Abstract
Nolinospiroside F is a steroidal saponin isolated from Ophiopogon japonicus (O. japonicus). In this study, we found that nolinospiroside F significantly extends the replicative lifespan of K6001 yeast at doses of 1, 3 and 10 μM, indicating that it has an anti-aging effect. This may be attributed to its anti-oxidative effect, as nolinospiroside F could increase yeast survival under oxidative stress conditions and decrease the level of malondialdehyde (MDA), an oxidative stress biomarker. It could also increase anti-oxidative stress genes, SOD1 and SOD2, expression, and the activity of superoxide dismutase (SOD). It increase the activity of SIRT1, an upstream inducer of SOD2 expression. In sod1 and sod2 mutant yeast strains, nolinospiroside F failed to extend their replicative lifespan. These results indicate that SOD participates in the anti-aging effect of nolinospiroside F. Furthermore, nolinospiroside F inhibited the expression of UTH1, a yeast-aging gene that is involved in the oxidative stress of yeast, and failed to extend the replicative lifespan of uth1 or skn7 mutant yeast cells. SKN7 is the transcriptional activator of UTH1. We also demonstrate that SOD and UTH1 regulate each other’s expression. Together, these results suggest that SOD and UTH1 genes are required for and play interactive roles in nolinospiroside F-mediated yeast lifespan extension.
Keywords: nolinospiroside F, SOD, UTH1, anti-aging, anti-oxidative stress
Abbreviations
- AD
Alzheimer’s disease
- MS
mass spectrometry
- MDA
malondialdehyde
- NMR
nuclear magnetic resonance
- O. japonicus
Ophiopogon japonicus
- RES
resveratrol
- ROS
reactive oxygen species
- RT-PCR
real-time polymerase chain reaction
- SOD
superoxide dismutase
- YPD
yeast peptone dextrose
- YPGal
yeast peptone galactose
1. Introduction
Aging has become a worldwide problem, and increasing numbers of people are suffering from age-related diseases, such as Alzheimer’s disease (AD) and diabetes. Recent studies have shown a close relationship between aging and age-related disorders, especially between aging and AD. Small molecules, such as resveratrol (RES), have been shown to have significant anti-aging effects on yeast and positive effects on AD via the SIRT1 signaling pathway, a pathway that’s known to have important functions in aging and AD [1–7]. Since no effective treatment drugs are available for AD, studies on anti-aging substances have great significance in AD treatment.
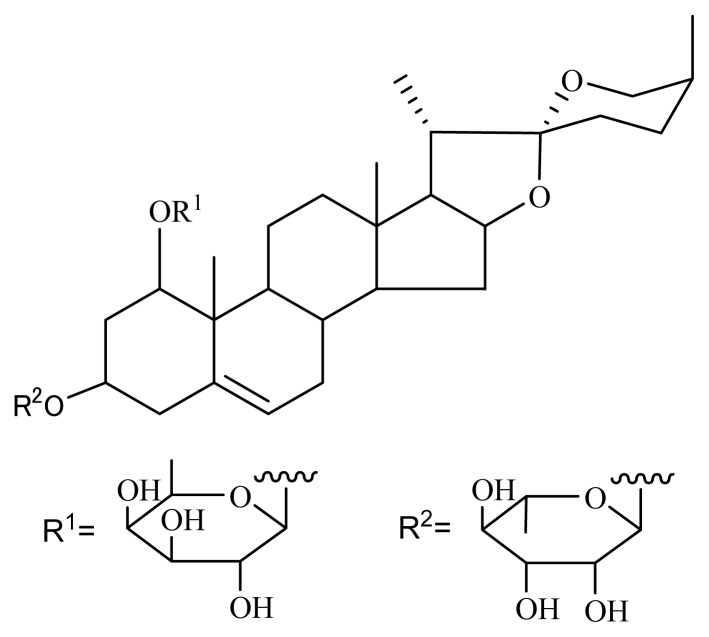
One of the best-known methods used in anti-aging studies is the yeast replicative lifespan assay. A new bioassay method was established with the K6001 yeast strain, which is more convenient than the traditional lifespan assay [8]. By using the new assay, we screened several anti-aging compounds, such as ganodermasides A–D, phloridzin and hesperidin [9–12]. In the present study, we have isolated a known steroidal saponin, nolinospiroside F, from O. japonicus (maidong in Chinese) (Figure 1) [13].
Figure 1.
Chemical structure of nolinospiroside F.
Steroidal saponins are molecules with various bioactivities. Steroidal saponins are mainly found in plants and some marine organisms, such as starfish [14–16]. Previous studies have shown that steroidal saponins have anti-AD, anti-tumor, anti-inflammatory, anti-thrombotic and anti-fungal activities [17–21]. Because of their diverse biological activities, steroidal saponins are extensively studied and have attracted commercial interest. However, few studies on the anti-aging effect of steroidal saponins have been reported. Although nolinospiroside F was isolated in 1983 [22], very few reports on its biological activity have been published. In the present paper, we report that nolinospiroside F possesses anti-aging effects on the yeast replicative lifespan by regulating each other’s expression of SOD and UTH1 genes.
2. Results and Discussion
2.1. Nolinospiroside F Has Anti-Aging Effects in Yeast
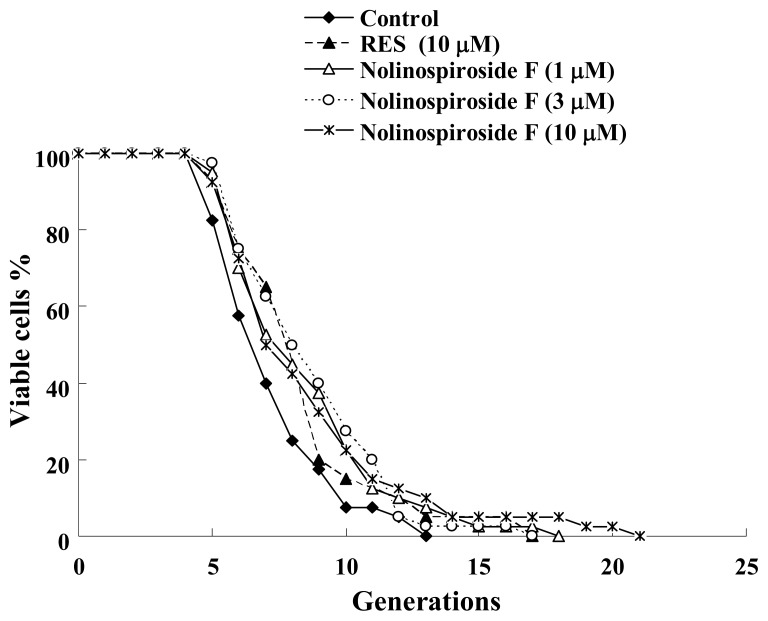
When screening natural products, the bioassay used must be simple, reliable and fast and have good reproducibility. The K6001 yeast strain meets these requirements, as proven in studies with a well-known small molecule, resveratrol (RES) [8]. In previous studies, anti-aging compounds were screened by using this bioassay, which included novel ganodermasides A–D isolated using this bioassay from the spores of the medicinal mushroom, Ganoderma lucidum, and phloridzin extracted from apple branch [9–11]. In this study, the bioassay was used to investigate the anti-aging effects of nolinospiroside F. The results show that nolinospiroside F extends the yeast replicative lifespan significantly at concentrations of 1, 3 and 10 μM (p < 0.05, p < 0.01 and p < 0.05, respectively) (Figure 2), indicating that it has anti-aging effects in yeast.
Figure 2.
Effects of nolinospiroside F on the replicative lifespan of K6001 yeast strain. The yeast cells grown in YPGal medium were spread on a glucose medium plate containing different nolinospiroside F concentrations. The daughter cells of 40 microcolonies of each experiment were counted. The assay was repeated at least thrice. The average lifespan of untreated K6001 was 6.43 generations; resveratrol (RES) at 10 μM, 7.55*; nolinospiroside F at 1 μM, 7.65*; at 3 μM, 7.88**; and at 10 μM, 7.80* (* p < 0.05, ** p < 0.01).
2.2. Nolinospiroside F Affects the Growth of Yeast under Oxidative Stress Conditions
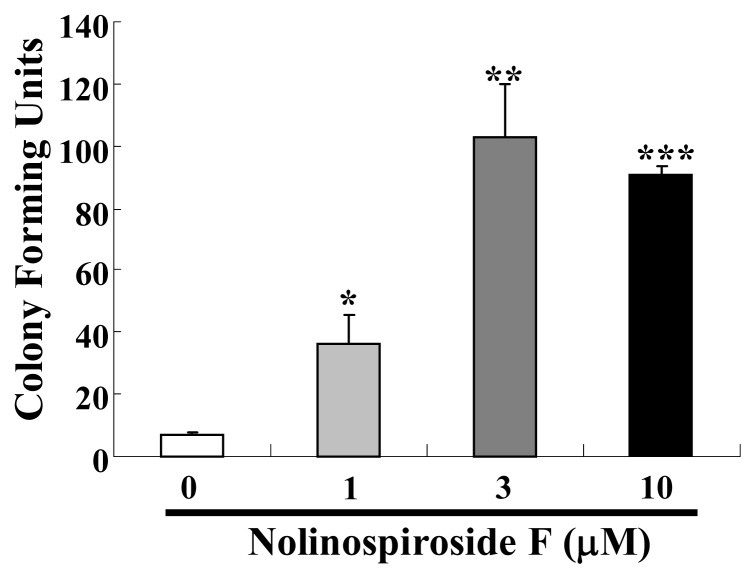
Among the various theories used to explain the aging process, the activity of free radicals, which was first proposed by Harman [23], has received great attention. The principle behind this theory is that the accumulation of reactive oxygen species (ROS), the by-products of aerobic activities in living organisms, shortens their lifespan [24]. The damage brought about by ROS contributes to aging. Thus, we investigated whether or not nolinospiroside F affects anti-oxidative stress. Under oxidative conditions (treatment with 7.5 mM H2O2), the yeast growth was significantly improved by nolinospiroside F at concentrations of 1, 3 and 10 μM (p < 0.05, p < 0.01 and p < 0.001, respectively) (Figure 3). These results suggest that nolinospiroside F may have an anti-oxidative effect, which may contribute to its anti-aging effect.
Figure 3.
Protection effect of nolinospiroside F on the growth of K6001 yeast under oxidative stress conditions. K6001 yeast was incubated for 36 h after treatment with 1, 3 or 10 μM nolinospiroside F. Then, 5 μL aliquots with the same number of cells were spotted onto plates containing 7.5 mM H2O2. The plates were incubated for 3 d at 28 °C, and the number of colonies was counted. The assay was repeated at least thrice. Vertical bars represent the mean ± S.E.M. of three assays. The average number of colonies of untreated K6001 was seven; nolinospiroside F at 1 μM, 37*; at 3 μM, 103**; and at 10 μM, 91*** (* p < 0.05, ** p < 0.01, *** p < 0.001).
2.3. Nolinospiroside F Decreases MDA Levels
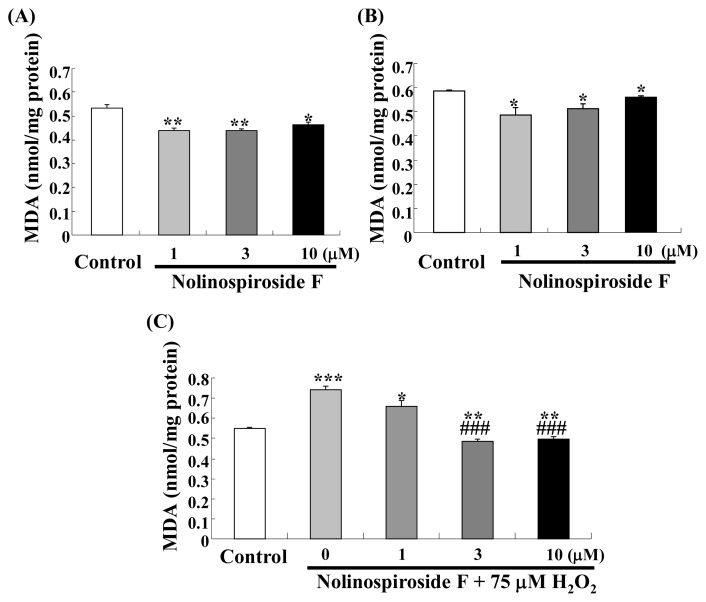
Malondialdehyde (MDA), the principal product of the degradation of polyunsaturated lipids by ROS [25], is an oxidative stress biomarker in organisms. MDA can cause membrane damage, add fluidity to cells and damage DNA [26–28]. Because nolinospiroside F has anti-oxidative effects, we determined the change in MDA after treatment with the steroid saponin in normal and oxidative stress condition. MDA levels decreased significantly after treatment with 1, 3 and 10 μM nolinospiroside F for 24 h (p < 0.01, p < 0.01, p < 0.05) (Figure 4A) and 48 h (p < 0.05, p < 0.05, p < 0.05) (Figure 4B). At the same time, the inhibition effects of nolinospiroside F on MDA under oxidative stress condition led by 75 μM H2O2 were observed (p < 0.001, p < 0.001) (Figure 4C). These results suggested that nolinospiroside F may have protective effects for cell membrane and DNA via inhibition of MDA level.
Figure 4.
Change in malondialdehyde (MDA) level in yeast after nolinospiroside F treatment at 24 h and 48 h (A, B) under normal and oxidative stress condition (C). K6001 yeast cells were incubated with various nolinospiroside F concentrations for 24 h and 48 h. In oxidative stress experiment, K6001 yeast cells were precultured with nolinospiroside F for 12 h, then 75 μM H2O2 was added and continuously incubated for 24 h. Changes in MDA level were measured with an MDA assay kit according to the protocol. Values shown are the mean ± S.E.M. of three independent experiments. *, ** and *** indicate significant difference relative to the control (p < 0.05, p < 0.01, p < 0.001); ### represents obvious difference among the group treated with 75 μM H2O2 (p < 0.001).
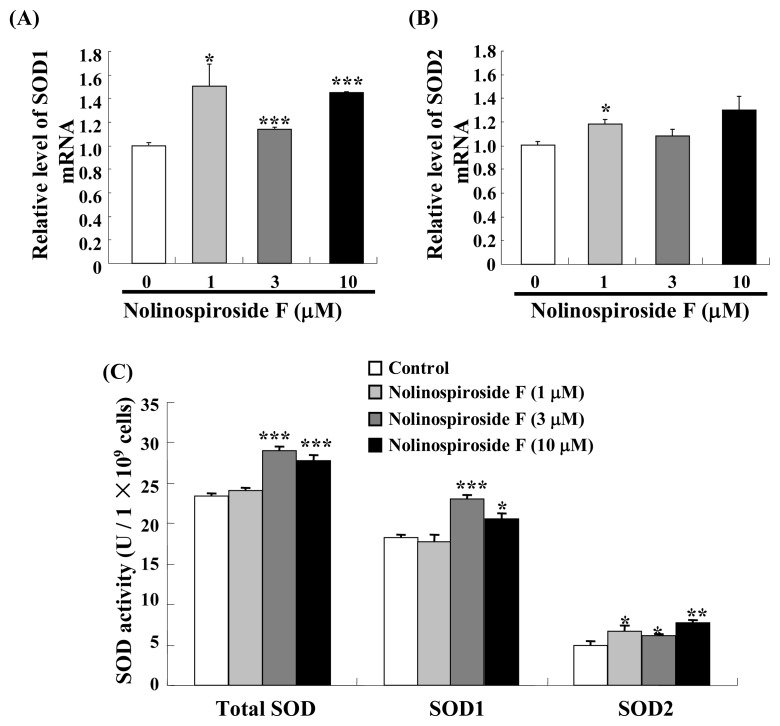
2.4. Treatment with Nolinospiroside F Increases SOD Gene Expression and SOD Enzyme Activity
SOD is an important anti-oxidative stress gene that participates in free radical scavenging. We hypothesized that SOD may participate in the anti-aging effects of nolinospiroside F. Thus, we evaluated SOD gene expression and superoxide dismutase (SOD) activity in yeast treated with nolinospiroside F. SOD1 expression was significantly increased in the treatment groups compared with the untreated group after treatment with 1, 3 and 10 μM nolinospiroside F (p < 0.05, p < 0.001 and p < 0.001, respectively) (Figure 5A). By contrast, only 1 μM nolinospiroside F resulted in a significant increase in SOD2 mRNA (p < 0.05) (Figure 5B). The total SOD (p < 0.001, p < 0.001) and SOD1 (p < 0.001, p < 0.05) activity significantly increased after yeast was treated with 3 and 10 μM nolinospiroside F (Figure 5C) for 48 h, whereas SOD2 activity increased significantly after treatment with 1, 3 and 10 μM nolinospiroside F (p < 0.05, p < 0.05 and p < 0.01, respectively) (Figure 5C). These results suggest that nolinospiroside F maybe extend the yeast lifespan and increase antioxidant defenses by increasing SOD gene expression and enzyme activity.
Figure 5.
Effects of nolinospiroside F on SOD gene expression (A, B) and enzyme activity (C). Amplification of TUB1 was used to normalize the relative levels of SOD1 and SOD2 mRNA. A superoxide dismutase (SOD) activity assay kit was used to measure SOD activity. Vertical bars represent the mean ± S.E.M. of three assays. Asterisks indicate significant differences compared with the corresponding controls (*p < 0.05, **p < 0.01, ***p < 0.001).
During SOD1 and SOD2 expression analysis, SOD1 expression increased significantly after treatment with 1, 3 and 10 μM nolinospiroside F. By contrast, SOD2 expression increased only with 1 μM nolinospiroside F. SOD1 located in the cytoplasm is believed to be very stable, whereas mitochondrial SOD2 is highly regulated by a number of environmental factors. However, our results are not consistent with the previous study; the SOD2 gene is more sensitive than SOD1 for hesperidin [12]. The difference between SOD1 and SOD2 expression may be due to the fact that SOD1 expression is easily triggered by saponins, as previously reported [29]. Total SOD and SOD1 enzyme activity did not change after treatment with 1 μM nolinospiroside F for 48 h. We determined changes in them 24, 48 and 72 h after treatment with 1 μM nolinospiroside F and found that total SOD and SOD1 activities increased 24 h after administration of 1 μM nolinospiroside F, but not 48 or 72 h later (data not shown). This result indicates that nolinospiroside F could be consumed by the yeast after long-term incubation.
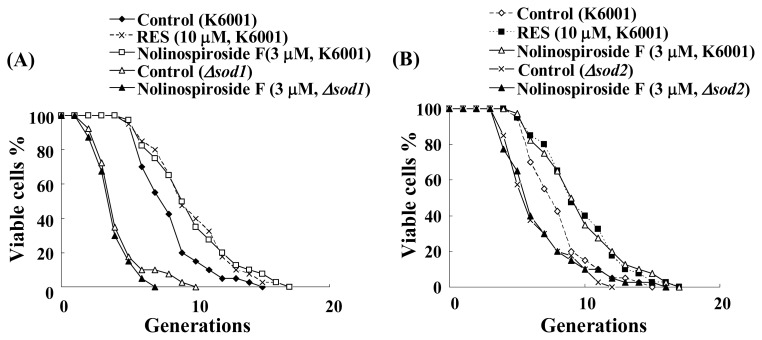
2.5. Nolinospiroside F Does Not Affect the Lifespans of sod1 and sod2 Yeast Mutant Strains
To confirm whether or not SOD participates in the anti-aging effect of nolinospiroside F, we used sod1 and sod2 mutant yeast strains to examine the effects of nolinospiroside F effects on replicative lifespan. Shorter lifespans of sod1 and sod2 mutant yeast strains with the K6001 background were observed in our experiment, and nolinospiroside F did not affect the lifespans of sod1 and sod2 mutant yeast strains (Figure 6). These results indicate that SOD plays an important role in the anti-aging effect of nolinospiroside F.
Figure 6.
Effects of nolinospiroside F on the replicative lifespan of sod1 (A) and sod2 (B) mutant yeast strains with a K6001 background. The assay is described in Figure 2. The daughter cells of 40 microcolonies of each experiment were counted. The assay was repeated at least thrice. The average lifespan of untreated K6001 was 6.35 generations; RES at 10 μM, 8.83***; nolinospiroside F at 3 μM, 8.75***; sod1 mutant with a K6001 background was 3.50; nolinospiroside F at 3 μM, 2.83; sod2 mutant with a K6001 background was 5.60; nolinospiroside F at 3 μM, 5.80. Δsod1 and Δsod2 represent the sod1 and sod2 mutants yeast strain with a K6001 background, respectively. (*** p < 0.001).
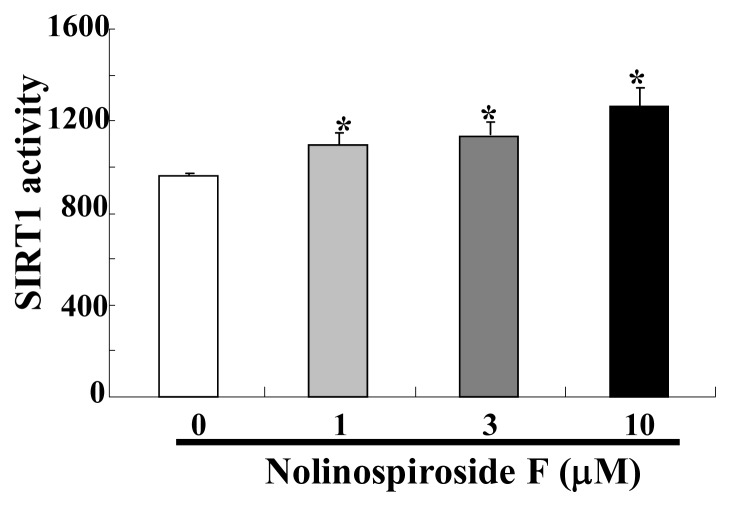
2.6. Nolinospiroside F Increased SIRT1 Activity
SIRT1, the homolog of yeast Sir2, has an important function in the lifespan of mammals. SIRT1 can deacetylate and activate PGC-1α and FOXOs to induce SOD2 expression [30,31]. It can also increase SOD2 expression to promote cell survival [32]. Thus, the effect of nolinospiroside F on SIRT1 activity was investigated. By using a SIRT1/Sir2 activity kit, we found SIRT1 activity increased obviously after treatment with 1, 3 and 10 μM nolinospiroside F (p < 0.05, p < 0.05, p < 0.05) (Figure 7). This result implied that Sir2 protein may be involved in the anti-aging effects of nolinospiroside F. To further confirm whether Sir2 participates in the anti-aging effect of nolinospiroside F, we tried to construct sir2 mutation yeast with K6001 background and did a replicative lifespan assay. However, the mother cells of K6001 yeast did not reproduce daughter cells after deleting the Sir2 gene. This result was consistent with other report [8]. Therefore, we could not utilize the lifespan assay of sir2 mutation yeast with a K6001 background to test the effects of nolinospiroside F on the Sir2 gene in this study.
Figure 7.
Effects of nolinospiroside F on SIRT1 activity. Values shown are the mean ± S.E.M. of three independent experiments. (*p < 0.05).
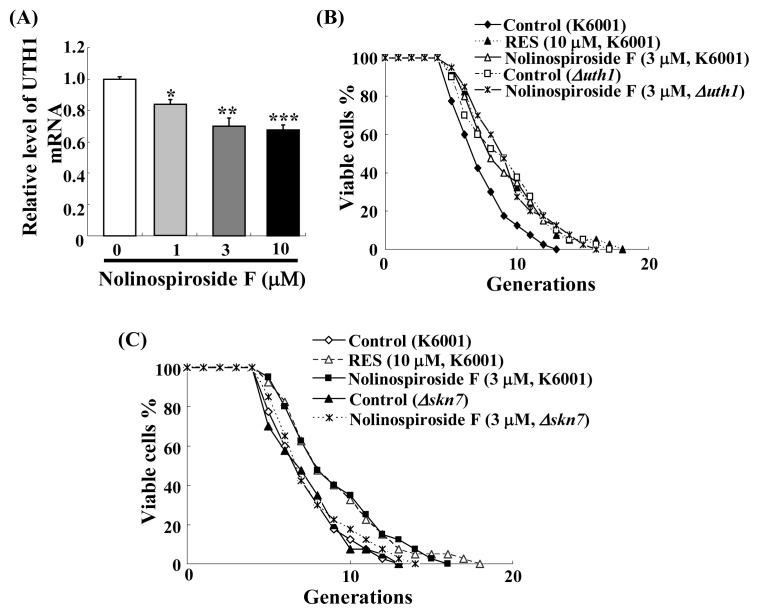
2.7. Nolinospiroside F Administration Inhibits UTH1 Gene Expression and UTH1, SKN7 Are Involved in Nolinospiroside F-Mediated Lifespan Extension
UTH1 is a yeast-aging gene that is reportedly involved in the oxidative stress of yeast [33]. Deletion of UTH1 increases the yeast lifespan [34,35]. Thus, we hypothesized that the UTH1 gene may be involved in nolinospiroside F-mediated yeast lifespan extension. Real-time polymerase chain reaction (RT-PCR) analysis and a lifespan assay of uth1 mutant were performed. UTH1 gene expression was inhibited significantly in groups treated with 1, 3 and 10 μM nolinospiroside F (p < 0.05, p < 0.01 and p < 0.001, respectively) (Figure 8A). The lifespan of the uth1 mutant was found to be longer than that of the K6001 yeast strain, which is consistent with other reports [34,35]. Nolinospiroside F did not affect the lifespan of uth1 mutants at a concentration of 3 μM (Figure 8B). These results suggest that UTH1 has an important role in nolinospiroside F-mediated yeast lifespan extension.
Figure 8.
Inhibition of UTH1 gene expression by nolinospiroside F (A) and effects of nolinospiroside F on the replicative lifespan of uth1 (B) and skn7 (C) mutants of K6001 yeast. Daughter cells of 40 microcolonies of each experiment were randomly counted. The assay was repeated at least thrice. The average lifespan of untreated K6001 was 6.50; RES at 10 μM, 8.20**; nolinospiroside F at 3 μM, 8.40**; uth1 mutant with a K6001 background was 8.20; nolinospiroside F at 3 μM, 8.50; untreated skn7 mutant with a K6001 background was 6.50 generations; nolinospiroside F at 3 μM, 6.88. Δuth1 and Δskn7 represents uth1 and skn7 mutant yeast strains with a K6001 background. (*p < 0.05, **p < 0.01, ***p < 0.001).
SKN7 is a transcriptional activator. In previous studies, we found that skn7 mutant yeast represses UTH1 expression significantly [12]. To observe whether or not SKN7 is important for the lifespan extension effect by nolinospiroside F, a lifespan assay of skn7 mutant yeast with a K6001 background was performed. Nolinospiroside F did not affect the lifespan of skn7 or uth1 mutant at 3 μM (Figure 8C), which indicates that SKN7 exerts effects on nolinospiroside F-mediated yeast lifespan extension by inhibiting UTH1 gene expression.
2.8. The Relationship between the SOD and UTH1 Gene
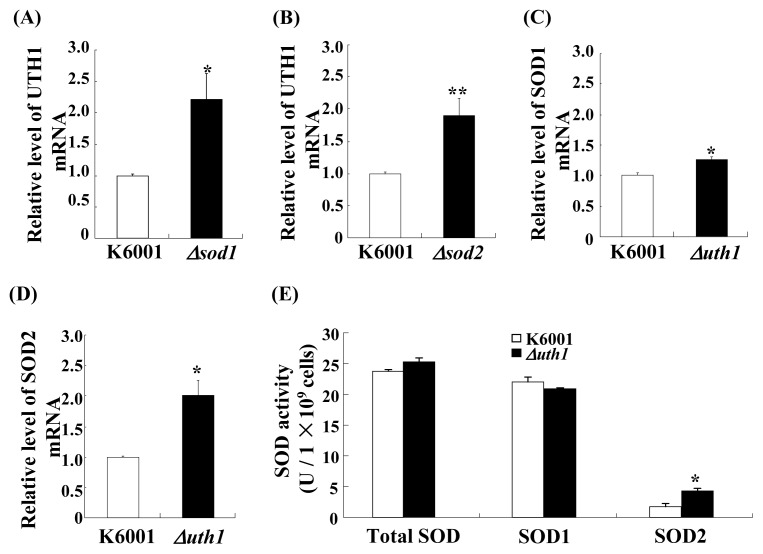
In this study, both UTH1 and SOD were found to be important for nolinospiroside F-mediated yeast lifespan extension, and these genes may have other important functions in yeast oxidative stress. UTH1 is reported to have a close relationship with SOD[36]. To explore this relationship further, SOD1 and SOD2 expressions in a uth1 yeast mutant strain and UTH1 expression in sod1 and sod2 mutants were investigated. The UTH1 expression in sod1 and sod2 mutants increased significantly compared to that in K6001 yeast (p < 0.05, p < 0.01, respectively); the SOD1 and SOD2 expressions in the uth1 mutant yeast strain also increased significantly (p < 0.05, p < 0.05) (Figure 9A–D). Furthermore, the significant increase of SOD2 activity in the uth1 mutant was observed (p < 0.05) (Figure 9E). These results suggest that UTH1 and SOD regulate expression with each other in yeast cells.
Figure 9.
The relationship between SOD and UTH1. UTH1 expression of sod1 and sod2 mutant yeast strains with a K6001 background was investigated (A and B). SOD1, SOD2 expressions and SOD enzyme activity of the uth1 mutant yeast strain with K6001 background were determined (C, D and E). UTH1 increased significantly when the SOD1 or SOD2 gene was deleted. The SOD1, SOD2 expressions and SOD2 enzyme activity of uth1 mutants were increased significantly, compared with the K6001 yeast strain. Values shown are the mean ± S.E.M. of three independent experiments (*p < 0.05, **p < 0.01).
3. Materials and Methods
3.1. Isolation of Nolinospiroside F
O. japonicus was bought in Zhejiang Province, China. Dried O. japonicus (dry wt: 10 kg) was extracted with MeOH (50 L). The supernatant was separated by filtration and concentrated to obtain 1.5 kg of extracts. The extract was then partitioned between H2O and EtOAc. The EtOAc layer was concentrated to obtain 8.3 g of the dried sample. Part of the EtOAc layer (0.73 g) was chromatographed on silica gel (200 to 300 mesh, Yantai Chemical Industry Research Institute) using CHCl3/MeOH (10:0, 9:1, 8:2, 5:5 and 0:10) to give five parts. The active sample eluted with CHCl3/MeOH (8:2 and 5:5) was separated through octadecylsilane (ODS) (Cosmosil 75 C18-OPN, Nacalai Tesque) and successively eluted with MeOH/H2O (4:6, 5:5, 7:3, 8:2, 9:1 and 10:0) and MeOH/CHCl3 (5:5) to obtain seven parts. The active sample (64.3 mg total) eluted with MeOH/H2O (7:3) was subjected to high-performance liquid chromatography (Develosil ODS-HG-5 (20/250 mm), Nomura Chemicals, flow rate: 8 mL/min, MeCN/H2O (5:5)) to yield the pure compound nolinospiroside F (21.5 mg, tR = 50 min). The chemical structure of the compound was determined by comparing 1H and 13C nuclear magnetic resonance (NMR), mass spectrometry (MS), and optical rotation data with those reported in the literatures [13,22]. The 13C NMR chemical shifts in ppm were referenced to the solvent peak of δc 135.91 for pyridine-d5. δ 14.98, 15.20, 16.66, 17.19, 17.78, 18.98, 24.20, 26.57, 26.74, 27.91, 32.34, 32.78, 33.38, 36.30, 39.72, 40.56, 40.75, 42.83, 43.32, 50.93, 57.35, 63.30, 65.40, 70.48, 71.50, 72.54, 72.87, 73.18, 73.18, 73.85, 74.51, 75.68, 81.58, 84.21, 100.31, 103.01, 110.11, 126.07 and 138.66; MS (m/z 745 (M + Na)+); [α]D25= −108.5 (c 0.21, pyridine).
3.2. Saccharomyces cerevisiae Strains, Media and Lifespan Assay
Strains were derived from K6001 yeast. Sod1, sod2, uth1 and skn7 mutants with a K6001 background were constructed using the kanamycin gene instead of the target gene. The yeast lifespan assay and media were described in a previous report [12]. Briefly, K6001 yeast strain was inoculated in 2 mL of galactose medium and incubated in a shaking incubator at 28 °C and 160 rpm for 48 h. Yeast culture was centrifuged at 1500 rpm for 3 min. After that, the yeast pellet was washed thrice and diluted with distilled water. Subsequently, yeast were counted with a hemocytometer, 5000 cells were plated on glucose medium agar plates containing various concentrations of the samples and plates were incubated at 28 °C for two days. Microcolonies that formed on agar plates were observed under a microscope, and daughter cells were counted.
3.3. Anti-Oxidative Stress Assay
After treatment with 0, 1, 3 or 10 μM nolinospiroside F, the K6001 yeast cells were cultured in 25 mL of galactose medium for 36 h. About 5 μL of the same OD600 aliquot of cultured yeast was spotted onto agar plates containing 7.5 mM H2O2. Incubation at 28 °C followed. After 3 d, the number of yeast colonies was counted.
3.4. MDA Assay
K6001 yeast cells were cultured in galactose medium after treatment with 0, 1, 3 or 10 μM nolinospiroside F for 24 or 48 h. To measure the MDA change in cells after administrating nolinospiroside F under oxidative stress condition, K6001 yeast cells were pretreated with 0, 1, 3 or 10 μM nolinospiroside F for 12 h. After that, 75 μM H2O2 was added into the cell culture, and yeast cells were incubated continuously for 24 h. The cells were washed thrice with PBS and collected after centrifugation at 3000 rpm for 10 min. Cells were resuspended in 200 μL of PBS and disintegrated by ultrasonication (1 min/time, 5 times) and freeze-thawing (5 min in liquid nitrogen, then 15 min in water bath at 37 °C) 5 times, followed by repeated ultrasonication 5 times. The cell lysate was centrifuged at 12,000 rpm at 4 °C for 15 min, after which the supernatant was removed. MDA was measured using an MDA assay kit following the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
3.5. RT-PCR Analysis
RT-PCR was performed as previously described [12]. Primers used were as follows: for SOD1, sense 5′-CAC CAT TTT CGT CCG TCT TT-3′ and antisense 5′-TGG TTG TGT CTC TGC TGG TC-3′; for SOD2, sense 5′-CTC CGG TCA AAT CAA CGA AT-3′ and antisense 5′-CCT TGG CCA GAA GAT CTG AG-3′; for UTH1, sense 5′-CGC CTC TTC TTC CTC CTC TT-3′ and antisense 5′-ACC ATC GGA AGG TTG TTC AG-3′; for TUB1, sense 5′-CCA AGG GCT ATT TAC GTG GA-3′ and antisense 5′-GGT GTA ATG GCC TCT TGC AT-3′. The amounts of UTH1, SOD1 and SOD2 mRNA were normalized to that of TUB1.
3.6. SOD Activity Assay
K6001 yeast cells were cultured in galactose medium after treatment with 0, 1, 3 or 10 μM nolinospiroside F for 48 h. K6001 or Δuth1 with K6001 background yeast cells were cultured in galactose medium for 48 h, respectively. Approximately 1 × 109 cells were washed thrice with PBS and resuspended in 1 mL of PBS. Cells were frozen and thawed (5 min in liquid nitrogen, then 15 min in water bath at 37 °C) thrice. Cell lysates were centrifuged at 10,000 rpm and 4 °C for 15 min, after which the supernatant was removed. Finally, SOD activity was measured using a SOD assay kit, according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
3.7. SIRT1 Assay
Different concentrations of nolinospiroside F were added to the reagents of an SIRT1/Sir2 assay kit, according to the manufacturer’s instructions (CycLex, Ina, Nagano, Japan). The detailed procedure for the SIRT1 assay was described in a previous report [12].
3.8. Statistical Analysis
Significant differences among groups in all experiments were determined by ANOVA, followed by two-tailed multiple t-tests with Bonferroni correction using SPSS biostatistics software. A p-value less than 0.05 was considered statistically significant.
4. Conclusion
In this study, we found that nolinospiroside F extends the replicative lifespan of the K6001 yeast strain significantly and improves yeast survival under oxidation conditions. It also increases SOD1 and SOD2 expressions, SOD enzyme activity and SIRT1 activity. MDA levels and UTH1 expression decreased after nolinospiroside F treatment. We also confirmed the function of SOD and UTH1 in the yeast lifespan extension effects of nolinospiroside F using a replicative lifespan assay with sod1, sod2 and uth1 mutant strains. SOD and UTH1 regulated expression with each other. The results suggest that the anti-aging effects of nolinospiroside F are a result of the multimodulation of mitochondrial functions via SOD and UTH1. Furthermore, the anti-aging effect of nolinospiroside F should be investigated in vivo in future experiments.
Acknowledgments
We are grateful to M. Breitenbach, Salzburg University, Austria for gifts of K6001 yeast strain. This work was supported by NSFC (30873152) and Qianjiang Talent Program of Zhejiang Province (2010R10052).
Conflict of Interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker J.A., Arango M., Abderrahmane S., Lambert E., Tourette C., Catoire H., Néri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat. Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- 3.Araki T., Sasaki Y., Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 4.Howitz K.T., Bitterman K.J., Cohen H.Y., Lamming D.W., Lavu S., Wood J.G., Zipkin R.E., Chung P., Kisielewski A., Zhang L.L., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 5.Pasinetti G.M., Wang J., Marambaud P., Ferruzzi M., Gregor P., Knable L.A., Ho L. Neuroprotective and metabolic effects of resveratrol: Therapeutic implications for Huntington’s disease and other neurodegenerative disorders. Exp. Neurol. 2011;232:1–6. doi: 10.1016/j.expneurol.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiozaki M., Hayakawa N., Shibata M., Koike M., Uchiyama Y., Gotow T. Closer association of mitochondria with lipid droplets in hepatocytes and activation of KupVer cells in resveratrol-treated senescence-accelerated mice. Histochem. Cell Biol. 2011;136:475–489. doi: 10.1007/s00418-011-0847-6. [DOI] [PubMed] [Google Scholar]
- 7.Valenzano D.R., Terzibasi E., Genade T., Cattaneo A., Domenici L., Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 8.Jarolim S., Millen J., Heeren G., Laun P., Goldfarb D.S., Breitenbach M. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004;5:169–177. doi: 10.1016/j.femsyr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Weng Y., Xiang L., Matsuura A., Zhang Y., Huang Q., Qi J. Ganodermasides A and B, two novel anti-aging ergosterols from spores of a medicinal mushroom Ganoderma lucidum on yeast via UTH1 gene. Bioorg. Med. Chem. 2010;18:999–1002. doi: 10.1016/j.bmc.2009.12.070. [DOI] [PubMed] [Google Scholar]
- 10.Weng Y., Lu J., Xiang L., Matsuura A., Zhang Y., Huang Q., Qi J. Ganodermasides C and D, two new anti-aging ergosterols from the spores of a medicinal mushroom Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2011;75:800–803. doi: 10.1271/bbb.100918. [DOI] [PubMed] [Google Scholar]
- 11.Xiang L., Sun K., Lu J., Weng Y., Taoka A., Sakagami Y., Qi J. Anti-aging effects of phloridzin, an apple polyphenol, on yeast via SOD and Sir2 genes. Biosci. Biotechnol. Biochem. 2011;75:854–858. doi: 10.1271/bbb.100774. [DOI] [PubMed] [Google Scholar]
- 12.Sun K., Xiang L., Ishihara S., Matsuura A., Sakagami Y., Qi J. Anti-aging effects of hesperidin on Saccharomyces cerevisiae via inhibition of reactive oxygen species and UTH1 gene expression. Biosci. Biotechnol. Biochem. 2012;76:640–645. doi: 10.1271/bbb.110535. [DOI] [PubMed] [Google Scholar]
- 13.Shevchuk G.V., Vollerner Y.S., Shashkov A.S., Chirva V.Y. Steroids of the furostan and spirostan series from nolina microcarpa II. Structures of nolinospiroside D and nolinofurosides D, E, and F. Chem. Nat. Compd. 1991;5:678–686. [Google Scholar]
- 14.Qi J., Ojika M., Sakagami Y. Linckosides A and B, two new neuritogenic steroid glycosides from the Okinawan starfish Linckia laevigata. Bioorg. Med. Chem. 2002;10:1961–1966. doi: 10.1016/s0968-0896(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 15.Qi J., Ojika M., Sakagami Y. Linckosides C–E, three new neuritogenic steroid glycosides from the Okinawan starfish Linckia laevigata. Bioorg. Med. Chem. 2004;12:4259–4265. doi: 10.1016/j.bmc.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 16.Han C., Qi J., Ojika M. Structure-activity relationships of novel neuritogenic steroid glycosides from the Okinawan starfish Linckia laevigata. Bioorg. Med. Chem. 2006;14:4458–4465. doi: 10.1016/j.bmc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Hua Z., Carcache D.A., Tian Y., Li Y.M., Danishefsky S.J. The synthesis and preliminary biological evaluation of a novel steroid with neurotrophic activity: NGA0187. J. Org. Chem. 2005;70:9849–9856. doi: 10.1021/jo051556d. [DOI] [PubMed] [Google Scholar]
- 18.Furuya S., Takayama F., Mimaki Y., Sashida Y., Satoh K., Sakagami H. Cytotoxic activity of saponins from Camassia leichtlinii against human oral tumor cell lines. Anticancer Res. 2001;21:959–964. [PubMed] [Google Scholar]
- 19.Shao B., Guo H.Z., Cui Y.J., Ye M., Han J., Guo D. Steroidal saponins from Smilax china and their anti-inflammatory activities. Phytochemistry. 2007;68:623–630. doi: 10.1016/j.phytochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Li H., Huang W., Wen Y.Q., Gong G.H., Zhao Q.B., Yu G. Anti-thrombotic activity and chemical characterization of steroidal saponins from Dioscorea zingiberensis C.H. Wright. Fitoterapia. 2010;81:1147–1156. doi: 10.1016/j.fitote.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Zhang Y.J., Jacob M.R., Li X.C., Yang C.R. Steroidal saponins from the stem of Yucca elephantipes. Phytochemistry. 2008;69:264–270. doi: 10.1016/j.phytochem.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe Y., Sanada S., Ida Y., Shoji J. Comparative studies on the constituents of ophiopogonis tuber and its congeners. I. Studies of the constituents of the subterranean part of Liriope platyphylla Wang et Tang. (1) Chem. Pharm. Bull. 1983;31:1980–1990. [Google Scholar]
- 23.Harman D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 24.Harman D. Origin and evolution of the free radical theory of aging: A brief personal history, 1954–2009. Biogerontology. 2009;10:773–781. doi: 10.1007/s10522-009-9234-2. [DOI] [PubMed] [Google Scholar]
- 25.Pryor W.A., Stanley J.P. Letter: A suggested mechanism for the production of malondialdehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J. Org. Chem. 1975;40:3615–3617. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- 26.Debouzy J.C., Fauvelle F., Vezin H., Brasme B., Chancerelle Y. Interaction of the malondialdehyde molecule with membranes. A differential scanning calorimetry, 1H-, 31P-NMR and ESR study. Biochem. Pharmacol. 1992;44:1787–1793. doi: 10.1016/0006-2952(92)90073-r. [DOI] [PubMed] [Google Scholar]
- 27.Ohyashiki T., Sakata N., Matsui K. A decrease of lipid fluidity of the porcine intestinal brush-border membranes by treatment with malondialdehyde. J. Biochem. 1992;111:419–423. doi: 10.1093/oxfordjournals.jbchem.a123772. [DOI] [PubMed] [Google Scholar]
- 28.Yang M., Schaich K.M. Factors affecting DNA damage caused by lipid hydroperoxides and aldehydes. Free Radic. Biol. Med. 1996;20:225–236. doi: 10.1016/0891-5849(95)02039-x. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y.H., Park K.H., Rho H.M. Transcriptional activation of the Cu, Zn-superoxide dismutase gene through the AP2 site by ginsenoside Rb2 extracted from a medicinal plant, Panax ginseng. J. Biol. Chem. 1996;271:24539–24543. doi: 10.1074/jbc.271.40.24539. [DOI] [PubMed] [Google Scholar]
- 30.St.-Pierre J., Drori S., Uldry M., Silvaggi J.M., Rhee J., Jager S., Handschin C., Zheng K., Lin J., Yang W., et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1α transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Daitoku H., Hatta M., Matsuzaki H., Aratani S., Ohshima T., Miyagishi M., Nakajima T., Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl. Acad. Sci. USA. 2004;101:10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanno M., Kuno A., Yano T., Miura T., Hisahara S., Ishikawa S., Shimamoto K., Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camougrand N., Kissová I., Velours G., Manon S. Uth1p: A yeast mitochondrial protein at the crossroads of stress, degradation and cell death. FEMS Yeast Res. 2004;5:133–140. doi: 10.1016/j.femsyr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Austriaco N.R., Jr Review: To bud until death: The genetics of ageing in the yeast Saccharomyces. Yeast. 1996;12:623–630. doi: 10.1002/(SICI)1097-0061(19960615)12:7%3C623::AID-YEA968%3E3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy B.K., Austriaco N.R., Jr, Zhang J., Guarente L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell. 1995;80:485–496. doi: 10.1016/0092-8674(95)90499-9. [DOI] [PubMed] [Google Scholar]
- 36.Bandara P.D., Flattery-O’Brien J.A., Grant C.M., Dawes I.W. Involvement of the Saccharomyces cerevisiae UTH1 gene in the oxidative-stress response. Curr. Genet. 1998;34:259–268. doi: 10.1007/s002940050395. [DOI] [PubMed] [Google Scholar]