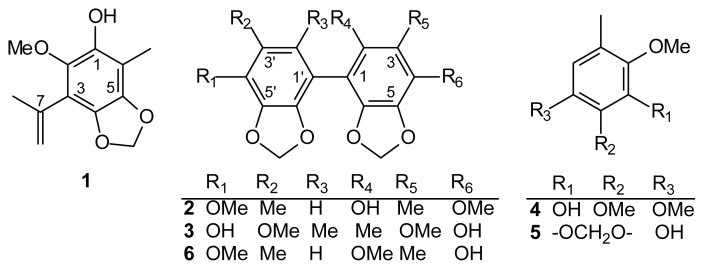
Abstract
Three new benzenoids, 3-isopropenyl-2-methoxy-6-methyl-4,5-methylenedioxyphenol (1), 2-hydroxy-4,4′-dimethoxy-3,3′-dimethyl-5,6,5′,6′-bimethylenedioxybiphenyl (2), 4,4′-dihydroxy-3,3′-dimethoxy-2,2′-dimethyl-5,6,5′,6′-bimethylenedioxybiphenyl (3), together with two known benzenoids, 2,3,6-trimethoxy-5-methylphenol (4) and 2,3-methylenedioxy-4-methoxy-5-methylphenol (5), were isolated from Antrodia camphorata. Our results support that compounds 1–5 potently inhibited LPS (lipopolysaccharide)-induced nitric oxide (NO) production in a dose-dependent manner. The IC50 values of compounds 1, 3 and 5 were 1.8 ± 0.2, 18.8 ± 0.6 and 0.8 ± 0.3 μg/mL, respectively.
Keywords: Antrodia camphorata, polyporaceae, benzenoid, anti-inflammatory
1. Introduction
Antrodia camphorata Wu, Ryvarden and Chang (synonym: Ganoderma camphoratum, Antrodia cinnamomea, Taiwanofungus camphoratus) (Polyporaceae) is a parasitic fungus on the inner wall of the heartwood of Cinnamomun kanehiria Hay (Lauraceae). The fruiting bodies of A. camphorata are called “chang-chih” or “niu-chang-chih” in Taiwan. Traditionally, the fungus has been used for the treatment of food and drug intoxication, diarrhea, abdominal pain, hypertension and liver cancer [1]. The components of this fungus have shown activities of anti-inflammation [2–12], immune-modulation [13], anti-Helicobacter pylori[14], neuroprotection from Aβ damage [15], anti-hepatitis-B virus [16,17] and anticancer [18–31]. Here, we present the result of chemical studies from a mixture of the fruiting body and mycelia of wood cultures of A. camphorata and three new benzenoids, 3-isopropenyl-2-methoxy-6-methyl-4,5-methylenedioxyphenol (1), 2-hydroxy-4,4′- dimethoxy-3,3′-dimethyl-5,6,5′,6′-bimethylenedioxybiphenyl (2), 4,4′-dihydroxy-3,3′-dimethoxy-2,2′- dimethyl-5,6,5′,6′-bimethylenedioxybiphenyl (3) together with two known benzenoids, 2,3,6-trimethoxy-5-methylphenol (4) and 2,3-methylenedioxy-4-methoxy-5-methylphenol (5) (Figure 1), which were isolated and elucidated.
Figure 1.
The chemical structures of compounds 1–5.
2. Results and Discussion
2.1. Isolation and Structure Elucidation
Extensive chromatographic purification of the EtOAc-soluble fraction (Fr. A) of the MeOH extract of A. camphorata afforded compounds 1–5.
Compound 1 was isolated as colorless oil. Its molecular formula, C12H14O4, was determined by High Resolution Fast Atom Bombardment Mass Spectrometry (HR-FABMS) ([M + 1]+, m/z 223.0963). The infrared (IR) spectral data showed the presence of the hydroxyl group (3440 cm−1) and the benzene ring (1618, 1510 cm−1). The 1H- and 13C-nuclear magnetic resonance (NMR) spectra (Table 1) of 1 showed the Heteronuclear Multiple-Quantum (HMQC) correlation, as follows: a OCH2O moiety [δH 5.93 (s), δC 101.7], a Me group [δH 2.27 (s), δC 13.3] and a MeO group [δH 3.94 (s), δC 60.4] on the phenol. The presence of an isopropenyl group was revealed by a Me group [δH 1.99 (s), δC 23.5], two olefinic protons of CH2 [δH 5.24 (br s), 5.36 (br s), δC 121.0] and a quaternary C-atom [δC 127.2 (C-7)]. On the basis of HMBC (Figure 2), cross-peaks [δH 5.93 (OCH2O) coupled to δC 133.0 (C-5) and 136.0 (C-4); δH 2.27 (Me) correlated to δC 110.0 (C-6), 132.0 (C-1) and 133.0 (C-5); δH 4.64 (OH) coupled to δC 132.0 (C-1); δH 3.94 (MeO) coupled to δC 138.5 (C-2); δH 1.99 (Me-7) coupled to δC 97.1 (C-3)] and a combination with the Nuclear Overhauser Effect Spectroscopy (NOESY) experiment (Figure 2) [MeO (δH 3.94) correlation with isopropenyl group (δH 1.99, 5.24, 5.36)] corroborated the locations of the functional groups on the benzene ring. On the basis of the 1H- and 13C-NMR (Table 1), NOESY (Figure 2), Distortionless Enhancement by Polarization Transfer (DEPT), HMQC and Heteronuclear Multiple Bond Correlation (HMBC) (Figure 2) experiments, 1 was characterized as 3-isopropenyl-2-methoxy-6-methyl-4,5-methylenedioxyphenol.
Table 1.
1H- and 13C-nuclear magnetic resonance (NMR) data (CDCl3, 500 and 125 MHz, resp.) of Compounds 1–3. Chemical shifts δ in ppm rel. to TMS, J in Hz. For atom numbering, see the Formulae.
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| δH | δC | δH | δC | δH | δC | |
| 1 | – | 132.0 | – | 129.3 | – | 123.7 |
| 2 | – | 138.5 | – | 135.6 | – | 114.6 |
| 3 | – | 97.1 | – | 116.8 | – | 136.0 |
| 4 | – | 136.0 | – | 135.0 | – | 133.2 |
| 5 | – | 133.0 | – | 136.0† | – | 138.9‡ |
| 6 | – | 110.0 | – | 133.4† | – | 133.3‡ |
| 7 | – | 127.2 | – | – | – | – |
| Me-2 | – | – | – | – | 1.82 (s) | 12.7 |
| Me-3 | – | – | 1.97 (s) | 9.4 | – | – |
| Me-6 | 2.27 (s) | 13.3 | – | – | – | – |
| MeO-2 | 3.94 (s) | 60.4 | – | – | – | – |
| MeO-3 | – | – | – | – | 3.88 (s) | 60.1 |
| MeO-4 | – | – | 3.88 (s) | 60.1 | – | – |
| Me-7 | 1.99 (s) | 23.5 | – | – | – | – |
| CH2-7 | 5.24 (br s) | 121.0 | – | – | – | – |
| 5.36 (br s) | ||||||
| 4-OCH2O-5 | 5.93 (s) | 101.7 | – | – | – | – |
| 5-OCH2O-6 | – | – | 5.96 (s) | 101.8 | 5.99 (s) | 101.7 |
| 1′ | – | – | – | 136.1 | – | 123.7 |
| 2′ | – | – | 5.94 (s) | 109.5 | – | 114.6 |
| 3′ | – | – | – | 124.1 | – | 136.0 |
| 4′ | – | – | – | 137.4 | – | 133.2 |
| 5′ | – | – | – | 138.6 | – | 138.9 |
| 6′ | – | – | – | 134.4 | – | 133.3 |
| Me-2′ | – | – | – | – | 1.82 (s) | 12.7 |
| Me-3′ | – | – | 2.03 (s) | 15.8 | – | – |
| MeO-3′ | – | – | – | – | 3.88 (s) | 60.1 |
| MeO-4′ | – | – | 3.87 (s) | 59.7 | – | – |
| 5′-OCH2O-6′ | – | – | 5.98 (s) | 101.7 | 5.99 (s) | 101.7 |
| OH | 4.64 (s) | – | – | – | 4.56 (s) | – |
exchangeable;
exchangeable.
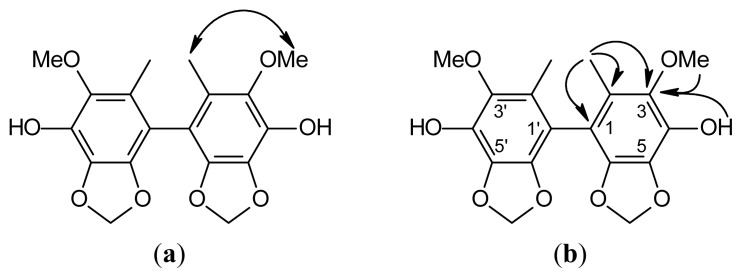
Figure 2.
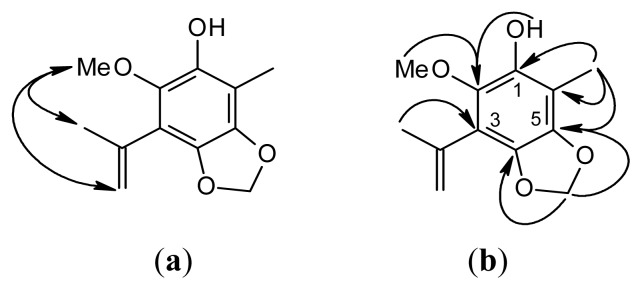
Nuclear Overhauser Effect Spectroscopy (NOESY) contacts (a) and key Heteronuclear Multiple Bond Correlation (HMBC) connectivities (b) of compound 1.
Compound 2 was isolated as an amorphous solid. Its molecular formula, C18H18O7, was determined by HR-FABMS ([M + 1]+, m/z 347.1128). The presence of phenolic moiety was revealed by IR spectral data (3481, 1615, 1512 cm−1). The above data combined with the NMR data (Table 1) revealed 2 to be a biphenyl compound. The 1H-NMR spectrum (Table 1) of 2 showed two OCH2O groups [δH 5.96 (s, 5-OCH2O-6), 5.98 (s, 5′-OCH2O-6′)], two MeO groups [δH 3.87 (s, MeO-4′), 3.88 (s, MeO-4)], two Me groups [δH 1.97 (s, Me-3), 2.03 (s, Me-3′)] and a single aromatic proton [δH 5.94 (s, H-2′)]. The 13C-NMR (Table 1) and DEPT spectra showed that 2 had a total of 18 C-atoms, accounting for two Me [δC 9.42 (Me-3), 15.8 (Me-3′)], two MeO [δC 59.7 (MeO-4′), 60.1 (MeO-4)], two OCH2O [δC 101.7 (5′-OCH2O-6′), 101.8 (5-OCH2O-6)], one aromatic CH [δC 109.5 (C-2′)] and 11 aromatic quaternary C-atoms [δC 116.8 (C-3), 124.1 (C-3′), 129.3 (C-1), 133.4 (C-6), 134.4 (C-6′), 135.0 (C-4), 135.6 (C-2), 136.0 (C-5), 136.1 (C-1′), 137.4 (C-4′), 138.6 (C-5′)]. These data also indicated a biphenyl skeleton. Assignment of chemical shifts of all protonated C-atoms and their associated H-atoms in the molecule can be finished according to HMQC data. On the basis of HMBC (Figure 3), cross-peaks of MeO-4 with C-4, of MeO-4′ with C-4′, of Me-3 with C-2, C-3 and C-4, of Me-3′ with C-2′, C-3′ and C-4′, of 5-OCH2O-6 with C-5 and C-6, of 5′-OCH2O-6′ with C-5′ and C-6′ and of H-2′ with C-1′, C-4′ and C-6′, the remaining C-atoms of the aromatic ring, C-1, were assigned. The NOESY experiment (Figure 2) showing Me-3 correlated with MeO-4 and Me-3′ correlated with H-2′ and MeO-4′ further supported the substitution pattern. According the above evidence, compound 2 can be assigned as structures 2 or 6 (Figure 1). The statistical calculation from a text book [32] suggested that the difference of 13C chemical shift between C-2 and C-4 is slight for structure 2 and larger for structure 6. Therefore, we assigned the compound 2, as structure 2 is more reasonable, and structure 6 will be excluded. On the basis of the 1H- and 13C-NMR (Table 1), NOESY (Figure 2), DEPT, HMQC and HMBC (Figure 3) experiments and comparison of 13C-NMR values between C-2 and C-4, compound 2 was characterized as 2-hydroxy-4,4′-dimethoxy-3,3′-dimethyl-5,6,5′,6′-bimethylenedioxybiphenyl (Figure 3).
Figure 3.
NOESY contacts (a) and key HMBC connectivities (b) of compound 2.
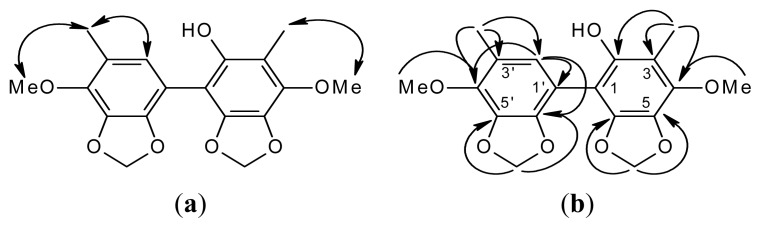
Compound 3 was isolated as an amorphous solid. Its molecular formula, C18H18O8, was determined by HR-FABMS ([M + 1]+, m/z 363.1076). The presence of phenolic moiety was revealed by IR spectral data (3421, 1605, 1508 cm−1). According to the molecular formula, IR spectrum combined with nine 13C-NMR signals indicated that compound 3 is a symmetrical biphenolic derivative. These data with the NMR data (Table 1) suggest a biphenyl compound. The 1H-NMR spectrum (Table 1) of 3 showed a OCH2O group [δH 5.99 (s, 5-OCH2O-6)], a MeO group [δH 3.88 (s, MeO-3)], a Me group [δH 1.82 (s, Me-2)] and a hydroxy group [δH 4.56 (s, HO-4)]. The 13C-NMR (Table 1) and DEPT spectra of 3 showed nine signals, accounting for a Me [δC 12.7 (Me-2)], a MeO [δC 60.1 (MeO-3)], a OCH2O [δC 101.7 (5-OCH2O-6)] and six aromatic quaternary C-atoms (δC 114.6 (C-2), 123.7 (C-1), 133.2 (C-4), 133.3 (C-6), 136.0 (C-3), 138.9 (C-5). Because HR-FABMS showed that the molecular formula is C18H18O8, 3 was suggested to be a symmetrical biphenolic compound. The HMBC data (Figure 4) showed that the H-atom signal of Me-2 correlated to the C-atom signals of C-1, C-2 and C-3 and the H-atom signals of MeO-3 and HO-4 correlated to the C-atom signal of C-3, suggesting that OCH2O group was positioned at C-5 and C-6. On the basis of the 1H- and 13C-NMR (Table 1), NOESY (Figure 4), DEPT, HMQC and HMBC (Figure 4) experiments, 3 was characterized as 4,4′-dihydroxy-3,3′-dimethoxy-2,2′-dimethyl-5,6,5′,6′-bimethylenedioxybiphenyl (Figure 4). The known isolates, 2,3,6-trimethoxy-5-methylphenol (4) [33] and 2,3-methylenedioxy-4-methoxy- 5-methylphenol (5) [33], were readily identified by comparison of physical and spectroscopic data (UV, IR, 1H NMR and mass spectrometry data) with values found in the literature.
Figure 4.
NOESY contacts (a) and key HMBC connectivities (b) of compound 3.
2.2. Anti-Inflammatory Activities
Compounds 1–5 were evaluated for anti-inflammatory activities and exhibited the potential inhibition against LPS (lipopolysaccharide)-induced NO in a dose-dependent manner (Table 2). The IC50 values of compounds 1, 3 and 5 were 1.8 ± 0.2, 18.8 ± 0.6 and 0.8 ± 0.3 μg/mL, respectively (Table 2).
Table 2.
Cell viability and effect of compounds 1–5 on LPS-induced NO production in macrophages a.
| Compound | Dose (μg/mL) | Cell viability (% of control) | NO level (μM) | IC50 (μg/mL) |
|---|---|---|---|---|
| control | (−) | 96.4 ± 4.3 | 2.5 ± 0.2 | |
| LPS | (+) | 97.0 ± 0.8 | 25.3 ± 3.0 ### | |
|
| ||||
| 1 | 0.312 | 92.0 ± 3.3 | 14.5 ± 2.2 ** | 1.8 ± 0.2 |
| 0.625 | 91.0 ± 3.7 | 14.1 ± 1.5 ** | ||
| 1.25 | 90.4 ± 2.4 | 13.0 ± 1.5 *** | ||
| 2.5 | 87.3 ± 2.3 | 12.0 ± 1.7 *** | ||
| 5 | 65.5 ± 1.7 | (−) | ||
|
| ||||
| 2 | 0.312 | 98.0 ± 1.5 | 16.4 ± 2.4 ** | |
| 0.625 | 96.6 ± 7.6 | 16.1 ± 1.3 ** | ||
| 1.25 | 96.6 ± 2.2 | 15.7 ± 2.0 ** | ||
| 2.5 | 92.7 ± 1.3 | 15.5 ± 1.9 ** | ||
| 5 | 71.4 ± 2.2 | (−) | ||
|
| ||||
| 3 | 3.12 | 95.1 ± 2.9 | 13.7 ± 0.1 *** | 18.8 ± 0.6 |
| 6.25 | 93.1 ± 2.7 | 13.4 ± 0.4 *** | ||
| 12.5 | 93.0 ± 2.6 | 13.2 ± 0.1 *** | ||
| 25 | 91.0 ± 7.7 | 12.1 ± 0.6 *** | ||
| 50 | 64.8 ± 2.2 | (−) | ||
|
| ||||
| 4 | 0.312 | 97.0 ± 1.1 | 20.6 ± 1.2 * | |
| 0.625 | 96.2 ± 2.2 | 19.4 ± 2.0 * | ||
| 1.25 | 95.0 ± 2.1 | 18.3 ± 0.3 ** | ||
| 2.5 | 94.6 ± 1.6 | 13.6 ± 0.6 *** | ||
| 5 | 69.3 ± 2.1 | (−) | ||
|
| ||||
| 5 | 0.312 | 93.8 ± 2.9 | 15.2 ± 1.4 ** | 0.8 ± 0.3 |
| 0.625 | 88.5 ± 1.5 | 12.8 ± 1.9 *** | ||
| 1.25 | 85.0 ± 2.9 | 12.1 ± 1.6 *** | ||
| 2.5 | 83.8 ± 1.9 | 10.4 ± 1.3 *** | ||
| 5 | 82.4 ± 2.7 | 10.0 ± 2.2 *** | ||
|
| ||||
| Indomethacin | 25 | 96.2 ± 1.1 | 19.2 ± 0.6 * | |
| 50 | 94.8 ± 1.3 | 14.3 ± 0.8 ** | ||
The data were presented as the mean ± SD for three different experiments performed in triplicate.
Compared with sample of the control group.
p < 0.05,
p < 0.01 and
p < 0.001 were compared with the LPS-alone group.
Compounds 5 is very potent (IC50 = 0.8) for the inhibition of NO production. We will study the anti-inflammatory activities of compound 5 further.
3. Experimental Section
3.1. General
Column chromatography (CC): silica gel 60 (Merck 70–230 mesh, 230–400 mesh, ASTM). Prep. HPLC: (LDC Analytical-III system; column: LiChrosorb Si 60, 7 μm, 250 × 10 mm). UV: Hitachi S-3200 spectrometer; λmax (log ɛ) in nm. IR spectra: Perkin-Elmer 983G spectrophotometer; ν in cm−1. 1H-, 13C- and 2D-NMR spectra: Bruker DMX-500 spectrometer; δ in ppm rel. to TMS, J in Hz. HR-FABMS: JEOL SX-102A spectrometer; m/z.
3.2. Plant Material
The solid cultural fruiting bodies of A. camphorata were identified and provided by Po-Zone Biotechnology Development, Taipei, Taiwan. A voucher specimen was deposited at Po-Zone Biotechnology Development Co. Ltd.
3.3. Extraction and Isolation
The fruiting bodies of wood culture A. camphorata (500 g) were extracted with MeOH (4 L) by maceration at room temperature (7 days × 3). After removal of MeOH under vacuum, the extract was partitioned into EtOAc (Fr. A, 113 g), n-BuOH (Fr. B, 15 g) and H2O-soluble (Fr. C, 27 g) fractions. The EtOAc fraction (Fr. A, 113 g) was subjected to CC (10 × 70 cm, silica gel, 230–400 mesh) using n-hexane, EtOAc and MeOH of increasing polarity as eluent to obtain 11 fractions: Frs. A1–A18. Fr. A4 (11 g, n-hexane/EtOAc 8:2) was subjected to HPLC (CH2Cl2/EtOAc 9:1) to yield 4 (11.9 mg) and 5 (73.2 mg). Fr. A5 (30 g, n-hexane/EtOAc 7:3) was subjected to HPLC (CH2Cl2/EtOAc 9:1) to yield 1 (4.2 mg), 2 (7.3 mg) and 3 (5.1 mg).
3.4. 3-Isopropenyl-2-methoxy-6-methyl-4,5-methylenedioxyphenol (1)
Colorless oil. UV (MeOH): 280 (3.92). IR (neat): 3440, 1618, 1510, 1470, 1230, 1061, 1026. 1H- and 13C-NMR: see Table 1. HR-FABMS m/z: 223.0963 [M + 1]+ (C12H15O4+, calc. 223.0970).
3.5. 2-Hydroxy-4,4′-dimethoxy-3,3′-dimethyl-5,6,5′,6′-bimethylenedioxybiphenyl (2)
Amorphous solid. UV (MeOH): 276 (3.86). IR (KBr): 3481, 1615, 1512, 1468, 1240, 1155. 1H- and 13C-NMR: see Table 1. HR-FABMS m/z: 347.1128 [M + 1]+ (C18H19O7+, calc. 347.1131).
3.6. 4,4′-Dihydroxy-3,3′-dimethoxy-2,2′-dimethyl-5,6,5′,6′-bimethylenedioxybiphenyl (3)
Amorphous solid. UV (MeOH): 270 (3.74). IR (KBr): 3421, 1605, 1508, 1472, 1233, 1130, 1089. 1H- and 13C-NMR: see Table 1. HR-FABMS m/z: 363.1076 [M + 1]+ (C18H19O8+, calc. 363.1080).
3.7. Chemicals
LPS (endotoxin from Escherichia coli, serotype 0127:B8), Carr (type IV), indomethacin, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and other chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
3.8. Cell Culture
A murine macrophage cell line, RAW264.7 (BCRC No. 60001), was purchased from the Bioresources Collection and Research Center (BCRC, Hsinchu, Taiwan) of the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were cultured in plastic dishes containing Dulbecco’s Modified Eagle Medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Sigma) in a CO2 incubator (5% CO2 in air) at 37 °C and subcultured every 3 days at a dilution of 1:5 using 0.05% trypsin-0.02% EDTA in Ca2+-, Mg2+-free phosphate-buffered saline (DPBS).
3.9. Cell Viability
Cells (2 × 105) were cultured in 96-well plate containing DMEM supplemented with 10% FBS for 1 day to become nearly confluent. Then, cells were cultured with compounds 1–5 in the presence of 100 ng/mL LPS (lipopolysaccharide) (Eschericha coli 026:B6; Sigma-Aldrich, St. Louis, Mo) for 24 h. After that, the cells were washed twice with DPBS and incubated with 100 μL of 0.5 mg/mL MTT for 2 h at 37 °C testing for cell viability. The medium was then discarded, and 100 μL dimethyl sulfoxide (DMSO) was added. After 30-min incubation, absorbance at 570 nm was read using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
3.10. Measurement of Nitric Oxide/Nitrite
NO production was indirectly assessed by measuring the nitrite levels in the cultured media and serum determined by a colorimetric method based on the Griess reaction. The cells were incubated with different concentrations of samples in the presence of LPS (100 ng/mL) at 37 °C for 24 h. Then, cells were dispensed into 96-well plates, and 100 μL of each supernatant was mixed with the same volume of Griess reagent (1% sulfanilamide, 0.1% naphthyl ethylenediamine dihydrochloride and 5% phosphoric acid) and incubated at room temperature for 10 min; the absorbance was measured at 540 nm with a Micro-Reader (Molecular Devices).
3.11. Statistical Analysis
IC50 values were estimated using a non-linear regression algorithm (Sigma Plot 8.0; SPSS Inc., Chicago, IL, USA). Statistical evaluation was carried out by one-way analysis of variance (ANOVA, followed by Scheffe’s multiple range tests).
4. Conclusions
3-Isopropenyl-2-methoxy-6-methyl-4,5-methylenedioxyphenol (1), 2-hydroxy-4,4′-dimethoxy-3,3′- dimethyl-5,6,5′,6′-bimethylenedioxybiphenyl (2) and 4,4′-dihydroxy-3,3′-dimethoxy-2,2′-dimethyl- 5,6,5′,6′-bimethylenedioxybiphenyl (3) are new compounds from A. camphorata. Compounds 1, 3 and 5 displayed a significant concentration-dependent inhibition of NO production with IC50 values 1.8 ± 0.2, 18.8 ± 0.6 and 0.8 ± 0.3 μg/mL, respectively.
Acknowledgments
This work was kindly supported by a grant from the China Medical University (CMU100-S-10), in part by the Taiwan Department of Heath Clinical Trial and Research Center of Excellence (DDH 102-TD-B-111-004).
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Tsai Z.T., Liaw S.L. The Use and the Effect of Ganoderma. Sang-Yun Press; Taichung, Taiwan: 1982. p. 116. [Google Scholar]
- 2.Shen Y.C., Chou C.J., Wang Y.H., Chen C.F., Chou Y.C., Lu M.K. Anti-inflammatory activity of the extracts from mycelia of Antrodia camphorata cultured with water-soluble fractions from five different Cinnamomum species. FEMS Microbiol. Lett. 2004;231:137–143. doi: 10.1016/S0378-1097(03)00953-4. [DOI] [PubMed] [Google Scholar]
- 3.Shen Y.C., Wang Y.H., Chou Y.C., Chen C.F., Lin L.C., Chang T.T., Tien J.H., Chou C.J. Evaluation of the anti-inflammatory activity of zhankuic acids isolated from the fruiting bodies of Antrodia camphorata. Planta Med. 2004;70:310–314. doi: 10.1055/s-2004-818941. [DOI] [PubMed] [Google Scholar]
- 4.Cheng J.J., Yang C.J., Cheng C.H., Wang Y.T., Huang N.K., Lu M.K. Characterization and functional study of Antrodia camphorata lipopolysaccharide. J. Agric. Food Chem. 2005;53:469–474. doi: 10.1021/jf049281a. [DOI] [PubMed] [Google Scholar]
- 5.Chen J.J., Lin W.J., Liao C.H., Shieh P.C. Anti-inflammatory benzenoids from Antrodia camphorata. J. Nat. Prod. 2007;70:989–992. doi: 10.1021/np070045e. [DOI] [PubMed] [Google Scholar]
- 6.Chien S.C., Chen M.L., Kuo H.T., Tsai Y.C., Lin B.F., Kuo Y.H. Anti-inflammatory activities of new succinic and maleic derivatives from the fruiting body of Antrodia camphorata. J. Agric. Food Chem. 2008;56:7017–7022. doi: 10.1021/jf801171x. [DOI] [PubMed] [Google Scholar]
- 7.Yang S.S., Wang G.J., Wang S.Y., Lin Y.Y., Kuo Y.H., Lee T.H. New constituents with iNOS inhibitory activity from mycelium of Antrodia camphorata. Planta Med. 2009;75:512–516. doi: 10.1055/s-0029-1185305. [DOI] [PubMed] [Google Scholar]
- 8.Wu S.J., Leu Y.L., Chen C.H., Chao C.H., Shen D.Y., Chan H.H., Lee E.J., Wu T.S., Wang Y.H., Shen Y.C., et al. Camphoratins A–J, potent cytotoxic and anti-inflammatory triterpenoids from the fruiting body of Taiwanofungus camphoratus. J. Nat. Prod. 2010;73:1756–1762. doi: 10.1021/np1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang G.J., Huang S.S., Lin S.S., Shao Y.Y., Chen C.C., Hou W.C., Kuo Y.H. Analgesic effects and the mechanisms of anti-inflammation of ergostatrien-3β-ol from Antrodia camphorata submerged whole broth in mice. J. Agric. Food Chem. 2010;58:7445–7452. doi: 10.1021/jf1013764. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y.C., Liu Y.L., Li F.Y., Chang C.I., Wang S.Y., Lee K.Y., Li S.L., Chen Y.P., Jinn T.R., Tzen J.T.C. Antcin A, a steroid-like compound from Antrodia camphorata, exerts anti-inflammatory effect via mimicking glucocorticoids. Acta Pharmacol. Sin. 2011;32:904–911. doi: 10.1038/aps.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung T.Y., Li F.Y., Chang C.I., Jinn T.R., Tzen J.T.C. Inhibition of Na+/K+-ATPase by antcins, unique steroid-like compounds in Antrodia camphorate. Am. J. Chin. Med. 2012;40:953–965. doi: 10.1142/S0192415X1250070X. [DOI] [PubMed] [Google Scholar]
- 12.Huang G.J., Deng J.S., Huang S.S., Shao Y.Y., Chen C.C., Kuo Y.H. Protective effect of antrosterol from Antrodia camphorata submerged whole broth against carbon tetrachloride-induced acute liver injury in mice. Food Chem. 2012;132:709–716. [Google Scholar]
- 13.Shen Y.C., Chen C.F., Wang Y.H., Chang T.T., Chou C.J. Evaluation of the immuno-modulating activity of some active principles isolated from the fruiting bodies of Antrodia camphorata. Chin. Pharm. J. 2003;55:313–318. [Google Scholar]
- 14.Geethangili M., Fang S.H., Lai C.H., Rao Y.K., Lien H.M., Tzeng Y.M. Inhibitory effect of Antrodia camphorata constituents on the Helicobacter pylori-associated gastric inflammation. Food Chem. 2010;119:149–153. [Google Scholar]
- 15.Chen C.C., Shiao Y.J., Lin R.D., Shao Y.Y., Lai M.N., Lin C.C., Ng L.T., Kuo Y.H. Neuroprotective diterpenes from the fruiting body of Antrodia camphorata. J. Nat. Prod. 2006;69:689–691. doi: 10.1021/np0581263. [DOI] [PubMed] [Google Scholar]
- 16.Lee I.H., Huang R.L., Chen C.T., Chen H.C., Hsu W.C., Lu M.K. Antrodia camphorata polysaccharides exhibit anti-hepatitis B virus effects. FEMS Microbiol. Lett. 2002;209:63–67. doi: 10.1111/j.1574-6968.2002.tb11110.x. [DOI] [PubMed] [Google Scholar]
- 17.Huang R.L., Huang Q., Chen C.F., Chang T.T., Chou C.J. Anti-viral effects of active compounds from Antrodia camphorata on wild-type and lamivudine-resistant mutant HBV. Chin. Pharm. J. 2003;55:371–379. [Google Scholar]
- 18.Nakamura N., Hirakawa A., Gao J.J., Kakuda H., Shiro M., Komatsu Y., Sheu C.C., Hattori M. Five new maleic and succinic acid derivatives from the mycelium of Antrodia camphorata and their cytotoxic effects on LLC tumor cell line. J. Nat. Prod. 2004;67:46–48. doi: 10.1021/np030293k. [DOI] [PubMed] [Google Scholar]
- 19.Liu J.J., Huang T.S., Hsu M.L., Chen C.C., Lin W.S., Lu F.J., Chang W.H. Antitumor effects of the partially purified polysaccharides from Antrodia camphorata and the mechanism of its action. Toxicol. Appl. Pharmacol. 2004;201:186–193. doi: 10.1016/j.taap.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Lee T.H., Lee C.K., Tsou W.L., Liu S.Y., Kuo M.T., Wen W.C. A new cytotoxic agent from solid-state fermented mycelium of Antrodia camphorata. Planta Med. 2007;73:1412–1415. doi: 10.1055/s-2007-990232. [DOI] [PubMed] [Google Scholar]
- 21.Yeh C.T., Rao Y.K., Yao C.J., Yeh C.F., Li C.H., Chuang S.E., Luong J.H., Lai G.M., Tzeng Y.M. Cytotoxic triterpenes from Antrodia camphorata and their mode of action in HT-29 human colon cancer cells. Cancer Lett. 2009;285:73–79. doi: 10.1016/j.canlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Deng J.Y., Chen S.J., Jow G.M., Hsueh C.W., Jeng C.J. Dehydroeburicoic acid induces calcium- and calpain-dependent necrosis in human U87MG glioblastomas. Chem. Res. Toxicol. 2009;22:1817–1826. doi: 10.1021/tx9002275. [DOI] [PubMed] [Google Scholar]
- 23.Tsai W.C., Rao Y.K., Lin S.S., Chou M.Y., Shen Y.T., Wu C.H., Geethangili M., Yang C.C., Tzeng Y.M. Methylantcinate A induces tumor specific growth inhibition in oral cancer cells via Bax-mediated mitochondrial apoptotic pathway. Bioorg. Med. Chem. Lett. 2010;20:6145–1648. doi: 10.1016/j.bmcl.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh Y.C., Rao Y.K., Wu C.C., Huang C.Y.F., Geethangili M., Hsu S.L., Tzeng Y.M. Methyl antcinate A from Antrodia camphorata induces apoptosis in human liver cancer cells through oxidant-mediated cofilin- and Bax-triggered mitochondrial pathway. Chem. Res. Toxicol. 2010;23:1256–1267. doi: 10.1021/tx100116a. [DOI] [PubMed] [Google Scholar]
- 25.Shi L.S., Chao C.H., Shen D.Y., Chan H.H., Chen C.H., Liao Y.R., Wu S.J., Leu Y.L., Shen Y.C., Kuo Y.H., et al. Biologically active constituents from the fruiting body of Taiwanofungus camphoratus. Bioorg. Med. Chem. 2011;19:677–683. doi: 10.1016/j.bmc.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh Y.C., Rao Y.K., Whang-Peng J., Huang C.Y.F., Shyue S.K., Hsu S.L., Tzeng Y.M. Antcin B and its ester derivative from Antrodia camphorata induce apoptosis in hepatocellular carcinoma cells involves enhancing oxidative stress coincident with activation of intrinsic and extrinsic apoptotic pathway. J. Agric. Food Chem. 2011;59:10943–10954. doi: 10.1021/jf202771d. [DOI] [PubMed] [Google Scholar]
- 27.Yu C.C., Chiang P.C., Lu P.H., Kuo M.T., Wen W.C., Chen P., Guh J.H. Antroquinonol, a natural ubiquinone derivative, induces a cross talk between apoptosis, autophagy and senescence in human pancreatic carcinoma cells. J. Nutr. Biochem. 2012;23:900–907. doi: 10.1016/j.jnutbio.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Tu S.H., Wu C.H., Chen L.C., Huang C.S., Chang H.W., Chang C.H., Lien H.M., Ho Y.S. In vivo antitumor effects of 4,7-dimethoxy-5-methyl-1,3-benzodioxole isolated from the fruiting body of Antrodia camphorata through activation of the p53-mediated p27/Kip1 signaling pathway. J. Agric. Food Chem. 2012;60:3612–3618. doi: 10.1021/jf300221g. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y.P., Tsai W.C., Ko C.J., Rao Y.K., Yang C.R., Chen D.R., Yang M.H., Yang C.C., Tzeng Y.M. Anticancer effects of eleven triterpenoids derived from Antrodia camphorata. Anticancer Res. 2012;32:2727–2734. [PubMed] [Google Scholar]
- 30.Lin T.Y., Chien S.C., Kuo Y.H., Wang S.Y. Distinguishing between R- and S-antcin C and their cytotoxicity. Nat. Prod. Commun. 2012;7:835–836. [PubMed] [Google Scholar]
- 31.Huang H.C., Liaw C.C., Yang H.L., Hseu Y.C., Kuo H.T., Tsai Y.C., Chien S.C., Amagaya S., Chen Y.C., Kuo Y.H. Lanostane triterpenoids and sterols from Antrodia camphorata. Phytochemistry. 2012;84:177–183. doi: 10.1016/j.phytochem.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Silverstein R.M., Webster F.X., Kiemle D.J. Spectrometric Identification of Organic Compounds. 7th ed. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2005. p. 223. [Google Scholar]
- 33.Chiu H.L., Wu J.H., Tung Y.T., Lee T.H., Chien S.C., Kuo Y.H. Triterpenoids and aromatics from Derris laxiflora. J. Nat. Prod. 2008;71:1829–1832. doi: 10.1021/np800253s. [DOI] [PubMed] [Google Scholar]