Abstract
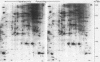
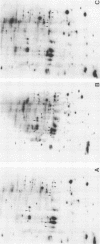
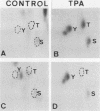
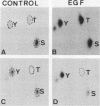
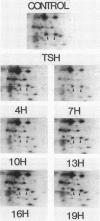
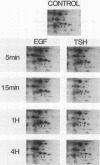
Protein phosphorylation was studied in primary cultures of thyroid epithelial cells after the addition of different mitogens: thyrotropin (TSH) acting through cyclic AMP, epidermal growth factor (EGF), or 12-O-tetradecanoylphorbol-13-acetate (TPA). EGF or TPA increased the phosphorylation of five common polypeptides. Among these, two 42-kilodalton proteins contained phosphotyrosine and phosphoserine with or without phosphothreonine. Their characteristics suggested that they are similar to the two 42-kilodalton target proteins for tyrosine protein phosphorylation demonstrated in fibroblasts in response to mitogens. No common phosphorylated proteins were detected in TSH-treated cells and in EGF- or TPA-treated cells. The differences in the protein phosphorylation patterns in response to TSH, EGF, and TPA suggested that the newly emerging cyclic AMP-mediated mitogenic pathway is distinct from the better known growth factor- and tumor promoter-induced pathways.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach L. K., Eggo M. C., Mak W. W., Burrow G. N. Phorbol esters stimulate growth and inhibit differentiation in cultured thyroid cells. Endocrinology. 1985 Apr;116(4):1603–1609. doi: 10.1210/endo-116-4-1603. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol lipids and cell proliferation. Biochim Biophys Acta. 1987 Apr 20;907(1):33–45. doi: 10.1016/0304-419x(87)90017-5. [DOI] [PubMed] [Google Scholar]
- Bertics P. J., Weber W., Cochet C., Gill G. N. Regulation of the epidermal growth factor receptor by phosphorylation. J Cell Biochem. 1985;29(3):195–208. doi: 10.1002/jcb.240290304. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Cirillo D. M., Gaudino G., Naldini L., Comoglio P. M. Receptor for bombesin with associated tyrosine kinase activity. Mol Cell Biol. 1986 Dec;6(12):4641–4649. doi: 10.1128/mcb.6.12.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contor L., Lamy F., Lecocq R. E. Use of electroblotting to detect and analyze phosphotyrosine containing peptides separated by two-dimensional gel electrophoresis. Anal Biochem. 1987 Feb 1;160(2):414–420. doi: 10.1016/0003-2697(87)90069-8. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Regulation of cell growth and transformation by tyrosine-specific protein kinases: the search for important cellular substrate proteins. Curr Top Microbiol Immunol. 1983;107:125–161. doi: 10.1007/978-3-642-69075-4_4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Diverse mitogenic agents induce the phosphorylation of two related 42,000-dalton proteins on tyrosine in quiescent chick cells. Mol Cell Biol. 1984 Jan;4(1):30–37. doi: 10.1128/mcb.4.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Ganong B. R., Bell R. M., Czech M. P. Structural requirements for diacylglycerols to mimic tumor-promoting phobol diester action on the epidermal growth factor receptor. J Biol Chem. 1985 May 10;260(9):5315–5322. [PubMed] [Google Scholar]
- Dumont J. E., Willems C., Van Sande J., Nève P. Regulation of the release of thyroid hormones: role of cyclic AMP. Ann N Y Acad Sci. 1971 Dec 30;185:291–316. doi: 10.1111/j.1749-6632.1971.tb45255.x. [DOI] [PubMed] [Google Scholar]
- Gilmore T., Martin G. S. Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature. 1983 Dec 1;306(5942):487–490. doi: 10.1038/306487a0. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Deery W. J., Ferdows M. S., Nielsen T. B., Field J. B. Role of cellular Ca++ in phosphorylation of 21 K and 19 K polypeptides in cultured thyroid cells: effects of phorbol ester, trifluoperazine, and 8-diethylamino-octyl-3,4,5-trimethoxybenzoate hydrochloride. Endocrinology. 1987 Jul;121(1):175–181. doi: 10.1210/endo-121-1-175. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Deery W. J., Nielsen T. B., Ferdows M. S., Field J. B. Dephosphorylation of 19K and 21K polypeptides in response to thyroid-stimulating hormone in cultured thyroid cells. Endocrinology. 1986 Aug;119(2):591–599. doi: 10.1210/endo-119-2-591. [DOI] [PubMed] [Google Scholar]
- Isacke C. M., Meisenhelder J., Brown K. D., Gould K. L., Gould S. J., Hunter T. Early phosphorylation events following the treatment of Swiss 3T3 cells with bombesin and the mammalian bombesin-related peptide, gastrin-releasing peptide. EMBO J. 1986 Nov;5(11):2889–2898. doi: 10.1002/j.1460-2075.1986.tb04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M. Diverse mitogenic agents induce rapid phosphorylation of a common set of cellular proteins at tyrosine in quiescent mammalian cells. J Biol Chem. 1985 Feb 10;260(3):1771–1779. [PubMed] [Google Scholar]
- Kohno M., Pouysségur J. Alpha-thrombin-induced tyrosine phosphorylation of 43,000- and 41,000-Mr proteins is independent of cytoplasmic alkalinization in quiescent fibroblasts. Biochem J. 1986 Sep 1;238(2):451–457. doi: 10.1042/bj2380451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Allemain G., Pouysségur EGF and insulin action in fibroblasts. Evidence that phosphoinositide hydrolysis is not an essential mitogenic signalling pathway. FEBS Lett. 1986 Mar 3;197(1-2):344–348. doi: 10.1016/0014-5793(86)80354-4. [DOI] [PubMed] [Google Scholar]
- Lamy F., Roger P. P., Lecocq R., Dumont J. E. Differential protein synthesis in the induction of thyroid cell proliferation by thyrotropin, epidermal growth factor or serum. Eur J Biochem. 1986 Mar 3;155(2):265–272. doi: 10.1111/j.1432-1033.1986.tb09485.x. [DOI] [PubMed] [Google Scholar]
- Lecocq R., Lamy F., Dumont J. E. Pattern of protein phosphorylation in intact stimulated cells: thyrotropin and dog thyroid. Eur J Biochem. 1979 Dec;102(1):147–152. doi: 10.1111/j.1432-1033.1979.tb06274.x. [DOI] [PubMed] [Google Scholar]
- Mockel J., Van Sande J., Decoster C., Dumont J. E. Tumor promoters as probes of protein kinase C in dog thyroid cell: inhibition of the primary effects of carbamylocholine and reproduction of some distal effects. Metabolism. 1987 Feb;36(2):137–143. doi: 10.1016/0026-0495(87)90007-2. [DOI] [PubMed] [Google Scholar]
- Nakamura K. D., Martinez R., Weber M. J. Tyrosine phosphorylation of specific proteins after mitogen stimulation of chicken embryo fibroblasts. Mol Cell Biol. 1983 Mar;3(3):380–390. doi: 10.1128/mcb.3.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parekh B. S., Mehta H. B., West M. D., Montelaro R. C. Preparative elution of proteins from nitrocellulose membranes after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1985 Jul;148(1):87–92. doi: 10.1016/0003-2697(85)90631-1. [DOI] [PubMed] [Google Scholar]
- Reuse S., Roger P. P., Vassart G., Dumont J. E. Enhancement of cmyc mRNA concentration in dog thyrocytes initiating DNA synthesis in response to thyrotropin, forskolin, epidermal growth factor and phorbol myristate ester. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1066–1076. doi: 10.1016/s0006-291x(86)80152-8. [DOI] [PubMed] [Google Scholar]
- Roger P. P., Dumont J. E. Factors controlling proliferation and differentiation of canine thyroid cells cultured in reduced serum conditions: effects of thyrotropin, cyclic AMP and growth factors. Mol Cell Endocrinol. 1984 Jun;36(1-2):79–93. doi: 10.1016/0303-7207(84)90087-x. [DOI] [PubMed] [Google Scholar]
- Roger P. P., Reuse S., Servais P., Van Heuverswyn B., Dumont J. E. Stimulation of cell proliferation and inhibition of differentiation expression by tumor-promoting phorbol esters in dog thyroid cells in primary culture. Cancer Res. 1986 Feb;46(2):898–906. [PubMed] [Google Scholar]
- Roger P. P., Servais P., Dumont J. E. Induction of DNA synthesis in dog thyrocytes in primary culture: synergistic effects of thyrotropin and cyclic AMP with epidermal growth factor and insulin. J Cell Physiol. 1987 Jan;130(1):58–67. doi: 10.1002/jcp.1041300110. [DOI] [PubMed] [Google Scholar]
- Roger P. P., Servais P., Dumont J. E. Regulation of dog thyroid epithelial cell cycle by forskolin, an adenylate cyclase activator. Exp Cell Res. 1987 Oct;172(2):282–292. doi: 10.1016/0014-4827(87)90387-9. [DOI] [PubMed] [Google Scholar]
- Roger P. P., Servais P., Dumont J. E. Stimulation by thyrotropin and cyclic AMP of the proliferation of quiescent canine thyroid cells cultured in a defined medium containing insulin. FEBS Lett. 1983 Jul 4;157(2):323–329. doi: 10.1016/0014-5793(83)80569-9. [DOI] [PubMed] [Google Scholar]
- Roger P. P., Van Heuverswyn B., Lambert C., Reuse S., Vassart G., Dumont J. E. Antagonistic effects of thyrotropin and epidermal growth factor on thyroglobulin mRNA level in cultured thyroid cells. Eur J Biochem. 1985 Oct 15;152(2):239–245. doi: 10.1111/j.1432-1033.1985.tb09189.x. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena A., Coombs M., Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5748–5752. doi: 10.1073/pnas.81.18.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEKEVITZ P. Uptake of radioactive alanine in vitro into the proteins of rat liver fractions. J Biol Chem. 1952 Apr;195(2):549–565. [PubMed] [Google Scholar]
- Tanabe A., Nielsen T. B., Rani C. S., Field J. B. Thyroid cell responses to thyrotropin and 12-O-tetradecanoyl-phorbol-13-acetate: translocation of protein kinase C and phosphorylation of thyroid cell polypeptide substrates. Arch Biochem Biophys. 1985 Nov 15;243(1):92–99. doi: 10.1016/0003-9861(85)90776-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y., Dekker A., Ferdows M., Rani C. S., Field J. B. Effects of phorbol esters on metabolic variables in the thyroid. Endocrinology. 1986 Nov;119(5):2018–2024. doi: 10.1210/endo-119-5-2018. [DOI] [PubMed] [Google Scholar]