Abstract
Augmented inositol 1,4,5-trisphosphate receptor (InsP3R) function has been linked to a variety of cardiac pathologies, including cardiac arrhythmia. The contribution of inositol 1,4,5-trisphosphate-induced Ca2+ release (IP3ICR) in excitation-contraction coupling (ECC) under physiological conditions, as well as under cellular remodelling, remains controversial. Here we test the hypothesis that local IP3ICR directly affects ryanodine receptor (RyR) function and subsequent Ca2+-induced Ca2+ release in atrial myocytes. IP3ICR was evoked by UV-flash photolysis of caged InsP3 under whole-cell configuration of the voltage-clamp technique in atrial myocytes isolated from C57/BL6 mice. Photolytic release of InsP3 was accompanied by a significant increase in the Ca2+ release event frequency (4.14 ± 0.72 vs. 6.20 ± 0.76 events (100 μm)−1 s−1). These individual photolytically triggered Ca2+ release events were identified as Ca2+ sparks, which originated from RyR openings. This was verified by Ca2+ spark analysis and pharmacological separation between RyR and InsP3R-dependent sarcoplasmic reticulum (SR)-Ca2+ release (2-aminoethoxydiphenyl borate, xestospongin C, tetracaine). Significant SR-Ca2+ flux but eventless SR-Ca2+ release through InsP3R were characterized using SR-Ca2+ leak/SR-Ca2+ load measurements. These results strongly support the idea that IP3ICR can effectively modulate RyR openings and Ca2+ spark probability. We conclude that eventless and highly efficient InsP3-dependent SR-Ca2+ flux is the main mechanism of functional cross-talk between InsP3Rs and RyRs, which may be an important factor in the modulation of ECC sensitivity.
Key points
Inositol 1,4,5-trisphosphate receptors (InsP3Rs) are functionally expressed in cardiac myocytes.
The influence of inositol 1,4,5-trisphosphate-induced sarcoplasmic reticulum (SR)-Ca2+release (IP3ICR) on atrial excitation-contraction coupling (ECC) under physiological and pathophysiological conditions remains elusive.
The present study focuses on local IP3ICR and its functional consequences for ryanodine receptor (RyR) activity and subsequent Ca2+-induced Ca2+ release in atrial myocytes.
Here we show significant SR-Ca2+ flux, but eventless SR-Ca2+ release through InsP3Rs.
We suggest a new mechanism based on eventless and highly efficient InsP3-dependent SR-Ca2+ flux as a crucial mechanism of functional cross-talk between InsP3Rs and RyRs, which may be an important factor in the modulation of ECC sensitivity.
Introduction
In cardiac myocytes two mechanisms for sarcoplasmic reticulum (SR)-Ca2+ release have been established, Ca2+-induced Ca2+ release (CICR) as the central mechanism for excitation-contraction coupling (ECC) and inositol 1,4,5-trisphosphate-induced Ca2+ release (IP3ICR), Ca2+ release via inositol 1,4,5-trisphosphate receptor (InsP3R) activation. However, although InsP3Rs are functionally expressed in cardiac preparations (Mackenzie et al. 2004; Zima & Blatter, 2004; Domeier et al. 2008; Harzheim et al. 2009), their role under physiological as well as under pathophysiological conditions in ECC and CICR is still a matter of an ongoing debate.
It has been suggested that SR-Ca2+ release via InsP3R may effectively modulate ECC in ventricular cardiomyocytes, even though the expression of InsP3Rs is only a fraction of ryanodine receptor (RyR) expression levels (Lipp et al. 2000; Kockskämper et al. 2008). These aspects gain even more importance in atrial myocytes, where InsP3Rs are expressed 3–10 times more compared with ventricle myocytes (Lipp et al. 2000; Domeier et al. 2008). In addition, this hypothesis is supported by two key observations. First, under pathophysiological conditions, during cellular remodelling, InsP3R expression is upregulated and may cause delayed after depolarizations, which lead to arrhythmogenicity (Zima & Blatter, 2004; Bootman et al. 2007). Second, InsP3R-deficient hearts are largely protected from pro-arrhythmogenic stress (Li et al. 2005). Supporting data that encourage a significant contribution of IP3ICR to ECC and CICR are colocalization studies of RyRs and InsP3Rs (Lipp et al. 2000; Mackenzie et al. 2002), the microarchitecture of atrial myocytes (Yamasaki et al. 1997), and functional studies that have been carried out (Mackenzie et al. 2002; Zima & Blatter, 2004; Li et al. 2005).
In atrial myocytes normal Ca2+ transient propagation by concentric Ca2+ waves, which are not pro-arrhythmogenic, may be facilitated by IP3ICR (Mackenzie et al. 2004). Local Ca2+ signalling between InsP3Rs/RyRs and vice versa might mediate a functional Ca2+ cross-talk between the two types of SR-Ca2+ release channels. This was conceptualized for smooth muscle cells, where local RyR Ca2+ release events, ‘Ca2+ sparks’, were activated via CICR subsequent to InsP3R activation (Gordienko & Bolton, 2002).
In atrial cells, RyR is the major Ca2+ release channel, organized in clusters and functionally coupled (Niggli & Shirokova, 2007). On a local scale, RyR openings give rise to ‘Ca2+ sparks’, the building blocks of global Ca2+ transients in cardiac cells (Cheng et al. 1993), while coordinated openings of clustered InsP3Rs will form ‘Ca2+ puffs’ with distinct properties (Yao et al. 1995). Along with microscopic Ca2+ release events (Ca2+ sparks and Ca2+ puffs), eventless Ca2+ release has been discovered (‘Ca2+ quarks’, ‘Ca2+ blips’; Lipp et al. 1996; Parker et al. 1996; Brochet et al. 2011). However, these largely invisible Ca2+ release events and concomitant Ca2+ fluxes may have a significant impact on Ca2+ signalling in subcellular microdomains, and may be involved in the functional cross-talk between RyRs and InsP3Rs, which is still enigmatic.
The present study focuses on local IP3ICR and its functional consequences for RyR activity in atrial myocytes. We examined local IP3ICR triggered by photoactivation of caged InsP3 in isolated atrial myocytes from mice under whole-cell voltage-clamp conditions. This highly specific approach bypasses sarcolemmal membrane receptor activation and subsequent signalling cascades. Surprisingly, we found that although no individual IP3ICR events could be visualized, InsP3 release significantly affected the Ca2+ spark probability and fosters that RyR Ca2+ release can be modulated by Ca2+ release via InsP3Rs in intracellular microdomains. This suggested a new mechanism based on highly efficient but ‘eventless’ InsP3-dependent SR-Ca2+ release. We conclude that invisible InsP3-dependent SR-Ca2+ events are the main mechanism of functional cross-talk between InsP3Rs and RyRs.
Methods
Atrial myocytes from adult male C57/BL6 mice, obtained from the Central Animal Facility, University Hospital, University of Bern, were freshly isolated by the Langendorff perfusion technique. Hearts were removed after animals were killed by cervical dislocation. The number of animals (N) and myocytes (n) are given in the figure legends. All experiments were performed at room temperature and approved by the State Veterinary Office of Bern, Switzerland, according to Swiss Federal Animal Protection Law. Whole-cell voltage-clamp was combined with confocal Ca2+ imaging and UV-flash photolysis. The pipette solution contained (in mmol l−1): CsAsp, 120; Hepes, 10; tetraethylammonium chloride, 20; potassium-ATP, 5; MgCl2, 1; K5-fluo-3 (Biotium), 0.1; GSH, 2; caged InsP3–6Na, (0.03, 0.06, 0.24; Sichem); pH 7.2 with CsOH. Cells were perfused with external solution containing (in mmol l−1): NaCl, 140; Hepes, 5; MgCl2, 1.1; KCl, 5.4; glucose, 10; CaCl2, 1.8; BaCl2, 0.5; CsCl, 1; pH 7.4 NaOH. Pharmacological experiments used 5 μm 2-aminoethoxydiphenyl borate (2APB; Fluka), 5 μm xestospongin C (Xesto; A.G Scientific Inc.), 1 mm tetracaine (TET; Sigma) or 10 mm caffeine (Sigma) added to the external solution. For Ca2+ leak/load experiments (Shannon et al. 2002), atrial myocytes were loaded with fluo-3 AM (Biotium).
Data are reported as means ± SEM. Where the data were normally distributed, paired or unpaired, where appropriate, Student's t test was applied. For not normally distributed data the Wilcoxon Matched-Pairs Signed-Ranks Test was applied. A detailed Materials and Methods section is available in the Online Data Supplement.
Results
Endothelin-1 (ET-1)-induced InsP3R Ca2+ release in atrial myocytes
Figure 1 shows that rapid administration of 100 nm ET-1, an InsP3 pathway activator, caused a significant increase in spontaneous local as well as global Ca2+ release events in resting mouse atrial cardiomyocytes. This increase was 2APB (InsP3R blocker) sensitive (Fig. 1B) and was also absent in control (Fig. 1C). Observations agreed with previously performed experiments from other groups (Mackenzie et al. 2004; Zima & Blatter, 2004; Li et al. 2005), that ET-1-mediated IP3ICR could be obtained in various mammalian species including mouse atrial myocytes. ET-1-triggered IP3ICR is based on a complex signalling cascade that involves G-protein-coupled phospholipase C activation followed by diacylglycerol and InsP3 production. To bypass this complex signal transduction pathway, InsP3Rs were activated by rapid and transient intracellular InsP3 elevation induced by photorelease of InsP3 from caged InsP3.
Figure 1. Endothelin-1 (ET-1)-induced InsP3R Ca2+ release in atrial myocytes.
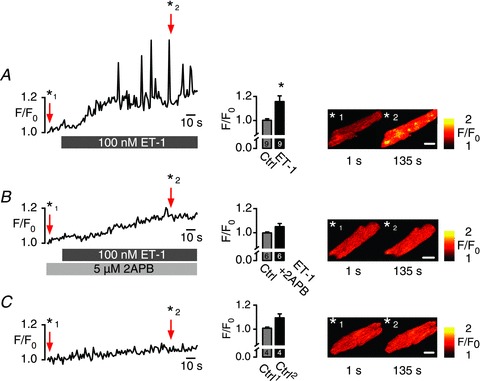
Time series of InsP3-induced Ca2+ release events expressed as F/F0 of fluo-3 fluorescence obtained in atrial myocytes. Cells were loaded with 5 μm fluo-3 AM (Biotium) for 20 min and left for de-esterification for 15 min before time series were collected in resting cells. A, the InsP3 pathway was stimulated with 100 nm ET-1 causing a substantial increase in SR-Ca2+ release event activity. B, inhibition of ET-1-triggered Ca2+ events with 5 μm 2-aminoethoxydiphenyl borate (2APB). C, spontaneous Ca2+ event activity under control conditions. SR-Ca2+ load was measured at the end of each experiment and found to be not significantly different in the various conditions (Ctrl: 3.45 ± 0.28 F/F0; ET-1: 3.89 ± 0.48 F/F0; 2APB + ET-1: 3.84 ± 0.53 F/F0). Bar graphs represent averaged F/F0 of the first 20 s (Ctrl) and 160–200 s; scale bars represent 10 μm, N= 3, n= 4–9.
InsP3-induced Ca2+ release triggered by UV-flash photolysis of caged InsP3
Atrial myocytes were voltage-clamped in the whole-cell configuration of the patch-clamp technique and held at –80 mV. Caged InsP3 in combination with the Ca2+ indicator fluo-3 were dialysed into the cell through the patch pipette. A SR-Ca2+ loading protocol based on L-type Ca2+ current activation elicited by depolarization steps from –80 mV to 0 mV (up to 10 times) was applied to ensure comparable SR-Ca2+ content, before line scan images were recorded (Fig. 2A). Under these conditions spontaneous Ca2+ event activity of about 3.7 ± 0.92 events (100 μm)−1 s−1 was observed.
Figure 2. Inositol 1,4,5-trisphosphate (InsP3)-induced Ca2+ release by UV-flash photolysis of caged InsP3 in atrial myocytes.
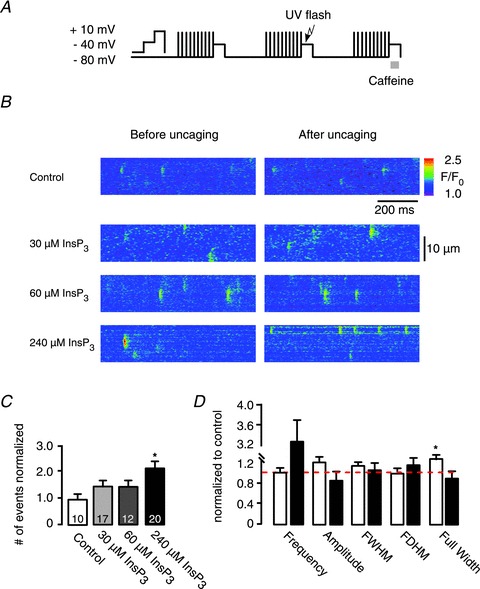
A, SR-Ca2+ loading protocol performed by ICa,L (10 times). B, representative line scan images of voltage-clamped atrial myocytes in whole-cell configuration before and after UV-flash photolysis in control and with 30, 60, 240 μm caged InsP3. C, the frequency of Ca2+ release events counted after UV-flash photolysis normalized to the spontaneous Ca2+ release events. D, averaged spatio-temporal Ca2+ spark properties after uncaging of InsP3 (240 μm) normalized to control. Cells were divided into two groups, white bars represent cells that showed little (<1.3) increase in Ca2+ release frequency (0.98 ± 0.08 events (100 μm)−1 s−1), black bars represent cells that showed >1.3 increase in Ca2+ release frequency (3.24 ± 0.55 events (100 μm)−1 s−1). Amplitude (ΔF/F0), full-width at half-maximum amplitude (FWHM; μm), full-duration at half-maximum amplitude (FDHM; ms) showed no significant difference after flash photolysis in both groups (see Supplementary Table 1). Full width (μm) showed a significant increase (from 3.80 ± 0.3 μm to 4.50 ± 0.4 μm) in cells with low Ca2+ event frequency in response to photolysis. *P < 0.05 vs. control, N= 6–7, n= 6–17, mean ± SEM.
In order to evoke IP3ICR in a highly specific manner, global UV-flashes were applied to photorelease InsP3 in the entire cytosol, which was followed by an increase of local Ca2+ event frequency triggered in a dose-dependent manner (Fig. 2C). The number of Ca2+ release events observed after photorelease of InsP3 was normalized to the number of spontaneous Ca2+ releases counted before InsP3 uncaging. Caged InsP3 concentrations of 30 μm and 60 μm caused a slight but not significant increase in the frequency of Ca2+ release events (30 μm: 2.73 ± 0.51 to 3.06 ± 0.71 events (100 μm)−1 s−1; 60 μm: 4.03 ± 0.73 to 4.20 ± 0.58 events (100 μm)−1 s−1). However, photolysis of 240 μm caged InsP3 triggered a significant (P= 0.02) increase in frequency of Ca2+ release events from 4.14 ± 0.72 to 6.20 ± 0.76 events (100 μm)−1 s−1 (Fig. 2B and C). It is to be mentioned that the effective InsP3 concentration after UV-flash application is orders of magnitude lower but in proportion to the loading concentration of the caged compound in the pipette solution (see online data supplement). Figure 2C shows control experiments lacking the caged compound, indicating that UV-flash application alone had no effect on the frequency of events. We expected that at least part of the total number of photolytically triggered Ca2+ events was based on IP3ICR (e.g. Ca2+‘puffs’). Although Ca2+ sparks show similarities with Ca2+ puffs, it has been shown that Ca2+ puffs exhibit significantly distinct spatio-temporal properties that are different from Ca2+ sparks (Cheng et al. 1993; Yao et al. 1995; Tovey et al. 2001; Niggli & Shirokova, 2007). At maximal event activity (240 μm caged InsP3), local Ca2+ release events were analysed in more detail for amplitude, full-duration at half-maximum amplitude (FDHM), full-width at half-maximum amplitude (FWHM), full duration, full width (fullwidth) and rise time (Picht et al. 2007). Cells were divided into two groups: one group showed little (<1.3 events (100 μm)−1 s−1) increase (frequency: 0.98 ± 0.08 events (100 μm)−1 s−1); a second group showed >1.3 events (100 μm)−1 s−1 increase in Ca2+ release events (frequency: 3.24 ± 0.55 events (100 μm)−1 s−1) after photolytic InsP3R activation. Ca2+ release events obtained in both groups showed no significant difference in their temporal characteristics. The fullwidth was significantly larger in the group of cells that showed little increase in the frequency of Ca2+ release events, from 3.80 ± 0.29 to 4.51 ± 0.38 μm (Fig. 2D), an indication for contribution of IP3ICR in Ca2+ spark formation. Nevertheless, Ca2+ event analysis did not show the anticipated two classes of local Ca2+ release events (i.e. Ca2+ sparks and Ca2+ puffs). The obtained parameters of Ca2+ events were comparable to those reported for Ca2+ sparks (Niggli & Shirokova, 2007) and are summarized in Supplementary Table 1 in the online material.
However, control measurements revealed photolytically triggered local InsP3 Ca2+ events (Ca2+ puffs) in neonatal rat cardiomyocytes. In other words, experimental conditions can be assumed to be appropriate for detecting microscopic subcellular Ca2+ events including Ca2+ puffs (see Supplementary Fig. 2S).
While photorelease of InsP3 lead to an increased Ca2+ spark frequency (Fig. 2), the precise mechanism by which this occurred still remained unclear. Therefore, pharmacological interventions were used to distinguish between Ca2+ sparks and Ca2+ puffs by selective inhibition of either RyRs or InsP3Rs. To inhibit IP3ICR, cells were incubated for at least 20 min in 5 μm Xesto, or 5 μm 2APB was acutely applied. TET (1 mm) was applied to inhibit RyR Ca2+ release (Fig. 3A). Photolytic increase of Ca2+ release events was inhibited by 2APB and Xesto, without affecting the spontaneous Ca2+ release event activity.
Figure 3. Identification of inositol 1,4,5-trisphosphate (InsP3)-evoked Ca2+ sparks.
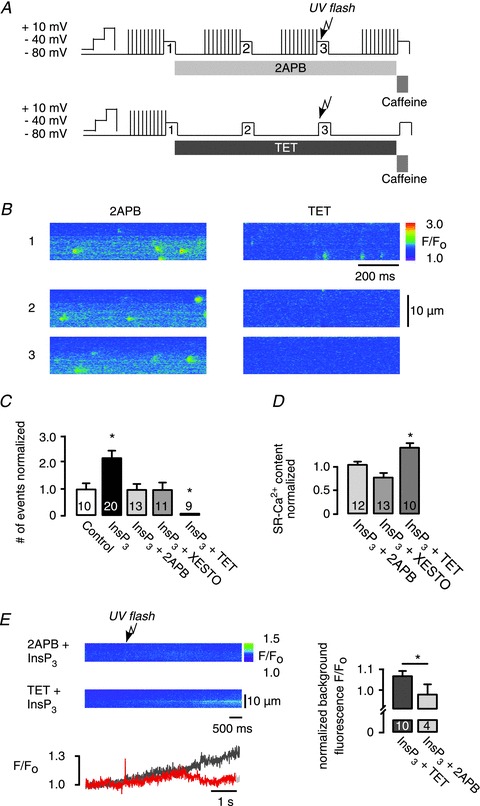
A, experimental protocol, sarcoplasmic reticulum (SR)-Ca2+ loading protocol performed by ICa,L (10 times); atrial myocytes were voltage-clamped under whole-cell conditions, the pipette solution contained 240 μm caged InsP3; line scans are numbered 1–3: (1) control; (2) pharmacological interventions; and (3) pharmacological interventions combined with photolytically released InsP3. Modified protocols were used for the application of 2-aminoethoxydiphenyl borate (2APB) and tetracaine (TET). B, representative data showing line scan images of fluo-3 fluorescence under whole-cell conditions taken at selected time points: (1) control; (2) in the presence of 2APB or TET; (3) pharmacology and photolytic release of InsP3. C, frequency of Ca2+ release events evoked by uncaging of caged InsP3 normalized to control (spontaneous frequency in rest). The increase in Ca2+ spark frequency was inhibited by the application of either 2APB or xestospongin (Xesto; InsP3R inhibitors) and by TET (RyR inhibitor). D, SR-Ca2+ content measured by rapid caffeine (10 mm) application. E, change in background fluo-3 fluorescence F/F0 after InsP3 uncaging, in the presence of TET (dark grey) or 2APB (red). Fluorescence F/F0 was normalized to background fluorescence before InsP3 release (right). Background fluorescence increase after UV-flash photolysis of caged InsP3 in the presence of TET was blocked by 2APB. *P < 0.05 vs. control, N= 2–5; n= 6–13, mean ± SEM.
Surprisingly, in the presence of TET a complete inhibition of all Ca2+ release events (Fig. 3B and C) was observed. This observation is in full agreement with the Ca2+ event analysis given in Fig. 2, showing that all provoked SR-Ca2+ release events can be classified as Ca2+ sparks. Taken together, photolytically activated IP3ICR efficiently facilitates RyR Ca2+ release.
SR-Ca2+ load and invisible InsP3R Ca2+ release (SR-Ca2+ leak)
Ca2+ spark probability strongly depends on luminal Ca2+ ([Ca2+]SR). RyRs and InsP3Rs may share, at least in part, the same luminal Ca2+ pool, suggesting that [Ca2+]SR may have a potential role in the functional cross-talk of both Ca2+ release channels, which was examined using caffeine-induced Ca2+ transients. InsP3R block (Fig. 3D) did not significantly affect the SR-Ca2+ content (2APB: 1 ± 0.18 to 1.09 ± 0.08 a.u., P= 0.8; and Xesto: from 1.13 ± 0.07 to 0.78 ± 0.09 a.u., P= 0.07), whereas TET caused a significant increase in the SR-Ca2+ content from 1 ± 0.07 to 1.4 ± 0.1 a.u., P= 0.02, presumably by suppressing Ca2+ leak (Shannon et al. 2002). Besides the SR-Ca2+ content, SR-Ca2+ leak is an important determinant for Ca2+ spark frequency. Indirect evidence that InsP3R stimulation may play a role in SR-Ca2+ leak came from the observation that in the presence of TET subsequent InsP3 release caused a slow but significant increase in background fluorescence, which was not seen in the presence of 2APB (Fig. 3E). This result suggests that ‘eventless’ Ca2+ flux through the InsP3R, which cannot be resolved as local Ca2+ release events, may be more pronounced in atrial cardiomyocytes than previously expected.
In Fig. 4 we addressed the question whether InsP3Rs may contribute to the SR-Ca2+ leak and thus affect the Ca2+ spark appearance by performing a modified SR-Ca2+ leak protocol introduced by Shannon et al. (2002). Atrial myocytes were field stimulated for 45 s at 1 Hz, followed by a rapid solution switch from 1.8 mm extracellular Ca2+ concentration ([Ca2+]o) to a nominally [Ca2+]o free and [Na+]o free solution. After 10 s, 10 μm cyclopiazonic acid (CPA; a potent SERCA blocker) was added and finally, 30 s later, the SR-Ca2+ content was measured with rapid caffeine application (Fig. 4A). Atrial myocytes were divided into two groups. The control group was recorded under conditions given above, the second group was pre-incubated with 100 nm ET-1 for at least 120 s before CPA was applied (Fig. 4A). CPA causes an inhibition of the SERCA function. Hence, in combination with the blocked Na+/Ca2+ exchanger function, any Ca2+ leaking from the SR is trapped in the cytoplasm and mirrored by an increase in the cytosolic Ca2+ concentration [Ca2+]i. This increase was measured and defined as SR-Ca2+ leak.
Figure 4. Invisible InsP3-dependent sarcoplasmic reticulum (SR)-Ca2+ release unmasked by SR-Ca2+ leak-load relationship.
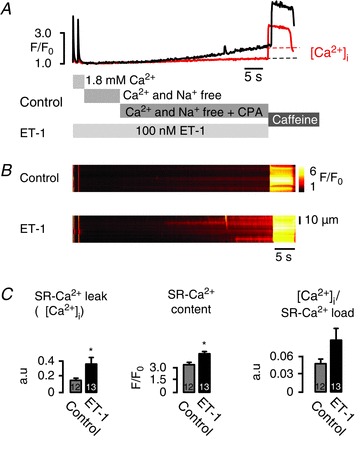
A, representative data showing averaged F/F0 of fluo-3 fluorescence changes (control: red; endothelin-1 (ET-1): black) and B, corresponding line scan images of fluo-3 fluorescence recorded in atrial myocytes. Cells were field stimulated for at least 45 s at 1 Hz before line scan images were recorded. After pre-pulse protocol, superfusion was rapidly switched from 1.8 mm[Ca2+]o to a nominally [Ca2+]o- and [Na+]o-free extracellular solution for 15 s, then 10 μm cyclopiazonic acid (CPA) was added for 20 s, before 10 mm caffeine was applied. The protocol was repeated in the presence of 100 nm ET-1 after pre-incubation with 100 nm ET-1 for at least 120 s. The increase in background fluorescence when CPA was applied represents SR-Ca2+ leak (Δ[Ca2+]i). C, averaged data, SR-Ca2+ leak (Δ[Ca2+]i), SR-Ca2+ content and SR-Ca2+ leak/SR-Ca2+ load relationship (Δ[Ca2+]i/SR-Ca2+ load) compared with control (no InsP3 pathway stimulation). In the presence of ET-1 there was a significant increase in the SR-Ca2+ leak (Δ[Ca2+]i) when CPA was added, unmasking a substantial contribution of the InsP3R opening to the total SR-Ca2+ leak. *P < 0.02–009 vs. control, N= 3; n= 12–13, mean ± SEM.
When CPA was added in the presence of ET-1 there was a significant increase in SR-Ca2+ leak Δ[Ca2+]i) compared with the leak in control (0.16 ± 0.03 to 0.38 ± 0.09 ΔF/F0, P= 0.02). The SR-Ca2+ leak/SR-Ca2+ load relationship increased accordingly, although not significantly (Δ[Ca2+]i/SR-Ca2+ load: 0.047 ± 0.007 to 0.086 ± 0.020, P= 0.09). This may be due to the additional Ca2+ influx induced by ET-1 (He et al. 2000), causing an increased SR-Ca2+ content (3.30 ± 0.24 to 4.53 ± 0.26 F/F0, P= 0.001). However, reduced SERCA activity unmasked a substantial contribution of InsP3R activity to the total SR-Ca2+ leak (Fig. 4), which was not seen before. Taken together, eventless InsP3R-Ca2+ release flux is part of SR-Ca2+ leak and facilitates SR-Ca2+ spark probability.
Discussion
The physiological role of atrial myocytes is to ensure ventricular blood refilling especially under conditions of physical stress and activity. This involves stimulation by direct autonomic nervous system innervation, neuro-humoral effects and interactions with hormones released by the vasculature that boost ECC on a cellular level. Similar to ventricular myocytes, the fundamental mechanism for ECC in atrial myocytes is driven by CICR. However, the contribution of IP3ICR in initiation, propagation and amplification of local and global SR-Ca2+ release and thus CICR in atrial myocytes remains controversial (Blatter et al. 2003; Mackenzie et al. 2004).
Our results obtained in atrial myocytes showed significant alterations in Ca2+ sparks (width and frequency) during InsP3R activation. This emphasizes that local Ca2+ release from InsP3Rs may play a significant role in the modulation of Ca2+-dependent RyR activity in atrial myocytes. Our approach, based on the combination of different biophysical methods, revealed some discrepancies between InsP3R activation and the appearance of local SR-Ca2+ release events, such as apparent invisible, ‘eventless’ Ca2+ release although InsP3R activation occurred. From these discrepancies we derived a novel conclusion regarding functional cross-talk between RyRs and InsP3Rs in atrial myocytes based on highly efficient but ‘eventless’ IP3ICR.
Identification of Ca2+ sparks
We analysed individual Ca2+ release events triggered by photorelease of InsP3. RyRs Ca2+ sparks are characterized by amplitudes of up to 2 ΔF/F0, widths ≍2 μm, FDHM ≍30 ms, whereas InsP3 Ca2+ puffs are characterized by smaller amplitudes, increased widths ≍6 μm and a prolonged FDHM of about 100–600 ms (Zima & Blatter, 2004; Niggli & Shirokova, 2007; Cheng & Lederer, 2008). Our spark analysis revealed Ca2+ sparks but did not show a second distinct class of Ca2+ release events that could be ad hoc assigned to Ca2+ puffs, respectively. In addition, selective pharmacological inhibition of either InsP3Rs or RyRs let us conclude that the detected InsP3-triggered Ca2+ release events can be classified as Ca2+ sparks. However, the absence of photolytically triggered IP3ICR events was surprising.
Functional cross-talk of InsP3R and RyR2 via invisible SR-Ca2+ release
Despite the evidence that IP3ICR occurs in cardiac cells (Zima & Blatter, 2004), reports of elementary Ca2+ signals arising from InsP3R openings have been rare, which may be due to the Ca2+ spark dominance. In atrial myocytes, InsP3Rs are colocalized with RyRs, preferentially in the subsarcolemmal space and around the nucleus (Lipp et al. 2000; Mackenzie et al. 2002). The expression of InsP3Rs is known to be regulated in a highly dynamic manner, notably during development and cellular remodelling in pathophysiological situations (Janowski et al. 2006).
RyRs are arranged in functional arrays (10–300 RyRs) and subject to stochastic cluster assembly processes (Baddeley et al. 2009). The synchronized openings of clustered RyRs result in local Ca2+ sparks. Under resting conditions, in diastole, spontaneous Ca2+ sparks reflect the finite open probability of RyRs, which is controlled by [Ca2+]i, luminal Ca2+ ([Ca2+]SR), phosphorylation state and numerous other factors (Eisner et al. 1998). A comparable configuration of spatially segregated clusters of 10–100 InsP3Rs is believed to be responsible for the generation of ‘Ca2+ puffs’, activated by InsP3 and Ca2+ (Foskett et al. 2007), but direct ultrastructural evidence is yet missing (Shuai et al. 2007). In addition, there are species differences in human/rat/rabbit versus mouse in the InsP3R cluster dynamics and microarchitecture that shape the InsP3 Ca2+ event characteristics (Diambra & Marchant, 2011). It has been suggested that InsP3Rs are mobile in the SR membrane and can form clusters upon stimulation (Pantazaka & Taylor, 2011). There is also evidence that InsP3R Ca2+ release sites represent pre-established, stable clusters of InsP3Rs (Smith et al. 2009). Under this view, brief exposure to InsP3 should trigger Ca2+ puffs, which was not the case in our study. We suggest that InsP3Rs in intact mouse atrial myocytes may not form sufficient cluster size to facilitate Ca2+ puffs due to a low level of InsP3R expression and/or intercluster distance. InsP3Rs may rather be distributed near RyRs to facilitate Ca2+ sparks. This view is supported by our immunohistochemistry study, showing that InsP3Rs seem to be rather homogeneously distributed (Supplementary Fig. 3S). According to our data, expression of functional InsP3Rs in atrial myocytes may not necessarily lead to the formation of distinguished Ca2+ puffs.
Thus, how can the increase in Ca2+ spark frequency after InsP3 photorelease be explained? The prevalent view that Ca2+ sparks and Ca2+ puffs are elementary events becomes more comprehensive, as for both types of local Ca2+ release events, even smaller elementary Ca2+ release events have been confirmed. Compared with Ca2+ sparks, openings of single RyRs may result in smaller but more frequent events (‘Ca2+ quarks’; Lipp et al. 1996; Brochet et al. 2011). At the same time, similar to Ca2+ quarks, InsP3R-dependent elementary Ca2+ release events arising from single InsP3R openings, termed ‘Ca2+ blips’, have been discovered (Parker et al. 1996). Opposed to triggered events, which are experimentally easy to detect, ‘blips’ and ‘quarks’ are largely invisible because these Ca2+ events are below detection threshold (Sobie et al. 2002; Williams et al. 2011). This is due to the low signal-to-noise ratio given by fluorescent Ca2+ measurements. The absence of visible Ca2+ puffs in our measurements, together with the observed increase in spark frequency mediated by InsP3Rs, suggests a significant contribution of ‘invisible’ or ‘eventless’ IP3ICR to the occurrence of Ca2+ sparks (and possible Ca2+ wave propagation). We propose that these eventless InsP3R openings may be responsible for microdomain [Ca2+]i increase that either sensitize RyRs for CICR or lead to direct RyR activation. Functional cross-talk between InsP3R and RyR can operate in both directions. Hence, microdomain [Ca2+]i elevations could sensitize InsP3R for InsP3, which would favour InsP3R openings (Foskett et al. 2007).
Thus, the contribution of eventless SR-Ca2+ release via InsP3R could be more substantial for the regulation of Ca2+ signalling in cellular microdomains than previously thought. This is consistent with a number of not entirely explained experimental reports regarding functional interactions of InsP3R and RyR. It has been reported that InsP3R activity increases [Ca2+]i in the vicinity of RyRs and thus facilitates CICR during ECC in adult cat atrial myocytes (Zima & Blatter, 2004), and that InsP3-dependent Ca2+ release has a positive inotropic effect on ECC by facilitating Ca2+ release through RyR clusters in rabbit ventricle myocytes (Domeier et al. 2008). Furthermore, there is evidence that Ca2+ leak through InsP3Rs is present at sites where RyRs are located and that this Ca2+ leak can modulate RyR Ca2+ release events (Gordienko & Bolton, 2002).
This ‘invisible’ SR-Ca2+ leak, respectively, SR-Ca2+ flux via InsP3R, was further examined in a series of SR-Ca2+ leak measurements. Here, InsP3R function was stimulated with ET-1. Compared with control, ET-1 may stimulate various Ca2+ influx pathways (e.g. via TRPC; Treves et al. 2004), which may have led to an increased SR-Ca2+ load before the nominally Ca2+- and Na+-free solution was added. This would lead per se to a transient increase in SR-Ca2+ leak until a new steady-state with matched leak/load relationship is reached. Nevertheless, increased InsP3R open probability will then concomitantly produce an ‘excess’ SR-Ca2+ leak in the Ca2+- and Na+-free solution compared with control as seen in Fig. 4A. This is largely independent of the total SR-Ca2+ content and results in an increased leak/load relationship.
Taken together, experiments using photorelease of InsP3 and SR-Ca2+ leak measurements suggest that ‘eventless’ InsP3-dependent SR-Ca2+ leak is the main mechanism of functional cross-talk between InsP3Rs and RyRs.
Pathophysiological implications
A change in the SR-Ca2+ leak/load relationship can effectively change [Ca2+]SR transiently or even under steady-state conditions. It is well established that [Ca2+]SR is involved in various forms of cellular instability and affected during cellular remodelling in response to pathophysiological stress (Bers, 2003; Shannon et al. 2003; Wehrens et al. 2005). Under chronic atrial fibrillation, InsP3Rs are targeted during cellular remodelling and were found to be upregulated, which may have positive inotropic effects on the global level (Zima & Blatter, 2004; Li et al. 2005; Bootman et al. 2007; Harzheim et al. 2009). Recently, subcellular mechanism(s) linking InsP3R activity to the development of atrial fibrillation and cardiac hypertrophy has been demonstrated (Higazi et al. 2009; Nakayama et al. 2010). Cardiac-specific blockage of the InsP3R pathway, therefore, could offer a new therapeutic strategy for treatment of atrial arrhythmogenicity.
Acknowledgments
The authors thank Ernst Niggli for valuable discussions and helpful comments on the manuscript. This work was supported by the Swiss National Science Foundation (31-111983), Berne University Research Foundation and Novartis Res. Foundation to M.E.
Glossary
- 2APB
2-aminoethoxydiphenyl borate
- CICR
Ca2+-induced Ca2+ release
- CPA
cyclopiazonic acid
- ECC
excitation-contraction coupling
- ET-1
endothelin-1
- FDHM
full-duration at half-maximum amplitude
- fullwidth
full width
- InsP3R
inositol 1,4,5-trisphosphate receptor
- IP3ICR
inositol 1,4,5-trisphosphate-induced Ca2+ release
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- TET
tetracaine
- Xesto
xestospongin C
Author contributions
All experiments were performed at the Department of Physiology, University of Bern, Switzerland. M.E. conceived and designed experiments. T.H. collected and analysed the experimental data. M.E., T.H. and N.D.U. drafted and revised the article. All authors approved the final version for publication.
Supplementary material
Supporting Information
References
- Baddeley D, Jayasinghe ID, Lam L, Rossberger S, Cannell MB, Soeller C. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc Natl Acad Sci U S A. 2009;106:22275–22280. doi: 10.1073/pnas.0908971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res. 2003;93:487–490. doi: 10.1161/01.RES.0000091871.54907.6B. [DOI] [PubMed] [Google Scholar]
- Blatter LA, Kockskämper J, Sheehan KA, Zima AV, Hüser J, Lipsius SL. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J Physiol. 2003;546:19–31. doi: 10.1113/jphysiol.2002.025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Harzheim D, Smyrnias I, Conway SJ, Roderick HL. Temporal changes in atrial EC-coupling during prolonged stimulation with endothelin-1. Cell Calcium. 2007;42:489–501. doi: 10.1016/j.ceca.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Brochet DXP, Xie W, Yang D, Cheng H, Lederer WJ. Quarky calcium release in the heart. Circ Res. 2011;108:210–218. doi: 10.1161/CIRCRESAHA.110.231258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Diambra L, Marchant JS. Inositol (1,4,5)-trisphosphate receptor microarchitecture shapes Ca2+ puff kinetics. Biophys J. 2011;100:822–831. doi: 10.1016/j.bpj.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H596–H604. doi: 10.1152/ajpheart.01155.2007. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Trafford AW, Diaz ME, Overend CL, O’Neill SC. The control of Ca2+ release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc Res. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung K-H, Mak D-OD. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordienko D, Bolton T. Crosstalk between ryanodine receptors and IP3 receptors as a factor shaping spontaneous Ca2+-release events in rabbit portal vein myocytes. J Physiol. 2002;542:743–762. doi: 10.1113/jphysiol.2001.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzheim D, Movassagh M, Foo RS-Y, Ritter O, Tashfeen A, Conway SJ, Bootman MD, Roderick HL. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106:11406–11411. doi: 10.1073/pnas.0905485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JQ, Pi Y, Walker JW, Kamp TJ. Endothelin-1 and photoreleased diacylglycerol increase L-type Ca2+ current by activation of protein kinase C in rat ventricular myocytes. J Physiol. 2000;3:807–820. doi: 10.1111/j.1469-7793.2000.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higazi DR, Fearnley CJ, Drawnel FM, Talasila A, Corps EM, Ritter O, McDonald F, Mikoshiba K, Bootman MD, Roderick HL. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol Cell. 2009;33:472–482. doi: 10.1016/j.molcel.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Janowski E, Cleemann L, Sasse P, Morad M. Diversity of Ca2+ signaling in developing cardiac cells. Ann NY Acad Sci. 2006;1080:154–164. doi: 10.1196/annals.1380.014. [DOI] [PubMed] [Google Scholar]
- Kockskämper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–147. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate (IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- Lipp P, Lüscher C, Niggli E. Photolysis of caged compounds characterized by ratiometric confocal microscopy: a new approach to homogeneously control and measure the calcium concentration in cardiac myocytes. Cell Calcium. 1996;19:255–266. doi: 10.1016/s0143-4160(96)90026-3. [DOI] [PubMed] [Google Scholar]
- Mackenzie L, Bootman M, Laine M, Berridge M, Thuring J, Holmes A, Li W, Lipp P. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol. 2002;541:395–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci. 2004;117:6327–6337. doi: 10.1242/jcs.01559. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Bodi I, Maillet M, DeSantiago J, Domeier T, Mikoshiba K, Lorenz J, Blatter L, Bers D, Molkentin J. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ Res. 2010;107:659–666. doi: 10.1161/CIRCRESAHA.110.220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli E, Shirokova N. A guide to sparkology: the taxonomy of elementary cellular Ca2+ signaling events. Cell Calcium. 2007;42:379–387. doi: 10.1016/j.ceca.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Pantazaka E, Taylor CW. Differential distribution, clustering, and lateral diffusion of subtypes of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2011;286:23378–23387. doi: 10.1074/jbc.M111.236372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I, Choi J, Yao Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium. 1996;20:105–121. doi: 10.1016/s0143-4160(96)90100-1. [DOI] [PubMed] [Google Scholar]
- Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol. 2007;293:C1073–C1081. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- Shuai J, Pearson JE, Foskett JK, Mak D-OD, Parker I. A kinetic model of single and clustered IP3 receptors in the absence of Ca2+ feedback. Biophys J. 2007;93:1151–1162. doi: 10.1529/biophysj.107.108795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IF, Wiltgen SM, Shuai J, Parker I. Ca2+ puffs originate from preestablished stable clusters of inositol trisphosphate receptors. Sci Signaling. 2009;2:1–7. doi: 10.1126/scisignal.2000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie E, Dilly K, Cruz J, Lederer J, Jafri M. Termination of cardiac Ca2+ sparks: an investigative mathematical model of calcium-induced calcium release. Biophys J. 2002;83:59–78. doi: 10.1016/s0006-3495(02)75149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey SC, de Smet P, Lipp P, Thomas D, Young KW, Missiaen L, De Smedt H, Parys JB, Berridge MJ, Thuring J, Holmes A, Bootman MD. Calcium puffs are generic InsP3-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. J Cell Sci. 2001;114:3979–3989. doi: 10.1242/jcs.114.22.3979. [DOI] [PubMed] [Google Scholar]
- Treves S, Franzini-Armstrong C, Moccagatta L, Arnoult C, Grasso C, Schrum A, Ducreux S, Zhu MX, Mikoshiba K, Girard T. Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J Cell Biol. 2004;166:537–548. doi: 10.1083/jcb.200404079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XHT, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- Williams GSB, Chikando AC, Tuan H-TM, Sobie EA, Lederer WJ, Jafri MS. Dynamics of calcium sparks and calcium leak in the heart. Biophys J. 2011;101:1287–1296. doi: 10.1016/j.bpj.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki Y, Furuya Y, Araki K, Matsuura K, Kobayashi M, Ogata T. Ultra-high-resolution scanning electron microscopy of the sarcoplasmic reticulum of the rat atrial myocardial cells. Anat Rec. 1997;248:70–75. doi: 10.1002/(SICI)1097-0185(199705)248:1<70::AID-AR8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol. 1995;482:533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


