Abstract
Duodenal epithelial cells need efficient defence strategies during gastric acidification of the lumen, while colonic mucosa counteracts damage by pathogens by building up a bacteria-free adherent mucus layer. Transport of HCO3− is considered crucial for duodenal defence against acid as well as for mucus release and expansion, but the transport pathways involved are incompletely understood. This study investigated the significance of the electroneutral Na+–HCO3− cotransporter NBCn1 for duodenal defence against acid and colonic mucus release. NBCn1 was localized to the basolateral membrane of duodenal villous enterocytes and of colonic crypt cells, with predominant expression in goblet cells. Duodenal villous enterocyte intracellular pH was studied before and during a luminal acid load by two-photon microscopy in exteriorized, vascularly perfused, indicator (SNARF-1 AM)-loaded duodenum of isoflurane-anaesthetized, systemic acid–base-controlled mice. Acid-induced HCO3− secretion was measured in vivo by single-pass perfusion and pH-stat titration. After a luminal acid load, NBCn1-deficient duodenocytes were unable to recover rapidly from intracellular acidification and could not respond adequately with protective HCO3− secretion. In the colon, build-up of the mucus layer was delayed, and a decreased thickness of the adherent mucus layer was observed, suggesting that basolateral HCO3− uptake is essential for optimal release of mucus. The electroneutral Na+–HCO3− cotransporter NBCn1 displays a differential cellular distribution in the murine intestine and is essential for HCO3−-dependent mucosal protective functions, such as recovery of intracellular pH and HCO3− secretion in the duodenum and secretion of mucus in the colon.
Key points
In the upper intestinal tract, luminal acidity due to intermittent release of gastric juice needs to be counteracted by basolateral HCO3− import. In the lower gastrointestinal tract, the build-up of a thick mucus layer is a major defence mechanism against pathogens, and HCO3− is of the utmost importance for this process. The pathways for HCO3− transport that play a role in these mucosal defence strategies are, however, unknown.
We recently identified the electroneutral Na+–HCO3− cotransporter NBCn1 as a major regulator of intracellular pH in duodenal villous enterocytes. The present study shows that the murine duodenocytes, whose intracellular pH was monitored by in vivo two-photon confocal microscopy in anaesthetized NBCn1 knock-out mice, are unable to recover rapidly from intracellular acidification imposed by a short pulse of low-pH solution in the duodenal lumen. Likewise, they are not able to respond to contact of the surface with low pH by a protective HCO3− secretory response.
The cotransporter NBCn1 is also expressed in the basolateral membrane of colonic crypt cells, many of which stain positive for mucin granules. We found only a minor role for NBCn1 in colonic epithelial HCO3− secretion, but the build-up of a mucus layer, measured in the exteriorized colon of anaesthetized mice by in vivo microscopy, was significantly delayed in the absence of NBCn1 expression.
Therefore, NBCn1 plays major but different roles in mucosal protective functions in the upper and lower intestine.
Introduction
Bicarbonate ions play a major role in intestinal epithelial homeostasis, epithelial transport and mucosal defence against gastric acid in the upper gastrointestinal (GI) tract (Allen & Flemström, 2005; Kaunitz & Akiba, 2006; Seidler & Sjoblom, 2012). Recently, a novel role for HCO3− ions has been delineated in the expulsion and expansion of mucus granules in the airways, intestine and reproductive tract (Quinton, 2010b). A defective mucus barrier has been shown to be a risk factor for pathogen-mediated mucosal damage in the colon by both a normal (Johansson et al. 2008; Petersson et al. 2011) and a pathogenic intestinal flora (Bergstrom et al. 2010; Hasnain et al. 2010).
The ionic pathways for intestinal HCO3− transport, which are activated during acid challenge and are important for acid defence in the upper GI tract and for formation of a mucus layer in the lower GI tract, have been only partly delineated. A crucial role of the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel in the coordination of agonist-stimulated HCO3− secretion has been established in both rodent and human intestine (Seidler et al. 1997; Pratha et al. 2000). In addition, apically expressed members of the Slc26 family of anion exchangers are involved in apical HCO3− extrusion (Tuo et al. 2006; Walker et al. 2009). However, the rate-limiting step for HCO3− secretion may be the uptake and/or generation of HCO3− ions rather than apical HCO3− transporter activation (Jacob et al. 2000), and recent studies are in support of this concept (Xiao et al. 2012b).
It has been demonstrated that HCO3−-dependent mucosal defence against luminal acid does not necessarily require HCO3− to be secreted into the lumen, but does require intact basolateral HCO3− uptake mechanisms, which are rapidly able to neutralize the protons that have accumulated in the enterocytes during luminal acid exposure (Akiba et al. 2001b). Thus, the first aim of this study was to assess intracellular pH (pHi) recovery after luminal acid exposure in vivo and the importance of NBCn1 for this regulation. The second aim was to study the importance of NBCn1 expression for the luminal acid-induced HCO3− secretory response. Expression of NBCn1 has also been detected in the colonic mucosa, albeit at lower levels than in the duodenum (Chen et al. 2012). The third aim of the study was therefore to obtain insight into the physiological function of colonic mucosal NBCn1.
The experimental strategies employed in this study were predominantly in vivo experiments, in which we measured the intracellular and the surface epithelial pH and the build-up of the colonic mucus layer using two-photon microscopic techniques in exteriorized, vascularly perfused duodenal and mid-distal colonic mucosa of anaesthetized mice. In addition, we assessed duodenal and mid-colonic fluid absorption and/or HCO3− secretory rates in luminally perfused, anaesthetized, acid–base status-controlled NBCn1 knock-out (KO) and wild-type (WT) mice. These experiments were supplemented by pHi-stat titration of HCO3− secretory rates in isolated colonic mucosa and immunohistochemical investigations.
Methods
Animals
Mice of the Slc4a7-gene-deleted strain, whose establishment and characteristics have been described elsewhere (Boedtkjer et al. 2011), were bred at Hannover Medical School in standard temperature and light conditions and were allowed free access to food and water, as described previously (Chen et al. 2012). The mice were age and sex matched and used in full adulthood, between 10 and 16 weeks of age. All experiments involving animals were approved by the Hannover Medical School Committee on investigations involving animals and an independent committee assembled by the local authorities.
Measurement of in vivo fluid absorption and bicarbonate secretion
Surgical procedures
The procedure for duodenal cannulation has been described in detail (Singh et al. 2012). The mice were anaesthetized by a spontaneous inhalation of isoflurane (Forene; Abbott Germany, Wiesbaden, Germany). The inhalation gas contained a mixture of ∼10–15% oxygen, ∼85–90% air, and 2.0 ± 0.2% isoflurane with the use of an isoflurane pump (Univentor 1250 Anaesthesia Unit; AgnTho, Lidingö, Sweden) and, after tracheal intubation, they were mechanically ventilated (MiniVent Type 845; Hugo Sachs Electronik, March-Hugstetten, Germany) and a catheter was placed in the left carotid artery for continuous infusion of a solution of the following composition (mm) to correct the systemic acid–base balance: 200 Na+, 100 HCO3−, 0.005 K+ and 0.005 Cl−, at a rate of 0.3 ml h−1. Animals were maintained at 37°C using a heating pad. After making a mid-line incision in the abdomen, the middle (starting ∼2.5 cm away from caecocolonic junction) and distal colon (last 1.5 cm) with intact blood supply were selected for the experiments. The middle colon was opened about 2 cm distal to the caecocolonic junction. A small polyethylene tube (PE-100; inner diameter 1 mm) with a distal flange was inserted and secured by a ligature, then the middle and distal colon was perfused gently to remove the contained faeces. The outlet tube (PE-200; inner diameter 2 mm) was put through the rectum and fixed at the anal canal. The middle and distal colon was perfused (Gilson minipuls evolution, Villiers, France) at a rate of 30 ml h−1 with normal saline using a single pass. The abdomen was closed after the surgery, and then the mice were left to stabilize for 20 min for fluid equilibration after the surgical trauma.
Measurement of colonic fluid absorption and HCO3− secretion
Measurement of fluid absorption started after the initial stabilization period of 20 min. Before perfusion, the perfusate and the collecting tubes were weighed after adjusting the pH of the perfusate to 7.0 in a 37°C water bath. After perfusion for 20 min, the perfusate and the collecting tubes were weighed again. The reduction in weight after perfusion was taken as the total fluid loss during the perfusion period. After each experiment, the blank fluid loss due to water evaporation, adhesion on the tube tip and other non-intestinal absorptive reasons was measured in the same perfusion conditions (37°C water bath, 30 ml h−1 perfusion speed, 20 min period) but without intestinal perfusion. The difference between total fluid loss and blank fluid loss was taken as the absorptive fluid loss. After the experiment, the mice were killed by cervical dislocation and the colonic segment was measured. The fluid absorption rates (in microlitres per centimetre per hour) were calculated from the absorptive fluid loss (assuming a fluid density of 1 ml mg−1), colonic length and perfusion time.
The measurement of in vivo colonic HCO3− secretion was very similar to that for duodenal HCO3− secretion (Xiao et al. 2012a).
Two-photon in vivo measurements
Measurement of pHi in the duodenocytes
The duodenum of the anaesthetized mouse was exteriorized with an intact blood supply, opened near the mesenteric axis and mounted on a custom-made perfusion chamber as previously described (Atuma et al. 2001; Sjöblom et al. 2009). After surgery, the exposed duodenal segment was incubated with 1 ml of 6 mm acetylcysteine (ACC) solution in saline for 15 min. This was followed by forced washing with the saline using a 10 ml syringe to get rid of the accumulated mucus. The loading of the duodenocytes of the villi was achieved by incubation with 20 μm SNARF-1 AM for 10 min in saline containing 6 mm ACC. The loading solution was gently taken away using a syringe and substituted with unbuffered saline, pH ∼6. The basal intracellular pH was measured for the next 10 min (every 5 min) followed by exposure of the villi to saline of pH 2.5 for another 5 min. The pH was recorded every 5 min. The low-pH saline was substituted with unbuffered saline after the 5 min exposure, and pH was measured for another 25 min. The intracellular pH was measured at 100, 200 and 300 μm from the villus tip. SNARF-1 was excited at 780 nm, and the emission was collected at 580 nm (523–605 nm) and 640 nm (610–700 nm), using a two-photon laser scanning microscope with an upright Leica TCS SP2 confocal microscope with a ×20 water immersion objective and a MaiTai Ti:sapphire-pulsed laser (Spectra-Physics, Darmstadt, Hessen, Germany). An in vitro calibration curve was made using solutions of different pH, as has been described previously (Sjöblom et al. 2009).
Measurement of epithelial surface pH in the colon
Surgery was performed in similar way to that for the duodenal segment. The middle colon of the anaesthetized mouse was exteriorized with an intact blood supply, opened near the mesenteric axis, and the distal part of the middle colon, corresponding to the descending colon in man, which we call ‘mid-distal’ in the text, was mounted on a custom-made perfusion chamber. After surgery, the exposed colonic mucosa was incubated with the unbuffered saline containing 5 μm SNARF-1 free acid. The pH measurement was started 10 min after the surgery had been completed (the time lag to move the mouse from the room where surgery was performed to the microscope and to start the measurement). The measurement was taken every 10 min for 1 h near the epithelial surface and 100 μm above the surface in the mucus layer.
Measurement of the thickness of the accumulated mucus
After surgery, the exposed mucosal surface was covered with 1 ml of unbuffered saline, and fluorescent polystyrene beads (1.0 × 106 beads ml−1; FluoSpheres® Polystyrene Microspheres (Darmstadt, Hessen, Germany), 15 μm, Crimson Fluorescent 625/645 Invitrogen, F-8839) were gently placed over the saline and allowed to settle by gravity for about 5 min. These large beads were chosen so that they would stick to the surface of the mucus and would not sink in. The beads were placed at different time points to assess the build-up of the mucus over time (0, 30 and 60 min post-surgery) and the surface was measured by simultaneous scanning at 488 and 633 nm in x–y planes along the z-axis using an upright Leica TCS SP2 confocal microscope with a ×20 water-immersion objective. A total of 50 stacks were taken to cover the distance in the z-direction, starting from the visualization of beads at the top followed by the surface at the bottom. The 488 nm wavelength was used in reflection mode to visualize the tissue, while 633 nm was used to excite the Alexa 633-labelled fluorescent beads. Further details and illustration of the technique can be found in Fig. 6.
Figure 6. Colonic NBCn1 and mucin 2 (Muc2) staining pattern and method for mucus layer build-up.
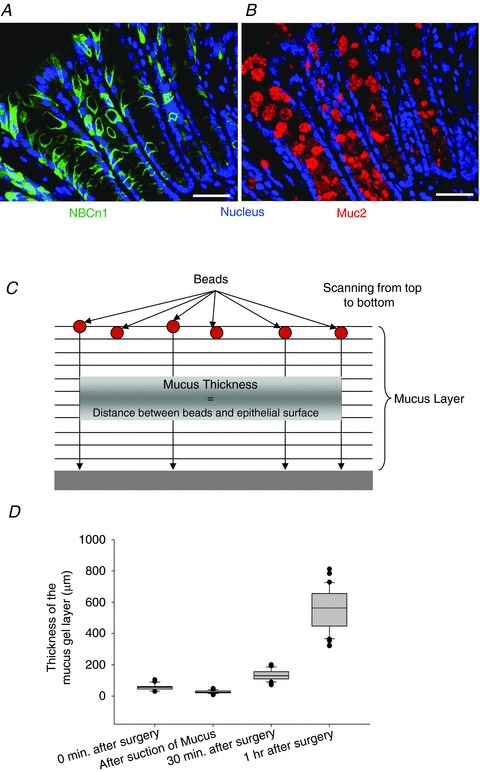
Staining for NBCn1 (A) and Muc2 (B) in murine distal colon suggests expression in the same cell compartment. Scale bars represent 50 μm. C, schematic image of the fluorometric assessment of colonic mucus layer build-up on the exteriorized colonic mucosal surface in vivo. Fluorescent beads were allowed to settle onto the surface of the mucus layer, and laser scanning was performed in parallel planes down to the epithelial surface, which can be detected by reflection of light from the epithelial surface. The mean distance between each bead and the epithelium in the z-axis was taken as a measurement of the accumulated mucus layer. D, the box plots represent the thickness of the accumulated mucus at different time points during the study. After mounting the colonic mucosa in the perfusion chamber, a little loose mucus remains attached (leftmost bar). The next column indicates the thickness of the mucus when the loosely adherent layer was gently sucked off. This represents the mucus layer thickness of the firmly adherent layer. As time progressed, the thickness of the accumulated mucus increased.
Ussing chamber experiments
Isolated colonic mucosa from NBCn1-deficient mice and WT littermates was placed in Ussing chambers and secretory studies were performed according to protocols identical to those described before (Tuo et al. 2006), except that open-circuit conditions were used, potential difference and electrical resistance were continuously recorded, and the short-circuit current was calculated as described before (Xiao et al. 2012b).
Fluorometric pHi measurements and determination of base uptake rates into NBCn1 KO and NBCn1 WT colonic enterocytes
Preparation of colonic crypts
Murine colonic crypts were isolated and pH measurement in the base of the glands was performed as previously described (Bachmann et al. 2006). To inhibit the Na+–H+ exchangers NHE1, NHE2 and NHE3, 1 μm (for NHE1) or 50 μm (for NHE1 and NHE2) cariporide mesilate (HOE642) and 20 μm S1611 (for NHE3 inhibition; both from Sanofi Aventis, Frankfurt, Germany) were used.
Immunohistochemistry
Immunohistochemistry for duodenal and colonic NBCn1 was performed as previously described (Chen et al. 2012). For the NBCn1 and mucin 2 (Muc2) staining, mouse mid-distal colon was flushed with PBS, fixed with 4% paraformaldehyde, paraffin embedded, and cut in serial sections of 2 μm thickness. For the NBCn1 antibody, heat-induced epitope retrieval was performed with Dako Target Retrieval Solution pH9 (Dako, Glostrup, Denmark) for 20 min at 96°C. The Muc2 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) slides were stored in PBS until both were blocked in 10% natural horse serum. The first antibodies were applied overnight at 4°C, and the corresponding secondary antibody with 0.5 μg ml−1 4′,6-diamidino-2-phenylindole (DAPI) fluorescent stain for 1 h at room temperature.
Mucosal thickness
For measurements of the thickness of the firm mucus layer, the protocol was followed as described by Johansson et al. (2008). The excised colon was directly fixed in Methacarn (methanol:chloroform:glacial acetic acid 6:3:1), paraffin embedded without contact with water and cut into serial sections of 5 μm thickness. The Muc2-stained slides of three NBCn1 KO and three control mice were viewed at ×10 magnification. Two images of three different colonic sections from each mouse within similar colonic segments were taken, and the thickness of the mucus was measured with the ‘line’ tool of ImageJ Software (NIH, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/) at six random positions per image (108 data points per group). The many data points allow for some compensation of the occasional very obvious difficulty in defining the borders of the firmly adherent layer (see lower right panel of Fig. 8A).
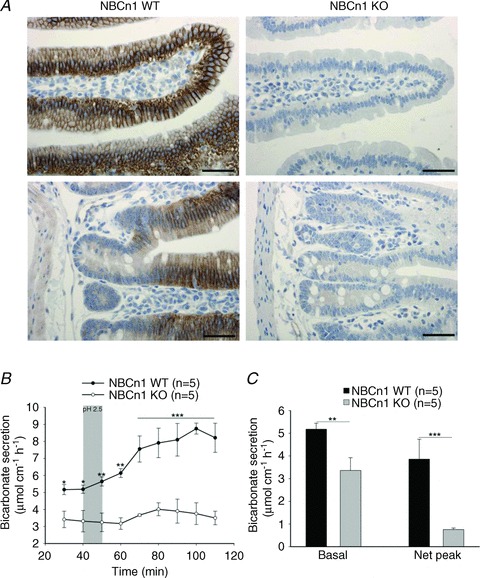
Figure 8. Assessment of the adherent mucus layer in Carnoy-fixed mid-distal colon.
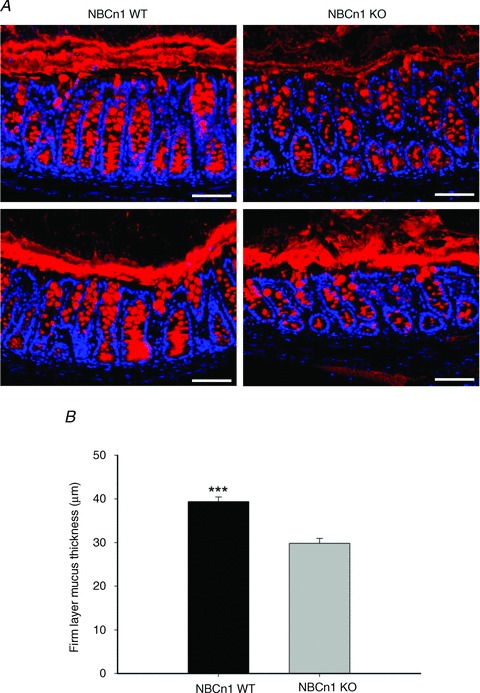
A, Muc2 staining of the colonic mucus layer in NBCn1 KO and NBCn1 WT mice. Two examples of mucus layer staining for WT (two left panels) and for NBCn1-deficient mice (two right panels) are displayed, although the majority of images looked like the upper two images. Scale bars represent 75 μm. B, systematic assessment of the thickness of the mucus (see Methods) in sex- and age-matched NBCn1 KO and WT mice revealed a slight but significant reduction in mucus layer thickness in NBCn1 KO mid-distal colon compared with WT littermates. n= 3. ***P≤ 0.001
Statistical evaluation
Descriptive statistics are expressed as the means ± SEM, with the number of mouse pairs (KO and WT) or individual mice, if applicable, given in parentheses. Statistical analyses were performed using the two-tailed Student's t test for unpaired data. Results were considered significant at P < 0.05.
Results
Recovery of pHi from intracellular acidification after luminal acid exposure is severely compromised in NBCn1-deficient duodenal mucosa
Duodenal enterocyte pHi was assessed at different distances from the tip of the villus. Interestingly, pHi decreased in the enterocytes, even in the lower part of the villi, in response to a short exposure of the duodenal surface to a pH of 2.5 (Fig. 1A–C), which has been found in previous experiments not to result in any increase in duodenal permeability (Sjöblom et al. 2009) and which did not result in any histologically visible acid damage to the villi in this study as well (see Fig. 1D). In WT enterocytes, rapid acidification was observed at all three distances from the duodenal villus tips, followed by rapid pHi recovery after removal of luminal acid (Fig. 1A–C). NBCn1-deficient enterocytes acidified with the same speed in the upper part of the villi, while in the mid and lower part acidification appeared somewhat delayed, but this did not reach the level of significance. However, while pHi recovery was observed in WT enterocytes after removal of the luminal acid load, this was not observed in the NBCn1 KO enterocytes during the period of observation. The results demonstrate that basolateral NBCn1 expression is essential for swift pHi recovery after the cellular acidification that follows the exposure of the mucosa to a low luminal pH. Furthermore, the results demonstrate that even in WT mice with maintenance of systemic acid–base status in the physiological range (see Table 1) during the experiment, luminal acid nevertheless results in acidification, albeit of brief duration, of the enterocytes even in the deeper areas of the villi.
Figure 1. NBCn1 knock-out (KO) mice failed to recover after luminal acid-induced intracellular acidification.
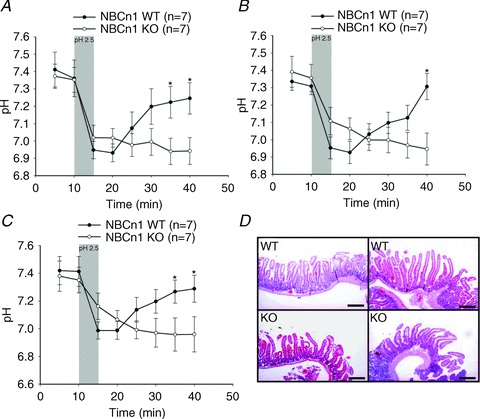
A–C show time course experiments for enterocyte pHi measured at different distances from the villus tip (A, 100 μm; B, 200 μm; and C, 300 μm) in SNARF-1 AM-loaded villi of luminally perfused, exteriorized duodenum in anaesthetized NBCn1 WT and NBCn1 KO mice. Villous enterocyte pHi recovered quickly after the removal of acidic saline in WT duodenum, while almost no pHi recovery was observed in NBCn1 KO duodenocytes during the observation period. The shaded bars indicate the time period for the application of low pH. The numbers of mice are given in parentheses. *P≤ 0.05 between the groups. D, Haematoxylin and Eosin staining of the duodenum after the experiments, indicating that the short exposure of the mucosa to pH 2.5 did not result in discernible villous tip damage and that no histological difference was detected in the duodenum of NBCn1 WT and NBCn1 KO mice. Scale bars represent 500 μm.
Table 1.
Blood gas analysis of NBCn1 wild-type and NBCn1 knock-out mice taken at the end of the experiment
| Genotype | pH |
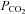 (mmHg) (mmHg) |
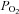 (mmHg) (mmHg) |
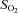 (%) (%) |
HCO3− (mmol l−1) | ABE (mmol l−1) | SBE (mmol l−1) | SBC SBC (mmol l−1) |
|---|---|---|---|---|---|---|---|---|
| NBCnl (+/+; n=6) | 7.43 ± 0.02 | 41.50 ± 2.47 | 98.17 ± 9.79 | 97.00 ± 0.63 | 27.00 ± 0.97 | 3.00 ± 0.86 | 2.83 ± 0.87 | 26.83 ± 0.79 |
| NBCnl (–/–; n=8) | 7.43 ± 0.02 | 40.50 ± 1.99 | 103.50 ± 8.25 | 97.63 ± 0.42 | 26.25 ± 1.08 | 2.38 ± 1.03 | 2.50 ± 1.07 | 27.88 ± 1.59 |
Both the wild-type group and the knock-out group of mice were infused with same carotid solution for the correction of systemic acidosis caused by anaesthesia (see Methods). There was no significant difference between the two groups during the experiment. SO2, Oxygen saturation; ABE, Actual base excess; SBE, Standard base excess and SBC, Standard bicarbonate content.
Acid-induced stimulation of duodenal bicarbonate secretion is severely compromised in NBCn1-deficient duodenum in vivo
Figure 2A demonstrates the strong NBCn1 expression exclusively in the duodenal villous region. This is also the area of the strongest acid exposure. We therefore assessed the ability of the duodenal mucosa to mount a HCO3− secretory response to a brief exposure to luminal acid [the same pH (pH 2.5) and exposure time (5 min) used in the experiments described in the previous section]. While 5 min acid exposure stimulated a long-lasting increase of HCO3− secretory rate for the full hour of experimental observation in WT mice, only a mild and delayed stimulation was observed in the NBCn1-deficient mucosa (Fig. 2B and C). This is interesting because the duodenum of these mice had been able to respond to forskolin (FSK) with a HCO3− secretory response both in vivo and in vitro, albeit reaching peak values that were significantly below those of the WT response (Chen et al. 2012). Given that steady-state pHi was not significantly different in the enterocytes of NBCn1-deficient mice compared with the WT enterocytes in vivo, we assume that the virtual absence of a HCO3− secretory response to luminal acid in the NBCn1-deficient mice in this study may be due to an insufficient capacity of the enterocytes to import HCO3− via the basolateral membrane after the intracellular acidification due to low luminal pH (see Fig. 1A–C). However, an additional effect of NBCn1 deficiency in the vasculature or even the nervous system (Boedtkjer et al. 2008) cannot be ruled out, because a short pulse of luminal acid-induced HCO3− secretion requires intact neural circuitry (Singh et al. 2012).
Figure 2. Acid-induced duodenal bicarbonate secretion was strongly reduced in the NBCn1 KO mice.
A, the strong NBCn1-dependent immunofluorescence signal was seen only in the basolateral membrane of duodenal villous cells (top panels), not of crypt enterocytes (bottom panels). Scale bars represent 100 μm. B and C, application of acid of pH 2.5 for 5 min elicited an almost 2fold increase in the duodenal bicarbonate secretion in NBCn1 WT mice, whereas the HCO3− secretory response was virtually absent in NBCn1 KO duodenum. Time course (B) and bar graph representation of the results (C), as basal duodenal bicarbonate secretion rate and net peak HCO3− secretory response. Net peak was calculated by subtracting the average basal response from the peak value for each experiment. The shaded bar in B indicates the time of acid exposure (5 min), followed by 5 min wash with unbuffered saline. The numbers of mice are given in parentheses. *P≤ 0.05, **P≤ 0.01 and ***P≤ 0.001 between the groups.
Recovery of pHi after NH4Cl-induced acidification is not significantly different from WT in the basal crypt cells of isolated NBCn1-deficient colonic enterocytes
Studies in NBCn1 promoter-driven LacZ-expressing mice had indicated that the colonic mucosa also expresses NBCn1 (Boedtkjer et al. 2008), which was confirmed by Chen et al. (2012) by real-time PCR (qPCR). Here we show immunohistochemical evidence for NBCn1 expression in the cryptal region of the mid-colonic mucosa (Fig. 3A). However, when pHi recovery was measured after acidification by the ammonium prepulse technique in the presence of bicarbonate buffer (CO2/HCO3−), base import rates after complete inhibition of NHEs with HOE642 [which inhibits murine intestinal NHE1 and NHE2 (Bachmann et al. 2004) and is said also to inhibit NHE8 (Xu H and Ghishan FK) personal communication] as well as S1611 [which inhibits NHE3 as well as NHE8 (Xu et al. personal communication)] were low and not different in NBCn1 WT and KO mice (Fig. 3B and C).
Figure 3. NBCn1 is expressed in the basolateral membrane of colonic crypt cells but appears not to be significantly involved in pHi recovery from intracellular acid loads.
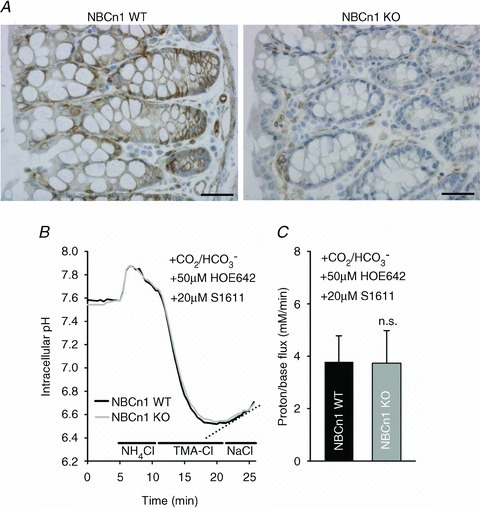
A, NBCn1 staining in colonic mucosa is markedly weaker than in the duodenum, and is crypt predominant. Scale bars represent 100 μm.B, representative pHi trace of cells at the base of the colonic crypts from mid-distal colon during an ammonium prepulse and recovery phase in the presence of inhibitors for the colonic NHEs (Bachmann et al. 2004). C, the low non-NHE-mediated pHi recovery rate from intracellular acidification was not different in NBCn1 KO and WT crypts. n= 5.
Experiments were also performed to delineate other important pHi regulatory mechanisms in colonic crypt cells. As shown in detail previously, the predominant part of pHi recovery from an intracellular acid load is mediated by Na+–H+ exchange even in the presence of CO2/HCO3− (Bachmann et al. 2003). A minor part is CO2/HCO3− dependent and inhibited by the NBC inhibitors S0859 (Bachmann et al. 2003) or DIDS (Cinar A and Seidler U unpublished). Given the fact that the presence or absence of NBCn1 expression does not alter this CO2/HCO3−-dependent, non-NHE-mediated pHi recovery (Fig. 3B and C), it is likely to be mediated by NBCe1, which is also expressed in the crypt basolateral membrane (Yu et al. 2009).
We therefore performed experiments in the absence of CO2/HCO3−, and used different concentrations of the NHE inhibitor HOE642 to inhibit sequentially NHE1, and NHE1 and NHE2, assessing pHi recovery individually in the surface cells near cryptal mouth and the basal regions of the crypts, as demonstrated visually by Cinar et al. (2007). Figure 4 shows that in the basal regions of the crypt, pHi recovery after an acid load is mediated ∼40% by NHE1 and ∼60% by NHE2, and no residual proton flux remains with addition of 50 μm HOE642. In the surface regions, NHE1 and NHE2 mediate <30% of pHi recovery. Given that the selective NHE3 inhibitor S1611 has been shown to inhibit rat NHE1 at higher concentrations, we used NHE3 KO mice to assess how much of the residual HOE642-insensitive pHi recovery in the WT was mediated by NHE3. When identical experiments were performed in NHE3 KO mice, the proton flux in the near-surface cells of the cryptal mouth region in the presence of 50 μm HOE642 was 4.2 mm min−1, in comparison to ∼25 mm min−1 in WT mice. The remaining ∼15% of Na+-dependent proton efflux, which is only seen in the surface colonocytes, is due to non-identified transport mechanisms.
Figure 4. Differential involvement of the NHE isoforms in pHi recovery from an acid load in the apical and basal portion of WT colonic crypts.
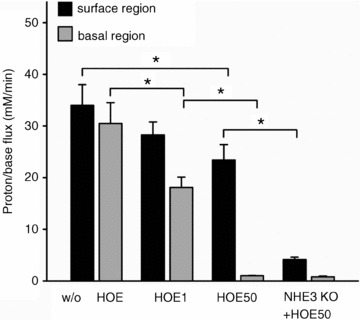
Proton efflux rates after ammonium prepulse-induced acidification in the surface and the basal portions of WT colonic crypts with no added inhibitor (w/o HOE; left two bars), with an NHE1-selective concentration of HOE642 (1 μm), and with 50 μm HOE642, which inhibits NHE1 and NHE2 but not NHE3 (Bachmann et al. 2004). In the absence of CO2/HCO3−, pHi recovery is completely inhibited by 50 μm HOE642 in the basal part of the crypts, demonstrating that NHE1 and NHE2 are responsible for NHE-mediated proton extrusion. In the cryptal mouth region near the surface, the major part of proton extrusion was not HOE sensitive. When the identical experiments were performed in NHE3-deficient colonic crypts, proton extrusion rates in the presence of 50 μm HOE642 were very low in the surface and basal regions. This suggests that the major acid extruder in the surface region is NHE3, but a residual ∼15% of Na+-dependent proton extrusion is performed by unidentified transporters. *P < 0.05, n= 6.
Basal and agonist-stimulated colonic mucosal HCO3− secretory rates in vitro show only very subtle differences between KO and WT mice
We next measured the HCO3− secretory rates in the different segments of isolated murine colonic mucosa. As we had previously observed that basal alkalinization rates in colonic mucosa are strongly Cl− dependent and mediated by the chloride anion exchanger DRA (Slc26a3), we first measured basal HCO3− secretory rates in WT and NBCn1-deficient proximal and mid-distal colonic mucosa, but performed luminal Cl− substitution before stimulation with agonists (Fig. 5A and B). The removal of luminal Cl− results in inhibition of Cl−–HCO3− exchange-mediated HCO3− export, a strong reduction in basal colonic mucosal alkalinization rates and an enhancement of the agonist-stimulated HCO3− secretory response (Xiao et al. 2012b). The basal, carbachol (CCh)- and FSK-stimulated colonic HCO3− secretory rates were not significantly different in the proximal or the mid-distal isolated colonic mucosa of NBCn1-deficient and WT mice (Fig. 5A and B). We next assessed the fluid absorptive rate in the mid-distal colon in anaesthetized, ventilated and acid–base-controlled mice by a luminal perfusion technique, as well as the HCO3− secretory rate in these conditions (Fig. 5C and D). No significant differences in the fluid absorptive rates (Fig. 5D) were observed between NBCn1-deficient and WT mice during luminal perfusion with unbuffered iso-osmolar saline, pH 7.0 (as well as after stimulation of fluid absorption by switching to an iso-osmolar CO2/HCO3−-containing solution of identical pH 7.0; data not shown). Luminal application of 10−5 m FSK resulted in a similar inhibition of fluid absorption in NBCn1-deficient and WT mice, but the concomitant stimulation of HCO3− secretion was slightly but significantly lower in NBCn1-deficient mice (Fig. 5C, right bars). Thus, the in vivo experiments were able to unmask a mild HCO3− secretory deficit in NBCn1-deficient mid-distal colon.
Figure 5. Colonic HCO3− secretion and fluid absorption in NBCn1 KO and WT mid-distal colon.
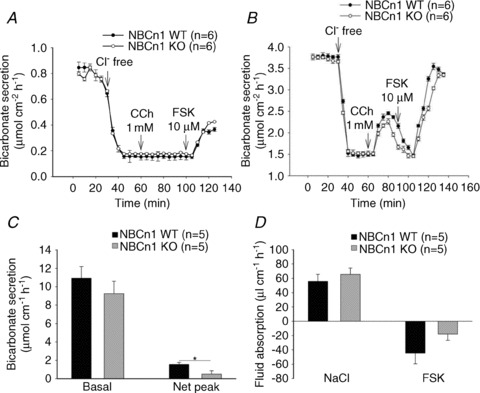
Bicarbonate secretory rates in isolated proximal (A) and mid-distal isolated colonic mucosa (B) in the Ussing chamber. After determination of the basal rate, luminal Cl− was removed to inhibit Cl−–HCO3− exchange and enhance agonist-mediated HCO3− secretion (Vidyasagar et al. 2004). No differences in basal, Cl−-free and agonist-stimulated HCO3− secretary rates were observed between NBCn1 KO and WT colonic mucosa. C, measurement of HCO3− secretory rates in the basal state (left bars) and forskolin (FSK; 10 μm)-induced change in HCO3− secretory rates (right bars) in luminally perfused mid-distal colon in anaesthetized mice. D, measurement of net fluid transport in the same experimental set-up. Net absorptive rates are shown in the basal state, and the right bars indicate the fluid secretory state after luminal FSK application. No difference was seen in basal HCO3− secretory rate or in the fluid absorptive and fluid secretory response to FSK in NBCn1 KO and WT colon, but a slight but significant difference was observed in the HCO3− secretory output after FSK. The numbers of mice are given in parentheses. *P≤ 0.05 between the groups.
NBCn1 appears to be strongly expressed in the basolateral membrane of colonic goblet cells, and its deletion results in delayed and reduced mucus layer build-up
Staining of the mid-distal colonic tissue for NBCn1 as well as for Muc2 resulted in basolateral staining of cells by the anti-NBCn1 antibody that showed a similar distribution to that for Muc2-positive cells. The similarity of the staining patterns suggests that NBCn1 is expressed in a particularly strong manner in colonic goblet cells (Fig. 6A and B). We therefore designed a fluorometric method to assess the build-up of a mucus layer on exteriorized, vascularly perfused, mid-distal colonic mucosa in anaesthetized mice. After complete removal by gentle suction of all the mucus that was not very firmly adherent, we used fluorescent beads that settled on top of the mucus layer that built up over time. Assessing the distance of the beads at different time periods after removal of mucus from the epithelial surface was used to study time-dependent build-up of the colonic mucus layer. Figure 6C demonstrates the technique, and Fig. 6D shows the time-dependent build-up of a mucus layer in WT mid-distal colon. The absence of NBCn1 expression resulted in a significant delay in mucus build-up both in non-stimulated conditions (Fig. 7A, left bars) and in FSK-stimulated conditions (Fig. 7A, right panels). This suggests that basolateral HCO3− import is conducive to mucus secretion and/or mucus expansion.
Figure 7. Basal as well as FSK-stimulated mucus accumulation was significantly reduced in NBCn1 KO mice, while colonic surface pH was not different from WT.
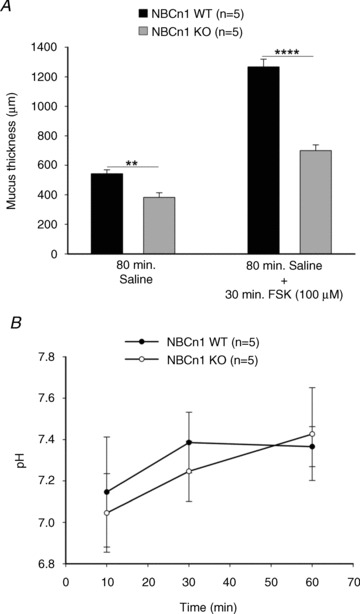
A, the rate of mucus accumulation was measured. In basal conditions, the thickness of the accumulated layer of mucus was significantly lower in case of NBCn1 KO mice than in their WT counterparts (left bars). The right bars show that after FSK stimulation, the rate at which KO colon secreted mucus was significantly lower than in the WT mice. The numbers of mice are given in parentheses. **P≤ 0.01 and ****P≤ 0.0001 between the groups. B shows the pH measured at the indicated time points near the epithelial surface after adding unbuffered saline with a pH of ∼6 at time zero. No significant differences were observed between NBCn1 KO and WT surface pH, and the rate of alkalization of the mucus layer was not different between the two groups.
Epithelial surface pH alkalinization was not significantly different in NBCn1 KO and WT colon in vivo
Current hypotheses envisage a potential role of the HCO3− secreted by the colonocytes directly adjacent to the goblet cells into the colonic lumen for the expansion and proper gel formation of mucin molecules once released from the goblet cell secretory granules (Garcia et al. 2009). In order to assess whether the presence of NBCn1 was important for the alkalinization of the pH directly at the epithelial surface, we studied the surface pH alkalinization after exposure of the epithelial surface to an unbuffered solution of pH ∼6. The initial reading was taken 10 min after addition of the luminal solution to allow complete stabilization of the luminal fluid layer, and then at regular intervals up to 1 h after application of the unbuffered solution. Surface pH rapidly rose to values around neutrality and stabilized at values around 7.4, which is a result of HCO3− export as well as CO2 diffusion into the unbuffered solution. No significant differences were observed in the alkalinization rate and steady-state surface pH between NBCn1-deficient and WT mid-distal colonic mucosa (Fig. 7B).
Immunohistochemical staining of the firmly adherent mucus reveals a thinner mucus layer in the absence of NBCn1 expression
The fluorometric method of assessing mucus layer build-up gives no indication about the quality of the mucus. One method to study the intactness of the firmly adherent mucus layer in the colon is to fix the excised colon in Carnoy solution and to cut perpendicular sections through the entire colon in the areas where faecal pellets can be observed in the lumen (Johansson et al. 2008). Mucin 2 immunofluorescence outlines the firmly adherent colonic mucus layer. The mucus layer in the NBCn1-deficient colon had an overall thinner appearance (right upper panel in Fig. 8A shows a representative image), and more frequent disruption of the continuity was observed. Even when the layer was of normal thickness, the structure appeared less smooth (Fig. 8A, lower right panel). Despite variability, the assessment of all sections as described in the Methods yielded a statistically thinner firmly adherent mucus layer in NBCn1-deficient mice compared with sex-matched WT littermates.
Discussion
In this study, we report defects in the mucosal protective responses to luminal acid, both in the pHi recovery of enterocytes after luminal acid exposure and in the acid-induced HCO3− secretory defect, in the duodenum of mice deficient in the electroneutral Na+–HCO3− cotransporter NBCn1 (Slc4a7) in in vivo conditions. The functional findings correspond to a very prominent expression of NBCn1 in the basolateral membrane of duodenal (but not jejunal or ileal; Boedtkjer et al. 2008; Chen et al. 2012) villous enterocytes. NBCn1 is also expressed in the colon, albeit at lower levels than in the duodenum, with a predominant (but not exclusive) expression of NBCn1 in the basolateral membrane of the goblet cells. Two-photon confocal assessment of dynamic mucus layer build-up in vivo revealed significantly slower rates in NBCn1-deficient colon. A corresponding decrease in the thickness and a more irregular structure of the firmly adherent colonic mucus layer was observed immunohistochemically in NBCn1-deficient mice compared with WT littermates. These findings give new insight into the rate-limiting factors for duodenal enterocyte pHi recovery after acidification by a luminal acid load. Furthermore, they show that, contrary to expectation, the villous enterocytes in the duodenum are the cells that mount the duodenal HCO3− response to acid. Lastly, the experiments implicate a dependence of colonic mucus layer build-up on the basolateral uptake of HCO3− via NBCn1, which is an interesting, novel aspect of the physiology of the mucus and underlines the importance of the cellular HCO3− concentration for proper secretion of mucus (Quinton, 2010b).
NBCn1 in duodenum
The so-called gastrointestinal protective barrier is composed of pre-epithelial (mucus–bicarbonate barrier), epithelial and sub-epithelial components (blood flow, mucosal nerves and immune system), which work together in a complex and poorly understood fashion to protect the mucosa from injury (Allen & Flemström, 2005; Kaunitz & Akiba, 2006). The relative importance of these different components for mucosal protection from luminal acid is disputed, in part due to lack of understanding of the cellular origin of the acid-induced HCO3− secretory response (for reviews see Allen & Flemström 2005, Kaunitz & Akiba 2006, Seidler & Sjoblom 2012). This study adds new insight into the molecular basis for this protective defence mechanism by giving an indication of its cellular origin. As a result of its strong dependence on the villus-expressed NBCn1, the long-lasting HCO3− secretory response to a short exposure of the mucosa to acid originates from the villus rather than the crypt region of the duodenum. Recent evidence suggests that the CFTR anion channel, originally believed to be very strongly crypt located in all parts of the intestine (Kälin et al. 1999; Ameen et al. 2000; Doucet et al. 2003), is also found with high expression levels in the apical membrane of the duodenal villous enterocytes (Jakab et al. 2011) and is associated with cAMP-stimulated Cl− efflux also in villous cells (Odes et al. 2003). Therefore, the findings of this paper do not contradict but complement previous findings of a strong dependence of the acid-induced duodenal HCO3− secretory response on the presence of CFTR (Hogan et al. 1997; Singh et al. 2008).
The murine duodenum expresses other NBC members of the Slc4 gene family, and it is particularly interesting that the electrogenic sodium bicarbonate cotransporter NBCe1, which has similar expression levels and a similar cellular distribution to NBCn1 (Praetorius et al. 2001; Chen et al. 2012) does not seem to be involved in the acid-induced HCO3− secretory response and cannot compensate for NBCn1. Possibly, the signalling mechanisms elicited by contact of the duodenal mucosa with luminal acid, which are to a large part dependent on neural transmission and are likely to involve the release of multiple effector molecules at the level of the duodenocyte, also result in a hyperpolarization of the basolateral membrane, thus making an electrogenic HCO3− import energetically less favourable than an electroneutral one. Carbonic anhydrase-facilitated CO2 hydration in conjunction with basolateral H+ export via NHE1 is another mechanism for duodenocyte HCO3− loading, and its importance for duodenal HCO3− secretion has been demonstrated in rabbit duodenum in the Ussing chamber (Jacob et al. 2000). Owing to the premature death of NHE1-deficient mice and the inability to inhibit NHE1 selectively in the intestine in the in vivo experiments necessary to explore the mechanisms involved in luminal acid-induced HCO3− secretion, we can only speculate but not test the assumption that this mechanism serves as one of the alternative pathways for HCO3− loading during acid-induced HCO3− secretion.
We next measured the enterocyte pHi in the villi during and after contact with a luminal pH of 2.5 for 5 min, an acid exposure which is not associated with cellular damage (Fig. 1) or an increase in epithelial permeability (Sjöblom et al. 2009). We observed a significant decrease in duodenocyte pHi not only in the villus tip region, but also in the lower parts of the villi. We had not observed the same degree of acidification of the lower villi in previous investigations, despite the similar age and background of the mice (Sjöblom et al. 2009). The major difference between that study and the present one is that the mice are now artificially ventilated via tracheal intubation during imaging. This avoids spontaneous hyperventilation. As a result, the alkaline blood pH, but low HCO3− concentration, a situation which is associated with low duodenal HCO3− secretory rates, is prevented. This has resulted in a visibly faster release of mucus and surface pH alkalinization in WT mice. Thus, it may be speculated that the better availability of extracellular bicarbonate allows more CO2 generation and diffusion deeper into the unstirred fluid layer and into the lower parts of the duodenocyte villi.
In NBCn1-deficient duodenum, no pHi recovery was observed within the period of observation (which was limited in length by release of fresh mucus, resulting in light scattering). Akiba et al. (2001a,b) have demonstrated that the pHi recovery from a short exposure of the duodenal mucosa to luminal acid is compromised by substances that may inhibit anion transporters such as, but not restricted to, NBCs, and that the application of these substances during luminal acidification results in a compromise of cell survival during acidification. However, the luminal acid concentration used for assessment of cell damage had to be higher than for pHi measurement, because in that study luminal pH values above pH 2 did not result in sufficient damage during the experimental period (Akiba et al. 2001b). We found pH 2.5 for 5 min not to cause damage, so we did not suspect more damage in the NBCn1-deficient than in WT mucosa in the experimental conditions. Nevertheless, a similar loss of the duodenal secretory response to acid was recently observed in mice deficient for cGMP-dependent kinase I in the nervous system, and those mice did develop spontaneous duodenal ulcers in the area near the major duodenal papilla (Singh et al. 2012). It is likely that differences during the chronic phase of gastric acid exposure between those mice and the NBCn1-deficient mice are present, such as motility disturbances or the fluid secretory response of the crypt region, which may be necessary to flush the epithelium. However, as the development of peptic duodenal damage is multifactorial, we assume that defective expression and/or function of NBCn1 in human duodenum, where it is also strongly expressed (Damkier et al. 2007), may increase the susceptibility to peptic damage to the epithelium.
NBCn1 in colon
Given that qPCR of scraped colonic mucosa had demonstrated colonic NBCn1 expression (Chen et al. 2012), and that the transgenic mouse expressing the LacZ gene, coding for bacterial β-galactosidase, in control of the NBCn1 promoter, displayed a ‘blue’ colon, when histochemically investigated for β-galactosidase activity (Boedtkjer et al. 2008), we also tried to delineate colonic NBCn1 function. To our surprise, colonic crypt base cells did not demonstrate a significant involvement of NBCn1 in pHi recovery from an ammonium prepulse-generated acidification in a CO2/HCO3−-containing buffer, despite the fact that the crypts displayed some NBCn1 expression by immunohistochemistry. Base import rates in this preparation are clearly predominantly NHE1- and NHE2-mediated in the crypts (Fig. 4), but the low residual base import rate after NHE inhibition had previously been shown to be sensitive to NBC inhibitors (Bachmann et al. 2003, 2008), and is therefore most likely to be NBC mediated. Given that NBCe1 is also expressed in colonic crypts (Bachmann et al. 2003; Yu et al. 2009), this may be the NBC isoform mediating CO2/HCO3−-dependent acute pHi recovery in the murine colonic crypt base. Possibly, selective pHi-metry in the strongly NBCn1-expressing goblet cells (Fig. 6A) would have yielded different results, but we were not able to distinguish goblet cells from other enterocytes during video imaging.
Likewise, we did not observe a significant influence of NBCn1 deletion on basal HCO3− secretory rates, or agonist-stimulated rates after luminal Cl− removal, which is a method to activate crypt-localized CFTR-dependent HCO3− secretion (Xiao et al. 2012b), in isolated proximal and mid-distal colonic mucosa in Ussing chambers. When net fluid movements and HCO3− secretion were assessed in the mid-distal colon of NBCn1-deficient and WT littermates, no overall difference in colonic fluid absorptive or secretory rate was found; however, a very mild but significant reduction in FSK-stimulated HCO3− secretory rate was observed.
We wondered whether colonic cryptal NBCn1 expression may serve a cellular function other than acute pHi regulation and basolateral base uptake during epithelial HCO3− secretion. A large percentage of the epithelial cells in the crypts stained positive for mucus granules and at the same time for NBCn1 in the basolateral membrane. Thus, we explored whether HCO3− uptake via NBCn1 was necessary for mucus layer build-up and/or the surface pH in the mucus layer. The surface pH alkalinization over time of an unbuffered solution with a starting pH ∼6 layered above the colonic epithelial surface mucus layer was not significantly different between NBCn1-deficient and WT mid-distal colon, but the mucus layer build-up was significantly delayed. Although this method cannot assess the viscosity and hydration status of the mucus layer, it provides an assessment of mucus layer dynamics in vivo. The role of a HCO3− transporter in mucus layer build-up in this study is consistent with the role of HCO3− concentration in mucus expansion (Quinton, 2010a,b).
The results point to a previously unrecognized role of intracellular HCO3− for release of colonic mucus. Mucins are polyanions that are tightly packed within the goblet cell granules and shielded by cations, mostly Ca2+ and H+ (Verdugo, 1991; Perez-Vilar et al. 2005). A high pH had been shown to promote cervical mucus granule expansion, whereas acidic pH was preventive (Espinosa et al. 2002). Espinosa et al. (2002). studied the size expansion of mucin granules in cervical cells in primary culture prior to the fusion of the granule membrane with the apical membrane and exocytosis; thus, it has to be inferred that the intra- not the extracellular pH was the determinant of these changes in granule size. Recent results suggest that the cyclical oscillations in intragranular Ca2+ concentrations, which may signal the onset of mucus granule exocytosis, are accompanied by and likely to be the result of intragranular pH changes (Chin et al. 2002). Further evidence supporting this concept came from in vitro studies of Muc2 molecule expansion (Ambort et al. 2012). Given that H+ uptake into and release from the granule must play a critical role in these intragranular pH changes, and given that acidic pH has been shown to inhibit granule exocytosis (Espinosa et al. 2002), pHi regulatory mechanisms via the basolateral membrane need to be very efficient in colonic goblet cells. The electroneutral Na+–HCO3− cotransporter NBCn1 is particularly suitable for HCO3− accumulation even at fairly high intracellular HCO3− concentrations and negative basolateral membrane potentials (which will inhibit NBCe1-mediated HCO3− import), making it particularly suitable for base import when NBCe1 and the NHEs are not active (because the pHi is not low enough to activate them). So far, no functional data have been acquired regarding acid–base transport regulation in colonic goblet cells, due to the fact that these cells are not easily accessible for study. Further experiments to unravel the importance of HCO3− and its transport mechanisms in colonic goblet cells may provide insights into goblet cell function, as well as their dysfunction and mucus layer defects during intestinal diseases such as inflammatory bowel disease and infectious colitis, and in cystic fibrosis.
In summary, our data reveal previously unrecognized physiological functions for the electroneutral Na+–HCO3− cotransporter NBCn1 in the intestinal tract, where this transporter has a segment-specific expression, with particularly high expression in the duodenum, but substantial expression also in colonic mucosa. NBCn1 plays a critical role in pHi recovery and the mounting of a protective HCO3− secretory response after contact of the duodenal mucosa with acid. In the colon, loss of NBCn1 is associated with a defective mucus layer build-up. NBCn1 is therefore operative in important protective functions of the gut. A search for compromised NBCn1 transport in intestinal diseases seems warranted.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft grants Se460/13-4 and Se460/9-6 (to U.S.), SFB621-C9 (to U.S.) and SFB621-C10 (to O.B.). F.X. was funded in part by a grant from the German Mucoviscidosis Foundation (to U.S.). J.P. was supported by the Lundbeck Foundation, Karen Elise Jensens Fond, Aarhus University Research Foundation and the Novo Nordisk Foundation. C.A. was supported by the Danish Medical Research Council. We thank Brigitte Rausch and Regina Engelhardt for expert technical help.
Glossary
- ACC
acetylcysteine
- CCh
carbachol
- CFTR
cystic fibrosis transmembrane conductance regulator
- FSK
forskolin
- GI
gastrointestinal
- KO
knock-out
- Muc2
mucin 2
- NBCn1
sodium–bicarbonate cotransporter
- NHE
Na+–H+ exchanger
- Slc
solute carrier
- WT
wild-type
Author contributions
A.K.S., W.X., J.L., W.Z., F.X., M.J., B.R., J.P., C.A. and U.S. designed, performed and analysed experiments, P.S., O.B., J.P. and C.A. provided expert assistance and suggestions, and U.S. wrote the manuscript. All the authors approved the final version of the manuscript.
Author's present address
W. Zheng: Department of Gynaecology and Obstetrics, Hannover Medical School, Hannover, Germany.
References
- Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acute adaptive cellular base uptake in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol. 2001a;280:G1083–G1092. doi: 10.1152/ajpgi.2001.280.6.G1083. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Sassani P, Dukkipatis R, Pushkin A, Kurtz I, Kaunitz JD. Cellular bicarbonate protects rat duodenal mucosa from acid-induced injury. J Clin Invest. 2001b;108:1807–1816. doi: 10.1172/JCI12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A, Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:C1–C19. doi: 10.1152/ajpcell.00102.2004. [DOI] [PubMed] [Google Scholar]
- Ambort D, Johansson ME, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, Koeck PJ, Hebert H, Hansson GC. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci U S A. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen N, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol. 2000;114:69–75. doi: 10.1007/s004180000164. [DOI] [PubMed] [Google Scholar]
- Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- Bachmann O, Franke K, Yu H, Riederer B, Li HC, Soleimani M, Manns MP, Seidler U. cAMP-dependent and cholinergic regulation of the electrogenic intestinal/pancreatic Na+/HCO3− cotransporter pNBC1 in human embryonic kidney (HEK293) cells. BMC Cell Biol. 2008;9:70. doi: 10.1186/1471-2121-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann O, Reichelt D, Tuo B, Manns MP, Seidler U. Carbachol increases Na+-HCO3− cotransport activity in murine colonic crypts in a M3−, Ca2+/calmodulin-, and PKC-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2006;291:G650–G657. doi: 10.1152/ajpgi.00376.2005. [DOI] [PubMed] [Google Scholar]
- Bachmann O, Riederer B, Rossmann H, Groos S, Schultheis PJ, Shull GE, Gregor M, Manns MP, Seidler U. The Na+/H+ exchanger isoform 2 is the predominant NHE isoform in murine colonic crypts and its lack causes NHE3 upregulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G125–G133. doi: 10.1152/ajpgi.00332.2003. [DOI] [PubMed] [Google Scholar]
- Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3− cotransporter subtype pNBC1. Am J Physiol Gastrointest Liver Physiol. 2003;284:G37–G45. doi: 10.1152/ajpgi.00209.2002. [DOI] [PubMed] [Google Scholar]
- Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtkjer E, Praetorius J, Füchtbauer EM, Aalkjaer C. Antibody-independent localization of the electroneutral Na+-HCO3− cotransporter NBCn1 (slc4a7) in mice. Am J Physiol Cell Physiol. 2008;294:C591–C603. doi: 10.1152/ajpcell.00281.2007. [DOI] [PubMed] [Google Scholar]
- Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Füchtbauer AC, Simonsen U, Füchtbauer EM, Aalkjaer C. Disruption of Na+, HCO3− cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+ sensitivity, and hypertension development in mice. Circulation. 2011;124:1819–1829. doi: 10.1161/CIRCULATIONAHA.110.015974. [DOI] [PubMed] [Google Scholar]
- Chen M, Praetorius J, Zheng W, Xiao F, Riederer B, Singh AK, Stieger N, Wang J, Shull GE, Aalkjaer C, Seidler U. The electroneutral Na+:HCO3− cotransporter NBCn1 is a major pHi regulator in murine duodenum. J Physiol. 2012;590:3317–3333. doi: 10.1113/jphysiol.2011.226506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin WC, Quesada I, Nguyen T, Verdugo P. Oscillations of pH inside the secretory granule control the gain of Ca2+ release for signal transduction in goblet cell exocytosis. Novartis Found Symp. 2002;248:132–141. discussion 141–139, 277–182. [PubMed] [Google Scholar]
- Cinar A, Chen M, Riederer B, Bachmann O, Wiemann M, Manns M, Kocher O, Seidler U. NHE3 inhibition by cAMP and Ca2+ is abolished in PDZ-domain protein PDZK1-deficient murine enterocytes. J Physiol. 2007;581:1235–1246. doi: 10.1113/jphysiol.2007.131722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2136–R2146. doi: 10.1152/ajpregu.00356.2007. [DOI] [PubMed] [Google Scholar]
- Doucet L, Mendes F, Montier T, Delépine P, Penque D, Férec C, Amaral MD. Applicability of different antibodies for the immunohistochemical localization of CFTR in respiratory and intestinal tissues of human and murine origin. J Histochem Cytochem. 2003;51:1191–1199. doi: 10.1177/002215540305100909. [DOI] [PubMed] [Google Scholar]
- Espinosa M, Noé G, Troncoso C, Ho SB, Villalón M. Acidic pH and increasing [Ca2+] reduce the swelling of mucins in primary cultures of human cervical cells. Hum Reprod. 2002;17:1964–1972. doi: 10.1093/humrep/17.8.1964. [DOI] [PubMed] [Google Scholar]
- Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A, Grencis RK, Thornton DJ, Khan WI. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology. 2010;138:1763–1771. doi: 10.1053/j.gastro.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology. 1997;113:533–541. doi: 10.1053/gast.1997.v113.pm9247473. [DOI] [PubMed] [Google Scholar]
- Jacob P, Christiani S, Rossmann H, Lamprecht G, Vieillard-Baron D, Müller R, Gregor M, Seidler U. Role of Na+HCO3− cotransporter NBC1, Na+/H+ exchanger NHE1, and carbonic anhydrase in rabbit duodenal bicarbonate secretion. Gastroenterology. 2000;119:406–419. doi: 10.1053/gast.2000.9358. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300:G82–G98. doi: 10.1152/ajpgi.00245.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kälin N, Claaß A, Sommer M, Puchelle E, Tümmler B. ΔF508 CFTR protein expression in tissues from patients with cystic fibrosis. J Clin Invest. 1999;103:1379–1389. doi: 10.1172/JCI5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunitz JD, Akiba Y. Review article: duodenal bicarbonate – mucosal protection, luminal chemosensing and acid-base balance. Aliment Pharmacol Ther. 2006;24(Suppl 4):169–176. doi: 10.1111/j.1365-2036.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Odes HS, Smirnoff P, Guberman R, Pollak-Charcon S, Sperber AD, Fich A, Fraser GM, Schwartz B. Cystic fibrosis transmembrane conductance regulator and Na+ channel subunits mRNA transcripts, and Cl− efflux, show a different distribution in rat duodenum and colon. Acta Physiol Scand. 2003;178:231–240. doi: 10.1046/j.1365-201X.2003.01138.x. [DOI] [PubMed] [Google Scholar]
- Perez-Vilar J, Olsen JC, Chua M, Boucher RC. pH-dependent intraluminal organization of mucin granules in live human mucus/goblet cells. J Biol Chem. 2005;280:16868–16881. doi: 10.1074/jbc.M413289200. [DOI] [PubMed] [Google Scholar]
- Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO, Roos S, Holm L, Phillipson M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G327–G333. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius J, Hager H, Nielsen S, Aalkjaer C, Friis UG, Ainsworth MA, Johansen T. Molecular and functional evidence for electrogenic and electroneutral Na+-HCO3− cotransporters in murine duodenum. Am J Physiol Gastrointest Liver Physiol. 2001;280:G332–G343. doi: 10.1152/ajpgi.2001.280.3.G332. [DOI] [PubMed] [Google Scholar]
- Pratha VS, Hogan DL, Martensson BA, Bernard J, Zhou R, Isenberg JI. Identification of transport abnormalities in duodenal mucosa and duodenal enterocytes from patients with cystic fibrosis. Gastroenterology. 2000;118:1051–1060. doi: 10.1016/s0016-5085(00)70358-1. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Birth of mucus. Am J Physiol Lung Cell Mol Physiol. 2010a;298:L13–L14. doi: 10.1152/ajplung.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton PM. Role of epithelial HCO3− transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Cell Physiol. 2010b;299:C1222–C1233. doi: 10.1152/ajpcell.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO3− secretion. J Physiol. 1997;505:411–423. doi: 10.1111/j.1469-7793.1997.411bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler U, Sjoblom M. Gastroduodenal bicarbonate secretion. In: Leonard RJ, Fayez KG, Jonathan DK, Juanita LM, Hamid MS, Jackie DW, editors. Physiology of the Gastrointestinal Tract. 5th edn. Amsterdam: Elsevier Academic Press; 2012. pp. 1311–1339. [Google Scholar]
- Singh AK, Sjöblom M, Zheng W, Krabbenhöft A, Riederer B, Rausch B, Manns MP, Soleimani M, Seidler U. CFTR and its key role in in vivo resting and luminal acid-induced duodenal HCO3− secretion. Acta Physiol (Oxf) 2008;193:357–365. doi: 10.1111/j.1748-1716.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- Singh AK, Spiessberger B, Zheng W, Xiao F, Lukowski R, Wegener JW, Weinmeister P, Saur D, Klein S, Schemann M, Krueger D, Seidler U, Hofmann F. Neuronal cGMP kinase I is essential for stimulation of duodenal bicarbonate secretion by luminal acid. FASEB J. 2012;26:1745–1754. doi: 10.1096/fj.11-200394. [DOI] [PubMed] [Google Scholar]
- Sjöblom M, Singh AK, Zheng W, Wang J, Tuo BG, Krabbenhöft A, Riederer B, Gros G, Seidler U. Duodenal acidity “sensing” but not epithelial HCO3− supply is critically dependent on carbonic anhydrase II expression. Proc Natl Acad Sci U S A. 2009;106:13094–13099. doi: 10.1073/pnas.0901488106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo B, Riederer B, Wang Z, Colledge WH, Soleimani M, Seidler U. Involvement of the anion exchanger SLC26A6 in prostaglandin E2- but not forskolin-stimulated duodenal HCO3− secretion. Gastroenterology. 2006;130:349–358. doi: 10.1053/j.gastro.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Verdugo P. Mucin exocytosis. Am Rev Respir Dis. 1991;144:S33–S37. doi: 10.1164/ajrccm/144.3_pt_2.S33. [DOI] [PubMed] [Google Scholar]
- Vidyasagar S, Rajendran VM, Binder HJ. Three distinct mechanisms of HCO3− secretion in rat distal colon. Am J Physiol Cell Physiol. 2004;287:C612–C621. doi: 10.1152/ajpcell.00474.2003. [DOI] [PubMed] [Google Scholar]
- Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology. 2009;136:893–901. doi: 10.1053/j.gastro.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Juric M, Li J, Riederer B, Yeruva S, Singh AK, Zheng L, Glage S, Kollias G, Dudeja P, Tian DA, Xu G, Zhu J, Bachmann O, Seidler U. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3− secretion in murine ileocolonic inflammation. Inflamm Bowel Dis. 2012a;18:101–111. doi: 10.1002/ibd.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Li J, Singh AK, Riederer B, Wang J, Sultan A, Park H, Lee MG, Lamprecht G, Scholte BJ, De Jonge HR, Seidler U. Rescue of epithelial HCO3− secretion in murine intestine by apical membrane expression of the cystic fibrosis transmembrane conductance regulator mutant F508del. J Physiol. 2012b;590:5317–5334. doi: 10.1113/jphysiol.2012.232124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Riederer B, Stieger N, Boron WF, Shull GE, Manns MP, Seidler UE, Bachmann O. Secretagogue stimulation enhances NBCe1 (electrogenic Na+/HCO3− cotransporter) surface expression in murine colonic crypts. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1223–G1231. doi: 10.1152/ajpgi.00157.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


