoparticles (Fe3O4) were prepared by hydrothermal co-precipitation of ferric and ferrous salts in an alkaline solution, followed by washing the precipitates with hot water [54]. Briefly, iron(II) chloride and iron(III) chloride (1:2) were dissolved in nanopure water at a concentration of 0.25 M iron ions and chemically precipitated at ambient temperature by repeatedly adding 1 M NaOH to keep a constant pH of 10.0. The precipitates were subsequently heated at 80 °C for 30 min under vigorous stirring and washed 4 times with water and several times with ethanol. During washing, the magnetic nanoparticles were separated from the washing liquid by a magnetic separator of N48M (MAG City Co., LTD., Taiwan) of 48 mega-oersteds. The nanoparticles were finally dried in a vacuum oven at 70 °C.
To prepare APES-bound magnetic nanoparticles, 0.5 g Fe3O4 was dispersed in 9.7 mL of ethanol by sonication. After adding 0.3 mL of APES, the reaction mixture was homogenized by a sonicator for 10 min, and the sample bottle was then rolled up and down overnight at 25 °C. The APES-bound magnetic nanoparticles were subsequently recovered from the reaction mixture by placing the bottle on a permanent magnet with a surface magnetization of 48 mega-oersteds. The resulting supernatant was discarded, and the precipitates were washed several times with ethanol.
The purified BlGGT was essentially immobilized on APES-bound magnetic nanoparticles through glutaraldehyde linkage [38]. The binding percentage of BlGGT was estimated by determining the amount of protein in the unreacted fraction. Protein concentration was determined by the colorimetric method at 595 nm with a Bio-Rad protein assay kit using bovine serum albumin as the reference standard.
3.5. Characterization of Magnetic Nanoparticles
The morphology and average size of the prepared nanoparticles were determined by transmission electron microscopy (TEM) using a JEM-1400 transmission electron microscope (JEOL Ltd., Tokyo, Japan). Samples for TEM analyses were prepared by placing a drop of the nanoparticle-ethanol suspension onto a Formvar covered copper grid and evaporated in air at ambient temperature.
The magnetic property was measured using Super-conducting Quantum Interference Device Magnetometer (SQUID, MPMS7, quantum design) at the temperature of 300 K and in a magnetic field up to 50 kOe.
The covalent immobilization of BlGGT on the aldehyde-functionalized magnetic nanoparticles was checked using FTIR spectra with KBr discs in the range of 2000–500 cm−1 on a Shimadzu FTIR-8400 spectrometer (Shimadzu Corporation, Kyoto, Japan).
3.6. Effects of Temperature and pH
The effect of temperature on free and immobilized enzymes was evaluated by incubating free BlGGT (8.7 U/mL) and BlGGT-conjugated magnetic nanoparticles (34.1 mg; wet weight) in 10 mL of 20 mM Tris-HCl buffer (pH 8.0) at various temperatures (30–80 °C) for 10 min. The amount of GGT activity was determined according to the assay procedure described above. The thermal stability of free and immobilized enzymes was performed in 10 mL of 20 mM Tris-HCl buffer (pH 8.0) at different temperatures (30–80 °C). After 30 min of incubation, the residual activity was determined under the standard assay conditions. The experiments were performed in triplicate, and the data were expressed as mean values.
To investigate the pH effect on free and immobilized enzymes, BlGGT (8.7 U/mL) and BlGGT-conjugated magnetic nanoparticles (34.1 mg; wet weight) were incubated with 10 mL of 20 mM Tris-maleate buffer (pH 4.5–6.0), 20 mM potassium phosphate buffer (pH 6.0–8.0), 20 mM Tris-HCl buffer (pH 8.0–9.0) or 20 mM glycine-NaOH buffer (pH 9.0–11.0) at 60 °C, and the amount of GGT activity was determined according to the standard assay conditions. For the measurement of pH stability, both free and immobilized enzymes were kept at 4 °C for 30 min in different buffers. The residual GGT activity was determined under the standard assay conditions. The experiments were performed in triplicate, and the data were expressed as mean values.
3.7. Reusability of the Immobilized Enzyme
The immobilized BlGGT was repeatedly used to catalyze the transpeptidation of l-γ-Glu-p-NA in the batch process. Enzyme-conjugated magnetic nanoparticles (12 mg) in 1 mL of 20 mM Tris-HCl buffer (pH 8.0) containing 1.25 mM l-γ-Glu-p-NA, 30 mM Gly-Gly, 1 mM MgCl2 and 20 mM Tris-HCl buffer (pH 8.0) were shaken (100 rpm) at 50 °C for 10 min each time. The GGT activity was immediately determined under the standard assay conditions. After each cycle of process, the enzyme-matrix complex was washed twice with 1 mL of 20 mM Tris-HCl buffer (pH 8.0) and reused for the next run. The experiments were performed in triplicate, and the data were expressed as mean values.
3.8. Storage Stability of the Immobilized Enzyme
The operational stability was assessed for the immobilized BlGGT (~1.2 g, wet weight) during storage in 100 mL of 20 mM Tris-HCl (pH 8.0) at 4 °C. At a specific time interval, aliquots (1 mL) were withdrawn to determine the GGT activity under the standard assay conditions. The experiments were performed in triplicate, and the data were expressed as mean values.
4. Conclusions
In this study, magnetic iron oxide nanoparticles are successfully synthesized by the thermal co-precipitation of ferric and ferrous chloride. The coating of Fe3O4 with APES has been proven to be efficient for the covalent immobilization of BlGGT. The immobilization process provides optimal recovery of activity and ideal conditions for the transpeptidation reaction. Moreover, the immobilized enzyme shows good durability and could be readily recovered by magnetic separation. To the best of our knowledge, this is the first report dealing with the covalent immobilization of a microbial GGT on surface-modified magnetic nanoparticles. The achieved stability makes the use of expensive enzymes economically viable and, therefore, opens new horizons for GGT-mediated catalysis in biotechnology.
Acknowledgments
This work was supported by the National Science Council of Taiwan (NSC 100-2313-B-415-003-MY3).
References
- 1.Vellard M. The enzyme as drug: Application of enzymes as pharmaceuticals. Curr. Opin. Biotechnol. 2003;14:444–450. doi: 10.1016/s0958-1669(03)00092-2. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C., Kim S.K. Application of marine microbial enzymes in the food and pharmaceutical industries. Adv. Food Nutr. Res. 2012;65:423–435. doi: 10.1016/B978-0-12-416003-3.00028-7. [DOI] [PubMed] [Google Scholar]
- 3.Koeller K.M., Wong C.H. Enzymes for chemical synthesis. Nature. 2001;409:232–240. doi: 10.1038/35051706. [DOI] [PubMed] [Google Scholar]
- 4.Schmid A., Dordick J.S., Hauer B., Kiener A., Wubbolt M., Witholt B. Industrial biocatalysts today and tomorrow. Nature. 2001;409:258–268. doi: 10.1038/35051736. [DOI] [PubMed] [Google Scholar]
- 5.Toscano M.D., Woycechowsky K.J., Hilvert D. Minimalist active-site redesign: Teaching old enzymes new tricks. Angew. Chem. Int. Ed. 2007;46:3212–3236. doi: 10.1002/anie.200604205. [DOI] [PubMed] [Google Scholar]
- 6.Khan A.A., Alzohairy M.A. Recent advances and applications of immobilized enzyme technologies: A review. Res. J. Biol. Sci. 2010;5:565–575. [Google Scholar]
- 7.Polizzi K.M., Bommarius A.S., Broering J.M., Chaparro-Riggers J.F. Stability of biocatalysts. Curr. Opin. Chem. Biol. 2007;11:220–225. doi: 10.1016/j.cbpa.2007.01.685. [DOI] [PubMed] [Google Scholar]
- 8.Hanefeld U., Gardossi L., Magner E. Understanding enzyme immobilization. Chem. Soc. Rev. 2009;38:453–468. doi: 10.1039/b711564b. [DOI] [PubMed] [Google Scholar]
- 9.Mateo C., Palomo J.M., Fernandez-Lorente G., Guisan J.M., Fernandez-Lafuente R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007;40:1451–1463. [Google Scholar]
- 10.Cao L. Immobilized enzymes: Science or art? Curr. Opin. Chem. Biol. 2005;9:217–226. doi: 10.1016/j.cbpa.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Brady D., Jordaan J. Advances in enzyme immobilization. Biotechnol. Lett. 2009;31:1639–1650. doi: 10.1007/s10529-009-0076-4. [DOI] [PubMed] [Google Scholar]
- 12.Straathof A.J., Panke S., Schmid A. The production of fine chemicals by biotransformations. Curr. Opin. Biotechnol. 2002;13:548–556. doi: 10.1016/s0958-1669(02)00360-9. [DOI] [PubMed] [Google Scholar]
- 13.Spahn C., Minteer S.D. Enzyme immobilization in biotechnology. Recent Pat. Eng. 2008;2:195–200. [Google Scholar]
- 14.Tischer W., Wedekind F. Immobilized enzymes: Methods and applications. Top. Curr. Chem. 1999;200:95–126. [Google Scholar]
- 15.Varavinit S., Chaokasem N. Covalent immobilization of a glucoamylase to bagasse dialdehyde cellulose. World J. Microbiol. Biotechnol. 2001;17:721–725. [Google Scholar]
- 16.Pierre S.J., Thies J.C., Dureault A., Cameron N.R., van Hest J.C.M., Carette N., Michon T., Weberslcirch R. Covalent enzyme immobilization onto photopolymerized highly porous monliths. Adv. Mater. 2006;18:1822–1826. [Google Scholar]
- 17.Kumar V.R., Pundir C.S. Covalent immobilization of lipase onto onion membrane affixed on plastic surface: Kinetic properties and application in milk fat hydrolysis. Indian J. Biotechnol. 2007;6:479–484. [Google Scholar]
- 18.Tasso M., Cordeiro A.L., Salchert K. Covalent immobilization of subtilisin A onto thin films of maleic anhydride copolymers. Macromol. Biosci. 2009;9:922–929. doi: 10.1002/mabi.200900005. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z.G., Wan L.S., Liu Z.M., Huang X.J., Xu Z.K. Enzyme immobilization on electronspun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009;56:189–195. [Google Scholar]
- 20.Li Y.X., Wang P., Li F., Huang X., Wang L., Lin X.Q. Covalent immobilization of single-walled carbon nanotubes and single-stranded deoxyribonucleic acid nanocomposites on glassy carbon electrode: Preparation, characterization, and applications. Talanta. 2008;77:833–838. [Google Scholar]
- 21.Jordan J., Kumar C., Chandra T. Preparation and characterization of cellulose-bound magnetic nanoparticles. J. Mol. Catal. B Enzym. 2011;68:139–146. [Google Scholar]
- 22.Liu H., Liu J., Tan B., Zhou F., Qin Y., Yang R. Covalent immobilization of Kluyveromyces fragilis β-galactosidase on magnetic nanosized epoxy support for synthesis of galacto-oligosaccharide. Bioproc. Biosyst. Eng. 2012;35:1287–1295. doi: 10.1007/s00449-012-0716-2. [DOI] [PubMed] [Google Scholar]
- 23.Brannigan J.A., Dodson G., Duggleby H.J., Moody P.C., Smith J.L., Tomchick D.R., Murzin A.G. A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature. 1995;90:416–419. doi: 10.1038/378416a0. [DOI] [PubMed] [Google Scholar]
- 24.Tate S.S., Meister A. γ-Glutamyl transpeptidase from kidney. Meth. Enzymol. 1985;113:400–419. doi: 10.1016/s0076-6879(85)13053-3. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H., Kumagai H. Autocatalytic processing of γ-glutamyl transpeptidase. J. Biol. Chem. 2002;277:43536–43543. doi: 10.1074/jbc.M207680200. [DOI] [PubMed] [Google Scholar]
- 26.Boanca G., Sand A., Barycki J.J. Uncoupling the enzymatic and autoprocessing activities of Helicobacter pylori γ-glutamyl transpeptidase. J. Biol. Chem. 2006;281:19029–19037. doi: 10.1074/jbc.M603381200. [DOI] [PubMed] [Google Scholar]
- 27.Castellano I., Merlino A., Rossi M., La Cara F. Biochemical and structural properties of γ-glutamyl transpeptidase from Geobacillus thermodenitrificans: An enzyme specialized in hydrolase activity. Biochimie. 2010;92:464–474. doi: 10.1016/j.biochi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki H., Hashimoto W., Kumagai H. Glutathione metabolism in Escherichia coli. J. Mol. Catal. B Enzym. 1999;6:175–184. [Google Scholar]
- 29.Dickinson D.A., Forman H.J. Glutathione in defense and signaling: Lessons from a small thiol. Ann. NY Acad. Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 30.Ubiyvovk V.M., Blazhenko O.V., Gigot D., Penninckx M., Sibirny A.A. Role of γ-glutamyl transpeptidase in detoxification of xenobiotics in the yests Hansenula polymorpha and Saccharomyces cerevisiae. Cell. Biol. Int. 2006;30:665–671. doi: 10.1016/j.cellbi.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H., Yamada C., Kato K. γ-Glutamyl compounds and enzymatic production using bacterial γ-glutamyl transpeptidase. Amino Acids. 2007;32:333–340. doi: 10.1007/s00726-006-0416-9. [DOI] [PubMed] [Google Scholar]
- 32.Lin L.L., Chou P.R., Hua Y.W., Hsu W.H. Overexpression, one-step purification, and biochemical characterization of a recombinant γ-glutamyl transpeptidase from Bacillus licheniformis. Appl. Microbiol. Biotechnol. 2006;73:103–112. doi: 10.1007/s00253-006-0440-4. [DOI] [PubMed] [Google Scholar]
- 33.Lin L.L., Yang L.Y., Hu H.Y., Lo H.F. Influence of N-terminal truncations on the functional expression of Bacillus licheniformis γ-glutamyl transpeptidase in recombinant Escherichia coli. Curr. Microbiol. 2008;57:603–608. doi: 10.1007/s00284-008-9250-5. [DOI] [PubMed] [Google Scholar]
- 34.Chang H.P., Liang W.C., Lyu R.C., Chi M.C., Wang T.Z., Su K.L., Hung H.C., Lin L.L. Effects of C-terminal truncation on autocatalytic processing of Bacillus licheniformis γ-glutamyl transpeptidase. Biochemistry (Mos. ) 2010;75:919–929. doi: 10.1134/s0006297910070151. [DOI] [PubMed] [Google Scholar]
- 35.Koneracká M., Kopčanský P., Timko M., Ramchand C.N., de Sequeira A., Treven M. Direct binding procedure of proteins and enzymes to fine magnetic particles. J. Magn. Magn. Mater. 2002;252:409–411. [Google Scholar]
- 36.Huang S.H., Liao M.H., Chen D.H. Direct binding and characterization of lipase onto magnetic nanoparticles. Biotechnol. Prog. 2003;19:283–291. doi: 10.1021/bp025587v. [DOI] [PubMed] [Google Scholar]
- 37.Shaw S.Y., Chen Y.J., Ou J.J., Ho L. Preparation and characterization of Pseudomonas putida esterase immobilized on magnetic nanoparticles. Enzyme Microb. Technol. 2006;39:1089–1095. [Google Scholar]
- 38.Kobayashi H., Matsunaga T. Amino-silane modified superparamagnetic particles with surface-immobilized enzyme. J. Colloid Interface Sci. 1991;141:505–511. [Google Scholar]
- 39.Wu C.W., Lee J.G., Lee W.C. Protein and enzyme immobilization on non-porous microspheres of polystyrene. Biotechnol. Appl. Biochem. 1998;27:225–230. [Google Scholar]
- 40.Ikediobi C.O., Stevens M., Latinwo L. Immobilization of linamarase on non-porous glass beads. Process Biochem. 1998;33:491–494. [Google Scholar]
- 41.Zhou J., Wu W., Caruntu D., Yu M.H., Martin A., Chen J.F., O’Connor C.J., Zhou W.L. Synthesis of porous magnetic hollow silica nanospheres for nanomedicine application. J. Phys. Chem. C. 2007;111:17473–17477. [Google Scholar]
- 42.Tsang S.C., Yu C.H., Gao X., Tam K. Silica-encapsulated nanomagnetic particle as a new recoverable biocatalyst carrier. J. Phys. Chem. B. 2006;110:16914–16922. doi: 10.1021/jp062275s. [DOI] [PubMed] [Google Scholar]
- 43.Yu C.C., Kuo Y.Y., Liang C.F., Chien W.T., Wu H.T., Chang T.C., Jan F.D., Lin C.C. Site-specific immobilization of enzymes on magnetic nanoparticles and their use in organic synthesis. Bioconjugate Chem. 2012;23:714–724. doi: 10.1021/bc200396r. [DOI] [PubMed] [Google Scholar]
- 44.Kouassi G.K., Irudayaraj J., McCarty G. Examination of cholesterol oxidase attachment to magnetic nanoparticles. J. Nanobiotechnol. 2005;3:1. doi: 10.1186/1477-3155-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren Y., Rivera J.G., He L., Kulkarni H., Lee D.K., Messersmith P.B. Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnol. 2011;11 doi: 10.1186/1472-6750-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao M.H., Chen D.H. Immobilization of yeast alcohol dehydrogenase on magnetic nanoparticles for improving its stability. Biotechnol. Lett. 2001;23:3234–3241. [Google Scholar]
- 47.Chen D.H., Liao M.H. Preparation and characterization of YADH-bound magnetic nanoparticles. J. Mol. Catal. B Enzym. 2002;16:283–291. [Google Scholar]
- 48.Huang C.L., Cheng W.C., Yang J.C., Chi M.C., Chen J.H., Lin H.P., Lin L.L. Preparation of carboxylated magnetic particles for the efficient immobilization of C-terminally lysine-tagged Bacillus stearothermophilus aminopeptidase II. J. Ind. Microbiol. Biotechnol. 2010;37:717–725. doi: 10.1007/s10295-010-0715-8. [DOI] [PubMed] [Google Scholar]
- 49.Blanco R.M., Calvete J.J., Guisan J.M. Immobilization stabilization of enzymes: Variables that control the intensity of the trypsin (amine)–agarose (aldehyde) multipoint attachment. Enzyme Microb. Technol. 1989;11:353–359. [Google Scholar]
- 50.Lei H., Wang W., Chen L.L., Li X.C., Yi B., Deng L. The preparation and catalytically active characterization of papain immobilized on magnetic composite microspheres. Enzyme Microb. Technol. 2004;35:15–21. [Google Scholar]
- 51.Pedroche J., del Mar Yust M., Mateo C., Fernadez-Lafuente R., Girón-Calle J., Alaiz M., Vioque J., Guisán J.M., Francisco M. Effect of the support and experimental conditions in the intensity of the multipoint covalent attachment of proteins on glyoxylagarose supports: Correlation between enzyme-support linkages and thermal stability. Enzyme Microb. Technol. 2007;40:1160–1166. [Google Scholar]
- 52.Kuroiwa T., Noguchi Y., Nakajima M., Sato S., Mukataka S. Production of chitosan oligosaccharides using chitosanase immobilized on amylase-coated magnetic nanoparticles. Process Biochem. 2008;43:62–69. [Google Scholar]
- 53.Orlowski M., Meister A. γ-Glutamyl transpeptidase: A new convenient substrate for determination and study of l- and d-glutamyl transpeptidase activities. Biochim. Biophys. Acta. 1963;73:679–681. doi: 10.1016/0006-3002(63)90348-2. [DOI] [PubMed] [Google Scholar]
- 54.Khalafalla S.E., Reimers G.W. Preparation of dilution-stable aqueous magnetic fluids. IEEE Trans. Magn. 1980;16:178–183. [Google Scholar]