Abstract
The role of nitric oxide (NO) in regulating lymphatic contractile function and, consequently, lymph flow has been the subject of intense study. Despite this, the precise effects of NO on lymphatic contractile activity remain unclear. Recent hypotheses posit that basal levels of endogenous NO increase lymphatic contraction strength as a consequence of lowering frequency (i.e. positive lusitropy), whereas higher agonist-evoked concentrations of NO exert purely inhibitory effects on contractile function. We tested both hypotheses directly by isolating and cannulating collecting lymphatic vessels from genetically modified mice for ex vivo study. The effects of basal NO and agonist-evoked NO were evaluated, respectively, by exposing wild-type (WT), endothelial NO synthase (eNOS)−/− and inducible NO synthase (iNOS)−/− lymphatic vessels to controlled pressure steps followed by ACh doses. To compare with pharmacological inhibition of eNOS, we repeated both tests in the presence of l-NAME. Surprisingly, genetic removal of basal NO enhanced contraction amplitude significantly without increasing contraction frequency. Higher levels of NO production stimulated by ACh evoked dilation, decreased tone, slowed contraction frequency and reduced fractional pump flow. We conclude that basal NO specifically depresses contraction amplitude, and that greater NO production then inhibits all other aspects of contractile function. Further, this work demonstrates definitively that mouse collecting lymphatic vessels exhibit autonomous, large-amplitude contractions that respond to pressure similarly to collecting lymphatics of other mammalian species. At least in the peripheral lymphatic vasculature, NO production depresses contractile function, which influences lymph flow needed for fluid regulation, humoral immunity and cancer metastasis.
Key points
Stimulation of nitric oxide (NO) production by lymphatic endothelium was originally thought to inhibit lymphatic contractile function.
However, recent studies have suggested that basal NO paradoxically increases the strength of contractions as a consequence of decreasing contraction frequency.
Here, we tested that hypothesis directly for the first time by establishing a new preparation where lymphatic vessels were isolated from transgenic mice and retained robust contractile activity.
Genetic removal of basal NO using endothelial NO synthase−/− mice led to an increase in contraction strength without increasing contraction frequency, opposing this hypothesis. In contrast, higher levels of NO production stimulated by ACh inhibited lymphatic contractile function in wild-type and inducible NO synthase−/− mice, consistent with previous studies.
Our results show that NO functions in the peripheral lymphatic vasculature to depress contractile function, which will ultimately depress lymph flow that determines fluid homeostasis, humoral immunity and cancer metastasis.
Introduction
Collecting lymphatic vessels must contract spontaneously, much like the heart, in order to generate pressure to propel lymph along the lymphatic vascular network to the lymph nodes. Thus, a detailed understanding of collecting lymphatic vessel contractile function is required before pharmacological approaches targeting lymph flow can be employed for the treatment of edema, autoimmune diseases or cancer metastasis. Several signalling molecules have been identified that modulate the spontaneous contractions of collecting lymphatic vessels, altering lymph flow either positively or negatively. The most widely studied of these is nitric oxide (NO), which has been examined recently as a possible topical treatment for envenomation (Saul et al. 2011).
Recent work has suggested that the actions of NO on lymphatic contractile activity are context dependent, in stark contrast to its consistent inhibition of basal tone in arterioles. The current working hypothesis is that large concentrations of NO evoked by agonists or continuous unidirectional flow inhibit contractile activity (Yokoyama & Ohhashi, 1993; Gashev et al. 2002), while lower (basal) levels of NO paradoxically increase contraction strength, or amplitude (Hagendoorn et al. 2004; Gasheva et al. 2006; Bohlen et al. 2009, 2011; Liao et al. 2011; Nagai et al. 2011; Kesler et al. 2012). Basal NO is defined here as the time-averaged level of NO produced in response to pulsatile flow generated by spontaneous contractions at a given pressure (Dixon et al. 2006), as NO has been shown to fluctuate periodically during individual contraction cycles (Bohlen et al. 2009, 2011). Collectively, interpretation of these results has led to the conclusion that basal NO increases contraction amplitude – relative to conditions of lower NO levels – by reducing the contraction frequency, thereby providing more time for the lymphangion to fill with fluid so that the next contraction becomes stronger (i.e. positive lusitropy). This hypothesis was originally formulated and tested for the isolated rat thoracic duct (Gasheva et al. 2006), the central lymphatic duct that possesses unique contractile and non-contractile regions specialized for pumping lymph into the bloodstream. In contrast, the aforementioned studies examined prenodal lymphatics in vivo under conditions where intralymphangion pressure and flow were unknown and uncontrolled (Hagendoorn et al. 2004; Bohlen et al. 2009, 2011; Liao et al. 2011). Importantly, pressure and flow exert profound and opposite effects on lymphatic contractile function that may confound the interpretation of in vivo observations (Scallan et al. 2012). Such interpretation is further limited by the use of non-specific NO synthase inhibitors (e.g. l-NAME), for which off-target or endothelium-independent effects have been demonstrated (Buxton et al. 1993; Suda et al. 2002; Murphy et al. 2007).
A direct way to test the effects of NO on collecting lymphatic contractile activity is to study mice in which the gene encoding endothelial NO synthase (eNOS) has been deleted. Using this approach circumvents many limitations of pharmacological tools, such as non-specific effects or lack of efficacy. Until now, genetic approaches have not been employed to study lymphatic contractile activity in isolated lymphatic vessels, where pressure and flow can be finely controlled. The reasons for this are mainly due to technical difficulties, but there has also been substantial controversy over whether or not lymphatic vessels in the mouse exhibit large-amplitude spontaneous contractions (Gashev et al. 2009, 2010), a general feature common to several other mammalian species that have been examined (McHale & Roddie, 1976; Johnston & Gordon, 1981; Zhang et al. 2007). Indeed, several groups still refer to lymphatic contractile behavior in the mouse as passive ‘pulses’ rather than active ‘contractions’ presumably due to an inability to measure vessel diameter accurately (Kwon & Sevick-Muraca, 2010; Zhou et al. 2010; Proulx & Detmar, 2012). Notably, only a single, recent study has demonstrated the possible existence of large-amplitude contractions of mouse collecting lymphatics in vivo (Liao et al. 2011).
Here we investigated the function of basal and stimulated NO production from lymphatic endothelium using an integrative approach combining physiology and genetics, in which murine popliteal collecting lymphatic vessels were removed from anaesthetized, transgenic mice and mounted on glass pipettes for ex vivo study. This model provides independent control over the hydrostatic pressures at either end of a collecting lymphatic vessel, flow through the vessel, and luminal/abluminal solutions. At the same time, lymphatic vessel internal diameter can be measured continuously over time, enabling comparison between wild-type (WT) and genetically-modified mouse vessels in a system where limited cell types are present (i.e. lymphatic endothelium and smooth muscle). We tested the prevailing hypotheses that: (1) basal NO production increases contraction amplitude due to a decreased contraction frequency; and (2) higher concentrations of stimulated NO production inhibit general contractile function. Experiments were performed using popliteal vessels from WT, eNOS−/−, and inducible NO synthase (iNOS)−/− mice, and tested the responses of single vessels to pressure steps and ACh, before and after treatment with l-NAME, a widely used pharmacological inhibitor of eNOS. We conclude that basal NO production depresses contraction amplitude without increasing frequency thus contradicting the prevailing hypothesis regarding the actions of basal NO, whereas higher concentrations of NO depress most common parameters of lymphatic pump function. Finally, this work demonstrates more definitively that mouse collecting lymphatic vessels exhibit large-amplitude (i.e. propulsive) spontaneous contractions – in the absence of external influences – that respond to pressure similarly to collecting lymphatics of other mammalian species and opens up the future application of transgenic models to quantitative studies of lymphatic physiology.
Methods
Mice
Male WT, eNOS−/− or iNOS−/− mice on the C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and studied between 5 and 7 weeks of age to limit variability in the contractile responses. All animal protocols were approved by the University of Missouri Animal Care and Use Committee, and conformed to the US Public Health Service policy for the humane care and use of laboratory animals (PHS Policy, 1996).
Vessel isolation procedure
Mice (18–25 g) were anaesthetized with pentobarbital sodium (Nembutal; 60 mg kg−1, i.p.) and placed in the prone position on a heating pad. A proximodistal incision (∼1 cm) was made in the skin beginning at the ankle of one leg to expose the superficial saphenous vein. Pilot experiments using India ink injected into the footpad confirmed that two afferent popliteal collecting lymphatic vessels ran consistently alongside this vein. After the connective tissue on either side of the vein was cleared away, the more superficial of two popliteal lymphatic vessels was then separated from the vein and placed in Krebs buffer containing albumin. Afterwards, the animal was killed by an overdose of pentobarbital sodium (200 mg kg−1, i.c.). Popliteal collecting lymphatic vessels (∼40–80 μm i.d.; 1–2 mm long) were pinned in a Sylgard dish and cleaned of connective and adipose tissue before transfer to a 3 mL chamber where the vessel was cannulated, pressurized, and trimmed of any remaining connective tissue prior to beginning the experimental protocol.
Solutions and chemicals
Krebs buffer contained (in mm): NaCl, 146.9; KCl, 4.7; CaCl2·2H2O, 2; MgSO4, 1.2; NaH2PO4·H2O, 1.2; NaHCO3, 3; sodium-Hepes, 1.5; d-glucose, 5 (pH 7.4 at 37°C). An identical buffer was prepared with the addition of 0.5% BSA. During cannulation the luminal and abluminal solutions contained Krebs with BSA, but during the experiment the abluminal solution was constantly exchanged with fresh Krebs lacking BSA. At the end of every experiment, a Ca2+-free physiological saline solution was used to obtain the passive diameter (Davis et al. 2011). All chemicals were obtained from Sigma (St Louis, MO, USA), with the exception of BSA (US Biochemicals; Cleveland, OH, USA), MgSO4 (Fisher Scientific; Pittsburgh, PA, USA) and sodium-Hepes (Fisher Scientific).
l-NAME was superfused at a concentration (1 × 10−4 m) that is routinely reported to inhibit eNOS activity maximally (Bohlen et al. 2009; Nagai et al. 2011). ACh dose–response curves were performed over a range of 1 × 10−9 m to 3 × 10−7 m, after pilot studies identified that a dose of 1 × 10−6 m produced a strong constriction followed by a maximal dilation that was difficult to wash out and repeat, requisite for this experimental design. Both l-NAME and ACh were diluted in Krebs buffer lacking BSA.
Pressure control and data acquisition
Vessels were cut to a length that contained only a single valve. Another group of vessels containing a single, complete lymphangion capable of generating its own systolic pressure-head to propel fluid was studied separately. To prevent continuous, but not pulsatile, flow through the vessel during the experiment, input and output pressures were kept equal, as it is well known that unidirectional flow elicits NO production from lymphatic endothelium (Gashev et al. 2002; Bohlen et al. 2009, 2011). Lymphatic vessel segments were tied onto two glass micropipettes (40 μm o.d.) mounted on a Burg-style V-track system (Duling et al. 1981). Polyethylene tubing (PE-190) attached to each micropipette was later connected to a valve that allowed pressure control to be switched between a manual reservoir and servo-controlled pumps (Davis et al. 2011). After the isolated vessel chamber was positioned on an inverted microscope, a suffusion line connected to a peristaltic pump maintained a constant superfusion of Krebs buffer at a rate of 0.4 ml min−1; a second line attached to the peristaltic pump in reverse orientation was used to remove excess buffer at the same rate. Input and output pressures were set briefly to the highest pressure used in this study (10 cmH2O) to facilitate the removal of axial slack, which minimized bowing of the vessel at high pressures that otherwise interfered with diameter tracking. Afterward, both pressures were lowered to 3 cmH2O to allow the vessel to warm up to 37°C and begin contracting. Spontaneous contractions usually began within the first 20 min of warm up and had stabilized completely by 1 h.
A computer was used to record the input and output pressure transducer signals, and displayed a video image of the vessel using a firewire camera (model A641FM Basler; Ahrensburg, Germany) at 30 Hz. A custom-written LabView program (National Instruments; Austin, TX, USA) measured the inner diameter (i.d.) of the vessel on the video image and recorded it as a function of time (Davis et al. 2011). All diameters reported here are inner diameters.
Protocols
Each vessel was equilibrated for approximately 1 h at 2–3 cmH2O and 37°C until a stable pattern of spontaneous contractions developed. Based on a pilot study, requirements for vessels to be included in this study were: (1) spontaneous contractions that developed within the 1 h equilibration period; (2) the development of spontaneous tone at the equilibration pressure; and (3) a contraction amplitude ≥30% of the end diastolic diameter at 1 cmH2O.
To test the response of each vessel to pressure, the input and output pressures were lowered together to 0.5 cmH2O. Both pressures were successively stepped to 1, 2, 3, 5, 7 and then 10 cmH2O for a total of seven cumulative pressure steps (Fig. 1B). Spontaneous contractions were recorded at each pressure for 2–6 min, a time sufficient to obtain at least three contractions at the lowest pressure of 0.5 cmH2O, at which some vessels did not contract (Supplemental Movie 1). After the last pressure step to 10 cmH2O, pressures were lowered to 3 cmH2O and the contraction pattern was allowed approximately 20 min to stabilize. To test the responses to stimulated NO production, an ACh dose–response curve was then performed over the range of 1 × 10−9 m to 3 × 10−7 m by adding a small, predetermined volume (≤90 μl) to the bath while pressure was held constant at 3 cmH2O (Fig. 1B). After a baseline period of 2 min, each dose was given in strict 2 min intervals to facilitate data analysis (Supplemental Movie 2). To evaluate the pharmacological inhibition of eNOS activity, the same vessel was treated for 20 min with a suffusion of an identical Krebs buffer that additionally contained 1 × 10−4 m l-NAME. After this period, the pressure steps and ACh dose–response protocols were repeated as before (Fig. 1B). At the end of the experiment, the vessel was suffused with Ca2+-free physiological saline solution for at least 20 min before passive diameters were recorded at every pressure used in the experiment in ascending order (Fig. 1B).
Figure 1. A video image of the isolated murine popliteal lymphatic vessel preparation (A) and experimental protocol designed to test the function of basal and stimulated NO production (B).
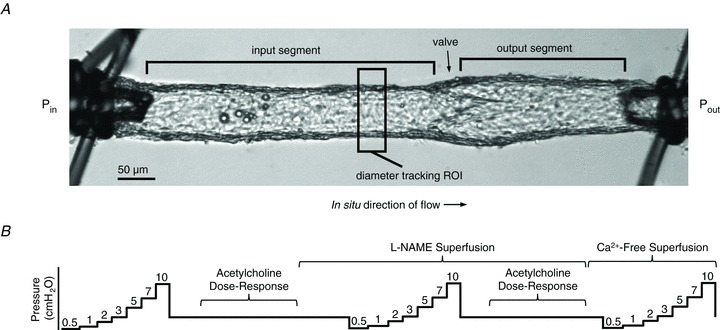
A, a typical popliteal collecting lymphatic vessel (∼60 μm i.d.) is tied onto two glass pipettes capable of pressure control. All vessels in this study contained either one or two valves (labelled). A custom computer program measured the inner diameter over time within the rectangular region of interest (ROI). B, a schematic diagram of the pressure steps and ACh dose–response tests before and after l-NAME application is shown. Pressure is plotted on the y-axis, while time is represented on the x-axis (not to scale).
Data analysis
After an experiment, custom-written analysis programs (LabView) were used to detect the end diastolic and end systolic diameters (EDD and ESD, respectively), contraction frequency (FREQ, computed on a contraction-by-contraction basis), contraction amplitude (AMP), and pipette pressures over time. These raw data were recorded during the experiment and used to calculate several commonly reported contraction parameters to assist with comparison to published data. From the pressure step protocols, the following were calculated and graphed:
| (1) |
| (2) |
| (3) |
| (4) |
where MaxD represents the maximum passive diameter obtained under Ca2+-free conditions at the same pressures at the end of every experiment. Raw EDD (μm) and FREQ (min−1) were additionally plotted. From the ACh dose–response protocols, the following were calculated and graphed:
| (5) |
| (6) |
| (7) |
| (8) |
where EDDavg, Toneavg and FREQavg represent the average EDD, Tone and FREQ during the 2 min baseline period prior to the addition of ACh to the bath. Additionally, EF and FPF were calculated as for the pressure step protocol.
Data obtained from the pressure step protocol were plotted as a function of pressure (cmH2O), while data from the dose–response protocol were plotted as a function of ACh concentration (m). Raw pressure/diameter traces were plotted against time using Igor Pro (Wavemetrics, Lake Oswego, OR, USA). To compare responses obtained within the same vessel (e.g. responses to l-NAME), a repeated-measures two-way ANOVA was used in conjunction with Bonferroni's post hoc test. To compare responses to pressure or ACh between vessels (e.g. of different genotypes), a two-way ANOVA was performed with Bonferroni's post hoc test to make multiple pairwise comparisons. For both pressure step and ACh protocols, Dunnett's multiple pairwise comparisons were used to test for significant differences from the first data point (at 0.5 cmH2O or 0 m ACh). Dose–response curves and functional responses to pressure were fit using curvilinear regression where appropriate. All data were tabulated using Excel, and statistical tests were performed using Prism 5 (Graphpad Software Inc., CA, USA), with significance for all tests set at P < 0.05 and reported as means (± SEM).
Results
A typical preparation used for this study is shown in Fig. 1A, where a single-valve popliteal collecting lymphatic vessel has been isolated, cleaned, and tied onto two glass micropipettes capable of independent pressure control. The successful isolation and cannulation of mouse collecting lymphatics that retain the ability to spontaneously generate high-velocity and large-amplitude contractions has not before been accomplished. The purpose of this study was to determine the effects of basal and ACh-stimulated NO production on collecting lymphatic contractile activity using a genetic approach, which allows these hypotheses to be tested directly for the first time.
Effects of basal NO production on murine lymphatic contractile activity
To determine the role of basal NO in lymphatic contractile activity, WT and eNOS−/− lymphatic vessels containing a single valve were allowed to contract spontaneously at a series of pressures. Representative traces are provided in Fig. 2. Both input and output pressures (top trace of Fig. 2A) were stepped simultaneously to 0.5, 1, 2, 3, 5, 7 and 10 cmH2O, while diameter (lower trace of Fig. 2A) was measured over time (x-axis). Because a pressure gradient for forward flow stimulates NO production from lymphatic endothelium (Gashev et al. 2002), holding input and output pressures equal allowed only pulsatile flow-induced NO production. Unlike previous reports of mouse lymphatic contraction strength in vivo (Ono et al. 2000; Liao et al. 2011), the AMP of this particular WT vessel at lower pressures (0.5–2 cmH2O) was approximately 50% of the active EDD (Fig. 2A). The same vessel was superfused with l-NAME for a 20 min period. This length of time (and concentration) was chosen based on prior work on rat collecting lymphatic vessels, where vessels were typically treated for 15–20 min prior to the commencement of experimental protocols (Shirasawa et al. 2000; Gasheva et al. 2006; Bohlen et al. 2009; Akl et al. 2011; Nagai et al. 2011). At least for this particular vessel, no visible differences were observed after l-NAME treatment, except for an insignificant tendency for tone to increase (Fig. 2B). A representative trace is shown for an eNOS−/− collecting lymphatic in Fig. 2C, which exhibited an even larger AMP than the WT vessel in Fig. 2A. When this eNOS−/− vessel was treated with l-NAME (Fig. 2D), no discernable differences were apparent, except for a decrease in FREQ. Notably, irregular pauses were sometimes observed in collecting lymphatic vessels after l-NAME treatment (of any genotype) and are especially evident in the diameter trace of Fig. 2D at a pressure of 7 cmH2O.
Figure 2. Raw traces of wild-type (WT; A and B) and endothelial nitric oxide synthase (eNOS)−/− (C and D) lymphatic contractions during the pressure step protocols in the absence and presence of l-NAME.
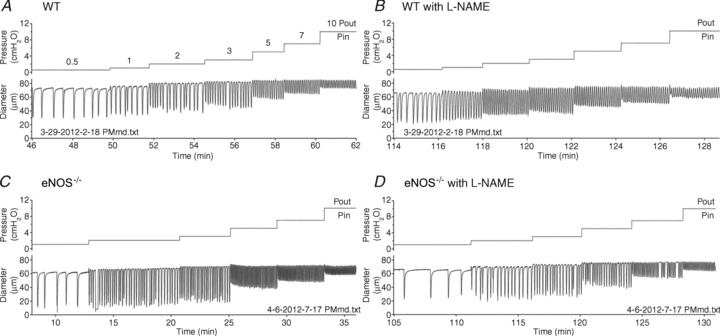
Input and output pressures (cmH2O) are displayed on the top trace and are overlaid because they were changed simultaneously from 0.5 to 1, 2, 3, 5, 7 and 10 cmH2O (labelled). The inner diameter (μm) was measured continuously over time and plotted on the bottom trace. Notice that the contraction amplitudes are approximately 50% of the resting EDD at low pressures. Corresponding traces from the same WT vessel are shown in A and B, and traces from a single eNOS−/− vessel are shown in C and D.
To gain insight into the effects associated with basal NO production, we plotted several common measures of collecting lymphatic contractile activity as a function of pressure before and after treatment with l-NAME. A total of 12 WT and 10 eNOS−/− single-valve vessels were studied, with their averaged contractile data plotted in Figs 3–5.
Figure 5. Effects of genetic deletion of endothelial nitric oxide synthase (eNOS) on lymphatic vessel contractile function.
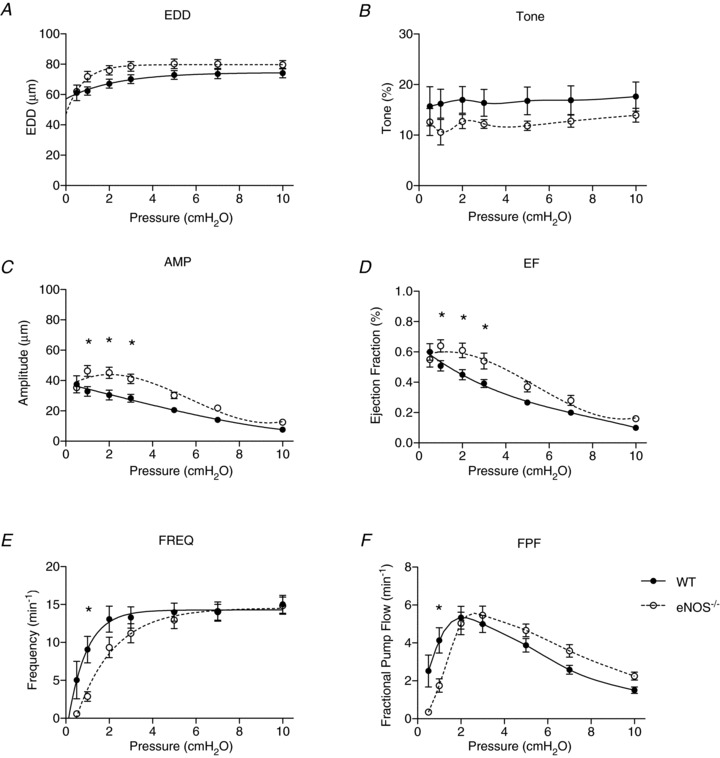
Lymphatic contractile function was directly compared between wild-type (WT; filled points) and eNOS−/− vessels (open points). End diastolic diameter (EDD; A), tone (B), contraction amplitude (AMP; C), ejection fraction (EF; D), contraction frequency (FREQ; E) and fractional pump flow (FPF; F) were compared between the two genotypes. All data are means (± SEM). When error bars appear missing, they are actually contained within the data points. Data in each graph were fit to a curve as appropriate, except for tone and FPF, which were necessarily splined. *Filled versus open data points differ significantly (P < 0.05).
Popliteal collecting lymphatics from WT mice exhibited a slight increase in EDD as pressure was elevated, which did not significantly differ from the EDD at the lowest pressure (Fig. 3A). Likewise, tone did not change significantly over the entire pressure range (Fig. 3B). As is well established for collecting lymphatics of other mammalian species, AMP declined significantly as pressure was raised (from 37.5 ± 5.6 μm to 7.7 ± 0.7 μm; Fig. 3C). Also as expected, FREQ increased significantly with pressure, from 5.0 ± 2.5 min−1 at 0.5 cmH2O to a plateau of 13.0 ± 1.7 min−1 at 2 cmH2O (Fig. 3E), although this relationship appears steeper than that of larger mammals, possibly reflecting an adaptation to lower pressures. The average FREQ at the highest pressure used in this study, 10 cmH2O, was 15.0 ± 1.2 min−1. Typically, EF is calculated from EDD and ESD, and represents the estimated fraction of lymph that is ejected during each contraction cycle, analogous to the cardiac EF. As pressure was increased, EF declined significantly and continuously, mirroring the AMP response to pressure (Fig. 3D). FPF is calculated as the product of EF and frequency, and serves as an index of theoretical lymph flow. FPF exhibited a biphasic response to pressure, increasing initially to a peak at 2 cmH2O and declining thereafter (Fig. 3F).
Figure 3. Effects of l-NAME on wild-type (WT) lymphatic vessel contractile function.
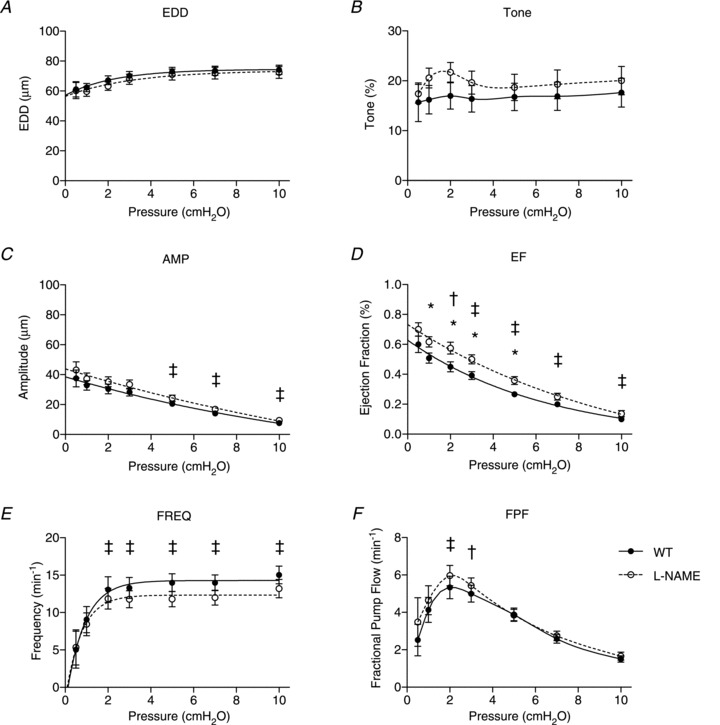
WT lymphatic contractile parameters are plotted against pressure on the x-axis, and include end diastolic diameter (EDD; A), tone (B), contraction amplitude (AMP; C), ejection fraction (EF; D), contraction frequency (FREQ; E) and fractional pump flow (FPF; F). Filled points indicate WT responses to pressure steps, while the open data points represent WT function in the presence of l-NAME (n= 12). All data are means (± SEM). When error bars appear missing, they are actually contained within the data points. Data in each graph were fit to a curve as appropriate, except for tone and FPF, which were necessarily splined. *Filled versus open data points differ significantly (P < 0.05); ‡filled and open data points both differ from their respective first data point at 0.5 cmH2O; †only filled data points differ significantly from the first data point at 0.5 cmH2O.
After treatment of the WT vessels with l-NAME to unmask the contribution of basal NO to contractile function, the same contractile parameters were reassessed at the same pressures (Fig. 3A–F, open circles). No significant differences were observed between the data from untreated (WT) and treated (+l-NAME) conditions with respect to EDD, tone, amplitude, frequency or FPF. Therefore, after l-NAME treatment the vessels exhibited the same responses to pressure as before with the exception of EF, which was significantly elevated over pressures ranging from 1 to 5 cmH2O.
Popliteal collecting lymphatics from eNOS−/− vessels were then exposed to the same protocol to determine their contractile activity as a function of pressure (Fig. 4A–F). In general, the eNOS−/− collecting lymphatics responded to increasing pressure similarly to WT vessels in that EDD rose slightly, but insignificantly (Fig. 4A), and tone did not change significantly at any pressure (Fig. 4B). AMP and EF (Fig. 4C and D) both increased somewhat from 0.5 to ∼2 cmH2O and then declined with pressure afterward (AMP dropped from 35.3 ± 1.5 μm to 12.5 ± 1.2 μm). FREQ increased gradually with pressure from 0.6 ± 0.3 min−1 to 14.9 ± 1.1 min−1 over the entire pressure range (Fig. 4E). FPF still exhibited a biphasic relationship with increasing pressure that peaked at 3 cmH2O and declined thereafter (Fig. 4F).
Figure 4. Effects of l-NAME on endothelial nitric oxide synthase (eNOS)−/− lymphatic vessel contractile function.
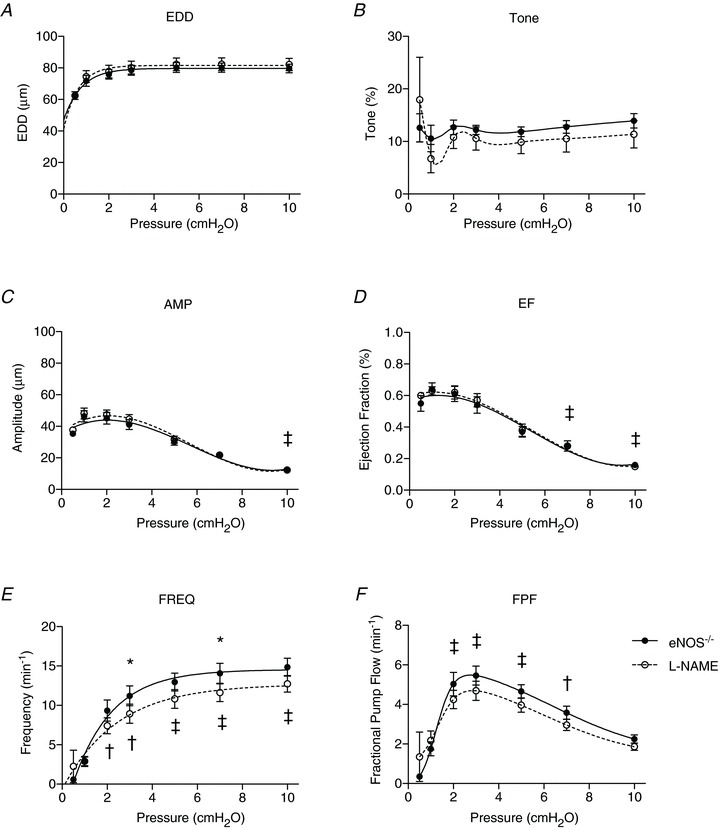
eNOS−/− lymphatic contractile parameters are plotted against pressure on the x-axis, and include end diastolic diameter (EDD; A), tone (B), contraction amplitude (AMP; C), ejection fraction (EF; D), contraction frequency (FREQ; E) and fractional pump flow (FPF; F). Filled points indicate eNOS−/− responses to pressure steps, while the open data points represent eNOS−/− function in the presence of l-NAME (n= 10). All data are means (± SEM). When error bars appear missing, they are actually contained within the data points. Data in each graph were fit to a curve as appropriate, except for tone and FPF, which were necessarily splined. *Filled versus open data points differ significantly (P < 0.05); ‡filled and open data points both differ from their respective first data point at 0.5 cmH2O; †only filled data points differ significantly from the first data point at 0.5 cmH2O.
The eNOS−/− vessels were treated with l-NAME for the same 20 min period before repeating the pressure steps; this served as a negative control as l-NAME was not expected to affect contractile function in vessels lacking eNOS unless this inhibitor displays non-specific actions in this preparation (Fig. 4A–F, open circles). As expected, no significant differences were found in EDD, tone, amplitude, EF or FPF when the responses were compared before and after l-NAME treatment. Unexpectedly, l-NAME significantly decreased FREQ at pressures of 3 and 7 cmH2O in eNOS−/− vessels, suggesting a non-specific action for this drug (Fig. 4E).
To determine the role of basal NO in the absence of chemical inhibitors, we compared the contractile function of WT and eNOS−/− vessels directly, in anticipation that this approach was more exact as the gene was completely deleted (Fig. 5A–F). As with treatment of the WT vessels with l-NAME, no significant differences were found with EDD, tone, FREQ or FPF. Also as expected based on the results in Fig. 3D–F, EF was significantly increased in the eNOS−/− vessels, but again only at low pressures (1–3 cmH2O; Fig. 5D). Interestingly, AMP was elevated significantly in the eNOS−/− vessels over the same pressure range as EF.
NO production stimulated by ACh depresses murine lymphatic contractile activity
To determine the effects of stimulated production of higher NO concentrations, the same single-valve WT and eNOS−/− vessels (n= 8 each) were exposed to six ACh doses before and after l-NAME treatment with hydrostatic pressure fixed at 3 cmH2O. Notably, this part of the protocol was performed after each series of pressure steps, so for the ACh responses in the presence of l-NAME the vessels had been exposed to the inhibitor for approximately 1 hour (refer to Fig. 1B).
Representative traces from ACh dose–responses of WT vessels are presented in Fig. 6A and B. In the absence of ACh, collecting lymphatic vessels (WT, eNOS−/− or iNOS−/−) contract stably such that the average EDD, amplitude and frequency do not change substantially for extended periods of time at a constant pressure. Thus, the increase in EDD and lower FREQ in Fig. 6A are due to ACh-stimulated NO production. At the highest dose of 3 × 10−7 m ACh, contractions returned in this particular vessel, indicative of muscarinic activation of the muscle layer. After this same vessel was treated with l-NAME (Fig. 6B), it did not respond to any dose of ACh in the range used, and the AMP appeared larger. Importantly, the vessel continued to gain tone over time as indicated by a constantly decreasing EDD. The same protocol was performed on eNOS−/− vessels, and representative traces are shown in Fig. 6C and D. In both traces ACh exerted no visible effect at any dose tested. In contrast to the WT vessel treated with l-NAME (Fig. 6B), the eNOS−/− vessel did not appear to gain tone over time, even when l-NAME was applied (Fig. 6D).
Figure 6. Raw traces of wild-type (WT; A and B) and endothelial nitric oxide synthase (eNOS)−/− (C and D) lymphatic vessel contractile activity during ACh dose–response tests, conducted in the absence and presence of l-NAME.
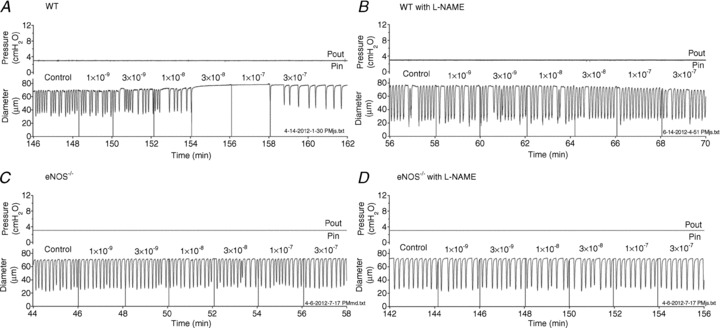
Input and output pressures, overlaid in the top trace, were fixed at 3 cmH2O for the entire protocol. The diameter traces are plotted over time, on the bottom. Vertical lines in the diameter traces mark the application of each dose (labelled) of ACh to the bath. Traces in A and B are from a single WT vessel, and traces in C and D are from a single eNOS−/− vessel.
The effects of ACh-stimulated NO production on WT and eNOS−/− vessels before and after l-NAME treatment were quantified in Figs 7–9 and plotted as a function of ACh concentration. As expected, progressively larger doses of ACh increasingly dilated the vessels so that EDD increased by 8.6 ± 2.2 μm at the highest dose (Fig. 7A). Tone was accordingly decreased by 10.3 ± 2.7% at the same dose (Fig. 7B). AMP and EF did not change significantly from baseline with any dose of ACh (Fig. 7C and D). FREQ decreased by approximately 50% over the entire range of doses (Fig. 7E). Primarily due to the reduction in FREQ, FPF also decreased significantly by ∼50% at the higher concentrations of ACh (Fig. 7F). In untreated WT vessels, the changes in EDD, tone, frequency, and FPF were significantly different from the baseline for the three highest doses of ACh.
Figure 9. Genetic deletion of endothelial nitric oxide synthase (eNOS) abolishes the ACh response of wild-type (WT) vessels.
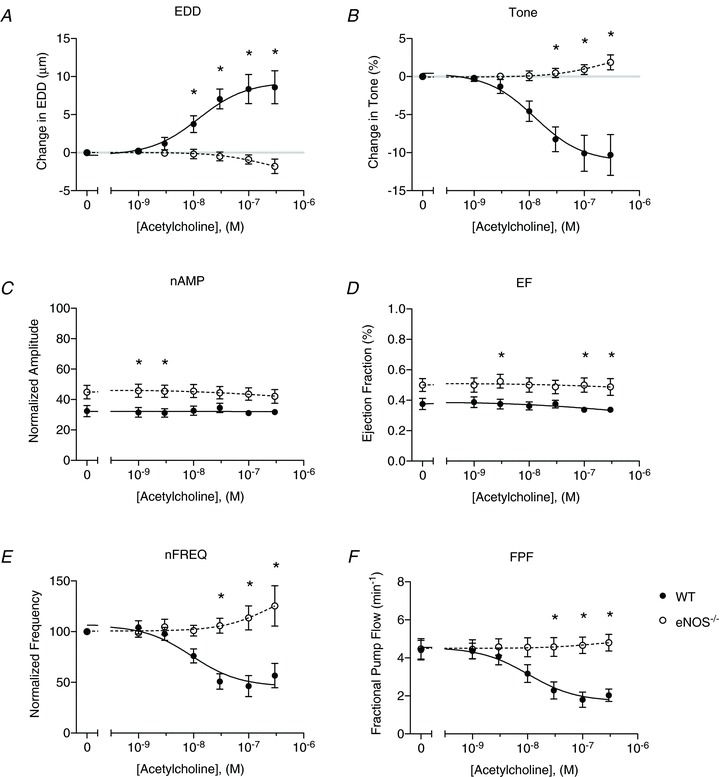
A direct comparison of lymphatic contractile function between WT (filled points) and eNOS−/− vessels (open points). A, end diastolic diameter (EDD); B, change in tone; C, normalized contraction amplitude (nAMP); D, ejection fraction (EF); E, normalized contraction frequency (nFREQ); and F, fractional pump flow (FPF). All data are means (± SEM). When error bars appear missing, they are actually contained within the data points. All data were fit with the same sigmoidal dose–response curve (3-parameter fit). *Filled versus open data points differ significantly (P < 0.05).
Figure 7. Effects of l-NAME on ACh-evoked NO production in wild-type (WT) vessels.
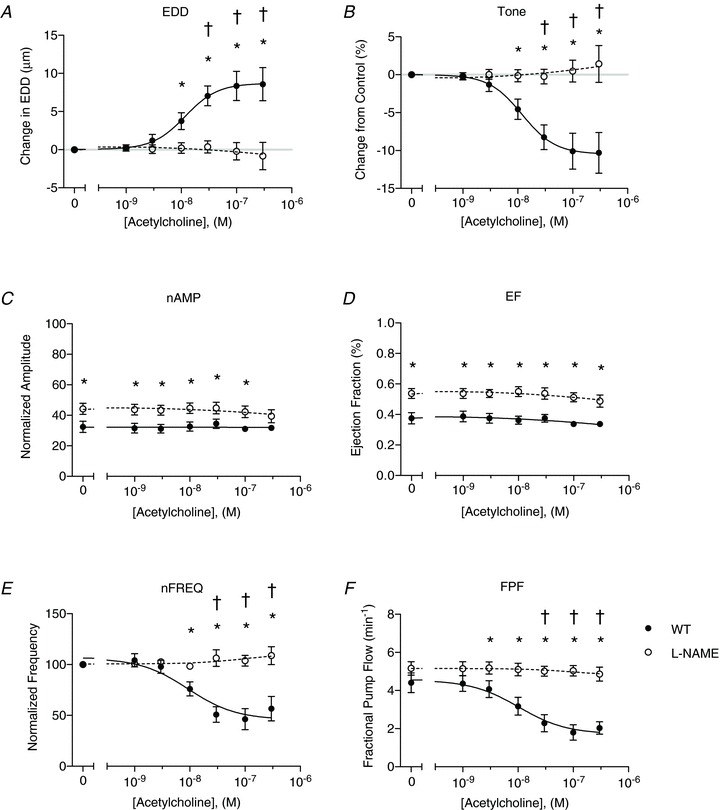
WT lymphatic contractile parameters are plotted as a function of ACh concentration (x-axis) and include the change in end diastolic diameter (EDD; A), change in tone (B), normalized contraction amplitude (nAMP; C), ejection fraction (EF; D), normalized contraction frequency (nFREQ; E) and fractional pump flow (FPF; F). Filled points indicate WT responses to ACh, while the open data points represent WT function in the presence of l-NAME (n= 8). All data are means (± SEM). When error bars appear missing, they are actually contained within the data points. All data were fit with the same sigmoidal dose–response curve (3-parameter fit). *Filled versus open data points differ significantly (P < 0.05); ‡filled and open data points both differ from their respective control data point at 0 m; †only filled data points differ significantly from the control data point at 0 m.
When the same WT vessels were then treated with l-NAME (Fig. 7A–F, open circles), the responses of EDD, tone, FREQ, and FPF to ACh were abolished completely. Further, AMP and EF were significantly elevated after l-NAME treatment, even at baseline, differing from the results in Fig. 3C where amplitude was not increased by l-NAME treatment to remove basal NO. This discrepancy most likely arises from the fact that the effects of l-NAME increased continuously over time, and that the ACh dose–response was performed after ∼1 h of l-NAME treatment, while the pressure step data were obtained after only 20 min of l-NAME treatment.
ACh dose–response curves were also performed on collecting lymphatics isolated from eNOS−/− mice as a control to determine whether deletion of this gene completely eliminated the response to ACh. Confirming that the ACh responses of WT vessels were due solely to endothelial production of NO, eNOS−/− collecting lymphatics did not respond to ACh with any significant changes relative to baseline (Fig. 8A–F). Some measures of lymphatic function, such as the change in EDD or tone, tended to deviate from the baseline at the highest dose of ACh, again suggesting muscarinic activation of the muscle layer. After the eNOS−/− vessels were treated with l-NAME, no significant changes were observed versus untreated conditions, as expected.
Figure 8. Effects of l-NAME on endothelial nitric oxide synthase (eNOS)−/− vessel responses to ACh.
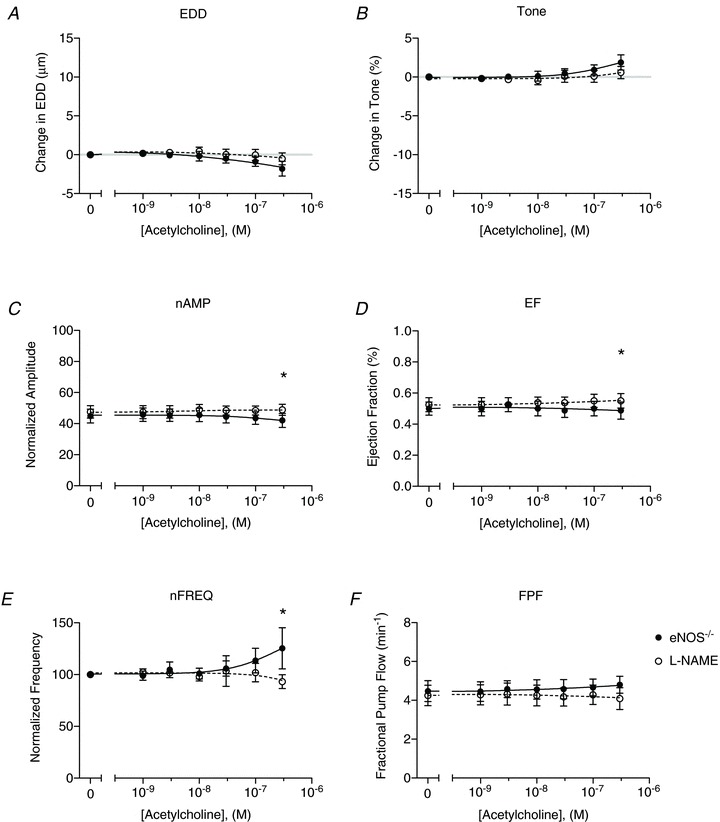
Lymphatic contractile parameters of eNOS−/− vessels in the absence (filled points) and presence (open points) of l-NAME are plotted as a function of ACh concentration (n= 8). A, end diastolic diameter (EDD); B, change in tone; C, normalized contraction amplitude (nAMP); D, ejection fraction (EF); E, normalized contraction frequency (nFREQ); and F, fractional pump flow (FPF). All data are means (± SEM). When error bars appear missing, they are actually contained within the data points. All data were fit with the same sigmoidal dose–response curve (3-parameter fit). *Filled versus open data points differ significantly (P < 0.05).
The effects of genetic removal of eNOS on the ACh dose–response curve were determined by comparing the WT and eNOS−/− data directly in Fig. 9. Deletion of the eNOS gene appeared to result in effects similar to pharmacological inhibition with l-NAME, as changes in EDD, tone, FREQ, and FPF were all completely abrogated. As with l-NAME treatment of WT vessels, AMP and EF of the eNOS−/− vessels were significantly elevated compared with WT.
Genetic deletion of iNOS recapitulates WT responses
As an added level of control, the same protocol (Fig. 1B) was performed on popliteal collecting lymphatics isolated from iNOS−/− mice (n= 7). These vessels were expected to yield similar data as the WT collecting lymphatics when basal and stimulated NO production were inhibited because iNOS is present in immune cells, which may have been present in limited numbers. Supplemental Fig. 1 contains representative traces from the same vessel exposed to the pressure step protocol (Supplemental Fig. 1A and B) and the ACh dose–response curves (Supplemental Fig. 1C and D). Similar to WT collecting lymphatics, iNOS−/− vessels exhibited a decrease in AMP and an increase in FREQ with increasing pressure (Supplemental Fig. 1A). After l-NAME treatment of this vessel (Supplemental Fig. 1B), no apparent differences were observed, except for an increase in FREQ at the lower pressures. Upon exposure to increasing doses of ACh (Supplemental Fig. 1C), a progressive increase in EDD accompanied a decrease in both AMP and FREQ. When the same doses were repeated in the presence of l-NAME, no substantial changes were observed (Supplemental Fig. 1D).
When the contractile parameters are summarized for the iNOS−/− lymphatics studied (Supplemental Fig. 2A–F), similar responses to pressure were obtained as for WT vessels. Briefly, EDD increased slightly, but not significantly, from the value at the lowest pressure of 0.5 cmH2O. Tone did not change significantly over the entire pressure range. AMP and EF both fell significantly as a function of pressure (AMP: from 32.1 ± 5.0 μm at 0.5 cmH2O to 10.4 ± 1.5 μm at 10 cmH2O). FREQ increased significantly with pressure from 3.2 ± 0.8 min−1 at 0.5 cmH2O to 13.0 ± 0.9 min−1 at 10.0 cmH2O. FPF remained biphasic as a function of pressure, with a peak occurring at approximately 3 cmH2O. When iNOS−/− vessels were treated with l-NAME to inhibit basal NO production, no significant differences were found with respect to EDD, tone, AMP or EF. At the two lowest pressures of 0.5 and 1 cmH2O, a significant increase in FREQ occurred, which corresponded to significant increases in FPF.
Supplemental Fig. 3 summarizes the contractile data from the ACh dose–response curves for the iNOS−/− lymphatics. As with the WT vessels, EDD increased significantly from baseline at the three highest doses of ACh, while tone, FREQ and FPF all declined significantly at the three highest doses of ACh. AMP and EF did not change significantly from baseline over the entire range of ACh doses. Upon treatment with l-NAME (again for ∼1 h), EDD, tone, FREQ and FPF did not respond to any dose of ACh. In the presence of l-NAME, AMP and EF did not change from baseline, but both were elevated significantly above the untreated iNOS−/− vessel data, indicating that the 20 min incubation with l-NAME prior to the pressure step protocols was insufficient to unmask the contribution of basal NO production in the iNOS−/− group.
Genetic removal of basal NO increases AMP and EF in single lymphangions
To ensure that the results regarding basal NO were not unique to vessel segments containing one valve, which have a limited ability to pump due to the open cannulation pipettes, we performed the same pressure step protocols on two-valve segments – otherwise known as single lymphangions – from six WT and nine eNOS−/− mice (Supplemental Figs 4–6). WT lymphangions treated with l-NAME for approximately 1 hour exhibited a significant decrease in EDD and a concomitant increase in tone over low pressures. Recall that EDD and tone of the single-valve WT vessels did not change after l-NAME treatment for 20 min, indicating a cumulative effect of l-NAME. No significant differences in AMP, EF or FREQ were obtained in WT lymphangions, similar to the WT vessels with one valve (Supplemental Fig. 4C–E). Only a significant increase in FPF at 0.5 cmH2O was observed, again largely consistent with the single-valve WT data in Fig. 3.
When eNOS−/− lymphangions were treated with l-NAME, no significant effects were detected for EDD, tone, AMP, EF or FPF (Supplemental Fig. 5), consistent with results from the single-valve eNOS−/− vessels. A significant reduction in FREQ occurred at 7 and 10 cmH2O in eNOS−/− lymphangions, suggesting a non-specific effect of l-NAME similar to that observed with single-valve eNOS−/− vessels (Fig. 4E).
A comparison of WT and eNOS−/− lymphangions (Supplemental Fig. 6) revealed that genetic removal of eNOS led to a significant increase in AMP and EF over low pressures (∼1–3 cmH2O), in line with the data obtained from the single-valve segments. No significant differences were detected for EDD or FPF between WT and eNOS−/− lymphangions, whereas WT lymphangions had a significantly higher tone at 7 and 10 cmH2O and significantly higher FREQ at 2 and 3 cmH2O.
Discussion
Combining the isolated lymphatic vessel preparation with genetic mouse models, we directly tested the hypotheses that: (1) basal NO production in lymphatic endothelium increases contraction amplitude/strength as a result of reduced frequency; and (2) greater NO production stimulated by ACh inhibits all aspects of contractile activity. Contrary to the first hypothesis, our results showed that genetic removal of basal NO production increases contraction amplitude and ejection fraction. Thus, we conclude that basal NO production depresses contraction amplitude without increasing frequency. Greater NO production evoked by ACh led to a concentration-dependent decline in all contractile parameters investigated, except for contraction amplitude and ejection fraction. The effects of ACh were completely abolished by pharmacological inhibition or genetic removal of eNOS, supporting the second hypothesis. In aggregate, NO appears to exert only an inhibitory effect on lymphatic contractile function at both basal and stimulated levels in this experimental model. This conclusion, discussed below, challenges the current understanding of the role of basal endothelial-derived NO in collecting lymphatic function.
Isolated murine collecting lymphatic vessels exhibit contractile activity
The notion that collecting lymphatics in the mouse undergo regular spontaneous contractions, unassisted by the movement of other tissues (e.g. contraction of skeletal muscle, peristalsis, etc.), has remained a controversial topic – even though murine lymphatic contractions were alluded to as early as 1949 (Smith, 1949). The delay in widespread acceptance of this idea is presumably due to the fact that no mouse lymphatic preparation had been established that reliably demonstrated large-amplitude contractions similar to those observed in other mammalian species. Here, for the first time, we have isolated and cannulated collecting lymphatics from the popliteal region of WT and genetically-engineered mice that exhibit large-amplitude spontaneous contractions (∼50% of EDD) and relatively high ejection fractions (∼60%). These murine popliteal collecting lymphatics respond to increased pressure in a manner similar to lymphatics of other mammalian species – including cow, guinea pig and rat – by decreasing contraction amplitude and increasing frequency (McHale & Roddie, 1976; Hargens & Zweifach, 1977; Zhang et al. 2007). In addition, murine popliteal lymphatics exhibit several other contractile features that have been identified using rat mesenteric lymphatic vessels, including myogenic constriction, rate-sensitive activation/inhibition, and characteristic responses to preload/afterload (unpublished observations). These findings contradict the speculation that murine collecting lymphatics possess fundamentally different contractile properties from those of other mammals (Gashev et al. 2009, 2010). That speculation was based on: (1) the assumption that mesenteric and iliac collecting lymphatics from the DDY strain of mice, which exhibit only small-amplitude contractions (∼10% of EDD), are representative of all murine lymphatic vessels (Ono et al. 2000; Mizuno et al. 2001; Nakaya et al. 2001); and (2) recent studies to image lymph flow in the mouse reported passive lymphatic ‘pulsing’ (Kwon & Sevick-Muraca, 2010; Zhou et al. 2010; Proulx & Detmar, 2012), which, based on our results, most likely represented active lymph propulsion generated by spontaneous contractions. Importantly, our study demonstrates the utility of using transgenic mouse models to study lymphatic physiology in the isolated vessel preparation by providing more definitive evidence for the existence of spontaneous lymphatic contractions in mice, particularly on a genetic background commonly used for maintaining transgenic lines (i.e. C57BL/6).
The role of basal NO in murine collecting lymphatic vessels
Using the isolated rat thoracic duct, Gasheva et al. (2006) first proposed and tested the hypothesis that basal NO, produced during spontaneous contractions as a result of pulsatile flow, reduces the contraction frequency to provide additional time for diastolic filling, which then enhances the strength of the next contraction. Importantly, the rat thoracic duct is the largest lymphatic duct in the body (rat: ∼575 μm diameter) and is specialized to perform more as a conduit, rather than a pump (Gasheva et al. 2006; Quick et al. 2007), consistent with it possessing contractile as well as non-contractile regions. Thus, it is uncertain whether the results obtained using this unique vessel apply to more peripheral prenodal lymphatic vessels of much smaller diameter (e.g. mouse: ∼70 μm diameter; rat: ∼150 μm diameter) that may produce much less NO per contraction cycle. Regardless, several recent studies of peripheral lymphatic vessels in vivo have interpreted their own data in light of this hypothesis.
Recently, it has been shown that the concentration of basal NO produced by rat lymphatic endothelium oscillates over individual contraction cycles due to shear stress caused by pulsatile flow (Bohlen et al. 2009). The same study demonstrated that l-NAME caused a decreased frequency in vivo, consistent with our data showing this non-specific effect of l-NAME in eNOS−/− vessels (Fig. 4; Supplemental Fig. 5). In another study of rat mesenteric lymphatic vessels, localized application of 1 mm l-NAME reduced the basal NO concentration by approximately 60% and increased the frequency by approximately 30% over control without substantially altering either EDD or ESD, reflecting no change in amplitude (see Eqn 1; Bohlen et al. 2011). The latter study stated, but did not show, that application of the same concentration of l-NAME to a longer (1 mm) length of vessel caused a reduction of both EDD and ESD concomitant with a decreased frequency of contractions, again suggesting that amplitude was minimally changed by pharmacological antagonism of eNOS. These results agree with our data presented in Fig. 4 and Supplemental Fig. 4 showing that l-NAME did not significantly change contraction amplitude, but decreased frequency. However, Bohlen et al. (2011) concluded that ‘the tubular region, [had] a greater ability to increase lusitropy and thus enhance stroke volume’ than the valve regions, based on experiments where relatively high doses of the vasoactive agonist, bradykinin, were applied.
Two studies using isolated rat lymphatic vessels have been cited in support of the hypothesis that basal NO increases contraction amplitude, but in those studies the endothelial layer was physically denuded instead of targeting eNOS selectively (Mizuno et al. 1997; Koller et al. 1999), removing all endothelial-mediated influences in addition to NO (e.g. myoendothelial coupling, endothelin-1, prostanoids, thromboxane A2, superoxide, etc.) and potentially damaging the muscle cell layer.
Using the in vivo mouse tail preparation, Hagendoorn et al. (2004) demonstrated that WT mice infused chronically with l-NMMA as well as eNOS−/− mice both exhibited lower lymphatic capillary flow rates. Although this was interpreted as a significant change in collecting lymphatic contractile function, the underlying collecting lymphatic vessels were neither directly imaged nor studied.
The only study directly examining mouse collecting lymphatics in vivo used chronic l-NMMA infusion as well as eNOS−/− mice (Liao et al. 2011). Systemic infusion of l-NMMA for 3 days in WT mice resulted in no significant change in popliteal lymphatic contraction frequency but a decrease in contraction amplitude. Examination of eNOS−/− mice in that study revealed an increase in frequency along with a decrease in contraction amplitude compared with WT controls, and led to the conclusion that ‘the necessary control of endothelial-regulated NO production is no longer able to induce strong lymphatic contractions’ in eNOS−/− vessels. More clearly, the same group (Kesler et al. 2012) restated this conclusion that deletion of eNOS ‘produces a decrease in contraction strength corresponding with an increase in the frequency of contractions in the afferent lymphatic vessels of the popliteal [lymph node],’ indicating a positive lusitropic effect. Surprisingly, Liao et al. (2011) reported a substantial increase, rather than a decrease, in EDD upon eNOS inhibition using either pharmacological or genetic means. These conclusions are completely opposite to our present finding that basal NO decreases amplitude without increasing frequency. However, caution must be used in interpreting data from a complex in vivo setting where many changes in unknown variables can occur, including local intravascular pressure, interstitial or vascular flow, sympathetic activation, oncotic pressures, immune cell activation, circulating metabolic factors, and capillary filtration. Of course the trade-off for precise control of pressure requires the removal of the vessel from many of the same in vivo cues. For the purpose of resolving the controversy regarding the effects of basal NO, this was necessary in order to control for pressure changes, which can increase frequency by >2-fold and lower EF by 30% for only a 1.5 cmH2O elevation in pressure (0.5–2 cmH2O; Figs 3 and 5). Indeed, intraluminal hydrostatic pressure and lymph flow were unknown and uncontrolled in the in vivo study of Liao et al. (2011), which is potentially problematic for the interpretation of some aspects of that study. For example, genetic deletion of eNOS increases mean arterial blood pressure and blood capillary filtration (Huang et al. 1995; Predescu et al. 2005) that would increase lymph volume and intraluminal pressure in vivo relative to WT. We propose that the elevated frequency, decreased contraction amplitude and increased diameter in response to eNOS ablation, as shown in Liao et al. (2011), are best explained by an increase in intraluminal hydrostatic pressure, in agreement with our previous reports that increased lymphatic preload leads to an increase in EDD and frequency while reducing contraction amplitude in proportion to the pressure change (Davis et al. 2012; Scallan et al. 2012; refer to Fig. 3A, C and E).
The role of large NO production stimulated by ACh
The few studies of the actions of ACh on isolated rat and cow collecting lymphatics have suggested that ACh elicits dilation (i.e. increased EDD) and reduces the contraction amplitude and frequency (Yokoyama & Ohhashi, 1993; Mizuno et al. 1998). Our results largely agree as we obtained a dilation that corresponded to a loss of tone, a decreased frequency and a decreased FPF. Further, the ACh responses that we showed were completely mediated by NO because eNOS inhibition or deletion blocked the entire response, except for the potential direct effect on the lymphatic muscle cells at the highest dose, in agreement with previous reports using pharmacological inhibitors (Yokoyama & Ohhashi, 1993; Mizuno et al. 1998). It is worth noting that the ACh responses and blockade also confirmed endothelial integrity in each of our preparations. However, one difference between our results and the literature is that a significant change in WT contraction amplitude did not occur at any dose of ACh tested. This may be attributed to the 10- and 100-fold higher doses of ACh (10−6 and 10−5 m) that were used in previous studies (Yokoyama & Ohhashi, 1993; Mizuno et al. 1998). Interestingly, we did obtain a significant decrease in contraction amplitude at the two highest doses in the iNOS−/− mice (Supplemental Fig. 3C), which were reported to have augmented eNOS protein expression (Liao et al. 2011), indicating that greater NO production does depress amplitude. Supporting this idea, contraction frequency and FPF were reduced more in the iNOS−/− vessels than in WT vessels at the highest dose of ACh (Supplemental Fig. 3E and F).
Physiological significance
The major conclusion of our work is that NO production, whether basal or stimulated, depresses murine collecting lymphatic contractile activity, at least in popliteal lymphatics that are peripherally located. Specifically, basal NO depresses only contraction amplitude, with larger concentrations of NO then inhibiting all other contractile parameters. If these results extend to other types of collecting lymphatics in the mouse, then a possible role for basal NO in collecting lymphatics might be to set contraction amplitude at a level that can be increased or decreased to modulate lymph flow, which in turn has implications for fluid homeostasis, humoral immunity, and cancer metastasis (Tammela et al. 2011; Thomas et al. 2012). Finally, this is the first study to demonstrate the utility of isolated lymphatic vessels from transgenic mice for the quantitative study of several aspects of lymphatic physiology, including contractile function.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Shan-Yu Ho. This work was supported by NIH grant HL-089784.
Glossary
- AMP
contraction amplitude
- BSA
bovine serum albumin
- EDD
end diastolic diameter
- EF
ejection fraction
- eNOS
endothelial nitric oxide synthase
- ESD
end systolic diameter
- FPF
fractional pump flow
- FREQ
contraction frequency
- iNOS
inducible nitric oxide synthase
- MaxD
maximal passive diameter
- NO
nitric oxide
- WT
wild-type
Author contributions
J.P.S. and M.J.D. both independently conceived the idea of establishing a preparation for isolated murine collecting lymphatic vessels that spontaneously contract. M.J.D. and J.P.S. designed and performed experiments in addition to collecting and compiling data. J.P.S. analysed and interpreted the data, and drafted and approved the final version of the manuscript. M.J.D. assisted with some data analysis, created the supplemental movies, and revised and approved the final version of the manuscript.
Supplementary material
Supplemental Movie 1
Supplemental Movie 2
Supplemental Fig. 1
Supplemental Fig. 2
Supplemental Fig. 3
Supplemental Fig. 4
Supplemental Fig. 5
Supplemental Fig. 6
References
- Akl TJ, Nagai T, Cote GL, Gashev AA. Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol. 2011;301:H1828–H1840. doi: 10.1152/ajpheart.00538.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol. 2011;301:H1897–H1906. doi: 10.1152/ajpheart.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol. 2009;297:H1319–H1328. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton IL, Cheek DJ, Eckman D, Westfall DP, Sanders KM, Keef KD. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993;72:387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE., Jr Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol. 2011;301:H48–H60. doi: 10.1152/ajpheart.00133.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol. 2012;303:H795–H808. doi: 10.1152/ajpheart.01097.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- Duling BR, Gore RW, Dacey RG, Jr, Damon DN. Methods for isolation, cannulation, and in vitro study of single microvessels. Am J Physiol Heart Circ Physiol. 1981;241:H108–H116. doi: 10.1152/ajpheart.1981.241.1.H108. [DOI] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ, Gasheva OY, Nepiushchikh ZV, Wang W, Dougherty P, Kelly KA, Cai S, Von Der Weid PY, Muthuchamy M, Meininger CJ, Zawieja DC. Methods for lymphatic vessel culture and gene transfection. Microcirculation. 2009;16:615–628. doi: 10.1080/10739680903120778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashev AA, Nagai T, Bridenbaugh EA. Indocyanine green and lymphatic imaging: current problems. Lymphat Res Biol. 2010;8:127–130. doi: 10.1089/lrb.2010.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in thoracic duct. J Physiol. 2006;15:821–832. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagendoorn J, Padera TP, Kashiwagi S, Isaka N, Noda F, Lin MI, Huang PL, Sessa WC, Fukumura D, Jain RK. Circ Res. 2004;95:204–209. doi: 10.1161/01.RES.0000135549.72828.24. [DOI] [PubMed] [Google Scholar]
- Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. Am J Physiol Heart Circ Physiol. 1977;233:H57–H65. doi: 10.1152/ajpheart.1977.233.1.H57. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Johnston MG, Gordon JL. Regulation of lymphatic contractility by arachidonate metabolites. Nature. 1981;293:294–297. doi: 10.1038/293294a0. [DOI] [PubMed] [Google Scholar]
- Kesler CT, Liao S, Munn LL, Padera TP. Lymphatic vessels in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2012;5:111–124. doi: 10.1002/wsbm.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller A, Mizuno R, Kaley G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1683–R1689. doi: 10.1152/ajpregu.1999.277.6.R1683. [DOI] [PubMed] [Google Scholar]
- Kwon S, Sevick-Muraca EM. Functional lymphatic imaging in tumor-bearing mice. J Immunol Methods. 2010;360:167–172. doi: 10.1016/j.jim.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D, Padera TP. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A. 2011;108:18784–18789. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol. 1976;261:255–269. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno R, Dörnyei G, Koller A, Kaley G. Myogenic responses of isolated lymphatics: modulation by endothelium. Microcirculation. 1997;4:413–420. doi: 10.3109/10739689709146805. [DOI] [PubMed] [Google Scholar]
- Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol Regul Integr Comp Physiol. 1998;274:R790–R796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- Mizuno R, Ono N, Ohhashi T. Parathyroid hormone-related protein-(1–34) inhibits intrinsic pump activity of isolated murine lymph vessels. Am J Physiol Heart Circ Physiol. 2001;281:H60–H66. doi: 10.1152/ajpheart.2001.281.1.H60. [DOI] [PubMed] [Google Scholar]
- Murphy TV, Kotecha N, Hill MA. Endothelium-independent constriction of isolated, pressurized arterioles by Nω-nitro-L-arginine methyl ester (L-NAME) Br J Pharmacol. 2007;151:602–609. doi: 10.1038/sj.bjp.0707262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation. 2011;18:463–473. doi: 10.1111/j.1549-8719.2011.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya K, Mizuno R, Ohhashi T. B16-BL6 melanoma cells release inhibitory factor(s) of active pump activity in isolated lymph vessels. Am J Physiol Cell Physiol. 2001;281:C1812–C1818. doi: 10.1152/ajpcell.2001.281.6.C1812. [DOI] [PubMed] [Google Scholar]
- Ono N, Mizuno R, Nojiri H, Ohhashi T. Development of an experimental apparatus for investigating lymphatic pumping activity of murine mesentery in vivo. Jpn J Physiol. 2000;50:25–31. doi: 10.2170/jjphysiol.50.25. [DOI] [PubMed] [Google Scholar]
- Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289:L371–L381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- Proulx ST, Detmar M. Watching lymphatic vessels grow by making them glow. Cell Research. 2012;22:12–13. doi: 10.1038/cr.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick CM, Venugopal AM, Gashev AA, Zawieja DC, Stewart RH. Intrinsic pump-conduit behaviour of lymphangions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1510–R1518. doi: 10.1152/ajpregu.00258.2006. [DOI] [PubMed] [Google Scholar]
- Saul ME, Thomas PA, Dosen PJ, Isbister GK, O’Leary MA, Whyte IM, McFadden SA, van Helden DF. A pharmacological approach to first aid treatment for snakebite. Nat Med. 2011;17:809–811. doi: 10.1038/nm.2382. [DOI] [PubMed] [Google Scholar]
- Scallan JP, Wolpers JH, Muthuchamy M, Zawieja DC, Gashev AA, Davis MJ. Independent and interactive effects of preload and afterload on the pump function of the isolated lymphangion. Am J Physiol Heart Circ Physiol. 2012;303:H809–H824. doi: 10.1152/ajpheart.01098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa Y, Ikomi F, Ohhashi T. Physiological roles of endogenous nitric oxide in lymphatic pump activity of rat mesentery in vivo. Am J Physiol Gastrointest Liver Physiol. 2000;278:G551–G556. doi: 10.1152/ajpgi.2000.278.4.G551. [DOI] [PubMed] [Google Scholar]
- Smith RO. Lymphatic contractility; a possible intrinsic mechanism of lymphatic vessels for the transport of lymph. J Exp Med. 1949;90:497–509. doi: 10.1084/jem.90.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda O, Tsutsui M, Morishita T, Tanimoto A, Horiuchi M, Tasaki H, Huang PL, Sasaguri Y, Yanagihara N, Nakashima Y. Long-term treatment with Nω-nitro-L-arginine methyl ester causes arteriosclerotic coronary lesions in endothelial nitric oxide synthase-deficient mice. Circulation. 2002;106:1729–1735. doi: 10.1161/01.cir.0000029749.16101.44. [DOI] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Holopainen T, Ylä-Herttuala S, Andersson LC, Virolainen S, Immonen I, Alitalo K. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci Transl Med. 2011;3:69ra11. doi: 10.1126/scitranslmed.3001699. [DOI] [PubMed] [Google Scholar]
- Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ, Swartz MA. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J Immunol. 2012;189:2181–2190. doi: 10.4049/jimmunol.1103545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Ohhashi T. Effects of acetylcholine on spontaneous contractions in isolated bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol. 1993;264:H1460–H1464. doi: 10.1152/ajpheart.1993.264.5.H1460. [DOI] [PubMed] [Google Scholar]
- Zhang R, Gashev AA, Zawieja DC, Lane MM, Davis MJ. Length-dependence of lymphatic phasic contractile activity under isometric and isobaric conditions. Microcirculation. 2007;14:613–625. doi: 10.1080/10739680701436160. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wood R, Schwarz EM, Wang YJ, Xing L. Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice. Arthritis Rheum. 2010;62:1881–1889. doi: 10.1002/art.27464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


