Abstract
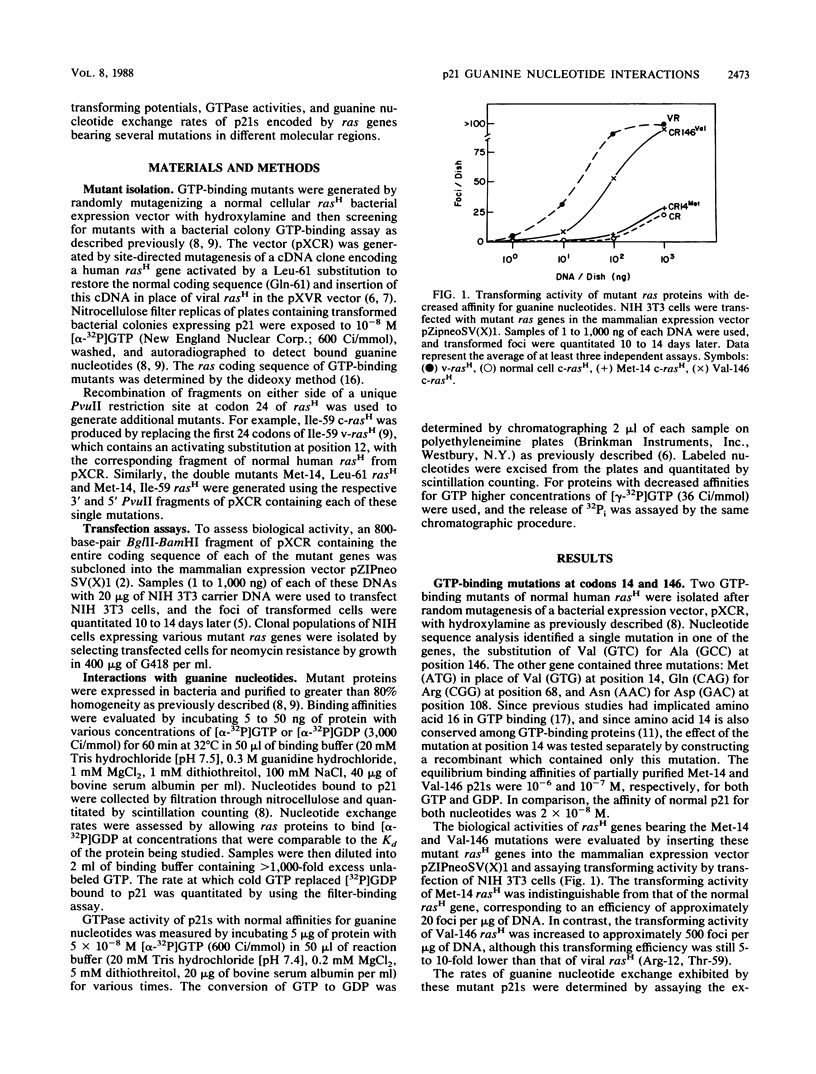
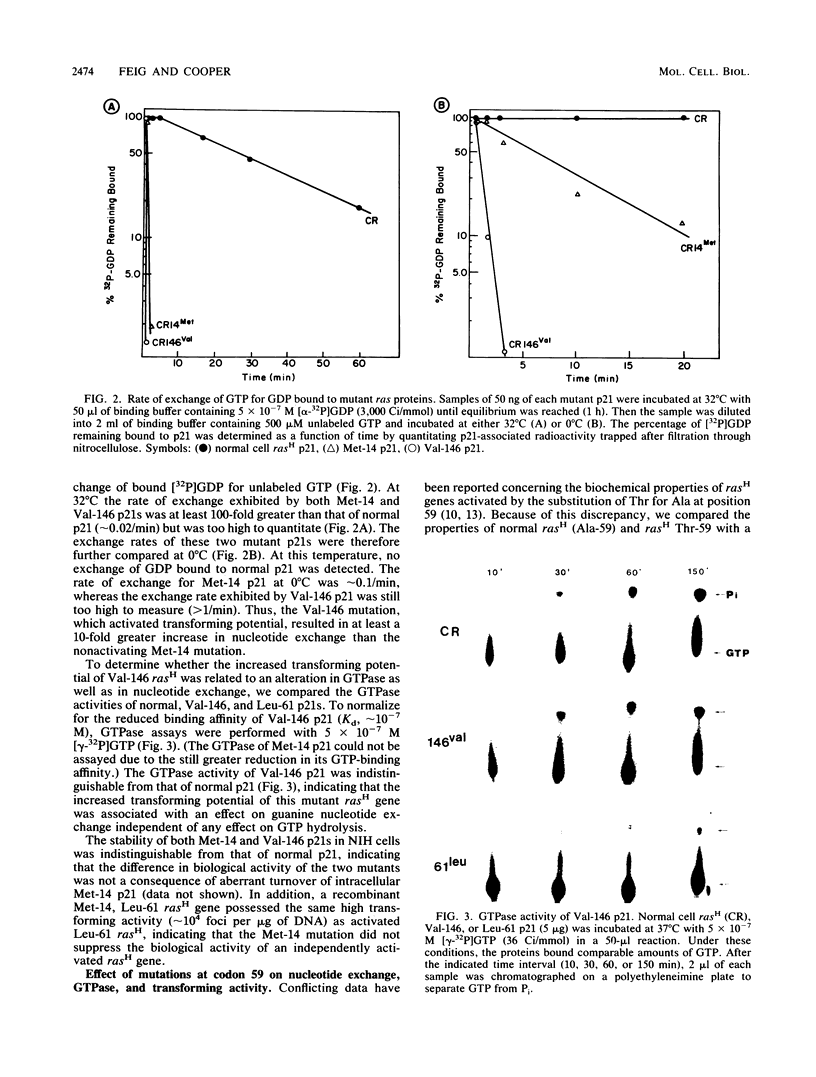
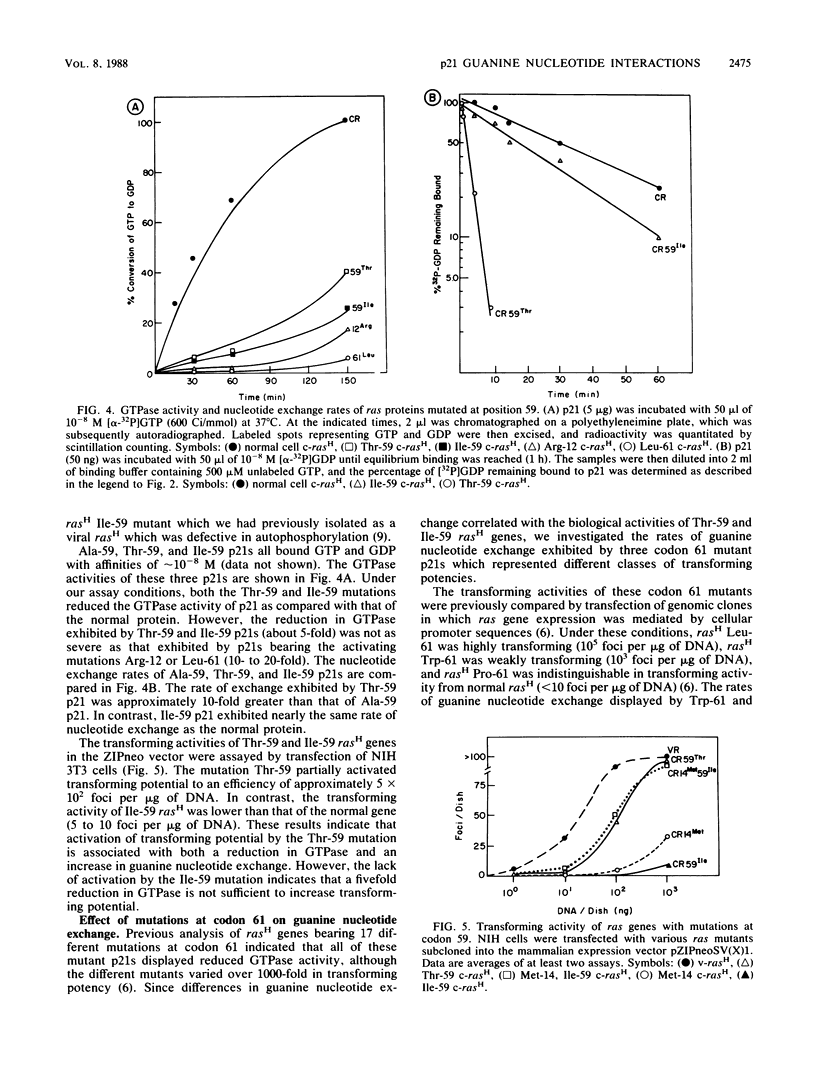
The effect of a series of mutations on the transforming potential of normal human rasH has been compared with their effects on GTPase and guanine nucleotide exchange rates of p21. The mutation Val-146 resulted in partial activation of transforming potential which could be attributed to a greater than 1,000-fold-increased rate of nucleotide exchange in the absence of an effect on GTPase. In contrast, the more modest enhancement of exchange rate (approximately 100-fold) which resulted from the mutation Met-14 did not affect biological activity. The partially activating mutation Thr-59 was found to result in both a 5-fold reduction in GTPase and a 10-fold increase in nucleotide exchange. However, the nontransforming mutant Ile-59 displayed a comparable decrease in GTPase without an effect on nucleotide exchange. The activating effect of the Thr-59 mutation may thus represent a combined effect of reduced GTPase and increased exchange. Similarly, the strongly activating mutation Leu-61 resulted in a fivefold increase in nucleotide exchange in addition to decreased GTPase, whereas weakly activating mutations at position 61 (Trp and Pro) resulted only in decreased GTPase without affecting nucleotide exchange rates. Finally, combining the two mutations Met-14 and Ile-59, which alone had no effect on biological activity, yielded a double mutant with a 20-fold increased transforming potential, demonstrating a synergistic effect of these two mutations. Overall, these results indicate that large increases in nucleotide exchange can activate ras transforming potential in the absence of decreased GTPase and that relatively modest increases in nucleotide exchange can act synergistically with decreased GTPase to contribute to ras activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Clanton D. J., Hattori S., Shih T. Y. Mutations of the ras gene product p21 that abolish guanine nucleotide binding. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5076–5080. doi: 10.1073/pnas.83.14.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanton D. J., Lu Y. Y., Blair D. G., Shih T. Y. Structural significance of the GTP-binding domain of ras p21 studied by site-directed mutagenesis. Mol Cell Biol. 1987 Sep;7(9):3092–3097. doi: 10.1128/mcb.7.9.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Cooper G. M. Transfection by exogenous and endogenous murine retrovirus DNAs. Cell. 1979 Feb;16(2):347–356. doi: 10.1016/0092-8674(79)90011-4. [DOI] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Der C. J., Pan B. T., Cooper G. M. rasH mutants deficient in GTP binding. Mol Cell Biol. 1986 Sep;6(9):3291–3294. doi: 10.1128/mcb.6.9.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig L. A., Corbley M., Pan B. T., Roberts T. M., Cooper G. M. Structure/function analysis of ras using random mutagenesis coupled with functional screening assays. Mol Endocrinol. 1987 Feb;1(2):127–136. doi: 10.1210/mend-1-2-127. [DOI] [PubMed] [Google Scholar]
- Feig L. A., Pan B. T., Roberts T. M., Cooper G. M. Isolation of ras GTP-binding mutants using an in situ colony-binding assay. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4607–4611. doi: 10.1073/pnas.83.13.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., Aaronson S. A. Activation of ras p21 transforming properties associated with an increase in the release rate of bound guanine nucleotide. Mol Cell Biol. 1986 Dec;6(12):4214–4220. doi: 10.1128/mcb.6.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Srivastava S. K., Anderson P. S., Aaronson S. A. Ras p21 proteins with high or low GTPase activity can efficiently transform NIH/3T3 cells. Cell. 1986 Feb 28;44(4):609–617. doi: 10.1016/0092-8674(86)90270-9. [DOI] [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Temeles G. L., Wolanski B. S., Socher S. H., Scolnick E. M. Mutant ras-encoded proteins with altered nucleotide binding exert dominant biological effects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):952–956. doi: 10.1073/pnas.83.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Trahey M., Milley R. J., Cole G. E., Innis M., Paterson H., Marshall C. J., Hall A., McCormick F. Biochemical and biological properties of the human N-ras p21 protein. Mol Cell Biol. 1987 Jan;7(1):541–544. doi: 10.1128/mcb.7.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Clark S. G., Levinson A. D. The oncogenic activation of human p21ras by a novel mechanism. Science. 1986 Aug 8;233(4764):649–652. doi: 10.1126/science.3487832. [DOI] [PubMed] [Google Scholar]