Abstract
Efferent signals from the vagus nerve are thought to mediate both basal and meal-induced gastric acid secretion, and provide trophic support of the mucosa. However, the underlying mechanisms are incompletely understood. Neurturin, signalling via glial cell line-derived neurotrophic factor (GDNF)-family receptor α2 (GFRα2), is essential for parasympathetic innervation of many target tissues but its role in gastric innervation is unknown. Here we show that most nerve fibres in wild-type mouse gastric mucosa, including all positive for gastrin-releasing peptide, are cholinergic. GFRα2-deficient (KO) mice lacked virtually all cholinergic nerve fibres and associated glial cells in the gastric (oxyntic and pyloric) mucosa but not in the smooth muscle, consistent with the selective expression of neurturin mRNA in the gastric mucosa. 2-Deoxyglucose and hexamethonium failed to affect acid secretion in the GFRα2-KO mice indicating the lack of functional innervation in gastric mucosa. Interestingly, basal and maximal histamine-induced acid secretion did not differ between wild-type and GFRα2-KO mice. Moreover, circulating gastrin levels in both fasted and fed animals, thickness of gastric mucosa, and density of parietal and different endocrine cells were similar. Carbachol-stimulated acid secretion was higher in GFRα2-KO mice, while atropine reduced basal secretion similarly in both genotypes. We conclude that cholinergic innervation of gastric mucosa depends on neurturin-GFRα2 signalling but is dispensable for gastrin secretion and for basal and maximal acid output. Basal acid secretion in the KO mice appears to be, at least partly, facilitated by constitutive activity of muscarinic receptors.
Key points
Nervous control over gastric function is mediated via intrinsic neurons in the gastric myenteric ganglia. The majority of these neurons are cholinergic and are innervated by preganglionic efferents from the vagus nerve. Intact vagal innervation is crucial for gastric acid secretion and mucosal maintenance since vagotomy is known to abolish both basal and stimulated acid secretion and unilateral vagotomy causes gastric mucosal atrophy.
Neurturin, a neurotrophic factor signalling via GDNF-family receptor α2 (GFRα2) is an important factor for parasympathetic innervation of many target tissues, but its role in gastric innervation is unknown.
GFRα2-deficient (KO) mice lack virtually all cholinergic nerve fibres and associated glial cells in the gastric mucosa, yet have normal gastric morphology, gastrin secretion, and basal and maximal histamine-stimulated acid secretion.
Blocking of myenteric ganglia with hexamethonium severely decreased basal acid secretion in wild-type mice but had no effect on the GFRα2-KO animals. Carbachol-stimulated acid secretion was higher in GFRα2-KO mice. Blocking of muscarinic receptors with atropine inhibited basal acid secretion in both genotypes suggesting that constitutive activity of muscarinic receptors may facilitate basal acid secretion.
Introduction
The two most important determinants of gastric acid secretion from the parietal cells are central input via the vagus and meal-stimulated gastrin release from the antrum. The stimulation of gastric acid secretion via the gastrin–enterochromaffin-like (ECL) cell–histamine pathway and its inhibition by somatostatin (from D-cells) is quite well established but the mechanisms underlying neuronal control of gastric acid secretion are complex and incompletely understood (Black & Shankley, 1987; Debas & Carvajal, 1994; Chen et al. 2005; Schubert & Peura, 2008; Ericsson et al. 2010b).
The vagus nerve controls gastric secretion via the intrinsic neurons in the gastric myenteric ganglia that provide the efferent innervation of the gastric mucosa. Most of these intramural neurons are cholinergic in all mammalian species studied (Pfannkuche et al. 1998; Nakajima et al. 2000; Pimont et al. 2003) and many of them coexpress neuropeptides including vasoactive intestinal peptide (VIP) and gastrin-releasing peptide (GRP) (Ekblad et al. 2000). In addition, gastric myenteric ganglia in both rodents and humans contain non-cholinergic neurons, including nitrergic inhibitory interneurons (Nakajima et al. 2000; Berthoud et al. 2001; Pimont et al. 2003). Studies in rats suggest that virtually all gastric myenteric neurons may be innervated by preganglionic vagal nerve fibres (Holst et al. 1997; Berthoud et al. 2001). Acetylcholine released from the postganglionic cholinergic nerve fibres in the gastric mucosa can increase gastric acid secretion directly by stimulating M3 muscarinic receptors on parietal cells and by inhibiting somatostatin release from D-cells. In addition, the neuropeptides released from the intrinsic nerves can regulate gastric acid secretion via the endocrine cells, and vice versa, somatostatin can regulate the activity of the intrinsic neurons in gastric mucosa (Schubert & Peura, 2008). The vagus and M3 receptors are required for basal acid secretion in different in vivo animal models and in humans (Schirmer, 1989; Debas & Carvajal, 1994; Aihara et al. 2003) and are also thought to provide trophic support to gastric mucosa in rats (Håkanson et al. 1984).
Plasticity and redundancy of gastric acid regulatory mechanisms have been reported in knockout mouse models (Chen et al. 2004; Zhao et al. 2008). For example, in gastrin–cholecystokinin double-mutant mice, basal and food-stimulated gastric acid secretion is normalized apparently by the cholinergic vagal pathway. In these mice, carbachol paradoxically reduces gastric acid secretion, possibly via an increased secretion of somatostatin in the oxyntic mucosa (Chen et al. 2004). Importantly, it is still unknown why vagotomy (or atropine) blocks both basal and gastrin- or histamine-stimulated acid response, as is the mechanism by which the vagus controls the mucosal cells (Chen et al. 2005).
Neurturin, signalling via the GDNF-family receptor α2 (GFRα2), is essential for the target innervation of many parasympathetic and a small subset of enteric neurons (Rossi et al. 2003, 2005). However, the role of neurturin-GFRα2 signalling in the stomach is unknown. Here, we show that GFRα2-deficient (KO) mice have a specific loss of the efferent cholinergic innervation in the gastric mucosa. This allowed us to investigate whether basal and stimulated gastric acid secretion, gastrin secretion and trophic support of the gastric mucosa requires the intrinsic cholinergic innervation.
Methods
Ethical approval
All animal experiments were carried out in accordance with the Guidelines laid down with the European Communities Council Directive (86/609/EEC) and were approved by the County Administrative Board of Southern Finland.
Animals
Two- to ten-month-old, age-matched GFRα2-KO and wild-type mice of both sexes in a congenic C57BL/6JOlaHsd background were used. All mice used for histology, PCR and gastrin measurements were littermates from heterozygous matings. Most mice used to study acid output were the F1 offspring of several pairs of wild-type and GFRα2-KO parents. Mice were genotyped as described earlier (Rossi et al. 1999) and kept in standard, specific pathogen-free conditions under a constant dark/light cycle. The average body weight of GFRα2-KO animals used for gastric acid measurements was ∼20% (range 17–25%) less than that of their age-matched wild-type controls. However, there was no correlation between the body weight and the gastric pH, acid content or secretion.
Immunohistochemistry and microscopy
Mice were anaesthetized with an overdose of pentobarbital (200 mg/kg, I.P.; Orion, Espoo, Finland) and transcardially perfused first with cold PBS, pH 7.4, followed by 4% paraformaldehyde or 4% carbodiimide (for histamine-immunostaining) (Panula et al. 1988) in PBS. The stomachs were removed and postfixed at +4°C for 2–3 h or overnight (depending on antibody used), cryoprotected in 30% sucrose and mounted in Tissue Tek (Sakura Finetek, Torrance, CA, USA). Stomachs were cut into 10–20 μm sagittal sections and mounted on Super Frost Ultraplus microscopic slides (Thermo Fisher Scientific, Rockford, IL, USA) and stained using standard immunofluorescence techniques.
Primary polyclonal antibodies were against bombesin/GRP (rabbit, a kind gift from Dr Panula; Panula et al. 1982), galanin (rabbit, Millipore), GFRα2 (goat; R&D Systems Europe, Abingdon, UK), ghrelin (goat; Santa Cruz Biotechnology, Santa Cruz, CA, USA), H+,K+-ATPase β-subunit (rabbit, Thermo Fisher), histamine (rabbit; Panula et al. 1988), protein gene product 9.5 (PGP9.5; rabbit; Millipore, Billerica, MA, USA), S100β (rabbit; Swant, Bellizona, Switzerland), substance P (SP) (rabbit, Millipore), tyrosine hydroxylase (TH) (rabbit; Millipore), vesicular acetylcholine transporter (VAChT) (rabbit; Phoenix Pharmaceuticals, Burlingame, CA, USA), vasoactive intestinal peptide (VIP) (rabbit; Progen Biotechnik, Heidelberg, Germany). Secondary antibodies were from Jackson ImmunoResearch Europe (Suffolk, UK). Confocal microscopy images were taken using a ×25 water immersion objective, and maximum intensity projections were generated using Zeiss LSM5 software (Carl Zeiss, Jena, Germany).
The density of nerve fibres and cells was quantified by a researcher blind to the genotype from microscopic images taken with ×20 magnification from nine sections through the stomach of each animal using systematic random sampling. The density of S100β-, TH-, VAChT- and VIP-positive fibres in gastric oxyntic mucosa was estimated by counting fibres crossing a standardized grid. The volume density of VAChT- and VIP-positive fibres in the inner, circular muscle layer of the oxyntic mucosa was estimated by counting the number of cut nerve profiles inside a circular probe randomly positioned on the muscle layer. The density of parietal cells and other cell types in the gastric mucosa was expressed as the number of clearly positive cell profiles per gland and length of mucosal surface, respectively.
Neurturin and GDNF mRNA in situ hybridization on cryosections from mouse stomach was performed as described (Rossi et al. 1999). No specific labelling above background was seen in adjacent sections hybridized with a sense probe.
Gastric juice pH
The animals were fasted overnight and killed by cervical dislocation. The stomachs were immediately removed and the gastric juice pH was measured with a flat-top pH probe (Blue Line 27 pH, Schott, Germany).
Acute fistula method
Briefly, the mice were fasted overnight with free access to water and anaesthetized with urethane (1.75 g kg−1, i.p.; Sigma-Aldrich, St Louis, MO, USA) and kept on a heating pad while their body temperatures were monitored with a rectal probe. An incision was made in the abdomen to expose the duodenum and stomach. A flexible cannula was inserted into the stomach through a small incision made in the duodenum and secured in place around the pylorus. The cannula was attached to a small syringe that allowed the stomach to be flushed.
The gastric acid content was determined from the combined first (approximately five) washes with 0.3 ml of warm (39°C) saline until pH was >6.0. Basal secretion was then collected for 2 × 30 min by rinsing with 3 × 0.3 ml of saline. After the basal period, the mice were given 2-deoxyglucose (2-DG, 400 mg kg−1, i.p.), histamine (10 mg kg−1, i.p.) or carbachol (30 μg kg−1, s.c.). These doses were chosen based on previous studies and our pilot experiments. For example, the histamine dose of 10 mg kg−1 was previously shown to lead to maximal acid stimulation (Tanaka et al. 2002). All drugs were from Sigma-Aldrich. Drug-stimulated acid secretions were collected up to 60 min after which the stomach was removed and the animals were killed by cervical dislocation. Gastric acid content in the samples was determined as above and expressed as micromoles of H+ per hour.
Gastric acid output without anaesthesia
The mice were fasted overnight with free access to water. Drugs (or saline) were injected subcutaneously 40 min (histamine 10 mg kg−1; pentagastrin 0.5 mg kg−1) or 70 min (hexamethonium 20 mg kg−1; atropine 3 mg kg−1) before the mice were killed by cervical dislocation. The gastric acid content was determined by manual titration to pH 7.0 against 10 mm NaOH and expressed as μmol H+ (g body weight)−1).
Plasma gastrin levels
Blood samples (∼100 μl) were collected in the morning from the saphenous vein of both fed and overnight (∼14 h)-fasted mice (3- to 5-months-old, both sexes). Terminal blood samples (∼0.5 ml) were collected through a cardiac puncture. The blood was centrifuged (13,000 g, 4°C) for 2 min and the separated plasma was stored at –80°C until analysis. Plasma gastrin levels were determined by using a commercially available RIA kit (MD302, Euro-Diagnostica, Malmö, Sweden) according to the manufacturer's instructions.
Real-time PCR
To assess mRNA expression of histidine decarboxylase (HDC) and different muscarinic receptor subtypes in the stomach, freely fed animals were killed and stomachs were dissected, washed and immediately frozen in liquid nitrogen and stored at –80°C. The tissues were lysed with QIAzol Lysis Reagent (Qiagen, Hilden, Germany) and the mRNA was isolated according to the product protocol. Two micrograms of mRNA was used to generate first-strand cDNA using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and random primers. SYBR Green-based real-time PCR was performed in triplicate with a Bio-Rad CFX96 system (Bio-Rad Laboratories) using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the control. GAPDH expression in the stomach did not differ between the genotypes. Analysis of data was done using the comparative Ct method. The primers were (5′-3′): HDC-fw: CTTTCTCATCCCGGCTACTATCCA, HDC-rev: ACCGCGTTGTCTTCCTCCTGT, M1-fw: TTCTGATCCC TGCTGTGTGG GAAT, M1-rev: TTGGCTCCTG ACTTCCTGCC TAAA, M2-fw: TACTTCTTGT TCAGCCTGGC CTGT, M2-rev: AGGCCAGTAG CCAATCACAG TGTA, M3-fw: TTGGTCCAAT GCCAATTCAG CAGG, M3-rev: AGACCCAGGC AGACCAATTT CTGA, M4-fw: ATGTGTGACT GCCATCGAGA TCGT, M4-rev: GATGAAGGCC AGCAGAATGG CAAA, M5-fw: TTCCCAGTGT CCAAAGACCC TTCA, M5-rev: ACAGAAGGTG GAAACCAGGA CCAT.
Statistical analysis
Values are presented as mean ± SEM. Data between two groups was compared using the two-tailed Student's t test, assuming unequal variance. Differences between multiple groups were determined by one-way or two-way ANOVA, followed by a Student–Newman–Keuls multiple comparison test. P < 0.05 was considered statistically significant.
Results
Profound loss of gastric mucosal cholinergic innervation and associated glial cells in GFRα2-KO mice
Intrinsic cholinergic nerve fibres comprise the bulk of gastric mucosal innervation in rats (Ekblad et al. 2000). We used neuronal and glial markers to compare innervation of the gastric wall in adult wild-type and GFRα2-KO mice. Numerous VAChT- and VIP-positive nerve fibres were seen in the basal part of wild-type mouse gastric (oxyntic and pyloric) mucosa (Fig. 1A and C and not shown). Double staining showed that most, if not all, VAChT-positive nerve fibres in wild-type mouse gastric mucosa were bombesin/GRP-positive (Supplemental Fig. 1A–C), and most of them also coexpressed VIP (Supplemental Fig. 1D–F). Importantly, the VAChT-, GRP- and VIP-positive innervation of the mucosa was profoundly reduced throughout the glandular part of the stomach in GFRα2-KO mice (Fig. 1B and D and Supplemental Fig. 2A and B). Quantification revealed that the density of VAChT- and GRP-positive nerve fibres of the oxyntic mucosa was reduced by more than 90% in GFRα2-KO compared with wild-type mice (Fig. 1E, P= 0.004 and Supplemental Fig. 2C). Also the density of VIP-positive nerve fibres was reduced by ∼80% (Fig. 1F, P= 0.02). A qualitatively similar loss of VAChT- and VIP-positive fibres was also seen in the pyloric mucosa (data not shown). In contrast, the density of VAChT- and VIP-positive nerve fibres in the gastric smooth muscle layer (Fig. 1C and F) was not different between the genotypes.
Figure 1. Loss of cholinergic innervation in GFRα2-KO mouse gastric mucosa.
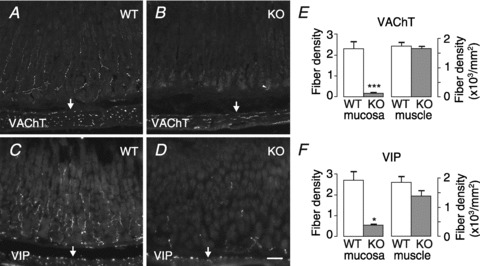
Representative sections from wild-type (WT) and GFRα2-KO (KO) mouse gastric mucosa (oxyntic part) immunostained for VAChT (A and B) and VIP (C and D). A, numerous VAChT-positive cholinergic fibres are seen in wild-type mouse gastric mucosa and in the underlying circular smooth muscle layer. B, density of VAChT-positive nerve fibres is profoundly reduced in the GFRα2-KO mouse gastric mucosa but not in the muscle layer (arrows). Arrowhead marks unspecific staining of gland lumen. C and D, density of VIP-positive nerve fibres is profoundly reduced in the GFRα2-KO mouse gastric mucosa but not in the underlying muscle layer (arrows). The density of VAChT- (E) and VIP- (F) positive fibres in the mucosa (in arbitrary units) and muscle was estimated from wild-type (n= 4–5) and GFRα2-KO (n= 3–4) mice using systematic random sampling. *P= 0.02; ***P= 0.004, using a t test. Scale bar: 50 μm.
Interestingly, the number of glial cells was also reduced in the gastric mucosa but not in the gastric smooth muscle or in the myenteric ganglia of GFRα2-KO mice (Fig. 2A and B). Quantification revealed that the density of S100β-positive glial cells (not shown) and their processes in the gastric mucosa was reduced by ∼80% in GFRα2-KO as compared with wild-type mice (WT: 1.8 ± 0.15, n= 4 vs. KO: 0.39 ± 0.06, n= 3, arbitrary units, P= 0.002).
Figure 2. Lack of enteric glia in GFRα2-KO gastric mucosa and expression of neurturin mRNA in postnatal mouse stomach.
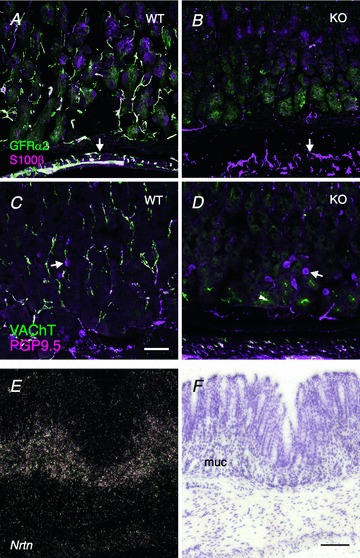
A and B, double-staining with GFRα2 (green) and the glial marker S100β (magenta). A, in wild-type mouse stomach, S100β-positive glial cells and their processes coexpress GFRα2 both in the oxyntic mucosa and in the muscle layer (arrows). B, density of S100β-positive glial processes is greatly reduced in the GFRα2-KO mouse gastric mucosa but not in muscle layer (arrows). C and D, double-staining with cholinergic marker VAChT (green) and the pan-neuronal marker PGP9.5 (magenta). C, PGP9.5-positive nerve fibres in wild-type mouse gastric mucosa are concentrated in the basal half of the mucosa and most of them are cholinergic (white). Arrows point at PGP9.5-positive and VAChT-negative (magenta) putative endocrine cells in the mucosa. D, in GFRα2-KO mice, the PGP9.5-positive nerve fibres are virtually absent in the gastric mucosa, but the PGP9.5-positive endocrine cells remain. Note many PGP9.5-positive nerve fibres in the submucosa and muscle layer. Arrowhead marks faint unspecific staining of gastric gland lumen by the VAChT antibody. E, in situ hybridization of a stomach section from adult mice shows that neurturin (Nrtn) mRNA is selectively expressed in the basal half of the gastric mucosa but not in the muscle layers. F, corresponding bright-field image of the dark-field image (E). muc, mucosa. Scale bar: 50 μm.
In contrast to the dense intrinsic cholinergic innervation, PGP9.5-positive and VAChT-negative, putative non-cholinergic nerve fibres in wild-type mouse gastric mucosa were relatively sparse and appeared unaffected in the GFRα2-KO mice (Fig. 2C and D). Few sympathetic TH-positive nerve fibres were seen in the mouse gastric mucosa (Supplemental Fig. 1K) and their density was similar between the genotypes (WT: 0.50 ± 0.01, n= 4 vs. KO: 0.46 ± 0.03, n= 3, arbitrary units, P= 0.3). Finally, a few galanin-positive nerve fibres were present similarly in both genotypes in the gastric submucosa and muscle layers (but not in the mucosa) (Supplemental Fig. 2D and E).
Localization of GFRα2 and neurturin in mouse gastric wall
Consistent with our previous results that myenteric cholinergic neurons and associated glial cells express GFRα2 (Rossi et al. 2003), GFRα2 immunoreactivity was colocalized with VAChT-positive nerve fibres (Supplemental Fig. 1G–I) and with S100β-positive enteric glial cells in the gastric mucosa and muscle layers (Fig. 2A). The few sympathetic (TH+) nerve fibres in the stomach mucosa were GFRα2 negative (Supplemental Fig. 1J–L), agreeing with the lack of GFRα2 expression in noradrenergic sympathetic neurons and nerve fibres in other tissues (Rossi et al. 2005).
A previous study reported that the preferred GFRα2 ligand neurturin is expressed in adult mouse gastric mucosa (Golden et al. 1999). Extending this observation, we found that neurturin mRNA was expressed selectively in the basal part of the gastric mucosa (in the oxyntic and pyloric parts but not in the gastric smooth muscle layers) both in 2-week-old (Supplemental Fig. 3) and adult mice (Fig. 2E and F). No expression of GDNF mRNA above the background was found in the gastric mucosa in adjacent sections (Supplemental Fig. 3). Thus, neurturin–GFRα2 receptor signalling is needed for the development and/or maintenance of the intrinsic cholinergic innervation and associated glia in the mouse gastric mucosa.
Normal morphology of gastric mucosa in GFRα2-KO mice
The overall morphology and the thickness of gastric (oxyntic and pyloric) mucosa in GFRα2-KO mice appeared normal in haematoxylin and eosin (H&E)-stained sections (Fig. 3A and not shown). Immunostaining of cryostat sections revealed a similar distribution and density of H+,K+-ATPase-positive parietal (Fig. 3B) and histamine-positive ECL (Fig. 3C) cells in the gastric oxyntic mucosa between the genotypes. Histidine decarboxylase (HDC) mRNA expression levels in the stomach wall were comparable (WT: 0.8 ± 0.2, n= 5 vs. KO: 1.0 ± 0.1, n= 4, P= 0.1). Also the distribution and density of somatostatin- (Fig. 3D) and ghrelin- (Fig. 3E) positive cells in the gastric mucosa did not differ between the genotypes.
Figure 3. Normal morphology of the gastric mucosa in GFRα2-KO mice.
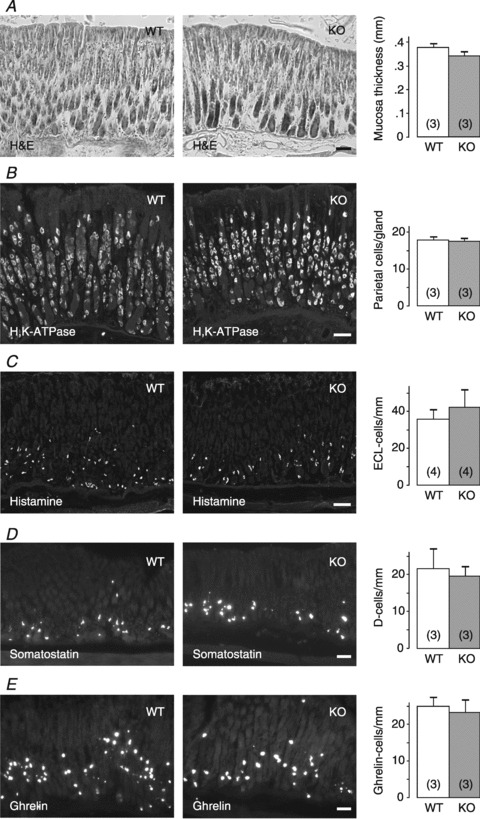
A, representative images of H&E-stained sections from wild-type (WT) and GFRα2-KO (KO) gastric mucosa (oxyntic part). Thickness of the mucosa is similar between the genotypes (P= 0.2). B–E, different cell types in the gastric mucosa were stained and quantified using antibodies for H+,K+-ATPase (B), histamine (C), somatostatin (D) and ghrelin (E): The distribution and density of the different cell types in the oxyntic mucosa is similar between the genotypes (P= 0.7–0.8). Number of animals per group is shown in parentheses. Scale bar: 50 μm.
Gastric pH, basal acid secretion and gastrin levels are not altered in GFRα2-KO mice
Surprisingly, despite the virtually complete lack of cholinergic innervation of the gastric mucosa, GFRα2-KO mice had a normal gastric pH after the overnight fast (Fig. 4A). Consistent with this finding, gastric acid content (Fig. 4B) and basal acid secretion under urethane anaesthesia (Fig. 4C) did not differ between GFRα2-KO and wild-type control mice. Moreover, plasma gastrin levels were similar between the genotypes both in fasted and in fed animals (Fig. 4D).
Figure 4. Similar gastric pH, acid content, basal acid secretion and gastrin levels in fasted wild-type and GFRα2-KO mice.
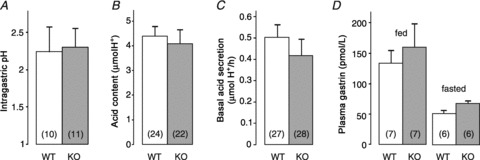
A, gastric pH of drug-naive animals (P= 0.8). B, acid content in the stomach (P= 0.7). C, basal gastric acid secretion measured using the gastric fistula method under urethane anaesthesia (P= 0.4). D, plasma gastrin levels (P= 0.6). Number of animals per group is shown in parentheses.
Maximal histamine-induced acid secretion is normal in GFRα2-KO mice
Administration of histamine at 10 mg kg−1 (i.p.) is known to induce a maximal acid output response in mice (Tanaka et al. 2002). Importantly, this dose of histamine produced a similar acid secretory response in both wild-type and GFRα2-KO mice (Fig. 5A), indicating that the gastric acid secretory capacity of GFRα2-KO mice was not impaired.
Figure 5. Drug-stimulated gastric acid secretion in fasted wild-type and GFRα2-KO mice.
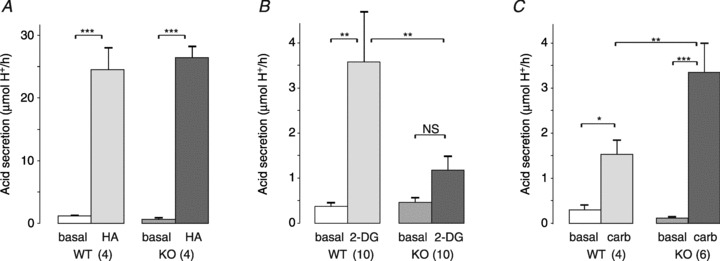
Acid secretion was measured under urethane anaesthesia using the gastric fistula method. Basal acid secretion was measured for 2 × 30 min before the stimulation. A, maximal acid output induced by histamine (HA, 10 mg kg−1, i.p.) was similar between the genotypes. B, 2-deoxyglucose (2-DG, 400 μg kg−1, i.p.) induced gastric acid secretion in wild-type but not in GFRα2-KO mice. C, carbachol (carb, 30 μg kg−1, s.c.) induced more acid secretion in GFRα2-KO mice than in the wild-type controls. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant. Number of animals per group is shown in parentheses.
Reduced vagally stimulated but increased carbachol-stimulated acid secretion in GFRα2-KO mice
Central hypoglycaemia induced by 2-DG stimulates gastric acid secretion via the vagus nerve (Becker et al. 1988). As expected, 2-DG induced a robust (∼8-fold) acid secretion in wild-type but not in the GFRα2-KO mice (Fig. 5B). Muscarinic receptor stimulation by carbachol (30 μg kg−1, s.c.) induced a 2-fold higher (P < 0.01) gastric acid secretion in GFRα2-KO mice than in wild-type controls (Fig. 5C). This result was supported by repetition with an independent cohort of mice using 20 μg kg−1 (i.p.) of carbachol (data not shown). Muscarinic receptor (M1 to M5) mRNA levels in the stomach (measured by real-time PCR) were similar between the genotypes (Supplemental Table 1).
Maximal gastric acid output without anaesthesia is similar between the genotypes
Urethane anaesthesia is known to inhibit basal and stimulated acid secretion by stimulating somatostatin release (Yang et al. 1990). To exclude the possibility that the anaesthesia (or the surgery) would affect acid secretion differently between the genotypes, we measured basal and secretagogue-stimulated acid output in intact, non-anaesthetized animals. Consistent with the results from the acute fistula model, basal acid secretion was similar in wild-type and GFRα2-KO mice, and stimulation by a maximal dose of histamine (10 mg kg−1, s.c.) produced a similar (about 3-fold) increase in gastric acid output in both genotypes (Fig. 6). Pentagastrin (0.5 mg kg−1, s.c.) increased acid output (by about 2-fold) in wild-type mice but not in GFRα2-KO mice (Supplemental Fig. 4).
Figure 6. Effect of histamine, hexamethonium and atropine on gastric acid output in fasted wild-type and GFRα2-KO mice without anaesthesia.
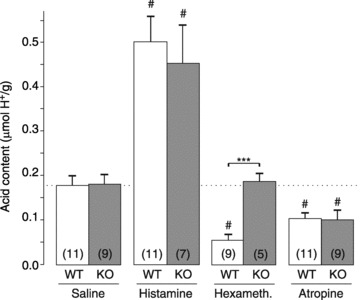
Histamine (10 mg kg−1), hexamethonium (20 mg kg−1), atropine (3 mg kg−1) or saline were administered subcutaneously 40–70 min before the animals were killed and gastric acid content analysed. ***P < 0.001 between genotypes; #P < 0.001 compared to saline. Number of animals per group is shown in parentheses.
Atropine but not hexamethonium reduces basal acid secretion in GFRα2-KO mice
The ganglion blocker hexamethonium is known to reduce basal acid secretion similarly to surgical vagotomy (Kay & Smith, 1951; Bunce & Parsons, 1977). Hexamethonium (20 mg kg−1, s.c.) reduced basal acid output in wild-type mice by ∼70% compared with saline treatment (P < 0.001). In contrast, hexamethonium treatment did not affect basal acid output in GFRα2-KO mice (Fig. 6). Interestingly, the muscarinic receptor antagonist atropine (3 mg kg−1, s.c.) reduced basal acid output similarly both in wild-type and in GFRα2-KO mice (Fig. 6).
Discussion
The main findings of this study are as follows. (1) GFRα2-KO mice lack virtually all efferent cholinergic innervation of the gastric oxyntic and pyloric mucosa resulting in a severely diminished acid secretory response to vagal stimulation by 2-deoxyglucose and no inhibition by hexamethonium. (2) Despite the loss of functional innervation gastric gland development, circulating gastrin levels, as well as basal and maximal histamine-stimulated gastric acid output were not impaired. (3) In comparison to wild-type mice, GFRα2-KO mice display a similar response to atropine but an increased sensitivity to carbachol suggesting that constitutive activity of muscarinic receptors facilitates basal acid secretion in the mice.
Cholinergic innervation of the gastric mucosa requires neurturin–GFRα2 signalling
Physiological stimuli from both inside and outside the stomach converge on the effector neurons in the gastric myenteric plexus that are the primary regulators of acid secretion (Schubert & Peura, 2008). Most of the effector neurons are cholinergic (60–65% of all myenteric neurons in rat) which provide the bulk of the gastric mucosal innervation but also project to the gastric muscle (Nakajima et al. 2000; Schicho et al. 2003). Subsets of the gastric cholinergic neurons coexpress neuropeptides including SP, VIP, GRP and pituitary adenylate cyclase-activating polypeptide (PACAP; Ekblad et al. 2000). The neuropeptides may mark putative functional specialization of gastric myenteric neurons: intrinsic neurons that coexpress GRP project predominantly to the mucosa, whereas intrinsic neurons that express SP project mainly to the muscle layer (Berthoud, 1996; Ekblad et al. 2000). We show here that the cholinergic innervation of gastric mucosa is profoundly (>90%) reduced in adult GFRα2-KO mice compared with wild-type animals, whereas cholinergic innervation of gastric muscle layers is not affected. We propose that the selective expression of neurturin mRNA in the gastric mucosa but not in the myenteric layer, explains the selective loss of cholinergic innervation only in the gastric mucosa in GFRα2-KO mice.
Gastric mucosal morphogenesis and maturation of secretory function takes place during the first postnatal weeks in rodents (for a review see Keeley & Samuelson, 2010). The selective expression of neurturin in the 2-week-old mouse gastric mucosa suggests that the development of gastric mucosal intrinsic innervation requires neurturin–GFRα2 signalling during first postnatal weeks. Consistent with this finding, our results indicate that the innervation deficit in gastric mucosa is present already in 2-week-old GFRα2-KO mice (Supplemental Fig. 5). Furthermore, the continuing expression of neurturin mRNA in the gastric mucosa (and its receptors in the myenteric neurons) in adult mice suggests that the maintenance of gastric mucosal innervation may also depend on neurturin–GFRα2 signalling. Although the exact identity of neurturin-expressing cells in the gastric mucosa is not known, the pattern of expression in the basal part of the mucosa suggests localization of neurturin mRNA in the endo/paracrine cells.
Non-cholinergic intramural neurons in the stomach include inhibitory nitrergic interneurons (20–25% of all intramural neurons in rat) many of which also express VIP and mainly innervate the gastric muscle and myenteric plexus (Pfannkuche et al. 1998; Nakajima et al. 2000; Berthoud et al. 2001; Schicho et al. 2003). The normal density of VIP- and PGP9.5-positive innervation of the gastric muscle layers in the GFRα2-KO mice suggests that the non-cholinergic myenteric neurons do not require GFRα2 signalling. Interestingly, enteric glial cells were also largely missing (∼80% reduction) in the gastric mucosa of the GFRα2-KO mice. In some parasympathetic target organs, such as salivary and lacrimal glands and pancreas, which also lack virtually all cholinergic innervation in GFRα2-KO mice (Rossi et al. 2003), the glial (Schwann) cells are not affected (Supplemental Fig. 6). Since the sympathetic and sensory innervation of these tissues is more abundant than in the gastric mucosa, we propose that the unaffected non-cholinergic innervation in these tissues may provide enough trophic support for the accompanying glial cells. Our results indicate that GFRα2 signalling is selectively needed for the development and/or maintenance of intrinsic cholinergic neurons and accompanying glial cells in the gastric mucosa. Since the cholinergic nerve fibres comprise the bulk of mucosal innervation, most of the intrinsic neurons that innervate the gastric mucosa require GFRα2 signalling for target innervation.
Intrinsic cholinergic innervation is not required for long term trophic support of gastric mucosa in mice
Gastrin is a major stimulant of gastric mucosa proliferation. In addition, the vagus nerve is thought to exert trophic support on the gastric mucosa since unilateral vagotomy in rats leads to atrophy of the gastric mucosa and reduces the density of ECL cells without altering gastrin levels (Håkanson et al. 1984). Since the gastric mucosa is not directly innervated by the efferent vagus, it is thought that the vagal control of ECL cells is mediated via the intramural neurons (Chen et al. 1999). One candidate is the neuropeptide PACAP that can regulate ECL cell proliferation (Lauffer et al. 1999) and gastric mucosal growth (Lu et al. 2011). Since PACAP is coexpressed in the VIP- and GRP-positive cholinergic nerve fibres in wild-type mouse gastric mucosa (Sundler et al. 1992), most if not all PACAP-containing innervation is expected to be missing in GFRα2-KO mouse gastric mucosa. However, since the thickness of gastric mucosa and the density of ECL cells were similar between GFRα2-KO mice and their wild-type littermates, trophic support of gastric mucosa does not seem to require cholinergic innervation in mice. Consistent with this, the importance of vagus on ECL cell proliferation has been questioned in other species including humans (Wangberg et al. 1996; Peghini et al. 2002). Yet, since the trophic effect of gastrin seems to depend on the M3 receptor (Aihara et al. 2003) and that the cholinergic innervation and muscarinic signalling have trophic effects on other epithelia (Rossi et al. 1999; Knox et al. 2010), further studies using the GFRα2-KO mice seem warranted.
Cholinergic innervation of the gastric mucosa is dispensable for gastrin secretion
Gastrin secretion from pyloric G-cells is stimulated by the luminal food as reflected by 2- to 3-fold higher serum gastrin levels in fed mice (Feng et al. 2010). In addition to the food-related luminal stimuli, gastrin secretion is thought to be regulated by messengers from other endocrine/paracrine cells and local neurons (Schubert & Makhlouf, 1992). However, the role of gastric mucosal innervation on basal and food-stimulated gastrin secretion has been controversial. Although GRP/bombesin is a potent stimulator of gastrin secretion (Schubert & Makhlouf, 1992; Ericsson et al. 2010a), studies using GRP receptor antagonists in humans indicate that GRP is dispensable for normal basal and food-induced gastrin secretion (Hildebrand et al. 2001). A recent antrum microdialysis study in rats concluded that food-stimulated gastrin release depends on a complex interplay between local neurons and the vagus (Ericsson et al. 2010b). Food-induced gastrin release was reduced by local infusion of neuronal blocker TTX but was paradoxically increased by unilateral vagotomy. This suggested that gastrin release depends on ‘local’ neurons and is predominantly inhibited by the vagus nerve. It should be noted that TTX and vagotomy also block the afferent (sensory) innervation of the gastric mucosa. In contrast, the GFRα2-KO mice lack only the efferent cholinergic innervation but have an apparently normal afferent innervation of the gastric mucosa. Since both fed and fasted circulating gastrin levels in the GFRα2-KO mice were similar to those in wild-type littermates, the efferent cholinergic nerve fibres in the gastric mucosa and their neurotransmitters (including GRP/bombesin) may not be necessary (and thus may not be physiologically important) in the control of normal gastrin secretion. Nevertheless, further studies on the role of local neurons in gastrin release seem warranted, e.g., whether anacidity-induced hypergastrinaemia that is proposed to be mediated by GRP/bombesin or by sensory neurons (Nojima et al. 2000) is affected in the GFRα2-KO mice.
Lack of efferent cholinergic innervation in the gastric mucosa does not affect basal and maximal histamine-stimulated acid secretion in GFRα2-KO mice: putative compensation by constitutive muscarinic receptor activity
Tonic activity of the efferent vagus is thought to be necessary for basal gastric acid secretion since vagotomy reduces basal gastric acid secretion by 70–90% in experimental animals as well as in humans (Håkanson et al. 1982; Debas & Carvajal, 1994). Basal acid secretion is also reduced by atropine and in M3 muscarinic receptor knockout mice (Aihara et al. 2003), consistent with the idea that acetylcholine, released from the intrinsic cholinergic mucosal nerve fibres following vagus nerve activity, stimulates basal gastric acid secretion via M3 receptors on parietal cells. In contrast, we found that basal gastric acid secretion was not reduced in the GFRα2-KO mice that lack virtually all cholinergic innervation of the gastric mucosa indicating that the basal acid secretion may not require efferent cholinergic innervation (and release of acetylcholine) in the gastric mucosa.
Cholinergic stimulation of gastric acid secretion is mediated by M3 and M5 muscarinic receptors in mice (Aihara et al. 2005), whereas activation of M2 or M4 receptors is thought to inhibit gastric acid secretion (Schubert & Peura, 2008). The increased carbachol-response in the GFRα2-KO mice suggests net sensitization of muscarinic receptor signalling in the gastric mucosa, although the underlying mechanism remains unclear. The possibility that residual acetylcholine release from the muscle layers (combined with the muscarinic receptor sensitization) would explain the maintained basal secretion can be excluded since hexamethonium treatment did not reduce basal acid secretion in the GFRα2-KO mice. This result also suggests that the KO mice have a complete loss of functional efferent innervation of the gastric mucosa.
Most if not all G protein-coupled receptors, including muscarinic receptors, are constitutively active, i.e., they can signal in the absence of agonist, although the physiological significance of constitutive receptor activity is less clear (Casarosa et al. 2010). Thus, muscarinic receptor blockers such as atropine are in fact negative antagonists (= inverse agonists) that inhibit both agonist-stimulated and constitutive, agonist-independent activity of the receptors (Burstein et al. 1997). Our finding that atropine reduced the basal acid output in the GFRα2-KO mice (to an extent similar to that in wild-type mice) suggests that constitutively active muscarinic receptors on parietal and/or D-cells may facilitate basal acid secretion in vivo. Confirmation of this hypothesis will require data from isolated gastric glands or cells. Interestingly, the lack of cholinergic innervation in GFRα2-KO mice is also associated with increased responsiveness to muscarinic agonists in urogenital organs (Nangle & Keast, 2006). Thus, we suggest that the increased carbachol responses in GFRα2-KO mice may reflect a compensatory upregulation of constitutive muscarinic receptor activity. However, we cannot exclude the possibility that there is also an increased sensitivity to histamine, which would indicate a more general sensitization of the system. In other words, this phenotype may reflect a functional adaptation to the life-long loss of a cholinergic/peptidergic input to ECL, D- and parietal cells. Assuming that GFRα2 signalling is also required for the maintenance of mucosal cholinergic innervation, a inducible GFRα2-knockout might allow the time course of the adaptation to be addressed.
Our results indicate that in vivo parietal cells can secrete normal amounts of gastric acid without the efferent cholinergic innervation in the mucosa during the basal state (Fig. 7). This conclusion is supported by previous studies using the isolated mouse stomach preparation (Black & Shankley, 1985; Schubert et al. 1987), as well as antrum microdialysis in conscious rats (Ericsson et al. 2010b) in which neuronal blocker TTX had no (or only a small) effect on basal gastric acid secretion. Understanding why basal acid secretion is reduced by blocking the vagal input but apparently not by blocking the myenteric neurons will require a more detailed knowledge of the gastric myenteric network function. Since gastric myenteric neurons that are encircled by a dense ring of vagal preganglionic varicosities are presumably more easily exited than others (Schemann & Grundy, 1992; Holst et al. 1997) tonic vagus activity may stimulate basal acid secretion via a selective subpopulation of gastric myenteric neurons. Future studies to directly monitor and manipulate the activity of the intramural nerve plexus in vivo, e.g., by optogenetic tools, may allow the underlying network mechanisms to be dissected.
Figure 7. Efferent innervation of gastric mucosa is dispensable for basal acid secretion in GFRα2-KO mice.
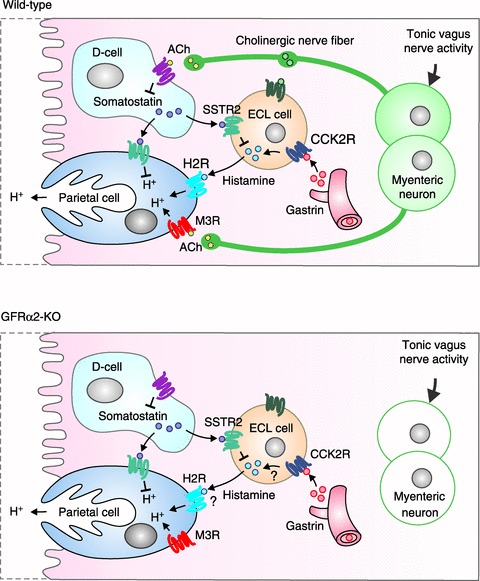
Upper panel, a model of basal gastric acid secretion in wild-type mice. Circulating gastrin (from antral G-cells, not shown) is thought to stimulate basal acid secretion largely by releasing histamine from ECL cells. Somatostatin from D-cells appears to exert tonic inhibition on acid secretion primarily by inhibiting histamine from ECL cells. Acetylcholine released from the intrinsic neurons in response to tonic vagus activity is thought to promote acid secretion via M3 receptors (M3R) on parietal cells but also by inhibiting tonic somatostatin release from D-cells. Lower panel, lack of cholinergic innervation in the gastric mucosa of GFRα2-KO mice does not impair basal acid secretion presumably partly because of compensatory upregulation of muscarinic receptor constitutive activity. The gastrin–histamine pathway may also be altered in the KO mice (as indicated by question marks; see Discussion for details). The role of neuropeptides (VIP, PACAP and GRP that are coexpressed in the mucosal cholinergic nerve fibres) in basal acid secretion is not clear. CCK2R, gastrin receptor; H2R, histamine H2 receptor, M3R, muscarinic M3 receptor; SSTR2, somatostatin receptor.
Vagotomy is also known to rapidly and permanently inhibit the maximal histamine-stimulated gastric acid secretion (Vallgren et al. 1983) but the underlying mechanism has remained unclear (Chen et al. 2005). Our observation that the maximal histamine-induced acid secretion was not affected in GFRα2-KO mice suggests that the parietal cells can maintain a normal acid secretory capacity to histamine without the intrinsic cholinergic innervation of the gastric mucosa. This is consistent with the previous studies in isolated mouse stomach preparations in which TTX treatment did not affect the maximal histamine-stimulated acid response (Angus & Black, 1982). We cannot exclude possible changes in histamine sensitivity or in the histamine–muscarinic signalling interaction in the GFRα2-KO mice at this stage. Further studies are also warranted to address the relative contribution of constitutive activity of different muscarinic (and possibly other) receptors to gastric functions.
A previous in vivo microdialysis study in rats suggested that the vagus nerve controls ECL cell sensitivity to gastrin (Norlen et al. 2005). This may explain why gastrin stimulated less acid output in the GFRα2-KO mice compared with wild-type controls. Confirmation of this hypothesis will require additional dose–response studies for gastrin-induced histamine mobilization and acid output.
In conclusion, the present work demonstrates that the efferent cholinergic innervation of the gastric mucosa requires neurturin–GFRα2 signalling but is not necessary for normal gastrin secretion, for basal or maximal acid secretion, or for trophic support of the gastric mucosa by the vagus. Our results suggest that basal acid output from the parietal cells in the GFRα2-KO mice can be maintained without innervation possibly in part by constitutively active muscarinic receptors (Fig. 7). Other possible compensatory mechanisms, such as sensitization to histamine, remain to be studied. In contrast, meal-induced acid secretion depends on the efferent cholinergic innervation consistent with the lack of gastric acid secretory responses to vagal stimulation in the GFRα2-KO mice. The GFRα2-KO mouse offers a unique model of a selective loss of the efferent cholinergic innervation in the gastric mucosa for future studies on the role of intrinsic neurons in gastric mucosal functions.
Acknowledgments
We thank Pertti Panula for antibodies, Vootele Voikar for statistical help and Kaija Berg for technical assistance. This study was supported by grants from the Sigrid Jusélius Foundation, Academy of Finland, University of Helsinki, and the Novo Nordisk Foundation. The authors have no conflicts of interest to disclose.
Glossary
- 2-DG
2-deoxyglucose
- ECL
enterochromaffin-like
- GDNF
glial cell line-derived neurotrophic factor
- GFRα2
GDNF family receptor alpha-2
- GRP
gastrin-releasing peptide
- HDC
histidine decarboxylase
- KO
knockout
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PGP9.5
protein gene product 9.5
- SP
substance P
- TH
tyrosine hydroxylase
- VAChT
vesicular acetylcholine transporter
- VIP
vasoactive intestinal peptide
Author contributions
The experiments were performed in the lab of M.S.A. J.K., J.R. and K.-H.H. acquired and analysed the data, M.S.A. designed and supervised the study. All authors contributed to the interpretation of data and writing the manuscript with intellectual consent. All authors approved the final version of the manuscript.
Supplementary material
Supplemental Fig. 1A--C
Supplemental Fig. 2A and B
Supplemental Fig. 3
Supplemental Table 1
Supplemental Fig. 4
Supplemental Fig. 5
Supplemental Fig. 6
References
- Aihara T, Fujishita T, Kanatani K, Furutani K, Nakamura E, Taketo MM, Matsui M, Chen D, Okabe S. Impaired gastric secretion and lack of trophic responses to hypergastrinemia in M3 muscarinic receptor knockout mice. Gastroenterology. 2003;125:1774–1784. doi: 10.1053/j.gastro.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Aihara T, Nakamura Y, Taketo MM, Matsui M, Okabe S. Cholinergically stimulated gastric acid secretion is mediated by M3 and M5 but not M1 muscarinic acetylcholine receptors in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1199–G1207. doi: 10.1152/ajpgi.00514.2004. [DOI] [PubMed] [Google Scholar]
- Angus JA, Black JW. The interaction of choline esters, vagal stimulation and H2-receptor blockade on acid secretion in vitro. Eur J Pharmacol. 1982;80:217–224. doi: 10.1016/0014-2999(82)90057-7. [DOI] [PubMed] [Google Scholar]
- Becker S, Niebel W, Singer MV. Nervous control of gastric and pancreatic secretory response to 2-deoxy-D-glucose in the dog. Digestion. 1988;39:187–196. doi: 10.1159/000199624. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Morphological analysis of vagal input to gastrin releasing peptide and vasoactive intestinal peptide containing neurons in the rat glandular stomach. J Comp Neurol. 1996;370:61–70. doi: 10.1002/(SICI)1096-9861(19960617)370:1<61::AID-CNE6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Zheng H. Vagal-enteric interface: vagal activation-induced expression of c-Fos and p-CREB in neurons of the upper gastrointestinal tract and pancreas. Anat Rec. 2001;262:29–40. doi: 10.1002/1097-0185(20010101)262:1<29::AID-AR1008>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Black JW, Shankley NP. How does gastrin act to stimulate oxyntic cell secretion. Trends Pharmacol Sci. 1987;8:486–490. [Google Scholar]
- Black JW, Shankley NP. The isolated stomach preparation of the mouse: a physiological unit for pharmacological analysis. Br J Pharmacol. 1985;86:571–579. doi: 10.1111/j.1476-5381.1985.tb08933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce KT, Parsons ME. The effect of hexamethonium on gastric acid secretion in the conscious rat. Agents Actions. 1977;7:507–511. doi: 10.1007/BF02111122. [DOI] [PubMed] [Google Scholar]
- Burstein ES, Spalding TA, Brann MR. Pharmacology of muscarinic receptor subtypes constitutively activated by G proteins. Mol Pharmacol. 1997;51:312–319. doi: 10.1124/mol.51.2.312. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Kiechle T, Sieger P, Pieper M, Gantner F. The constitutive activity of the human muscarinic M3 receptor unmasks differences in the pharmacology of anticholinergics. J Pharmacol Exp Ther. 2010;333:201–209. doi: 10.1124/jpet.109.163188. [DOI] [PubMed] [Google Scholar]
- Chen D, Friis-Hansen L, Håkanson R, Zhao CM. Genetic dissection of the signaling pathways that control gastric acid secretion. Inflammopharmacology. 2005;13:201–207. doi: 10.1163/156856005774423872. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao CM, Håkanson R, Samuelson LC, Rehfeld JF, Friis-Hansen L. Altered control of gastric acid secretion in gastrin-cholecystokinin double mutant mice. Gastroenterology. 2004;126:476–487. doi: 10.1053/j.gastro.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao CM, Lindstrom E, Håkanson R. Rat stomach ECL cells up-date of biology and physiology. Gen Pharmacol. 1999;32:413–422. doi: 10.1016/s0306-3623(98)00221-3. [DOI] [PubMed] [Google Scholar]
- Debas HT, Carvajal SH. Vagal regulation of acid secretion and gastrin release. Yale J Biol Med. 1994;67:145–151. [PMC free article] [PubMed] [Google Scholar]
- Ekblad E, Mei Q, Sundler F. Innervation of the gastric mucosa. Microsc Res Tech. 2000;48:241–257. doi: 10.1002/(SICI)1097-0029(20000301)48:5<241::AID-JEMT2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ericsson P, Håkanson R, Norlén P. Gastrin response to candidate messengers in intact conscious rats monitored by antrum microdialysis. Regul Pept. 2010a;163:24–30. doi: 10.1016/j.regpep.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Ericsson P, Håkanson R, Rehfeld JF, Norlén P. Gastrin release: Antrum microdialysis reveals a complex neural control. Regul Pept. 2010b;161:22–32. doi: 10.1016/j.regpep.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Feng J, Petersen CD, Coy DH, Jiang JK, Thomas CJ, Pollak MR, Wank SA. Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci U S A. 2010;107:17791–17796. doi: 10.1073/pnas.1009078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Håkanson R, Hedenbro J, Liedberg G, Vallgren S. Effect of vagotomy on gastric acid secretion in the rat. Acta Physiol Scand. 1982;115:135–139. doi: 10.1111/j.1748-1716.1982.tb07055.x. [DOI] [PubMed] [Google Scholar]
- Håkanson R, Vallgren S, Ekelund M, Rehfeld JF, Sundler F. The vagus exerts trophic control of the stomach in the rat. Gastroenterology. 1984;86:28–32. [PubMed] [Google Scholar]
- Hildebrand P, Lehmann FS, Ketterer S, Christ AD, Stingelin T, Beltinger J, Gibbons AH, Coy DH, Calam J, Larsen F, Beglinger C. Regulation of gastric function by endogenous gastrin releasing peptide in humans: studies with a specific gastrin releasing peptide receptor antagonist. Gut. 2001;49:23–28. doi: 10.1136/gut.49.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;381:81–100. doi: 10.1002/(sici)1096-9861(19970428)381:1<81::aid-cne7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kay AW, Smith AN. The effect of the ganglion-blocking methonium salts on gastric secretion and motility. Gastroenterology. 1951;18:503–517. [PubMed] [Google Scholar]
- Keeley TM, Samuelson LC. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1241–G1251. doi: 10.1152/ajpgi.00239.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329:1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer JM, Modlin IM, Hinoue T, Kidd M, Zhang T, Schmid SW, Tang LH. Pituitary adenylate cyclase-activating polypeptide modulates gastric enterochromaffin-like cell proliferation in rats. Gastroenterology. 1999;116:623–635. doi: 10.1016/s0016-5085(99)70184-8. [DOI] [PubMed] [Google Scholar]
- Lu Y, Germano P, Ohning GV, Vu JP, Pisegna JR. PAC1 deficiency in a murine model induces gastric mucosa hypertrophy and higher basal gastric acid output. J Mol Neurosci. 2011;43:76–84. doi: 10.1007/s12031-010-9440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Tooyama I, Yasuhara O, Aimi Y, Kimura H. Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats. J Chem Neuroanat. 2000;18:31–40. doi: 10.1016/s0891-0618(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Nangle MR, Keast JR. Loss of nitrergic neurotransmission to mouse corpus cavernosum in the absence of neurturin is accompanied by increased response to acetylcholine. Br J Pharmacol. 2006;148:423–433. doi: 10.1038/sj.bjp.0706760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima K, Sumii K, Sumii M, Okahara S, Haruma K, Yoshihara M, Kajiyama G. Acid-sensitive and alkaline-sensitive sensory neurons regulate pH dependent gastrin secretion in rat. Dig Dis Sci. 2000;45:1217–1226. doi: 10.1023/a:1005570507166. [DOI] [PubMed] [Google Scholar]
- Norlen P, Ericsson P, Kitano M, Ekelund M, Håkanson R. The vagus regulates histamine mobilization from rat stomach ECL cells by controlling their sensitivity to gastrin. J Physiol. 2005;564:895–905. doi: 10.1113/jphysiol.2005.082677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Häppölä O, Airaksinen MS, Auvinen S, Virkamäki A. Carbodiimide as a tissue fixative in histamine immunohistochemistry and its application in developmental neurobiology. J Histochem Cytochem. 1988;36:259–269. doi: 10.1177/36.3.3343510. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HY, Costa E. Neuronal location of the bombesin-like immunoreactivity in the central nervous system of the rat. Regul Pept. 1982;4:275–283. doi: 10.1016/0167-0115(82)90120-3. [DOI] [PubMed] [Google Scholar]
- Peghini PL, Annibale B, Azzoni C, Milione M, Corleto VD, Gibril F, Venzon DJ, Delle Fave G, Bordi C, Jensen RT. Effect of chronic hypergastrinemia on human enterochromaffin-like cells: insights from patients with sporadic gastrinomas. Gastroenterology. 2002;123:68–85. doi: 10.1053/gast.2002.34231. [DOI] [PubMed] [Google Scholar]
- Pfannkuche H, Reiche D, Sann H, Schemann M. Different subpopulations of cholinergic and nitrergic myenteric neurones project to mucosa and circular muscle of the guinea-pig gastric fundus. Cell Tissue Res. 1998;292:463–475. doi: 10.1007/s004410051075. [DOI] [PubMed] [Google Scholar]
- Pimont S, Bruley Des Varannes S, Le Neel JC, Aubert P, Galmiche JP, Neunlist M. Neurochemical coding of myenteric neurones in the human gastric fundus. Neurogastroenterol Motil. 2003;15:655–662. doi: 10.1046/j.1350-1925.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- Rossi J, Herzig KH, Voikar V, Hiltunen PH, Segerstråle M, Airaksinen MS. Alimentary tract innervation deficits and dysfunction in mice lacking GDNF family receptor α2. J Clin Invest. 2003;112:707–716. doi: 10.1172/JCI17995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Luukko K, Poteryaev D, Laurikainen A, Sun YF, Laakso T, Eerikäinen S, Tuominen R, Lakso M, Rauvala H, Arumäe U, Pasternack M, Saarma M, Airaksinen MS. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFRα2, a functional neurturin receptor. Neuron. 1999;22:243–252. doi: 10.1016/s0896-6273(00)81086-7. [DOI] [PubMed] [Google Scholar]
- Rossi J, Santamäki P, Airaksinen MS, Herzig KH. Parasympathetic innervation and function of endocrine pancreas requires the glial cell line-derived factor family receptor α2 (GFRα2) Diabetes. 2005;54:1324–1330. doi: 10.2337/diabetes.54.5.1324. [DOI] [PubMed] [Google Scholar]
- Schemann M, Grundy D. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am J Physiol Gastrointest Liver Physiol. 1992;263:G709–G718. doi: 10.1152/ajpgi.1992.263.5.G709. [DOI] [PubMed] [Google Scholar]
- Schicho R, Schemann M, Pabst MA, Holzer P, Lippe IT. Capsaicin-sensitive extrinsic afferents are involved in acid-induced activation of distinct myenteric neurons in the rat stomach. Neurogastroenterol Motil. 2003;15:33–44. doi: 10.1046/j.1365-2982.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- Schirmer BD. Current status of proximal gastric vagotomy. Ann Surg. 1989;209:131–148. doi: 10.1097/00000658-198902000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert ML, Edwards NF, Arimura A, Makhlouf GM. Paracrine regulation of gastric acid secretion by fundic somatostatin. Am J Physiol Gastrointest Liver Physiol. 1987;252:G485–G490. doi: 10.1152/ajpgi.1987.252.4.G485. [DOI] [PubMed] [Google Scholar]
- Schubert ML, Makhlouf GM. Neural, hormonal, and paracrine regulation of gastrin and acid secretion. Yale J Biol Med. 1992;65:553–560. discussion 621–623. [PMC free article] [PubMed] [Google Scholar]
- Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134:1842–1860. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Sundler F, Ekblad E, Absood A, Håkanson R, Koves K, Arimura A. Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience. 1992;46:439–454. doi: 10.1016/0306-4522(92)90064-9. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Hamada K, Yamada N, Sugita Y, Tonai S, Hunyady B, Palkovits M, Falus A, Watanabe T, Okabe S, Ohtsu H, Ichikawa A, Nagy A. Gastric acid secretion in L-histidine decarboxylase-deficient mice. Gastroenterology. 2002;122:145–155. doi: 10.1053/gast.2002.30312. [DOI] [PubMed] [Google Scholar]
- Vallgren S, Ekelund M, Håkanson R. Mechanism of inhibition of gastric acid secretion by vagal denervation in the rat. Acta Physiol Scand. 1983;119:77–80. doi: 10.1111/j.1748-1716.1983.tb07308.x. [DOI] [PubMed] [Google Scholar]
- Wängberg B, Nilsson O, Theodorsson E, Modlin IM, Dahlström A, Ahlman H. The effect of vagotomy on enterochromaffin-like cells in Mastomys natalensis. J Auton Nerv Syst. 1996;59:133–139. doi: 10.1016/0165-1838(96)00016-1. [DOI] [PubMed] [Google Scholar]
- Yang H, Wong H, Wu V, Walsh JH, Tache Y. Somatostatin monoclonal antibody immunoneutralization increases gastrin and gastric acid secretion in urethane-anesthetized rats. Gastroenterology. 1990;99:659–665. doi: 10.1016/0016-5085(90)90952-w. [DOI] [PubMed] [Google Scholar]
- Zhao CM, Martinez V, Piqueras L, Wang L, Tache Y, Chen D. Control of gastric acid secretion in somatostatin receptor 2 deficient mice: shift from endocrine/paracrine to neurocrine pathways. Endocrinology. 2008;149:498–505. doi: 10.1210/en.2007-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


