Abstract
Compromised pregnancies such as those associated with gestational diabetes mellitus, intrauterine growth retardation, preeclampsia, maternal undernutrition, and maternal stress may negatively affect fetal development. Such pregnancies may induce oxidative stress to the fetus and alter fetal development through the epigenetic process that may affect development at a later stage. Melatonin is an oxidant scavenger that reverses oxidative stress during the prenatal period. Moreover, the role of melatonin in epigenetic modifications in the field of developmental programming has been studied extensively. Here, we describe the physiological function of melatonin in pregnancy and discuss the roles of melatonin in fetal programming in compromised pregnancies, focusing on its involvement in redox and epigenetic mechanisms.
Keywords: melatonin, epigenetic, fetal programming, redox, pregnancy
Abbreviations
- AGE
Advanced glycation end product
- CREB
cAMP response element-binding protein
- DNMT1
DNA (cytosine-5)-methyltransferase 1
- GDM
gestational diabetis mellitus
- GR
glucocorticoid receptor
- IUGR
intrauterine growth retardation
- MeCP2
methyl CpG binding protein 2
- mGlu
metabotropic glutamate receptor
- MT
melatonin receptor
- NF-E2-related factor
nuclear factor erythroid 2-related factor 2
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Pdx-1
pancreatic and duodenal homeobox 1
- Per1
period circadian protein homolog 1
- PPAR
peroxisome proliferator-activated receptor
- ROS
reactive oxygen species
- REM
rapid eye movement
- SCN
suprachiasmatic nucleus
1. Introduction
Melatonin (N-acetyl-5-methoxytryptamine), an endogenously produced indoleamine of the pineal gland, is an antioxidant, free radical scavenger, anti-inflammatory molecule, and is produced and secreted in a circadian fashion [1–4]. Melatonin was first identified during the late 1950s by Lerner et al. in the pineal gland [5]. It was first identified and named for its skin-lightening properties observed in fish and frog melanocytes. Melatonin is a small lipophilic indoleamine that crosses the placenta and blood-brain barrier and enters cells rapidly. Melatonin acts as a free radical scavenger and protects nuclear and mitochondrial DNA from the damage induced by free radicals [6]. In addition, melatonin stimulates the expression of antioxidant enzyme [7,8] and anti-inflammatory genes [4]. Recent studies involving the role of melatonin in the epigenetic modifications associated with developmental programming are emerging rapidly [8,9]. Moreover, melatonin can stimulate the immune system, protecting organisms against bacterial and viral infections [1,2].
Melatonin has been used clinically in cancer [10], neurodegenerative diseases [11,12], sleep disorders [13,14], aging [11,15], and in neonatal and pediatric diseases of neonates and children [16,17]; however, its utility in pregnancy has rarely been discussed [17–21].
2. Melatonin Synthesis and Its Receptors
In mammals, the melatonin rhythm is generated by an endogenous circadian clock in the suprachiasmatic nucleus (SCN) of the hypothalamus [22]. An individual must predict the upcoming seasonal changes to adapt both physiological and behavioral functions. The biosynthesis and metabolism of melatonin are regulated by the light/dark cycle. Once synthesized, melatonin is not stored in pineal cells but is rapidly released into the bloodstream. Circulating melatonin provides circadian and seasonal timing cues. Melatonin is locally found in various cells, tissues, and organs, including lymphocytes, bone marrow, thymus, gastrointestinal tract, skin, the Harderian gland, and the retina [1–3]. Melatonin production has also been detected in invertebrates, bacteria, and plants [22].
Melatonin is synthesized primarily in the pineal gland from the amino acid precursor tryptophan. At least four enzymes are involved in melatonin biosynthesis, among which serotonin N-acetyltransferase is considered the rate-limiting enzyme. The half-life of melatonin in serum varies between less than 30 min and 60 min. Seventy percent of serum melatonin is bound to albumin, and the remaining 30% diffuses in the surrounding tissues [23]. It is metabolized primarily in the liver and secondarily in the kidney [22]. Melatonin is catabolized to 6-sulfoxy-melatonin exclusively by hepatic P450 monooxygenase; after conjugation, 6-sulfoxy melatonin then forms the urinary 6-sulfoxy-melatonin [23,24].
Two types of membrane-associated melatonin receptors have been cloned in mammals [25]; these receptors, MT1 and MT2, which belong to the G-protein-coupled receptor superfamily, have seven transmembrane domains. The MT1 and MT2 melatonin receptors show 60% homology at the amino acid level. A third membrane-associated receptor, MT3, has been identified as the quinone reductase 2. MT3 belongs to a group of reductases that are involved in the protection against oxidative stress by preventing the electron transfer reactions of quinones.
3. Melatonin Possesses Both Antioxidant and Epigenetic Modifications Abilities
Melatonin has pleiotropic bioactivities and is involved in the regulation of the circadian rhythm, reproductive physiology, anti-inflammation, sleep promotion, and body temperature regulation [2,3]. In addition, melatonin and its metabolites have been reported to have important antioxidant properties owing to their direct and indirect antioxidant activities [2,3].
Oxidative stress is defined as an imbalance between prooxidant and antioxidant forces leading to an overall prooxidant insult. Melatonin and its byproducts are capable of scavenging both ROS and reactive nitrogen species (RNS), including the hydroxyl radical (•OH), hydrogen peroxide (H2O2), singlet oxygen (1O2), hypochlorous acid (HClO), peroxynitrite anion (ONOO−), and/or peroxynitrous acid (ONOOH) [11]. Melatonin can protect both humans and animals from oxidative stress [26,27].
Melatonin also acts as a potent endogenous antioxidant by scavenging free radicals and upregulating antioxidant pathways. The activity and expression of antioxidant enzymes such as superoxide dismutase, glutathione, catalase, glutathione peroxidase, and glutathione reductase are increased by melatonin, which supports data concerning its indirect antioxidant action [11]. Notably, melatonin upregulates superoxide dismutase 2 mRNA expression via an epigenetic mechanism [9]. Further evidence of the antioxidant effect of melatonin is provided by its ability to reduce lipid peroxidation, a degradative phenomenon involved in the pathogenesis of many diseases [11].
Melatonin is a much more potent antioxidant than many traditionally used antioxidants and has two unique features. First, melatonin does not undergo redox cycling and once oxidized, cannot be regenerated to its reduced form [28]. Second, the antioxidant action of melatonin involves the donation of two electrons instead of one; therefore, melatonin does not become a free radical in the antioxidant process [28]. Furthermore, melatonin can be metabolized to both kynurenic acid and to N1-acetyl-N2-formyl-5-methoxykynuramine and N1-acetyl-5-methoxykynuramine [26]. These metabolites are very powerful antioxidants and cyclooxygenase-2 inhibitors. Therefore, they are considered as potentially selective anti-inflammatory agents [1–3].
Mitochondria produce high amounts of ROS during their biogenesis. Mitochondria, which are characterized by a double membrane, contain several hundreds of proteins and 2–10 copies of mitochondrial DNA in the matrix enclosed by the mitochondrial inner membrane. Mitochondria play a critical role in generating energy in most eukaryotic cells. In this context, melatonin can stimulate mitochondrial biogenesis and increase the efficiency of the electron transport chain, thereby limiting electron leakage and free radical generation. In addition, the antioxidant effect of melatonin and its ability to increase mitochondrial glutathione levels is of great importance for mitochondrial physiology [29].
Epigenetic modifications refer to stable and heritable gene expression changes that are not mediated by DNA sequence alterations. Unlike genetic information, which is stable, epigenetic modifications occur in response to early environmental signals and instantly respond to transient stimuli, resulting in modified gene expression patterns and phenotypes later in life. Melatonin may regulate antioxidant gene and enzyme expression through epigenetic mechanisms [9]. Melatonin induces the expression of the Nrf2 gene and suppresses that of NF-κB through epigenetic processes [9]. In addition, certain anti-cancer effects of melatonin are the result of its effect on epigenetic changes [30].
4. Physiological Functions of Melatonin during Pregnancy
Melatonin plays an important role in pregnancy and parturition [18,19]. Maternal plasma melatonin levels are elevated during pregnancy, reaching a maximum at term, and returning to basal levels immediately after delivery [18]. The placenta expresses melatonin receptors and melatonin easily crosses the placenta without being altered [18]. During normal pregnancy, melatonin acts as an antioxidant and appears to be essential for a successful pregnancy. Sandyk et al. had found the association of melatonin and spontaneous abortion which was presumed to be due to melatonin’s role in diminishing uterine contractions by decreasing the production of prostaglandins and in prevention of the immunologic rejection of trophoblast by stimulating the progesterone production [31]. Further, Matsuzuka et al. showed that alleviation of the embryo death might be achieved by administration of melatonin in mice [32]. Moreover, maternal melatonin also plays a key role in the regulation of development of fetal organs that are critical for the successful adaptation of the neonate to extrauterine life [33].
In rodents, melatonin-binding sites are observed in the fetal pituitary gland by gestational day 15 and in the SCN by gestational day 18 [34,35]. Melatonin receptors are present in the human fetal SCN [36] and in many areas of the fetal human brain [37]. Therefore, maternal melatonin may be involved in various fetal functions.
Predicting the upcoming seasons to adapt physiological and behavioral functions is important for the survival of individuals and the perpetuity of species. Information about day length and circadian phase is transferred to the fetus prenatally [38]. Maternal melatonin crosses the placenta freely and enters the fetal circulation easily, playing a critical role in providing photoperiodic information to the fetus. In sheep and ewes, reproductive activity is initiated during the fall and inhibited during summer; in such organisms, melatonin has a stimulatory effect on the reproductive axis and influences the photoperiod through pulsatile secretion of luteinizing hormone [39]. This has been observed after the removal of the pineal gland, which disrupts the photoperiod-induced reproductive responses to seasonal changes according to the duration of day and night [40].
During pregnancy, circadian variations in melatonin levels in the maternal circulation have been reported in sheep [41,42] and rats [43]. The passage of maternal melatonin through the placenta exposes the fetus to a daily melatonin rhythm of low concentrations during the day and high concentrations at night [44,45]. The maternal melatonin circadian rhythm is linked to the generation of the circadian rhythms in the fetal adrenal gland [38].
Torres-Farfan et al. reported that maternal melatonin decreased cortisol production in the fetal adrenal gland of the capuchin monkey [46]. In a subsequent study on sheep, they found that melatonin had direct inhibitory effects on noradrenaline-stimulated fetal cerebral artery contraction, the release of glycerol by brown adipose tissue, and on ACTH-induced secretion of cortisol by the fetal adrenal gland [33]. The lack of maternal melatonin during the early stages of gestation was found to disrupt drinking behavior of rat pups, and this effect is reversed by the administration of exogenous melatonin to the mother [47]. In addition, low levels or a lack of a circadian rhythm of fetal corticosterone may result in intrauterine growth retardation [48]. Taken together, these findings indicate that maternal melatonin plays a key role in both the regulation of the development of fetal organs critical for the successful adaptation of the neonate to extrauterine life and prevention of pregnancy loss.
5. Safety Profiles and Side Effects of Melatonin in Pregnancy
There is general agreement that melatonin therapy has a remarkably benign safety profile. Melatonin has shown no obvious detrimental effects on mouse and rat embryo development in toxicity tests [49,50]. In pregnant rats, administration of high doses of melatonin (200 mg·kg−1·day−1) from gestational days 6–19 did not adversely affect the development of rat pups [51]. In a study by Sadowsky et al., high-dose melatonin had no apparent effect on fetal or maternal well-being, and it did not affect myometrial activity during late gestation [52]. However, melatonin has been shown to inhibit the activity of prostaglandin synthases, and prostaglandins have important circulatory and endocrine functions in the fetus [53].
6. The Concept of Fetal Programming
The plasticity of the developmental process allows the organism to respond to the surrounding environment. Programming is defined as the induction, silencing, or restriction of the development of somatic structures or a physiological system, which results in long-term effects. Human epidemiological studies have provided convincing support for the concept of developmental programming by showing a strong association between low birth weight and an increased risk of adverse outcomes in adulthood, such as coronary heart disease, stroke, high blood pressure, and type 2 diabetes [54].
Perturbations of the developmental adaptation process can also have adverse consequences on organ function and disease risk later in life. The placenta plays a critical role in fetal programming. Maternal complications of pregnancy such as gestational diabetes mellitus (GDM), intrauterine growth restriction (IUGR), preeclampsia, maternal undernutrition, and maternal stress are associated with an increased risk of brain dysfunction, cardiovascular disease, and metabolic syndrome in the offspring through fetal programming mechanisms [55–60]. It is now evident that epigenetic regulation plays a critical role in developmental programming.
7. Redox and Epigenetic Mechanisms in Fetal Programming
Several mechanisms contribute to fetal programming. In this review, we discuss two important mechanisms underlying fetal programming, redox and epigenetic alterations [55–60]. Detailed reviews of the mechanisms of fetal programming can be found in the literature [61,62].
7.1. The Role of Redox Alterations in Fetal Programming
ROS are produced within the follicle; these play a physiological role in the process of ovulation [63]. Pregnancy is associated with physiologically increased oxidative stress in the mother [19,64]. The placenta is of utmost importance for intrauterine fetal development and growth and is susceptible to oxygen tension changes [65].
Extensive increases in oxidative stress have been observed in association with various conditions, including smoking, diabetes, hypertension, and hypoxia, which are not uncommon in pregnant women. These conditions can cause increased oxidative stress either in the mother or the fetus, which may result in the programming of diseases in the offspring at a later stage [58,66].
7.2. The Role of Epigenetic Modifications in Fetal Programming
Epigenetic modifications refer to stable and heritable gene expression changes without alterations of the DNA sequence. The mechanisms of epigenetic change include posttranslational histone modifications and DNA methylation, imprinting, and small-RNA mediated controls. Epigenetic modifications perceive the effect of early environmental signals and play a role in programming.
In the first stages of placental vascular development, endothelial specialization and blood vessel formation are controlled by epigenetic mechanisms [67]. Moreover, the expression of genes implicated in trophoblast invasion, such as maspin, is regulated by histone modifications [68]. Recent studies have shown that placental dysfunction owing to maladaptation to external stressors during pregnancy plays a critical role in fetal programming, as evidenced by changes in the placental size, molecular components, and histopathology, which may result in dismal maternal and fetal outcomes [69]. Deregulation of placentation can lead to adverse outcomes for both the mother and fetus, as evidenced by alterations of the epigenetic profile in cultured human trophoblasts [70]. Taken together, these findings indicate that environmental cues in utero might produce long-term consequences through the epigenetic mechanism [71].
7.3. Redox Alterations in Utero Play a Role in Epigenetic Modifications
Increased levels of ROS are important in epigenetic modification of DNA or chromatin and influences gene expression and cell differentiation [72,73]. The effect of ROS on epigenetic alterations has been documented in cancer studies [74]. The glucocorticoid receptor (GR) is susceptible to redox influences and epigenetic modifications and is therefore an example of the involvement of oxidative stress in epigenetic modifications and programming [59].
8. The Roles of Melatonin in Redox and Epigenetic Alterations in Fetal Programming
8.1. Melatonin Has a Role in Redox Modifications in Fetal Programming
Human follicular fluid contains high concentrations of melatonin [75]. Melatonin has a direct role in oocyte maturation and embryo development because it decreases oxidative stress in ovarian follicles and protects oocytes from free radical damage [65]. In addition, melatonin can increase glutathione peroxidase activity in the human chorion [76].
Shift work is common during pregnancy and may disrupt the maternal melatonin rhythm. Epidemiological studies in women show that shift work increases the risk of premature delivery and the incidence of low birth weight [77,78], which are both strongly related to redox-related fetal programming. In animal studies, the suppression of maternal melatonin inhibited fetal adrenal maturation with a subsequent IUGR [48] and glucose intolerance in the offspring [79]. Melatonin thus plays a role in redox regulation and fetal programming.
8.2. Melatonin Has a Role in Epigenetic Modifications in Fetal Programming
Exposure of dams to a high-fat diet has been shown to increase fetal liver lipid accumulation and to increase the expression of genes involved in the liver gluconeogenic pathway [80,81]. Alterations in the expression of the clock genes Per1 and Npas2 are caused by increased occupancy of H3K14 at the histone acetylase sites in the Npas2 promoter; these alterations suggested that melatonin plays a role in epigenetic programming [80,81].
Melatonin has been suggested to regulate the expression of antioxidant genes and enzymes through epigenetic mechanisms [9]. Sun et al.[82] showed that both its gene expression and Nrf2-dependent antioxidant enzyme expression are dependent on Nrf2 acetylation by CBP/p300. Kawai et al.[83] demonstrated that acetylation and deacetylation of Nrf2 regulate its transcriptional activity. Therefore, melatonin has the dual role of regulating Nrf2 gene induction by acetylation and recruiting the basal transcriptional machinery to the promoter region of Nrf2-related genes. Therefore, melatonin may play a role in epigenetic modifications in fetal programming through the regulation of antioxidant enzymes [9].
9. The Roles of Melatonin in Compromised Pregnancies
Pregnancy is a physiological state accompanied by a high metabolic demand and elevated requirements for oxygen and hence prone to oxidative stress-induced organ damage. Furthermore, the placenta is a major source of oxidative stress because it is rich in polyunsaturated fatty acids. As gestation progresses, there is a gradual favoring of antioxidant activity over oxidant activity. In parallel, maternal plasma melatonin levels increase during pregnancy, reaching a maximum at term.
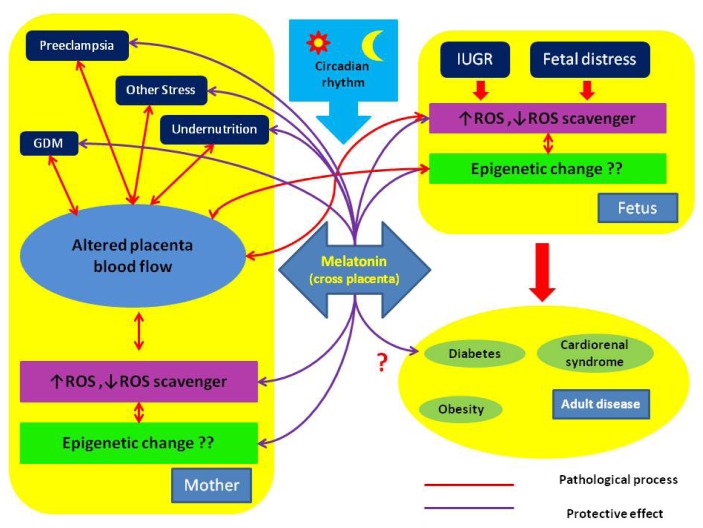
Fetal organs, especially the SCN, are vulnerable to environmental insults via the mother [33,46,84]. In compromised pregnancies, melatonin homeostasis between mother and fetus may be affected [19,33,84]. Furthermore, environmental cues during early development may influence the circadian clock system, which consists of oscillating molecular pacemakers in the hypothalamus, most peripheral tissues, and the hypothalamic-pituitary-adrenal axis and therefore affect the responses to environmental challenges in adult life [85,86]. In addition, premature infants have altered circadian rhythmicity as compared with full-term infants [87,88]. Taken together, these findings indicate that melatonin is involved in fetal programming in compromised pregnancies. Figure 1 depicts the role of melatonin in modulation of pregnancy and fetal programming.
Figure 1.
The proposed pathways showing how melatonin can affect normal and compromised pregnancies and result in different adult phenotypes. Melatonin can reduce ROS in both mother and fetus and alters fetal programming in compromised pregnancies through epigenetic changes. GDM (gestational diabetes mellitus); IUGR (intrauterine growth retardation); ROS (reactive oxygen species).
9.1. Melatonin and GDM
GDM is a syndrome characterized by glucose intolerance leading to maternal hyperglycemia, endothelial dysfunction, and abnormal regulation of vascular tone [89]. Depending on the diagnostic and screening criteria, the prevalence of gestational diabetes has been reported to range from 1.3% to 19.9% [90]. Placentas from GDM pregnancies are larger than normal [91] and show decreased formation of terminal villi and increased numbers of intermediate villi compared to those from normal pregnancies [92]. These vascular changes are likely to affect placental vascular resistance and vascular volume, leading to metabolic changes in the feto-placental microvascular and macrovascular endothelium [93].
Hyperglycemia increases the activity of the polyol pathway, which decreases antioxidant defenses and increases oxidative stress [94]. Oxidative stress is also increased in the hyperglycemic state by increased glucose auto-oxidation and protein glycation, which upregulate the production of oxidative factors [95]. ROS formation caused by the hyperglycemic state is associated with the progression of vascular complications [96]. ROS can activate numerous pathways that damage cells, and these pathways are often linked to complications that occur in the later stages of diabetes. Brownlee et al. reported that hyperglycemia may result in the non-enzymatic glycation of proteins called advanced glycation end products (AGEs), which can interfere with signal transduction and thus change the soluble levels of cytokines, hormones, and free radicals, and these proteins can alter the function of the glycated proteins [97].
In addition to its function as a ROS scavenger involving the amelioration of oxidative damage and the proinflammatory state present in high-risk pregnancies, recent studies have shown that melatonin also plays an important role in the regulation of body weight and adiposity, which could be related to its involvement in the control of the circadian rhythm [98,99]. Moreover, disruption or alterations in endogenous melatonin secretion by the pineal gland have been found to be related to disturbances in glucose and lipid homeostasis [99]. Similarly, genetic variants of the MTNR1B gene, a functional melatonin receptor, have been reported to be associated with gestational glucose intolerance in the Chinese population [100]. Therefore, melatonin may function as an antioxidant as well as a metabolic modulator in the context of GDM.
GDM may predispose offspring to many disorders such as obesity, type 2 diabetes mellitus, and cardiorenal metabolic syndrome. The underlying pathophysiology may include epigenetic modifications and alterations in the balance between glucose, insulin, and other regulatory hormones involved in glucose homeostasis during intrauterine and perinatal life [101]. In fetal metabolic programming, an imbalance between leptin and adiponectin leads to obesity, while altered beta-cell proliferation and compensatory islet leads to type 2 diabetes, and beta-cell remodeling and endothelial cell dysfunction leads to cardiorenal syndrome [102]. Pdx-1 is a pancreatic and duodenal homeobox 1 transcription factor that regulates pancreatic development and cell differentiation. There is increased methylation of the CpG island proximal promoter of the Pdx-1 gene and a subsequent blunting of its transcription, and the development of diabetes during adulthood, explaining an epigenetic mechanism for fetal programming. Recently, Miehle et al. conducted a human study to find an association between the percentage of DNA methylation of the leptin gene in the placenta and glycemia using the 2 h post-oral glucose tolerance test at 24–28 weeks of pregnancy [103]. In the same study, DNA methylation in the placenta was inversely correlated with the placental leptin mRNA levels and serum leptin levels in the mother [103]. However, little is known about the role of melatonin in epigenetic modifications in the context of GDM, which warrants further investigation.
9.2. Melatonin and Intrauterine Growth Restriction
IUGR is defined as a condition in which the fetus has an estimated body weight and/or length below the 10th percentile for gestational age [104]. IUGR affects most organ systems by interrupting developmental processes and is associated with insulin resistance [105], obesity [106], and cardiovascular disease [107] in adulthood.
Women diagnosed with IUGR show increased values of the indices of oxidative stress in the serum, suggesting the presence of oxidative stress [108,109]. During follow-up, children born with growth retardation show increased levels of lipid peroxidation and have higher blood pressure than age-matched children of normal birth weight [110]. Pregnant rats fed a low-protein diet have offspring with elevated arterial blood pressure and increased vasoconstrictor responsiveness to angiotensin II [111]. Circulating melatonin in pregnant animals was shown to be affected by IUGR [112]. Using a mid- to late-gestation ovine model of IUGR, Lemley et al. showed that melatonin might negate the consequences of IUGR in the presence of specific abnormalities in umbilical blood flow as long as sufficient uterine blood perfusion is maintained during pregnancy [113]. Therefore, melatonin may work as an antioxidant in the context of IUGR.
IUGR has been shown to induce epigenetic modification of selected genes in the placenta [114], as well as the liver [115,116], heart [117], pancreas [118], adrenal gland [119], and pulmonary arteries [120]. In rats with IUGR, global decreases in DNA methylation and increases in histone H3 acetylation on lysine 9 (K9) and K14 are observed in the brain at birth [121]. These epigenetic changes are associated with a concomitant decrease in DNMT1, MeCP2, and HDAC1 protein expressions [121]. IUGR in rats induces histone code modifications affecting glut4 expression in skeletal muscle [122], causes increased acetylation of H3K9 and K14 [115], and reduced expression of DNMT1 in the liver [116]. However, little is known about the role of melatonin in epigenetic modifications in the context of IUGR, which warrants further investigation.
9.3. Melatonin and Preeclampsia
Preeclampsia is a multisystem disorder that is unique to human pregnancy, occurring in 5%–10% of pregnancies and is a leading cause of maternal and neonatal mortality and morbidity [123]. There is growing evidence that the physiologically immature fetus is highly susceptible to disruptions in placental blood flow, which may predispose an individual to an increased risk of disease beyond the immediate postnatal period. Epidemiological studies show that exposure of infants to preeclampsia during gestation is associated with an increased risk of diabetes and cardiovascular morbidity in adulthood [124].
In women with preeclampsia, lipid peroxide levels in maternal blood and placental tissue are significantly increased. In addition, total antioxidant activities are decreased. Hence, preeclampsia might be considered as an oxidative stress disorder during pregnancy [125]. Endogenous melatonin level is significantly decreased in women with severe preeclampsia [126], who also show alterations in placental melatonin production and melatonin receptor expression [127]. During normal pregnancy, melatonin directly functions as a free radical scavenger and indirectly functions as an antioxidant, and it appears to be essential for successful pregnancy. Besides, melatonin can be a desirable component of the antioxidant system in the human placenta because it significantly improves mitochondrial efficiency [128]. Therefore, melatonin may function as an antioxidant in the context of preeclampsia.
Maternal adversities leading to dysregulation of placental development originated from preimplantation affecting the course of pregnancy were shown in both animal and human studies to alter the epigenetic process (e.g., DNA methylation, histone modifications, and genome imprinting) and result in preeclampsia and dismal fetal outcomes [66,69]. A recent study showed altered global DNA methylation patterns in preeclampsia placentas and its association with blood pressure [129]. Chim et al. reported increased concentrations of unmethylated maspin concentrations in the plasma of women with preeclampsia compared with that of healthy pregnant controls [130]. However, little is known about the role of melatonin in epigenetic modifications in the context of preeclampsia, which should be studied further.
9.4. Melatonin and Maternal Undernutrition
Adequate nutrition during gestation is essential for fetal growth and development. Maternal undernutrition can significantly affect fetal growth and intrauterine programming. The placenta may act as a nutrient sensor, modifying nutrient and hormone availability to feto-placental tissues in relation to environmental challenges. There is growing evidence linking slow fetal growth with the developmental programming of cardiovascular and metabolic diseases and neuropsychiatric disorders [131].
Maternal undernutrition during pregnancy can result in asymmetric growth retardation and is associated with increased oxidative stress. In humans, fetal undernutrition is associated with significant oxidative stress in small-for-gestational-age neonates born at term to malnourished mothers [132]. Melatonin treatment in undernourished mothers during pregnancy has been shown to improve birth weight and protect the placenta from ischemia/reperfusion-induced oxidative stress [133,134]. Maternal dietary protein restriction during pregnancy was found to have adverse effects on the quality of the sleep-wake cycle in the adult rat offspring [135]. In rat offspring of mothers exposed to a low-protein diet, antenatal administration of antioxidants to the mother prevents the development of hypertension and vascular dysfunction during adulthood [111]. Therefore, melatonin may function as an antioxidant in the context of maternal undernutrition.
Maternal undernutrition can have long-lasting effects on gene expression in the fetus and therefore can extensively affect the phenotypic outcome of the progeny. Maternal undernutrition can result in marked epigenetic changes affecting the GR and proopiomelanocortin (POMC) gene expression in the fetal hypothalamus and contributes to fetal programming. The consequences of maternal undernutrition include altered regulation of food intake, energy expenditure, and glucose homeostasis later in life [136]. Studies have shown that modest dietary protein restriction during pregnancy induces an altered phenotype through epigenetic changes in specific genes. Decreased methylation of the GR and PPARα promoters was detected in the heart of the offspring [137] and the PPARα promoter was hypomethylated in the umbilical cord [138]. However, there are a few reports on the role of melatonin in epigenetic modifications in the context of maternal undernutrition and this requires further investigation.
9.5. Melatonin and Maternal Stress
The rat model of maternal stress is used to replicate putative factors implicated in the etiology of major depression [139]. It is well known that prenatal exposure to glucocorticoids and stress leads to programming of the hypothalamic-pituitary-adrenal function and behavior and has long-term effects on the offspring [59,139,140]. The effects of prenatal stress on fetal outcome are mediated in part by elevated fetal glucocorticoid exposure.
The maternal milieu may perturb the development of the fetal circadian clock through its effect on glucocorticoid receptors, which are already present in the SCN during early development [141]. Dugovic et al. reported that the offspring of stressed mothers showed increased rapid eye movement (REM), total sleep, and an increase in slow-wave sleep during the dark phase [142]. Agomelatine is a mixed MT1/MT2 melatonin receptor agonist and 5HT2C serotonin receptor antagonist. Mariesse et al. showed reduced duration of slow-wave sleep, increased duration of REM sleep, increased number of REM sleep events, and increased motor activity before the beginning of the dark phase of the light/dark cycle in adult offspring of mothers exposed to stress [143]. This report provided evidence that agomelatine corrects sleep architecture and restores circadian homeostasis in a rat model of maternal stress [143]. Stress can affect sleep behaviors through its effect on inflammatory cytokines [144], which are susceptible to redox alteration [145,146]. In addition, the anti-inflammatory properties of melatonin arise from the fact that it prevents the translocation of NF-κB to the nucleus, thus reducing the upregulation of proinflammatory cytokines [6]. Taken together, these findings indicate that sleep behavior is affected in the offspring of mothers exposed to stress. Melatonin plays a role in reverting sleep disorders in maternal stress offspring, possibly through the modulation of proinflammatory cytokines via a redox mechanism. The possible role of melatonin in other long-term sequelae in maternal stress offspring warrants further studies.
The effect of maternal stress on fetal programming may be mediated by epigenetic mechanisms, with resulting behavioral modifications and altered biological rhythms in adult offspring [147]. Morley-Fletcher et al. demonstrated that agomelatine could correct all biochemical, cellular, and behavioral abnormalities displayed by maternal stress rats in adult life [148]. These authors showed that agomelatine reversed the reduction in the levels of p-CREB, mGlu2/3 receptors, and mGlu5 receptors in the hippocampus of maternal stress rats [148]. Interestingly, the mGlu2/3 receptor was shown to be altered via an epigenetic mechanism in maternal stress adult offspring [149], suggesting that melatonin has a potential role in epigenetic modifications in the context of maternal stress.
10. Conclusions
Melatonin is a potent free radical scavenger, a broad-spectrum antioxidant, and an epigenetic modification agent. The role of melatonin in pregnancy and parturition is well established. Melatonin readily crosses the placenta and the fetal blood-brain barrier and plays a key role in the regulation of development of fetal organs to extrauterine life. Compromised pregnancies result in oxidative stress to the fetus and alter fetal development through the epigenetic process. In this regard, melatonin is beneficial for reversing the adverse programming effects associated with compromised pregnancies via a redox mechanism; however, the potential role of melatonin in epigenetic modifications requires further study. Additional studies exploring the role of melatonin as a target for other pregnancy-related diseases are warranted.
Acknowledgements
The study was supported by the grant CMRPG8B0301 from Chang Gung Memorial Hospital, Kaohsiung, Taiwan to Li-Tung Huang.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Reiter R.J., Calvo J.R., Karbownik M., Qi W., Tan D.X. Melatonin and its relation to the immune system and inflammation. Ann. N. Y. Acad. Sci. 2000;917:376–386. doi: 10.1111/j.1749-6632.2000.tb05402.x. [DOI] [PubMed] [Google Scholar]
- 2.Radogna F., Diederich M., Ghibelli L. Melatonin: A pleiotropic molecule regulating inflammation. Biochem. Pharmacol. 2010;80:1844–1852. doi: 10.1016/j.bcp.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 3.Hardeland R., Cardinali D.P., Srinivasan V., Spence D.W., Brown G.M., Pandi-Perumal S.R. Melatonin-a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Mauriz J.L., Collado P.S., Veneroso C., Reiter R.J., González-Gallego J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013;54:1–14. doi: 10.1111/j.1600-079X.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury I., Sengupta A., Maitra S.K. Melatonin: Fifty years of scientific journey from the discovery in bovine pineal gland to delineation of functions in human. Indian J. Biochem. Biophys. 2008;45:289–304. [PubMed] [Google Scholar]
- 6.Reiter R.J., Tan D.X., Osuna C., Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J. Biomed. Sci. 2000;7:444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez C., Mayo J.C., Sainz R.M., Antolin I., Herrera F., Martin V., Reiter R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 8.Korkmaz A., Reiter R.J. Epigenetic regulation: A new research area for melatonin? J. Pineal Res. 2008;44:41–44. doi: 10.1111/j.1600-079X.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 9.Korkmaz A., Rosales-Corral S., Reiter R.J. Gene regulation by melatonin linked to epigenetic phenomena. Gene. 2012;503:1–11. doi: 10.1016/j.gene.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 10.Mirick D.K., Davis S. Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol. Biomarkers Prev. 2008;17:3306–3313. doi: 10.1158/1055-9965.EPI-08-0605. [DOI] [PubMed] [Google Scholar]
- 11.Reiter R.J., Tan D.X., Burkhardt S. Reactive oxygen and nitrogen species and cellular and organismal decline: amelioration with melatonin. Mech. Ageing Dev. 2002;123:1007–1019. doi: 10.1016/s0047-6374(01)00384-0. [DOI] [PubMed] [Google Scholar]
- 12.Cardinali D.P., Pagano E.S., Scacchi Bernasconi P.A., Reynoso R., Scacchi P. Melatonin and mitochondrial dysfunction in the central nervous system. Horm. Behav. 2013 doi: 10.1016/j.yhbeh.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Jan J.E., Freeman R.D., Fast D.K. Melatonin treatment of sleep-wake cycle disorders in children and adolescents. Dev. Med. Child Neurol. 1999;41:491–500. [PubMed] [Google Scholar]
- 14.Dodge N.N., Wilson G.A. Melatonin for treatment of sleep disorders in children with developmental disabilities. J. Child Neurol. 2001;16:581–584. doi: 10.1177/088307380101600808. [DOI] [PubMed] [Google Scholar]
- 15.Okatani Y., Wakatsuki A., Reiter R.J., Miyahara Y. Hepatic mitochondrial dysfunction in senescence-accelerated mice: Correction by long-term, orally administered physiological levels of melatonin. J. Pineal Res. 2002;33:127–133. doi: 10.1034/j.1600-079x.2002.02109.x. [DOI] [PubMed] [Google Scholar]
- 16.Gitto E., Aversa S., Reiter R.J., Barberi I., Pellegrino S. Update on the use of melatonin in pediatrics. J. Pineal Res. 2011;50:21–28. doi: 10.1111/j.1600-079X.2010.00814.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y.C., Tain Y.L., Sheen J.M., Huang L.T. Melatonin utility in neonates and children. J. Formos. Med. Assoc. 2012;111:57–66. doi: 10.1016/j.jfma.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Tamura H., Nakamura Y., Terron M.P., Flores L.J., Manchester L.C., Tan D.X., Sugino N., Reiter R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008;25:291–303. doi: 10.1016/j.reprotox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Reiter R.J., Tan D.X., Manchester L.C., Paredes S.D., Mayo J.C., Sainz R.M. Melatonin and reproduction revisited. Biol. Reprod. 2009;81:445–456. doi: 10.1095/biolreprod.108.075655. [DOI] [PubMed] [Google Scholar]
- 20.Gitto E., Pellegrino S., Gitto P., Barberi I., Reiter R.J. Oxidative stress of the newborn in the pre- and post-natal period and the clinical utility of melatonin. J. Pineal Res. 2009;46:128–139. doi: 10.1111/j.1600-079X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 21.Aversa S., Pellegrino S., Barberi I., Reiter R.J., Gitto E. Potential utility of melatonin as an antioxidant during pregnancy and in the perinatal period. J. Matern. Fetal Neonatal. 2012;25:207–221. doi: 10.3109/14767058.2011.573827. [DOI] [PubMed] [Google Scholar]
- 22.Harderland R., Poeggeler B. Non-vertebrate melatonin. J. Pineal Res. 2003;34:233–241. doi: 10.1034/j.1600-079x.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 23.Hardeland R., Pandi-Perumal S.R., Cardinali D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006;38:313–316. doi: 10.1016/j.biocel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Facciola G., Hidestrand M., von Bahr C., Tybring G. Cytochrome P450 isoforms involved in melatonin metabolism in human liver microsomes. Eur. J. Clin. Pharmacol. 2001;56:881–888. doi: 10.1007/s002280000245. [DOI] [PubMed] [Google Scholar]
- 25.Dubocovich M.L., Delagrange P., Krause D.N., Sugden D., Cardinali D.P., Olcese J. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardeland R., Tan D.X., Reiter R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009;47:109–126. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 27.Reiter R.J., Korkmaz A., Paredes S.D., Manchester L.C., Tan D.X. Melatonin reduces oxidative/nitrosative stress due to drugs, toxins, metals, and herbicides. Neuro. Endocrinol. Lett. 2008;29:609–613. [PubMed] [Google Scholar]
- 28.Tan D.X., Manchester L.C., Reiter R.J., Qi W.B., Karbownik M., Calvo J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 2000;9:137–159. doi: 10.1159/000014635. [DOI] [PubMed] [Google Scholar]
- 29.Acuña-Castroviejo D., Martín M., Macías M., Escames G., Leon J., Khaldy H., Reiter R.J. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 2001;30:65–74. doi: 10.1034/j.1600-079x.2001.300201.x. [DOI] [PubMed] [Google Scholar]
- 30.Korkmaz A., Sanchez-Barcelo E.J., Tan D.X., Reiter R.J. Role of melatonin in the epigenetic regulation of breast cancer. Breast Cancer Res. Treat. 2009;115:13–27. doi: 10.1007/s10549-008-0103-5. [DOI] [PubMed] [Google Scholar]
- 31.Sandyk R., Anastasiadis P.G., Anninos P.A., Tsagas N. The pineal gland and spontaneous abortions: Implications for therapy with melatonin and magnetic field. Int. J. Neurosci. 1992;62:243–250. doi: 10.3109/00207459108999775. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzuka T., Sakamoto N., Ozawa M., Ushitani A., Hirabayashi M., Kanai Y. Alleviation of maternal hyperthermia-induced early embryonic death by administration of melatonin to mice. J. Pineal Res. 2005;39:217–223. doi: 10.1111/j.1600-079X.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 33.Torres-Farfan C., Valenzuela F.J., Mondaca M., Valenzuela G.J., Krause B., Herrera E.A., Riquelme R., Llanos A.J., Seron-Ferre M. Evidence of a role for melatonin in fetal sheep physiology: Direct actions of melatonin on fetal cerebral artery, brown adipose tissue and adrenal gland. J. Physiol. 2008;586:4017–4027. doi: 10.1113/jphysiol.2008.154351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seron-Ferre M., Torres-Farfan C., Forcelledo M.L., Valenzuela G.J. The development of circadian rhythms in the fetus and neonate. Semin. Perinatol. 2001;25:363–370. doi: 10.1053/sper.2001.29037. [DOI] [PubMed] [Google Scholar]
- 35.Serón-Ferré M., Mendez N., Abarzua-Catalan L., Vilches N., Valenzuela F.J., Reynolds H.E., Llanos A.J., Rojas A., Valenzuela G.J., Torres-Farfan C. Circadian rhythms in the fetus. Mol. Cell Endocrinol. 2012;349:68–75. doi: 10.1016/j.mce.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 36.Reppert S.M., Weaver D.R., Rivkees S.A., Stopa E.G. Putative melatonin receptor in a human biological clock. Science. 1998;242:78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y.H., Zhou J.N., Balesar R., Unmehopa U., Bao A., Jockers R., Van Heerikhuize J., Swaab D.F. Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: Colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J. Comp. Neurol. 2006;499:897–910. doi: 10.1002/cne.21152. [DOI] [PubMed] [Google Scholar]
- 38.Simonneaux V. Naughty melatonin: How mothers tick off their fetus. Endocrinology. 2011;152:1734–1738. doi: 10.1210/en.2011-0226. [DOI] [PubMed] [Google Scholar]
- 39.Karsch F.J., Bittman E.L., Foster D.L., Goodman R.L., Legan S.J., Robinson J.E. Neuroendocrine basis of seasonal reproduction. Recent Prog. Horm. Res. 1984;40:185–232. doi: 10.1016/b978-0-12-571140-1.50010-4. [DOI] [PubMed] [Google Scholar]
- 40.Malpaux B., Tricoire H., Mailliet F., Daveau A., Migaud M., Skinner D.C., Pelletier J., Chemineau P. Melatonin and seasonal reproduction: Understanding the neuroendocrine mechanisms using the sheep as a model. Reprod. Suppl. 2002;59:167–179. [PubMed] [Google Scholar]
- 41.McMillen I.C., Houghton D.C., Young I.R. Melatonin and the development of circadian and seasonal rhythmicity. J. Reprod. Fertil. Suppl. 1995;49:137–146. [PubMed] [Google Scholar]
- 42.Yellon S.M., Longo L.D. Melatonin rhythms in fetal and maternal circulation during pregnancy in sheep. Am. J. Physiol. 1987;252:E799–E802. doi: 10.1152/ajpendo.1987.252.6.E799. [DOI] [PubMed] [Google Scholar]
- 43.Deguchi T. Ontogenesis of a biological clock for serotonin: acetyl coenzyme A N-acetyltransferase in pineal gland of rat. Proc. Natl. Acad. Sci. USA. 1975;72:2814–2818. doi: 10.1073/pnas.72.7.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yellon S.M., Longo L.D. Effect of maternal pinealectomy and reverse photoperiod on the circadian melatonin rhythm in the sheep and fetus during the last trimester of pregnancy. Biol. Reprod. 1988;39:1093–1099. doi: 10.1095/biolreprod39.5.1093. [DOI] [PubMed] [Google Scholar]
- 45.Mcmillen I.C., Nowak R. Maternal pinealectomy abolishes the diurnal rhythm in plasma melatonin concentrations in the fetal sheep and pregnant ewe during late gestation. J. Endocrinol. 1989;120:459–464. doi: 10.1677/joe.0.1200459. [DOI] [PubMed] [Google Scholar]
- 46.Torres-Farfan C., Richter H.G., Germain A.M., Valenzuela G.J., Campino C., Rojas-García P., Forcelledo M.L., Torrealba F., Serón-Ferré M. Maternal melatonin selectively inhibits cortisol production in the primate fetal adrenal gland. J. Physiol. 2004;554:841–856. doi: 10.1113/jphysiol.2003.056465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellavía S.L., Carpentieri A.R., Vaqué A.M., Macchione A.F., Vermouth N.T. Pup circadian rhythm entrainment-effect of maternal ganglionectomy or pinealectomy. Physiol. Behav. 2006;89:342–349. doi: 10.1016/j.physbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Mendez N., Abarzua-Catalan L., Vilches N., Galdames H.A., Spichiger C., Richter H.G., Valenzuela G.J., Seron-Ferre M., Torres-Farfan C. Timed maternal melatonin treatment reverses circadian disruption of the fetal adrenal clock imposed by exposure to constant light. PLoS One. 2012;7:e42713. doi: 10.1371/journal.pone.0042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan W.Y., Ng T.B. Development of pre-implantation mouse embryos under the influence of pineal indoles. J. Neural Transm. Gen. Sect. 1994;96:19–29. doi: 10.1007/BF01277925. [DOI] [PubMed] [Google Scholar]
- 50.McElhinny A.S., Davis F.C., Warner C.M. The effect of melatonin on cleavage rate of C57BL/6 and CBA/Ca preimplantation embryos cultured in vitro. J. Pineal Res. 1996;21:44–48. doi: 10.1111/j.1600-079x.1996.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 51.Jahnke G., Marr M., Myers C., Wilson R., Travlos G., Price C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 1999;50:271–279. doi: 10.1093/toxsci/50.2.271. [DOI] [PubMed] [Google Scholar]
- 52.Sadowsky D.W., Yellon S., Mitchell M.D., Nathanielsz P.W. Lack of effect of melatonin on myometrial electromyographic activity in the pregnant sheep at 138–142 days gestation (term = 147 days gestation) Endocrinology. 1991;128:1812–1818. doi: 10.1210/endo-128-4-1812. [DOI] [PubMed] [Google Scholar]
- 53.Leach C.M., Thorburn G.D. A comparison of the inhibitory effects of melatonin and indomethacin on platelet aggregation and thromboxane release. Prostaglandins. 1980;20:51–56. doi: 10.1016/0090-6980(80)90005-2. [DOI] [PubMed] [Google Scholar]
- 54.Barker D.J., Winter P.D., Osmond C., Margetts B., Simmonds S.J. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 55.Chen M., Zhang L. Epigenetic mechanisms in developmental programming of adult disease. Drug Discov. Today. 2011;16:1007–1018. doi: 10.1016/j.drudis.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanson M., Godfrey K.M., Lillycrop K.A., Burdge G.C., Gluckman P.D. Developmental plasticity and developmental origins of non-communicable disease: Theoretical considerations and epigenetic mechanisms. Prog. Biophys. Mol. Biol. 2011;106:272–280. doi: 10.1016/j.pbiomolbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Hochberg Z., Feil R., Constancia M., Fraga M., Junien C., Carel J.C., Boileau P., Le Bouc Y., Deal C.L., Lillycrop K., et al. Child health, developmental plasticity, and epigenetic programming. Endocr. Rev. 2011;32:159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson L.P., Al-Hasan Y. Impact of oxidative stress in fetal programming. J. Pregnancy. 2012;2012:582748. doi: 10.1155/2012/582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiong F., Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front. Neuroendocrinol. 2012;12:62–63. doi: 10.1016/j.yfrne.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo Z.C., Fraser W.D., Julien P., Deal C.L., Audibert F., Smith G.N., Xiong X., Walker M. Tracing the origins of “fetal origins” of adult diseases: Programming by oxidative stress? Med. Hypotheses. 2006;66:38–44. doi: 10.1016/j.mehy.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 61.Luo Z.C., Xiao L., Nuyt A.M. Mechanisms of developmental programming of the metabolic syndrome and related disorders. World J. Diabetes. 2010;1:89–98. doi: 10.4239/wjd.v1.i3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tarry-Adkins J.L., Ozanne S.E. Mechanisms of early life programming: Current knowledge and future directions. Am. J. Clin. Nutr. 2011;94:1765S–1771S. doi: 10.3945/ajcn.110.000620. [DOI] [PubMed] [Google Scholar]
- 63.Tamura H., Takasaki A., Taketani T., Tanabe M., Kizuka F., Lee L., Tamura I., Maekawa R., Aasada H., Yamagata Y., et al. The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 2012;5:5. doi: 10.1186/1757-2215-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller S.L., Wallace E.M., Walker D.W. Antioxidant therapies: A potential role in perinatal medicine. Neuroendocrinology. 2012;96:13–23. doi: 10.1159/000336378. [DOI] [PubMed] [Google Scholar]
- 65.Nelissen E.C., van Montfoort A.P., Dumoulin J.C., Evers J.L. Epigenetics and the placenta. Hum. Reprod. Update. 2011;17:397–417. doi: 10.1093/humupd/dmq052. [DOI] [PubMed] [Google Scholar]
- 66.Huang L.T., Hsieh C.S., Chang K.A., Tain Y.L. Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int. J. Mol. Sci. 2012;13:14606–14622. doi: 10.3390/ijms131114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ribatti D., Nico B., Crivellato E. Morphological and molecular aspects of physiological vascular morphogenesis. Angiogenesis. 2009;12:101–111. doi: 10.1007/s10456-008-9125-1. [DOI] [PubMed] [Google Scholar]
- 68.Dokras A., Coffin J., Field L., Frakes A., Lee H., Madan A., Nelson T., Ryu G.Y., Yoon J.G., Madan A. Epigenetic regulation of maspin expression in the human placenta. Mol. Hum. Reprod. 2006;12:611–617. doi: 10.1093/molehr/gal074. [DOI] [PubMed] [Google Scholar]
- 69.Longtine M.S., Nelson D.M. Placental dysfunction and fetal programming: The importance of placental size, shape, histopathology, and molecular composition. Semin. Reprod. Med. 2011;29:187–196. doi: 10.1055/s-0031-1275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuen R.K., Chen B., Blair J.D., Robinson W.P., Nelson D.M. Hypoxia alters the epigenetic profile in cultured human placental trophoblasts. Epigenetics. 2013;8:192–202. doi: 10.4161/epi.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rugg-Gunn P.J. Epigenetic features of the mouse trophoblast. Reprod. Biomed. Online. 2012;25:21–30. doi: 10.1016/j.rbmo.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 72.Cerda S., Weitzman S.A. Influence of oxygen radical injury onDNA methylation. Mutat. Res. 1997;386:141–152. doi: 10.1016/s1383-5742(96)00050-6. [DOI] [PubMed] [Google Scholar]
- 73.Hitchler M.J., Domann F.E. An epigenetic perspective on the free radical theory of development. Free Radic. Biol. Med. 2007;43:1023–1036. doi: 10.1016/j.freeradbiomed.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ziech D., Franco R., Pappa A., Panayiotidis M.I. Reactive Oxygen Species (ROS)-induced genetic and epigenetic alterations in human carcinogenesis. Mutat. Res. 2011;711:167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 75.Brzezinski A., Seibel M.M., Lynch H.J., Deng M.H., Wurtman R.J. Melatonin in human preovulatory follicular fluid. J. Clin. Endocrinol. Metab. 1987;64:865–867. doi: 10.1210/jcem-64-4-865. [DOI] [PubMed] [Google Scholar]
- 76.Okatani Y., Wakatsuki A., Shinohara K., Kaneda C., Fukaya T. Melatonin stimulates glutathione peroxidase activity in human chorion. J. Pineal Res. 2001;30:199–205. doi: 10.1034/j.1600-079x.2001.300402.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhu J.L., Hjollund N.H., Andersen A.M., Olsen J. Shift work, job stress, and late fetal loss: The National Birth Cohort in Denmark. J. Occup. Environ. Med. 2004;46:1144–1149. doi: 10.1097/01.jom.0000145168.21614.21. [DOI] [PubMed] [Google Scholar]
- 78.Croteau A., Marcoux S., Brisson C. Work activity in pregnancy, preventive measures, and the risk of delivering a small-for-gestational-age infant. Am. J. Public Health. 2006;96:846–855. doi: 10.2105/AJPH.2004.058552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferreira D.S., Amaral F.G., Mesquita C.C., Barbosa A.P., Lellis-Santos C., Turati A.O., Santos L.R., Sollon C.S., Gomes P.R., Faria J.A., et al. Maternal melatonin programs the daily pattern of energy metabolism in adult offspring. PLoS One. 2012;7:e38795. doi: 10.1371/journal.pone.0038795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCurdy C.E., Bishop J.M., Williams S.M., Grayson B.E., Smith M.S., Friedman J.E., Grove K.L. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suter M., Bocock P., Showalter L., Hu M., Shope C., McKnight R., Grove K., Lane R., Aagaard-Tillery K. Epigenomics: Maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J. 2011;25:714–726. doi: 10.1096/fj.10-172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Z., Chin Y.E., Zhang D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell. Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawai Y., Garduno L., Theodore M., Yang J., Arinze I.J. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J. Biol. Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kennaway D.J. Programming of the fetal suprachiasmatic nucleus and subsequent adult rhythmicity. Trends Endocrinol. Metab. 2002;13:398–402. doi: 10.1016/s1043-2760(02)00692-6. [DOI] [PubMed] [Google Scholar]
- 85.Cagampang F.R., Poore K.R., Hanson M.A. Developmental origins of the metabolic syndrome: Body clocks and stress responses. Brain Behav. Immun. 2011;25:214–220. doi: 10.1016/j.bbi.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Kennaway D.J., Goble F.C., Stamp G.E. Factors influencing the development of melatonin rhythmicity in humans. J. Clin. Endocrinol. Metab. 1996;81:1525–1532. doi: 10.1210/jcem.81.4.8636362. [DOI] [PubMed] [Google Scholar]
- 87.Holditch-Davis D., Edwards L.J. Temporal organization of sleep-wake states in preterm infants. Dev. Psychobiol. 1998;33:257–269. doi: 10.1002/(sici)1098-2302(199811)33:3<257::aid-dev6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 88.Mirmiran M., Maas Y.G., Ariagno R.L. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med. Rev. 2003;7:321–334. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- 89.Muñoz-Hoyos A., Rodriguez-Cabezas T., Molina-Carballo A., Martinez-Sempere J.J., Ruiz-Cosano C., Acuña-Castroviejo D. Melatonin concentration in the umbilical artery and vein in human preterm and term neonates and neonates with acute fetal distress. J. Pineal Res. 1992;13:184–191. doi: 10.1111/j.1600-079x.1992.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 90.Simmons D. Epidemiology of Diabetes in Pregnancy. In: McCance D., Maresh M., editors. Practical Management of Diabetes in Pregnancy. Blackwell Publishing; London, UK: 2010. [Google Scholar]
- 91.Muñoz-Hoyos A., Bonillo-Perales A., Avila-Villegas R., González-Ripoll M., Uberos J., Florido-Navío J., Molina-Carballo A. Melatonin levels during the first week of life and their relation with the antioxidant response in the perinatal period. Neonatology. 2007;92:209–216. doi: 10.1159/000102957. [DOI] [PubMed] [Google Scholar]
- 92.Saito S., Tachibana T., Choi Y.H., Denbow D.M., Furuse M. ICV melatonin reduces acute stress responses in neonatal chicks. Behav. Brain Res. 2005;165:197–203. doi: 10.1016/j.bbr.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 93.Owens J.A., Witmans M. Sleep problems. Curr. Probl. Pediatr. Adolesc. Health Care. 2004;34:154–179. doi: 10.1016/j.cppeds.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 94.Chung S.S., Ho E.C., Lam K.S., Chung S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003;14:S233–S236. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 95.Giugliano D., Ceriello A., Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 96.Gao L., Mann G.E. Vascular NAD(P)H oxidase activation in diabetes: A double-edged sword in redox signalling. Cardiovasc. Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 97.Brownlee M. The pathological implications of protein glycation. Clin. Invest. Med. 1995;18:275–281. [PubMed] [Google Scholar]
- 98.Agil A., Navarro-Alarcón M., Ruiz R., Abuhamadah S., El-Mir M.Y., Vázquez G.F. Beneficial effects of melatonin on obesity and lipid profile in young zucker diabetic fatty rats. J. Pineal Res. 2011;50:207–212. doi: 10.1111/j.1600-079X.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- 99.Tan D.X., Manchester L.C., Fuentes-Broto L., Paredes S.D., Reiter R.J. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: Relation to human obesity. Obes. Rev. 2011;12:167–188. doi: 10.1111/j.1467-789X.2010.00756.x. [DOI] [PubMed] [Google Scholar]
- 100.Liao S., Liu Y., Tan Y., Gan L., Mei J., Song W., Chi S., Dong X., Chen X., Deng S. Association of genetic variants of melatonin receptor 1B with gestational plasma glucose level and risk of glucose intolerance in pregnant Chinese women. PLoS One. 2012;7:e40113. doi: 10.1371/journal.pone.0040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nistala R., Hayden M.R., Demarco V.G., Henriksen E.J., Lackland D.T., Sowers J.R. Prenatal programming and epigenetics in the genesis of the cardiorenal syndrome. Cardiorenal. Med. 2011;1:243–254. doi: 10.1159/000332756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garcia-Vargas L., Addison S.S., Nistala R., Kurukulasuriya D., Sowers J.R. Gestational diabetes and the offspring: implications in the development of the cardiorenal metabolic syndrome in offspring. Cardiorenal. Med. 2012;2:134–142. doi: 10.1159/000337734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miehle K., Stephan H., Fasshauer M. Leptin, adiponectin, and other adipokines in gestational diabetes mellitus and preeclampsia. Clin. Endocrinol. 2012;76:2–11. doi: 10.1111/j.1365-2265.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- 104.Mandruzzato G., Antsaklis A., Botet F., Chervenak F.A., Figueras F., Grunebaum A., Puerto B., Skupski D., Stanojevic M. Intrauterine restriction (IUGR) J. Perinat. Med. 2008;36:277–281. doi: 10.1515/JPM.2008.050. [DOI] [PubMed] [Google Scholar]
- 105.Forsén T., Eriksson J., Tuomilehto J., Reunanen A., Osmond C., Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann. Intern. Med. 2000;133:176–182. doi: 10.7326/0003-4819-133-3-200008010-00008. [DOI] [PubMed] [Google Scholar]
- 106.Pilgaard K., Hammershaimb Mosbech T., Grunnet L., Eiberg H., Van Hall G., Fallentin E., Larsen T., Larsen R., Poulsen P., Vaag A. Differential nongenetic impact of birth weight versus third-trimester growth velocity on glucose metabolism and magnetic resonance imaging abdominal obesity in young healthy twins. J. Clin. Endocrinol. Metab. 2011;96:2835–2843. doi: 10.1210/jc.2011-0577. [DOI] [PubMed] [Google Scholar]
- 107.Painter R.C., de Rooij S.R., Bossuyt P.M., Simmers T.A., Osmond C., Barker D.J., Bleker O.P., Roseboom T.J. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am. J. Clin. Nutr. 2006;84:322–327. doi: 10.1093/ajcn/84.1.322. [DOI] [PubMed] [Google Scholar]
- 108.Karowicz-Bilinska A., Kedziora-Kornatowska K., Bartosz G. Indices of oxidative stress in pregnancy with fetal growth restriction. Free Radic. Res. 2007;41:870–873. doi: 10.1080/10715760701291647. [DOI] [PubMed] [Google Scholar]
- 109.Biri A., Bozkurt N., Turp A., Kavutcu M., Himmetoglu O., Durak I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Invest. 2007;64:187–192. doi: 10.1159/000106488. [DOI] [PubMed] [Google Scholar]
- 110.Mohn A., Chiavaroli V., Cerruto M., Blasetti A., Giannini C., Bucciarelli T., Chiarelli F. Increased oxidative stress in prepubertal children born small for gestational age. J. Clin. Endocrinol. Metab. 2007;92:1372–1378. doi: 10.1210/jc.2006-1344. [DOI] [PubMed] [Google Scholar]
- 111.Cambonie G., Comte B., Yzydorczyk C., Ntimbane T., Germain N., Le N.L., Pladys P., Gauthier C., Lahaie I., Abran D., et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1236–R1245. doi: 10.1152/ajpregu.00227.2006. [DOI] [PubMed] [Google Scholar]
- 112.Maggioni C., Cornelissen G., Antinozzi R., Ferrario M., Grafe A., Halberg F. A half-yearly aspect of circulating melatonin in pregnancies complicated by intrauterine growth retardation. Neuro. Endocrinol. Lett. 1999;20:55–68. [PubMed] [Google Scholar]
- 113.Lemley C.O., Meyer A.M., Camacho L.E., Neville T.L., Newman D.J., Caton J.S., Vonnahme K.A. Melatonin supplementation alters uteroplacental hemodynamics and fetal development in an ovine model of intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R454–467. doi: 10.1152/ajpregu.00407.2011. [DOI] [PubMed] [Google Scholar]
- 114.Gheorghe C.P., Goyal R., Mittal A., Longo L.D. Gene expression in the placenta: Maternal stress and epigenetic responses. Int. J. Dev. Biol. 2010;54:507–523. doi: 10.1387/ijdb.082770cg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fu Q., McKnight R.A., Yu X., Callaway C.W., Lane R.H. Growth retardation alters the epigenetic characteristics of hepatic dual specificity phosphatase 5. FASEB J. 2006;20:2127–2129. doi: 10.1096/fj.06-6179fje. [DOI] [PubMed] [Google Scholar]
- 116.Lillycrop K.A., Slater-Jefferies J.L., Hanson M.A., Godfrey K.M., Jackson A.A., Burdge G.C. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br. J. Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patterson A.J., Xiao D., Xiong F., Dixon B., Zhang L. Hypoxia-derived oxidative stress mediates epigenetic repression of PKCɛ gene in foetal rat hearts. Cardiovasc. Res. 2012;93:302–310. doi: 10.1093/cvr/cvr322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pinney S.E., Simmons R.A. Metabolic programming, epigenetics, and gestational diabetes mellitus. Curr. Diab. Rep. 2012;12:67–74. doi: 10.1007/s11892-011-0248-1. [DOI] [PubMed] [Google Scholar]
- 119.Bogdarina I., Haase A., Langley-Evans S., Clark A.J.L. Glucocorticoid effects on the programming of AT1b angiotensin receptor gene methylation and expression in the rat. PLoS One. 2010;5:e9237. doi: 10.1371/journal.pone.0009237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rexhaj E., Bloch J., Jayet P.Y., Rimoldi S.F., Dessen P., Mathieu C., Tolsa J.F., Nicod P., Scherrer U., Sartori C. Fetal programming of pulmonary vascular dysfunction in mice: Role of epigenetic mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H247–H252. doi: 10.1152/ajpheart.01309.2010. [DOI] [PubMed] [Google Scholar]
- 121.Ke X., Lei Q., James S.J., Kelleher S.L., Melnyk S., Jernigan S., Yu X., Wang L., Callaway C.W., Gill G., et al. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol. Genom. 2006;25:16–28. doi: 10.1152/physiolgenomics.00093.2005. [DOI] [PubMed] [Google Scholar]
- 122.Raychaudhuri N., Raychaudhuri S., Thamotharan M., Devaskar S.U. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J. Biol. Chem. 2008;283:13611–13626. doi: 10.1074/jbc.M800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Noris M., Perico N., Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nat. Clin. Pract. Nephrol. 2005;1:98–114. doi: 10.1038/ncpneph0035. [DOI] [PubMed] [Google Scholar]
- 124.Simmons R.A. Developmental origins of adult disease. Pediatr. Clin. North. Am. 2009;56:449–466. doi: 10.1016/j.pcl.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seabra M.L., Bignotto M., Pinto L.R., Jr, Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000;29:193–200. doi: 10.1034/j.1600-0633.2002.290401.x. [DOI] [PubMed] [Google Scholar]
- 126.Gitto E., Reiter R.J., Amodio A., Romeo C., Cuzzocrea E., Sabatino G., Buonocore G., Cordaro V., Trimarchi G., Barberi I. Early indicators of chronic lung disease in preterm infants with respiratory distress syndrome and their inhibition by melatonin. J. Pineal Res. 2004;36:250–255. doi: 10.1111/j.1600-079X.2004.00124.x. [DOI] [PubMed] [Google Scholar]
- 127.Lanoix D., Guérin P., Vaillancourt C. Placental melatonin production and melatonin receptor expression are altered in preeclampsia: New insights into the role of this hormone in pregnancy. J. Pineal Res. 2012;53:417–425. doi: 10.1111/j.1600-079X.2012.01012.x. [DOI] [PubMed] [Google Scholar]
- 128.Gordon N. The therapeutics of melatonin: A paediatric perspective. Brain Dev. 2000;22:213–217. doi: 10.1016/s0387-7604(00)00120-0. [DOI] [PubMed] [Google Scholar]
- 129.Kulkarni A., Chavan-Gautam P., Mehendale S., Yadav H., Joshi S. Global DNA methylation patterns in placenta and its association with maternal hypertension in pre-eclampsia. DNA Cell Biol. 2011;30:79–84. doi: 10.1089/dna.2010.1084. [DOI] [PubMed] [Google Scholar]
- 130.Chim S.S., Tong Y.K., Chiu R.W., Lau T.K., Leung T.N., Chan L.Y., Oudejans C.B., Ding C., Lo Y.M. Detection of the placental epigenetic signature of the maspin gene in maternal plasma. Proc. Natl. Acad. Sci. USA. 2005;102:14753–14758. doi: 10.1073/pnas.0503335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fowden A.L., Giussani D.A., Forhead A.J. Intrauterine programming of physiological systems: Causes and consequences. Physiology (Bethesda) 2006;21:29–37. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- 132.Gupta P., Narang M., Banerjee B.D., Basu S. Oxidative stress in term small for gestational age neonates born to undernourished mothers: A case control study. BMC Pediatr. 2004;4:14. doi: 10.1186/1471-2431-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Richter H.G., Hansell J.A., Raut S., Giussani D.A. Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J. Pineal Res. 2009;46:357–364. doi: 10.1111/j.1600-079X.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- 134.Nagai R., Watanabe K., Wakatsuki A., Hamada F., Shinohara K., Hayashi Y., Imamura R., Fukaya T. Melatonin preserves fetal growth in rats by protecting against ischemia/reperfusion-induced oxidative/nitrosative mitochondrial damage in the placenta. J. Pineal Res. 2008;45:271–276. doi: 10.1111/j.1600-079X.2008.00586.x. [DOI] [PubMed] [Google Scholar]
- 135.Duran P., Cintra L., Galler J.R., Tonkiss J. Prenatal protein malnutrition induces a phase shift advance of the spontaneous locomotor rhythm and alters the rest/activity ratio in adult rats. Nutr. Neurosci. 2005;8:167–172. doi: 10.1080/10284150400026117. [DOI] [PubMed] [Google Scholar]
- 136.Stevens A., Begum G., Cook A., Connor K., Rumball C., Oliver M., Challis J., Bloomfield F., White A. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology. 2010;151:3652–3664. doi: 10.1210/en.2010-0094. [DOI] [PubMed] [Google Scholar]
- 137.Lillycrop K.A., Phillips E.S., Jackson A.A., Hanson M.A., Burdge G.C. Dietary protein restriction in the pregnant rat induces altered epigenetic regulation of the glucocorticoid receptor and peroxisomal proliferator-activated receptor alpha in the heart of the offspring which is prevented by folic acid. Proc. Nutr. Soc. 2006;65:65A. [Google Scholar]
- 138.Burdge G.C., Hanson M.A., Slater-Jefferies J.L., Lillycrop K.A. Epigenetic regulation of transcription: A mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br. J. Nutr. 2007;97:1036–1046. doi: 10.1017/S0007114507682920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Y., Gonzalez P., Zhang L. Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: Mechanisms and possible interventions. Prog. Neurobiol. 2012;98:145–165. doi: 10.1016/j.pneurobio.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lui C.C., Wang J.Y., Tain Y.L., Chen Y.C., Chang K.A., Lai M.C., Huang L.T. Prenatal stress in rat causes long-term spatial memory deficit and hippocampus MRI abnormality: Differential effects of postweaning enriched environment. Neurochem. Int. 2011;58:434–441. doi: 10.1016/j.neuint.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 141.Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog. Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 142.Dugovic C., Maccari S., Weibel L., Turek F.W., Van Reeth O. High corticosterone levels in prenatally stressed rats predict persistent paradoxical sleep alterations. J. Neurosci. 1999;19:8656–8664. doi: 10.1523/JNEUROSCI.19-19-08656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mairesse J., Silletti V., Laloux C., Zuena A.R., Giovine A., Consolazione M., van Camp G., Malagodi M., Gaetani S., Cianci S., et al. Chronic agomelatine treatment corrects the abnormalities in the circadian rhythm of motor activity and sleep/wake cycle induced by prenatal restraint stress in adult rats. Int. J. Neuropsychopharmacol. 2012;6:1–16. doi: 10.1017/S1461145711001970. [DOI] [PubMed] [Google Scholar]
- 144.Basta M., Chrousos G.P., Vela-Bueno A., Vgontzas A.N. Chronic insomnia and stress system. Sleep Med. Clin. 2007;2:279–291. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Allan S.M., Rothwell N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 146.Soumyarani V.S., Jayakumari N. Oxidatively modified high density lipoprotein promotes inflammatory response in human monocytes-macrophages by enhanced production of ROS, TNF-α, MMP-9, and MMP-2. Mol. Cell Biochem. 2012;366:277–285. doi: 10.1007/s11010-012-1306-y. [DOI] [PubMed] [Google Scholar]
- 147.Darnaudéry M., Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res. Rev. 2008;57:571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 148.Morley-Fletcher S., Mairesse J., Soumier A., Banasr M., Fagioli F., Gabriel C., Mocaer E., Daszuta A., McEwen B., Nicoletti F., et al. Chronic agomelatine treatment corrects behavioral, cellular, and biochemical abnormalities induced by prenatal stress in rats. Psychopharmacology (Berl) 2011;217:301–313. doi: 10.1007/s00213-011-2280-x. [DOI] [PubMed] [Google Scholar]
- 149.Matrisciano F., Tueting P., Maccari S., Nicoletti F., Guidotti A. Pharmacological activation of group-II metabotropic glutamate receptors corrects a schizophrenia-like phenotype induced by prenatal stress in mice. Neuropsychopharmacology. 2012;37:929–938. doi: 10.1038/npp.2011.274. [DOI] [PMC free article] [PubMed] [Google Scholar]