Abstract
Delayed cord clamping improves circulatory stability in preterm infants at birth, but the underlying physiology is unclear. We investigated the effects of umbilical cord clamping, before and after ventilation onset, on cardiovascular function at birth. Prenatal surgery was performed on lambs (123 days) to implant catheters into the pulmonary and carotid arteries and probes to measure pulmonary (PBF), carotid (CaBF) and ductus arteriosus blood flows. Lambs were delivered at 126 ± 1 days and: (1) the umbilical cord was clamped at delivery and ventilation was delayed for about 2 min (Clamp 1st; n = 6), and (2) umbilical cord clamping was delayed for 3–4 min, until after ventilation was established (Vent 1st; n = 6). All lambs were subsequently ventilated for 30 min. In Clamp 1st lambs, cord clamping rapidly (within four heartbeats), but transiently, increased pulmonary and carotid arterial pressures (by ∼30%) and CaBF (from 30.2 ± 5.6 to 40.1 ± 4.6 ml min−1 kg−1), which then decreased again within 30–60 s. Following ventilation onset, these parameters rapidly increased again. In Clamp 1st lambs, cord clamping reduced heart rate (by ∼40%) and right ventricular output (RVO; from 114.6 ± 14.4 to 38.8 ± 9.7 ml min−1 kg−1), which were restored by ventilation. In Vent 1st lambs, cord clamping reduced RVO from 153.5 ± 3.8 to 119.2 ± 10.6 ml min−1 kg−1, did not affect heart rates and resulted in stable blood flows and pressures during transition. Delaying cord clamping for 3–4 min until after ventilation is established improves cardiovascular function by increasing pulmonary blood flow before the cord is clamped. As a result, cardiac output remains stable, leading to a smoother cardiovascular transition throughout the early newborn period.
Key points
Delayed cord clamping improves circulatory stability in preterm infants at birth, but the underlying reason is not known.
In a new preterm lamb study we investigated whether delayed cord clamping until ventilation had been initiated improved pulmonary, cardiovascular and cerebral haemodynamic stability.
We demonstrated that ventilation prior to cord clamping markedly improves cardiovascular function by increasing pulmonary blood flow before the cord is clamped, thus further stabilising the cerebral haemodynamic transition.
These results show that delaying cord clamping until after ventilation onset leads to a smoother transition to newborn life, and probably underlies previously demonstrated benefits of delayed cord clamping.
Introduction
Traditionally, the umbilical cord is clamped and cut immediately after birth, but in 2010, the International Liaison Committee on Resuscitation (ILCOR) recommended that the cord should not be cut for at least 1 min after birth in infants not requiring resuscitation (Perlman et al. 2010). This recommended change in practice is to facilitate blood transfer from placenta to baby to reduce iron deficiency and later anaemia. However, the immediate cardiovascular consequences of leaving the umbilical cord intact after birth have not been explored.
Clamping the umbilical cord immediately increases systemic peripheral resistance, resulting in an increase in arterial pressure (afterload) (Dawes, 1968; Rudolph, 1977). However, as the placental circulation receives 30–50% of fetal cardiac output, cord clamping transiently reduces venous return (by 30–50%), which, combined with the increase in afterload, decreases cardiac output (Crossley et al. 2009). These changes significantly impact on cardiac function in the newborn as its circulation transitions into a neonatal phenotype. In adults, venous return to the left ventricle (LV; preload) is solely derived from pulmonary venous return. In the fetus, LV preload is mostly derived from the umbilical circulation via the ductus venosus and foramen ovale (Rudolph, 1979), because fetal pulmonary vascular resistance (PVR) is high and pulmonary blood flow (PBF) is low. Thus, following cord clamping, umbilical venous return is lost and left ventricular output (LVO) becomes dependent on PBF, as in adults. Any delay between umbilical cord clamping and the increase in PBF could therefore severely affect LVO and potentially result in organ injury.
Lung aeration at birth is central to the fetal to neonatal transition (Hooper & Harding, 2005). It triggers an 8- to 10-fold increase in PBF (Dawes et al. 1953; Rudolph, 1977; Polglase & Hooper, 2006), which is essential for both pulmonary gas exchange and maintenance of LVO. To avoid the loss of venous return and decrease in LVO caused by cord clamping, ideally pulmonary ventilation should precede cord clamping so that PVR can decrease first. This would enable pulmonary venous return to immediately replace umbilical venous return as the primary source for LV preload and to minimise swings in LVO caused by cord clamping. As large, rapid swings in LVO and arterial pressure increase the risk of intraventricular haemorrhage, this could be clinically important, particularly in very preterm infants who have poor autoregulatory control of their cerebral circulation (Del Toro et al. 1991; Greisen, 2005; Gilmore et al. 2011).
Most term infants cry and begin breathing soon after delivery and so probably decrease PVR and increase PBF before the umbilical cord is clamped. However, in infants born apnoeic, the cord is clamped before respiratory support is provided. Recent clinical trials indicate that delayed cord clamping improves cardiovascular function after birth (Meyer & Mildenhall, 2012; Sommers et al. 2012) and cerebral oxygenation (Takami et al. 2012), but the benefits are thought to result from improved blood transfer from placenta to infant, rather than other physiological factors. In particular, understanding how lung aeration and the decrease in PVR may contribute to the benefits of delayed cord clamping after birth has not been considered. Delayed cord clamping allows time for the infant to aerate its lungs and increase PBF before venous return from the placental circulation is lost. As a result, the venous return that sustains LVO can immediately switch from the umbilical to the pulmonary circulation, thereby avoiding large swings in cardiovascular function.
We have investigated the effect of commencing ventilation before and after clamping the umbilical cord on the transition of the cardiopulmonary circulation after preterm birth. We hypothesised that initiating ventilation before umbilical cord clamping will improve cardiovascular stability following preterm birth.
Methods
Ethics statement
The experimental protocol was performed in accordance with guidelines established by the National Health and Medical Research Council of Australia and was approved by the Monash Medical Centre (MMCA) animal ethics committee at Monash University.
Surgical preparation
Aseptic surgery was performed on 12 pregnant Border-Leicester/Merino ewes at 123 ± 1 days gestation (term is ∼147 days) as described previously (Crossley et al. 2009). Anaesthesia of ewe and fetus was induced with an i.v. bolus of 5% sodium thiopentone (Pentothal; 1 g in 20 ml) and, following intubation, maintained with inhalation of 1.5–3% halothane in air. The fetal head and neck were exposed via a hysterotomy and an ultrasonic flow probe (3 mm: Transonic Systems, Ithaca, NY, USA) was placed around a carotid artery. Polyvinyl catheters were inserted into the other carotid artery and into a jugular vein. Ultrasonic flow probes (4 mm) were also placed around the left main pulmonary artery and ductus arteriosus (DA) via a thoracotomy. A polyvinyl catheter was placed into the left pulmonary artery via direct puncture. The fetal chest and neck were sutured closed and the fetus returned to the uterus and the maternal incisions closed. The ewe recovered for 3 days and received post-operative analgesia (transdermal fentanyl patches, 75 μg h−1; Janssen-Cilag, North Ryde, NSW, Australia). Fetal well-being was monitored daily by measuring the partial pressure in arterial blood of oxygen (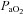 ) and carbon dioxide (
) and carbon dioxide (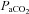 ), pH, arterial oxygen saturation of haemoglobin (
), pH, arterial oxygen saturation of haemoglobin (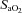 ; Rapidlab Massimo Frenchs Forest, NSW, Australia), glucose and lactate (ABL30; Radiometer, Copenhagen, Denmark). Instantaneous blood flows in the left main pulmonary artery (LPA) and DA were recorded digitally using a data acquisition system (Powerlab; ADInstruments, Castle Hill, NSW, Australia). Arterial pressures were measured using pressure transducers (PD10; DTX Plus Transducer; Becton Dickinson, Singapore) and also recorded digitally. Control recordings of all arterial pressures and flows were obtained 24 h before the experimental procedure.
; Rapidlab Massimo Frenchs Forest, NSW, Australia), glucose and lactate (ABL30; Radiometer, Copenhagen, Denmark). Instantaneous blood flows in the left main pulmonary artery (LPA) and DA were recorded digitally using a data acquisition system (Powerlab; ADInstruments, Castle Hill, NSW, Australia). Arterial pressures were measured using pressure transducers (PD10; DTX Plus Transducer; Becton Dickinson, Singapore) and also recorded digitally. Control recordings of all arterial pressures and flows were obtained 24 h before the experimental procedure.
Experimental procedure
At 126 ± 1 days, ewes were anaesthetised (as above) and fetuses exposed via hysterotomy. The fetal trachea was intubated with a 4.0 mm cuffed endotracheal tube and lung liquid was drained passively. At 126 days of gestation fetal sheep are in the early alveolar stage of lung development (Alcorn et al. 1981). However, functionally, their lungs more closely relate to a 26–28 week preterm infant as they develop severe respiratory distress soon after birth and are unable to survive without significant respiratory support. Each fetus was randomised to either umbilical cord clamping before ventilation (Clamp 1st) or cord clamping after the establishment of ventilation (Vent 1st) groups (n = 6 for each). In Clamp 1st lambs, the umbilical cord was immediately clamped and cut and ventilation commenced 2.1 ± 0.1 min later. In Vent 1st lambs, pulmonary ventilation commenced while the umbilical cord remained patent. Umbilical cord clamping was delayed (by 3.7 ± 0.3 min) until after PBF had increased, whereupon the cord was clamped and cut. In all lambs, ventilation began with a 20 s sustained inflation delivered by a Neopuff (Fisher & Paykel Healthcare, Panmure, Auckland, New Zealand) using a peak inflation pressure (PIP) of 35 cmH2O. Lambs then received positive pressure ventilation (Babylog 8000+; Dräger, Lübeck, Germany) in volume guarantee mode with a tidal volume of 7 ml kg−1 and a positive end-expiratory pressure of 5 cmH2O. Inspired gases were warmed and humidified, the inspired oxygen was adjusted to maintain transcutaneous arterial oxygen saturation (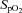 ) at 90–95% and a
) at 90–95% and a 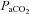 of 35–55 mmHg was targeted. The lamb's well-being was monitored by regular blood gas analysis (ABL30; Radiometer) and
of 35–55 mmHg was targeted. The lamb's well-being was monitored by regular blood gas analysis (ABL30; Radiometer) and 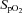 monitoring. All lambs remained anaesthetised and apnoeic throughout the experiments using an infusion of Alfaxane (i.v. 5–15 mg kg−1 h−1; Jurox, East Tamaki, Auckland, New Zealand) in 5% dextrose via the jugular vein catheter. Ewes and lambs were humanely killed (sodium pentobarbitone: >100 mg kg−1
i.v.) at the end of the study period.
monitoring. All lambs remained anaesthetised and apnoeic throughout the experiments using an infusion of Alfaxane (i.v. 5–15 mg kg−1 h−1; Jurox, East Tamaki, Auckland, New Zealand) in 5% dextrose via the jugular vein catheter. Ewes and lambs were humanely killed (sodium pentobarbitone: >100 mg kg−1
i.v.) at the end of the study period.
Data and statistical analysis
All pressure and flow data were averaged over 5 heart beats and analysed every 30 s before, during and after umbilical cord clamping and ventilation onset. Data were also obtained for each of the first 10 consecutive heart beats following umbilical cord clamping in both groups. Right ventricular output (RVO) was calculated as the sum of total PBF and blood flow through the DA, taking ductal flow from pulmonary to aorta as positive and flow from aorta to pulmonary artery as negative. Total PBF was estimated from LPA flow based on the weight difference between the left and right lungs as previously described (Crossley et al. 2009). All data were compared over time and between groups using either a two-way repeated-measures ANOVA for postnatal physiological data, or a one-way ANOVA for fetal data (Sigmastat v3.0; SPSS Inc., Chicago, IL, USA). Data are presented as mean ± SEM. Statistical significance was accepted for P < 0.05.
Results
Fetal body weights and arterial blood gas parameters were similar between groups prior to delivery (Table 1). By chance, four females were randomised to the Clamp 1st group and none to the Vent 1st group. We have previously shown no sex-related differences in the cardiopulmonary haemodynamic transition at birth in preterm lambs (Polglase et al. 2012).
Table 1.
Fetal characteristics
| Clamp 1st | Vent 1st | |
|---|---|---|
| n (males) | 6 (2) | 6 (6) |
| Weight (kg) | 3.3 ± 0.6 | 2.9 ± 0.5 |
| pH | 7.34 ± 0.06 | 7.47 ± 0.02 |
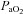 (mmHg) (mmHg) |
18.3 ± 2.9 | 24.5 ± 4.5 |
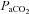 (mmHg) (mmHg) |
46.3 ± 4.5 | 43.4 ± 2.3 |
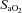 (%) (%) |
68.2 ± 7.7 | 68.1 ± 13.3 |
| Hb (g dl-1) | 9.3 ± 0.6 | 12.0 ± 1.2 |
Ventilation parameters
All lambs received a tidal volume of 7.0 ± 0.1 ml kg−1. PIPs (Clamp 1st, 42.3 ± 2.8; Vent 1st, 39.8 ± 3.0; P = 0.54), respiratory rates (Clamp 1st, 49.4 ± 3.4 breaths min-1; Vent 1st, 52.5 ± 3.3 breaths min-1; P = 0.53) and mean airway pressures (data not shown) were similar in both groups at all times.
Arterial blood gas parameters
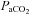 ,
, 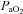 and
and 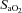 levels were not different between groups prior to or during ventilation (Tables 1 and 2). Oxygenation, determined by the alveolar arterial difference in oxygen (AaDO2), was not different between groups, although oxygenation tended (P = 0.059) to be lower in Vent 1st lambs at 5 min (Table 2). Similarly, there was a trend for higher
levels were not different between groups prior to or during ventilation (Tables 1 and 2). Oxygenation, determined by the alveolar arterial difference in oxygen (AaDO2), was not different between groups, although oxygenation tended (P = 0.059) to be lower in Vent 1st lambs at 5 min (Table 2). Similarly, there was a trend for higher 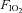 at 5 min in Vent 1st lambs (P = 0.059) but overall there was no difference between groups (data not shown). Haemoglobin tended to be higher after 30 min in Vent 1st than in Clamp 1st lambs but this was not statistically significant (Vent 1st: 11.4 ± 0.7 g dl−1; Clamp 1st: 10.4 ± 0.7 g dl−1; P = 0.10).
at 5 min in Vent 1st lambs (P = 0.059) but overall there was no difference between groups (data not shown). Haemoglobin tended to be higher after 30 min in Vent 1st than in Clamp 1st lambs but this was not statistically significant (Vent 1st: 11.4 ± 0.7 g dl−1; Clamp 1st: 10.4 ± 0.7 g dl−1; P = 0.10).
Table 2.
Arterial blood gas changes after birth in lambs ventilated before (Vent 1st) and after (Clamp 1st) cord clamping
| Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | |||||
| Clamp 1st | Vent 1st | Clamp 1st | Vent 1st | Clamp 1st | Vent 1st | Clamp 1st | Vent 1st | |
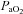 (mmHg) (mmHg) |
16.8 ± 3.2 | 32.9 ± 9.7 | 25.6 ± 11.4 | 29.4 ± 5.1 | 25.9 ± 3.7 | 24.4 ± 3.6 | 27.0 ± 4.1 | 30.6 ± 3.8 |
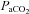 (mmHg) (mmHg) |
63.6 ± 4.2 | 70.0 ± 12.4 | 55.2 ± 4.2 | 55.2 ± 12.3 | 54.9 ± 5.7 | 50.2 ± 13.5 | 55.6 ± 6.1 | 48.3 ± 14.7 |
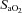 (%) (%) |
54.0 ± 8.1 | 59.3 ± 17.0 | 47.4 ± 8.2 | 76.5 ± 10.9 | 72.4 ± 6.0 | 75.1 ± 7.3 | 71.4 ± 8.1 | 76.4 ± 7.0 |
| AaDO2 | 267 ± 72 | 188 ± 40 | 311 ± 100 | 264 ± 69 | 220 ± 33 | 376 ± 95 | 221 ± 33 | 388 ± 87 |
Cardiovascular changes caused by umbilical cord clamping
Umbilical cord clamping caused major haemodynamic changes in unventilated (Clamp 1st) lambs, which were mitigated if ventilation preceded (Vent 1st group) umbilical cord clamping (Fig. 1).
Figure 1. Recordings in unventilated and ventilated lambs before and after umbilical cord occlusion.
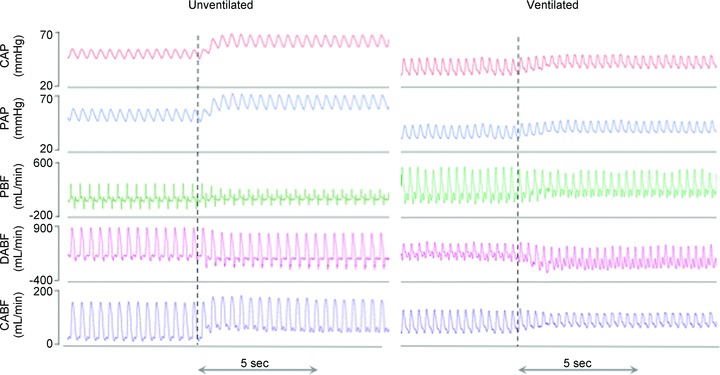
Carotid arterial pressure (PCA), pulmonary arterial pressure (PPA), pulmonary blood flow (PBF), blood flow through the ductus arteriosus (DABF) and carotid arterial blood flow (CaBF) in unventilated (left) and ventilated (right) lambs before and after umbilical cord occlusion (indicated by dotted line).
Heart rate
In Clamp 1st lambs, heart rates decreased from 171.0 ± 11.9 beats min-1 immediately before cord clamping to 102.0 ± 7.0 beats min-1 at 120 s after cord clamping. Heart rates remained unchanged in Vent 1st lambs (165.2 ± 15.5 vs. 164.8 ± 17.0 beats min-1; Fig. 2).
Figure 2. Parameter changes in ventilated and unventilated lambs before and after umbilical cord occlusion.
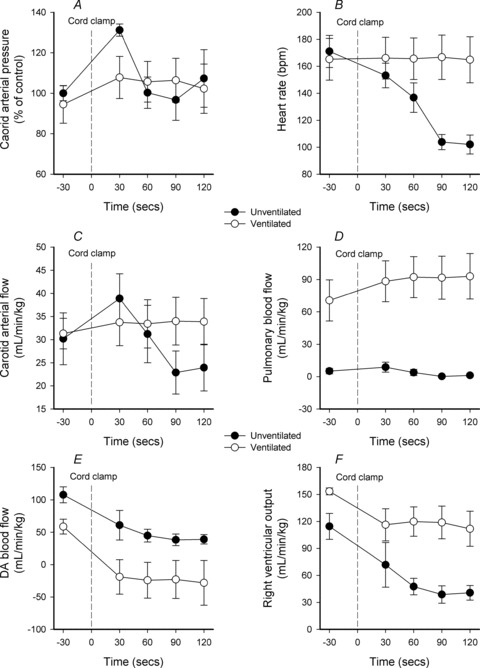
A–F, changes in mean (±SEM) carotid arterial blood pressure (A), heart rate (B), carotid arterial blood flow (C), pulmonary blood flow (D), ductus arteriosus (DA) blood flow (E) and right ventricular output (F) in ventilated (open circles) and unventilated lambs (filled circles) before and after umbilical cord occlusion (indicated by dotted line).
Carotid artery pressure (PCA) and carotid artery blood flow (CaBF)
In Clamp 1st lambs, cord clamping rapidly increased PCA from 34.8 ± 4.2 to 46.0 ± 5.4 mmHg (∼34% increase) after only 4 heart beats (Fig. 3). It remained elevated at 30 s (46.3 ± 6.0 mmHg) before decreasing to 31.0 ± 3.3 mmHg at 90 s (Fig. 2). Similarly, in Clamp 1st lambs, cord clamping increased CaBF from 30.2 ± 5.6 to 40.1 ± 4.6 ml min−1 kg−1 after 4 heart beats (Fig. 3). CaBF remained elevated at 30 s (38.8 ± 5.4 ml min−1 kg−1) before decreasing to 22.9 ± 7.7 ml min−1 kg−1 at 90 s (Fig. 2). The increase in CaBF coincided with increased diastolic flow, which increased from 13.9 ± 4.2 to 26.0 ± 3.8 ml min−1 kg−1 after 4 heart beats (Fig. 3). In contrast, cord clamping in Vent 1st lambs caused only a small increase in PCA (from 41.1 ± 4.2 to 43.8 ± 3.9 mmHg) and CaBF (from 31.3 ± 3.3 to 35.0 ± 4.8 ml min−1 kg−1) after 4 heat beats (Fig. 3), which remained stable at these levels for the next 120 s (Fig. 2).
Figure 3. Parameter changes in ventilated and unventilated lambs for the first 10 heart beats after umbilical cord occlusion.
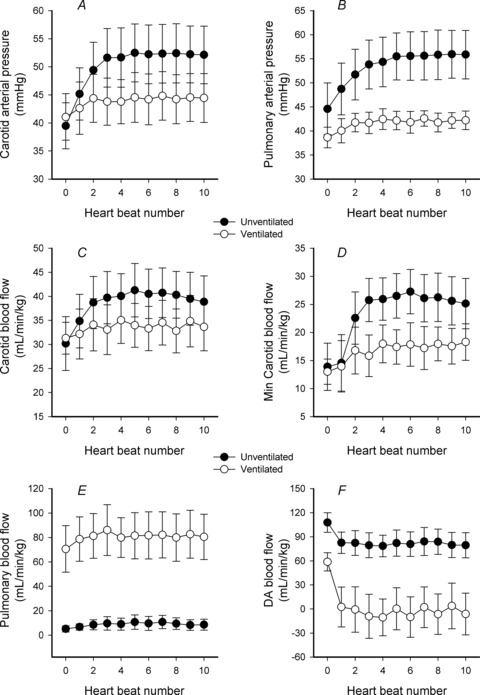
A–F, changes in mean (±SEM) carotid arterial blood pressure (A), pulmonary arterial pressure (B), carotid arterial blood flow (C), minimum carotid arterial blood flow (D), pulmonary blood flow (E) and ductus arteriosus (DA) blood flow (F) in ventilated (open circles) and unventilated lambs (filled circles) for the first 10 heart beats after umbilical cord occlusion (indicated by dotted line). ‘0’ represents the heart beat immediately before cord clamping.
Pulmonary artery pressure (PPA) and PBF
In Clamp 1st lambs, cord clamping also rapidly increased PPA from 44.6 ± 5.4 to 54.3 ± 5.1 mmHg after 4 heart beats (Fig. 3), which remained elevated at 30 s (51.5 ± 5.9 mmHg) before decreasing to 38.2 ± 3.2 mmHg at 90 s (Fig. 2). Despite these changes in PPA, cord clamping did not alter mean PBF because the minimum PBF increased (from −59.9 ± 9.7 to −36.6 ±12.6 ml min−1 kg−1) and the maximum PBF decreased (from 99.3 ± 21.9 to 70.1 ± 20.4 ml min−1 kg−1) by a similar degree (Fig. 1). In Vent 1st lambs, cord clamping caused only small increases in PPA (from 38.6 ± 2.2 to 42.5 ± 2.2 mmHg) and PBF (from 70.6 ± 19.0 to 82.8 ± 18.0 ml min−1 kg−1) (Fig. 2).
DA blood flow and RVO
In Clamp 1st lambs, cord clamping reduced DA blood flow from 107.8 ± 12.2 to 78.6 ± 13.3 ml min−1 kg−1 after 4 heart beats (Fig. 3), to 60.9 ± 15.7 ml min−1 kg−1 at 30 s and to 38.3 ± 9.0 ml min−1 kg−1 at 90 s (Fig. 2). In Vent 1st lambs, cord clamping reduced DA flow from 58.8 ± 11.4 to −10.5 ± 22.9 ml min−1 kg−1 within 4 heart beats (Fig. 3). The change from positive to ‘negative’ flows indicates a switch from right to left flow (positive value) through the DA to predominantly left to right flow (negative value). In Clamp 1st lambs, cord clamping reduced RVO by ∼65% within 90 s (from 114.6 ± 14.4 to 38.8 ± 9.7 ml min−1 kg−1), which accounts for the large decrease in DA flow. However, in Vent 1st lambs, cord clamping only reduced RVO by ∼22% (from 153.5 ± 3.8 to 119.2 ± 10.6 ml min−1 kg−1) and so the associated decrease in DA flow resulted from the increase in PBF (Fig. 2).
Cardiovascular changes caused by ventilation
PCA and CaBF
In Clamp 1st lambs, ventilation increased both PCA (from 40.2 ± 4.8 to 47.2 ± 4.3 mmHg) and CaBF (from 24.0 ± 7.1 to 33.4 ± 9.4 ml min−1 kg−1) within 30 s; CaBF continued to increase to 37.7 ± 9.0 ml min−1 kg−1 at 120 s after ventilation onset (Fig. 4). In Vent 1st lambs, ventilation caused a gradual decrease in PCA (from 53.7 ± 5.5 to 48.1 ± 4.6 mmHg) and CaBF (from 37.9 ± 6.4 to 31.3 ± 4.4 ml min−1 kg−1) over the first 120 s after ventilation onset (Fig. 4).
Figure 4. Parameter changes in response to ventilation onset.
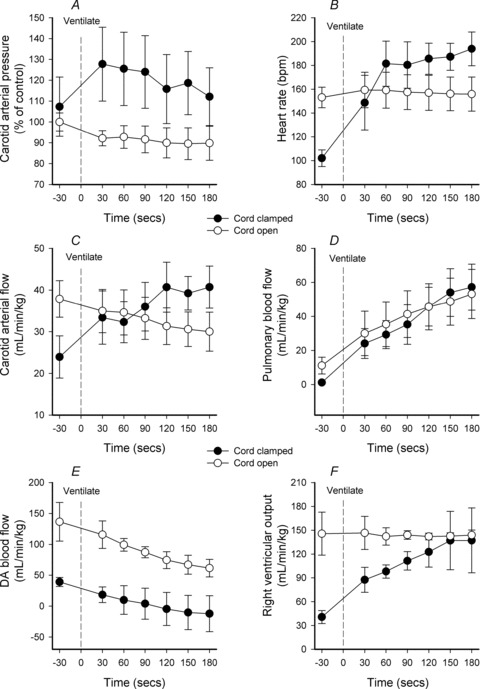
A–F, changes in mean (±SEM) carotid arterial blood pressure (A), heart rate (B), carotid arterial blood flow (C), pulmonary blood flow (D), ductus arteriosus (DA) blood flow (E) and right ventricular output (F) in response to ventilation onset (indicated by dotted line). Lambs either had an open, intact umbilical cord (open circles) or had a clamped umbilical cord (filled circles).
PPA and PBF
In Clamp 1st lambs, ventilation increased PPA (from 41.2 ± 4.2 to 46.5 ± 3.7 mmHg) and PBF (from 1.2 ± 0.7 to 24.1 ± 8.8 ml min−1 kg−1) after 30 s and PBF continued to increase to 45.7 ± 11.3 ml min−1 kg−1 after 120 s (Fig. 4). In Vent 1st lambs, ventilation caused a gradual decrease in PPA (from 40.0 ± 3.8 to 34.9 ± 0.8 mmHg at 120 s), whereas PBF increased from 11.1 ± 4.9 to 45.7 ± 13.5 ml min−1 kg−1 at 120 s after ventilation onset (Fig. 4).
DA blood flow and RVO
In both Clamp 1st and Vent 1st lambs, ventilation caused a gradual and similar reduction in DA blood flow although absolute values were very different between groups (Fig. 4). DA blood flow was reduced from 136.6 ± 31.3 and 39.1 ± 7.3 ml kg−1 min−1 to 74.5 ± 13.6 and −4.6 ± 26.8 ml min−1 kg−1 at 120 s after ventilation onset in Vent 1st and Clamp 1st lambs, respectively. In contrast, the changes in RVO were very different between groups (Fig. 4). Importantly, ventilation did not alter RVO in Vent 1st lambs (144.1 ± 5.4 ml min−1 kg−1) but markedly increased RVO in Clamp 1st lambs from 40.7 ± 8.2 to 122.7 ± 19.3 ml min−1 kg−1 at 120 s after ventilation onset.
Temporal changes in cardiovascular function after birth
Initiating ventilation before cord clamping had beneficial affects on cardiovascular function for at least 30 min after birth (Figs 5 and 6). In Clamp 1st lambs, cord clamping increased PCA, PPA and CaBF within 4–5 heart beats (Fig. 3). These parameters remained elevated for 30 s, were then reduced at 120 s before they rapidly (within 30–60 s) increased again following ventilation onset (Fig. 5) where they remained for at least 30 min after birth (Fig. 6). In contrast, PCA, PPA and CaBF remained relative constant in Vent 1st lambs throughout the 30 min study period after birth (Figs 5 and 6).
Figure 5. Time course of changes in Vent 1st and Clamp 1st lambs.
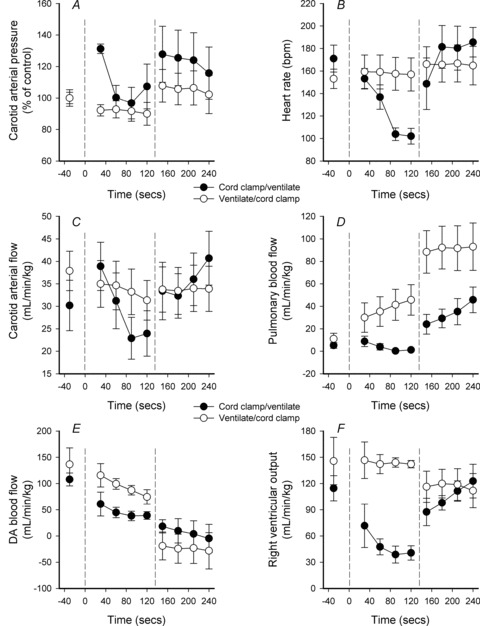
A–F, time course for changes in mean (±SEM) carotid arterial blood pressure (A), heart rate (B), carotid arterial blood flow (C), pulmonary blood flow (D), ductus arteriosus (DA) blood flow (E) and right ventricular output (F) from birth in Vent 1st (open circles) and Clamp 1st lambs (filled circles). The left dotted line (at time 0) indicates the time of cord clamping in Clamp 1st lambs and the time of ventilation onset in Vent 1st lambs. The right dotted line indicates the time of ventilation onset in Clamp 1st lambs and the time of cord clamping in Vent 1st lambs.
Figure 6. Time course of changes in for the first 30 min after birth in Vent 1st and Clamp 1st lambs.
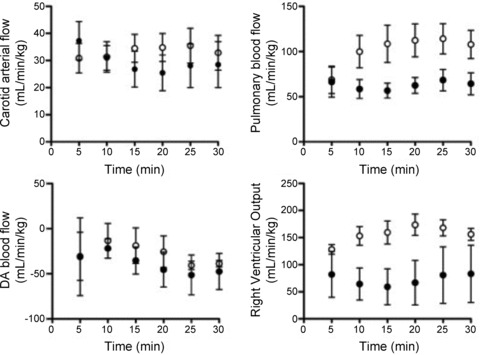
A–D, time course for changes in mean (±SEM) carotid arterial blood flow (A), pulmonary blood flow (B), ductus arteriosus (DA) blood flow (C) and right ventricular output (D) for the first 30 min after birth in Vent 1st (open circles) and Clamp 1st lambs (filled circles).
Although ventilation onset increased PBF to a similar degree in both Vent 1st and Clamp 1st lambs, initiating ventilation before cord clamping resulted in higher PBF levels for the first 4 min after birth (Fig. 5). At 5 min after birth, PBF values were similar between groups but by 10 min, PBF values were again higher in Vent 1st lambs and remained higher at 30 min after birth (Fig. 6). In contrast to Vent 1st lambs, RVO was markedly reduced by cord clamping in Clamp 1st lambs and remained reduced until ventilation began. RVO then markedly increased in Clamp 1st lambs and was not different from Vent 1st lambs 3–4 min after birth (Fig. 5). However, between 10 and 30 min after birth, RVO was significantly lower in Clamp 1st lambs than in Vent 1st lambs (Fig. 6).
Discussion
Current ILCOR guidelines recommend that cord clamping should be delayed by 1 min in babies not requiring resuscitation, but state that there is insufficient evidence to support or refute a recommendation to delay clamping in babies requiring resuscitation (Perlman et al. 2010). Our study shows that delaying cord clamping until after ventilation begins could have marked beneficial effects in infants requiring respiratory support. We have detailed the cardiovascular changes caused by umbilical cord clamping and how initiating ventilation prior to cord clamping modifies these changes. Specifically, umbilical cord clamping caused a profound disturbance in cardiovascular function, resulting in a rapid (within 4 heart beats), transient (∼30 s) increase in PCA, PPA and CaBF, but all of these then decreased below pre-clamping levels over the next minute. Similarly, heart rates and RVO were markedly decreased within 120 s of cord clamping and remain reduced until ventilation began. In contrast, if umbilical cord clamping was delayed until after ventilation had commenced, these large changes in heart rate, arterial pressures and flows were greatly reduced, resulting in a much more stable cardiovascular transition after birth. Furthermore, when ventilation precedes cord clamping, a secondary increase in PBF and RVO persists for at least 30 min after birth.
The increases (∼30%) in PCA caused by umbilical cord clamping in the absence of ventilation has been described previously (Dawes, 1968) and are accompanied by increases in PPA. These result from an increase in systemic arterial resistance, caused by the loss of the low resistance placental vascular bed when the cord is clamped. In contrast, ventilation prior to cord clamping reduces the increase in PCA and PPA from ∼30% to <10% (Figs 1–3 and 5). The probable reason for this reduction is that ventilation reduces PVR before cord clamping and as the two circulations (systemic and pulmonary) are joined via the DA, the low PVR helps to keep the overall circulatory resistance low upon cord clamping. As a result, the pulmonary circulation can immediately become an alternative route for systemic arterial flow when systemic resistance increases with cord clamping. Indeed, in ventilated (Vent 1st) lambs, umbilical cord clamping reduced right to left flow through the DA by over 95% within 1 heart beat and had reversed within 4 heat beats to become mainly left to right (−10.5 ± 22.9 ml min−1 kg−1) (Fig. 3). Thus, within 4 heat beats of cord clamping, the contribution of RVO to systemic arterial flow had virtually ceased in Vent 1st lambs and was redirected into the pulmonary circulation. In addition, flow from the systemic circulation began to contribute to PBF due to left to right shunting through the DA, as previously described (Crossley et al. 2009). However, the overall increase in PBF was relatively small because RVO was simultaneously reduced (Fig. 2). In contrast, if the cord was clamped before ventilation (Clamp 1st lambs), DA flow remained right to left because PVR remained high and downstream resistance in the systemic circulation was still lower despite the loss of the low resistance placental circulation. The consequence of this is a much more severe decrease in RVO following cord clamping due to the increase in afterload and the loss of preload.
When the cord was clamped before ventilation began, the initial increase in PCA was associated with a 25% increase in CaBF, mainly due to a large increase in diastolic flow (Fig. 3). These increases were quickly (within 30–60 s) followed by decreases in both PCA and CaBF (Figs 2 and 5), demonstrating that, at least over this short time, blood flows distal to the common carotid artery, including the cerebral circulation, were pressure passive. Thus, following cord clamping in the absence of ventilation, the cerebral circulation was exposed to large and rapid changes in flow caused by large changes in PCA (Fig. 5). This may increase the risk of cerebral haemorrhage, particularly in preterm infants (<30 weeks) who are commonly unable to maintain constant CaBF with changing systemic arterial pressures (Greisen, 2005; Soul et al. 2007). These rapid changes in CaBF and arterial pressures associated with cord clamping were minimised by ventilating lambs first (Fig. 5).
In the absence of ventilation, the decrease in PCA and CaBF 90–120 s after cord clamping probably resulted from a decrease in LVO caused by the loss of umbilical venous return, which is the main source of LV preload in the fetus (Rudolph, 1979). Although we were unable to estimate LVO, because the degree and direction of shunting through the foramen ovale was unknown, LVO must have been reduced in Clamp 1st lambs. This is because PBF remained low, heart rates were reduced and as RVO flow was reduced but flow through the DA was not reversed (i.e. not left to right), LVO must also have been reduced before ventilation began (Figs 2 and 5). Immediately following ventilation onset in Clamp 1st lambs, PCA increased and CaBF almost doubled within 120 s, most probably because LVO had increased, as previously described (Teitel et al. 1990). The increase in LVO probably results from increased PBF, which increases pulmonary venous return and LV preload (Figs 4 and 5).
As the umbilical circulation receives 30–50% of cardiac output, cord clamping before ventilation must immediately reduce venous return (preload) to both ventricles by a similar degree. Combined with the increased afterload caused by the increased systemic arterial resistance, it is not surprising that both LVO and RVO rapidly decreased after cord clamping. The effects of the increased afterload were immediate, increasing PCA within 1–4 heart beats (Figs 1 and 3), whereas the effects of reduced venous return on preload appeared to take longer, with the decrease in CaBF and PCA becoming evident by 60–120 s (Figs 2 and 5). It is possible that a baroreceptor-mediated response also contributed to the reductions in heart rates and blood pressures. When the cord was clamped before ventilation, it appeared that CaBF and PCA were beginning to stabilise by 120 s after clamping, but they rapidly increased again with ventilation onset (Fig. 5). This was undoubtedly due to the increase in PBF associated with ventilation, resulting in an increase in LV preload and LVO. Surprisingly RVO also rapidly increased in Clamp 1st lambs following ventilation onset, despite a simultaneous increase in afterload (PPA; Fig. 4). This may result from increased RV preload caused by left to right shunting through the foramen ovale. Although it is commonly believed that the foramen ovale closes when left atrial pressure exceeds right atrial pressure after birth (Dawes et al. 1955; Rudolph, 1979; Teitel et al. 1987), left to right shunting through the foramen ovale is commonly observed by Doppler ultrasound in human infants (Evans & Kluckow, 1996).
The effects of commencing ventilation before cord clamping on PBF and RVO persisted for up to 30 min after birth (Fig 6). As the higher PBF did not result from increased left to right flow through DA, it must have resulted from the increase in RVO. This indicates that LVO was also greater in Vent 1st than in Clamp 1st lambs over this time, otherwise left to right shunting through the DA would have been reduced or the flow would have reverted to right to left, if RVO was much greater than LVO. The reason for the persisting higher RVO in Vent 1st lambs is unknown, but likely possibilities include: (1) increased preload resulting from an increased systemic venous return arising from a higher LVO, (2) increased preload due to increased left to right shunting through the foramen ovale and (3) better preserved myocardial function in hearts not exposed to a transiently high afterload.
Delayed cord clamping also increases RVO and reduces the risk of low superior vena cava (SVC) flow states in preterm infants within 48 h of birth (Meyer & Mildenhall, 2012; Sommers et al. 2012). This is potentially important because low SVC flows are associated with intraventricular haemorrhage (IVH) in preterm infants and worse neurodevelopmental outcomes at 3 years of age (Kluckow & Evans, 2000, 2001; Hunt et al. 2004). Clinical trials have shown that delayed cord occlusion reduces the incidence of IVH in very low birth weight infants (Rabe et al. 2008) and improves motor development at 7 months (Mercer et al. 2010). However, no attempt was made in those trials to record time of first breath or to initiate ventilation before cord clamping. We suggest that the benefits of delayed cord clamping result from the improved cardiovascular stability afforded by commencing ventilation before cord clamping and not from delayed cord clamping per se. Unfortunately, however, we did not assess cerebral haemorrhage in this study.
Clinical trials examining the effects of delayed cord clamping in preterm infants are continuing and many cardiopulmonary benefits have already been reported (Baenziger et al. 2007; Kugelman et al. 2007; Rabe et al. 2008; Mercer et al. 2010; Sommers et al. 2012; Takami et al. 2012). However, based on our findings, the mechanisms for these improvements may not be due to increased neonatal blood volumes as proposed, but due to a more stable haemodynamic transition. Unfortunately, we did not measure blood volumes before and after cord clamping to determine whether blood volumes increased in Vent 1st lambs. In infants, a delay of 30–45 s can increase blood volumes 8–24% (Narenda et al. 1998) whereas 3 min delays can increase blood volumes by ∼50% (Chaparro & Lutter, 2007). Although not statistically different, haemoglobin levels tended to be higher in Vent 1st lambs at 30 min, which may indicate increased fetal blood transfer. Lambs were placed at the mid-abdominal level of the ewe to minimise blood transfer into the fetus, but it is unclear how this would affect blood transfer, as the effects of gravity are unclear. Further studies are warranted.
In conclusion, we have demonstrated that ventilation prior to umbilical cord occlusion improved cardiovascular function and stability during the immediate transition to neonatal life after birth in preterm lambs. We showed that the initiation of ventilation prior to cord clamping mitigated most of the adverse cardiovascular responses to cord clamping, indicating that the decrease in PVR prior to cord clamping has a profound influence on cardiovascular function after birth. We speculate that some of the benefits of delayed cord clamping are due to the circulatory transition initiated by air breathing as opposed to an increase in blood volume. In view of these findings, it is important that the timing of ventilation onset is recorded in current trials examining the benefits of delayed cord clamping and that a Vent 1st strategy is further evaluated in an appropriately designed trials.
Translational perspective
Numerous clinical trials have indicated that delayed umbilical cord clamping improves neonatal cardiovascular function, although it is commonly assumed that the benefits result from enhanced placental to infant blood transfer. Indeed, delays of 30–45 s reportedly increase neonatal blood volume by 8–24% (Narenda et al. 1998), whereas delays of up to 3 min can result in much larger increases (Chaparro & Lutter 2007). The reported cardiovascular benefits in the neonate include higher haematocrits, reduced blood transfusions, improved blood pressure and superior vena cava flow, increased blood volumes, improved respiratory function, reduced incidence of necrotising enterocolitis, reduced intraventricular haemorrhage and better cerebral oxygenation. However, there is much debate as to the length of time after birth that umbilical cord clamping should be delayed. This study provides new evidence to indicate a major benefit of delayed cord clamping is improved cardiovascular stability during the fetal to neonatal transition. The increased stability results from an increase in PBF before the cord is clamped and the umbilical venous contribution to LV proload is lost. This allows PBF to immediately replace umbilical venous return as the primary source of LV preload upon cord clamping. Thus, it is evident that the timing of delayed cord clamping should not be determined by an arbitrarily defined period of time, but should be based on whether physiological changes, such as the onset of pulmonary ventilation, have been established in the infant.
Acknowledgments
The authors would like to acknowledge the technical support of Alison Moxham, Valerie Zahra and Karyn Rodgers for their assistance with all experimental procedures described here. This research was supported by NHMRC Program Grant (606789), NH&MRC Research Fellowships (G.R.P. 1026890 and S.B.H. 545921), Rebecca L. Cooper Medical Research Foundation Fellowship (G.R.P.) and the Victorian Government's Operational Infrastructure Support Program. A.B.t.P. is recipient of a Veni-grant, The Netherlands Organisation for Health Research and Development (ZonMw), part of the Innovational Research Incentives Scheme Veni-Vidi-Vici, project number 91612027. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Glossary
- AaDO2
alveolar arterial difference in oxygen
- CaBF
carotid blood flow
- DA
ductus arteriosus
- ILCOR
International Liaison Committee on Resuscitation
- IVH
intraventricular haemorrhage
- (LPA
left main pulmonary artery
- LV
left ventricle
- LVO
left ventricular output
- PBF
pulmonary blood flow
- PCA
carotid arterial blood pressure
- PIP
peak inflation pressure
- PPA
carotid arterial blood pressure
- PVR
pulmonary vascular resistance
- RV
right ventricle
- RVO
right ventricular output
- SVC
superior vena cava
Author contributions
All animal and laboratory experiments were conducted at The Ritchie Centre, Monash University, Clayton, Victoria. Conception and design of the experiments: S.B., B.A., K.C., G.P., S.H. Collection, analysis and interpretation of data: S.B., B.A., E.W., K.C., A.G., M.K., A.tP., C.M., G.P., S.H. Drafting the article or revising it critically for important intellectual content: S.B., B.A., E.W., K.C., A.G., M.K., A.tP., C.M., G.P., S.H. All authors approved the final version of the manuscript.
References
- Alcorn DG, Adamson TM, Maloney JE, Robinson PM. A morphologic and morphometric analysis of fetal lung development in the sheep. Anat Rec. 1981;201:655–667. doi: 10.1002/ar.1092010410. [DOI] [PubMed] [Google Scholar]
- Baenziger O, Stolkin F, Keel M, von Siebenthal K, Fauchere JC, Das Kundu S, Dietz V, Bucher HU, Wolf M. The influence of the timing of cord clamping on postnatal cerebral oxygenation in preterm neonates: a randomized, controlled trial. Pediatrics. 2007;119:455–459. doi: 10.1542/peds.2006-2725. [DOI] [PubMed] [Google Scholar]
- Chaparro CM, Lutter C. Beyond survival: integrated delivery care practices for longterm maternal and infant nutrition, health and development. Washington, DC: Pan American Health Organization; 2007. [Google Scholar]
- Crossley KJ, Allison BJ, Polglase GR, Morley CJ, Davis PG, Hooper SB. Dynamic changes in the direction of blood flow through the ductus arteriosus at birth. J Physiol. 2009;587:4695–4704. doi: 10.1113/jphysiol.2009.174870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes GS. Foetal and neonatal physiology: a comparative study of the changes at birth. Chicago: Year Book Medical Publishers, Inc; 1968. [Google Scholar]
- Dawes GS, Mott JC, Widdicombe JG. Closure of the foramen ovale in newborn lambs. J Physiol. 1955;128:384–395. doi: 10.1113/jphysiol.1955.sp005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes GS, Mott JC, Widdicombe JG, Wyatt DG. Changes in the lungs of the new-born lamb. J Physiol. 1953;121:141–162. doi: 10.1113/jphysiol.1953.sp004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Toro J, Louis PT, Goddard-Finegold J. Cerebrovascular regulation and neonatal brain injury. Pediatr Neurol. 1991;7:3–12. doi: 10.1016/0887-8994(91)90098-6. [DOI] [PubMed] [Google Scholar]
- Evans N, Kluckow M. Early determinants of right and left ventricular output in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;74:F88–94. doi: 10.1136/fn.74.2.f88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore MM, Stone BS, Shepard JA, Czosnyka M, Easley RB, Brady KM. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J Perinatol. 2011;31:722–729. doi: 10.1038/jp.2011.17. [DOI] [PubMed] [Google Scholar]
- Greisen G. Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev. 2005;81:423–428. doi: 10.1016/j.earlhumdev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hooper SB, Harding R. Role of aeration in the physiological adaptation of the lung to air-breathing at birth. Curr Respir Med Rev. 2005;1:185–195. [Google Scholar]
- Hunt RW, Evans N, Rieger I, Kluckow M. Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J Pediatr. 2004;145:588–592. doi: 10.1016/j.jpeds.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2000;82:F188–194. doi: 10.1136/fn.82.3.F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluckow M, Evans N. Low systemic blood flow in the preterm infant. Semin Neonatol. 2001;6:75–84. doi: 10.1053/siny.2000.0035. [DOI] [PubMed] [Google Scholar]
- Kugelman A, Borenstein-Levin L, Riskin A, Chistyakov I, Ohel G, Gonen R, Bader D. Immediate versus delayed umbilical cord clamping in premature neonates born< 35 weeks: a prospective, randomized, controlled study. Am J Perinatol. 2007;24:307–315. doi: 10.1055/s-2007-981434. [DOI] [PubMed] [Google Scholar]
- Mercer JS, Vohr BR, Erickson-Owens DA, Padbury JF, Oh W. Seven-month developmental outcomes of very low birth weight infants enrolled in a randomized controlled trial of delayed versus immediate cord clamping. J Perinatol. 2010;30:11–16. doi: 10.1038/jp.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MP, Mildenhall L. Delayed cord clamping and blood flow in the superior vena cava in preterm infants: an observational study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F484–486. doi: 10.1136/adc.2010.199703. [DOI] [PubMed] [Google Scholar]
- Narenda A, Beckett CAT, Kyle E. Is it possible to promote placental transfusion at preterm delivery. Pediatr Res. 1998;44:453. [Google Scholar]
- Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, Guinsburg R, Hazinski MF, Morley C, Richmond S, Simon WM, Singhal N, Szyld E, Tamura M, Velaphi S. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122:S516–538. doi: 10.1161/CIRCULATIONAHA.110.971127. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Hooper SB. Role of intra-luminal pressure in regulating PBF in the fetus and after birth. Curr Pediatr Rev. 2006;2:287–299. [Google Scholar]
- Polglase GR, Hooper SB, Kluckow M, Gill AW, Harding R, Moss TJM. Cardiopulmonary hemodynamics in lambs after preterm birth: are they influenced by sex. Reprod Fertil Dev. 2012;24:510–516. doi: 10.1071/RD11121. [DOI] [PubMed] [Google Scholar]
- Rabe H, Reynolds G, Diaz-Rossello J. A systematic review and meta-analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology. 2008;93:138–144. doi: 10.1159/000108764. [DOI] [PubMed] [Google Scholar]
- Rudolph AM. Fetal and neonatal pulmonary circulation. Am Rev Respir Dis. 1977;115:11–18. doi: 10.1164/arrd.1977.115.S.11. [DOI] [PubMed] [Google Scholar]
- Rudolph AM. Fetal and neonatal pulmonary circulation. Annu Rev Physiol. 1979;41:383–395. doi: 10.1146/annurev.ph.41.030179.002123. [DOI] [PubMed] [Google Scholar]
- Sommers R, Stonestreet BS, Oh W, Laptook A, Yanowitz TD, Raker C, Mercer J. Hemodynamic effects of delayed cord clamping in premature infants. Pediatrics. 2012;129:e667–672. doi: 10.1542/peds.2011-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, Disalvo DN, Moore M, Akins P, Ringer S, Volpe JJ, Trachtenberg F, du Plessis AJ. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61:467–473. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- Takami T, Suganami Y, Sunohara D, Kondo A, Mizukaki N, Fujioka T, Hoshika A, Akutagawa O, Isaka K. Umbilical cord milking stabilizes cerebral oxygenation and perfusion in infants born before 29 weeks of gestation. J Pediatr. 2012;161:742–747. doi: 10.1016/j.jpeds.2012.03.053. [DOI] [PubMed] [Google Scholar]
- Teitel DF, Iwamoto HS, Rudolph AM. Effects of birth-related events on central blood flow patterns. Pediatr Res. 1987;22:557–566. doi: 10.1203/00006450-198711000-00017. [DOI] [PubMed] [Google Scholar]
- Teitel DF, Iwamoto HS, Rudolph AM. Changes in the pulmonary circulation during birth-related events. Pediatr Res. 1990;27:372–378. doi: 10.1203/00006450-199004000-00010. [DOI] [PubMed] [Google Scholar]


