Abstract
Bile acids (BAs) play important roles not only in lipid metabolism, but also in signal transduction. TGR5, a transmembrane receptor of BAs, is an immunomodulative factor, but its detailed mechanism remains unclear. Here, we aimed to delineate how BAs operate in immunological responses via the TGR5 pathway in human mononuclear cell lineages. We examined TGR5 expression in human peripheral blood monocytes, several types of in vitro differentiated macrophages (Mϕs) and dendritic cells. Mϕs differentiated with macrophage colony-stimulating factor and interferon-γ (Mγ-Mϕs), which are similar to the human intestinal lamina propria CD14+ Mϕs that contribute to Crohn's disease (CD) pathogenesis by production of pro-inflammatory cytokines, highly expressed TGR5 compared with any other type of differentiated Mϕ and dendritic cells. We also showed that a TGR5 agonist and two types of BAs, deoxycholic acid and lithocholic acid, could inhibit tumour necrosis factor-α production in Mγ-Mϕs stimulated by commensal bacterial antigen or lipopolysaccharide. This inhibitory effect was mediated by the TGR5–cAMP pathway to induce phosphorylation of c-Fos that regulated nuclear factor-κB p65 activation. Next, we analysed TGR5 levels in lamina propria mononuclear cells (LPMCs) obtained from the intestinal mucosa of patients with CD. Compared with non-inflammatory bowel disease, inflamed CD LPMCs contained more TGR5 transcripts. Among LPMCs, isolated CD14+ intestinal Mϕs from patients with CD expressed TGR5. In isolated intestinal CD14+ Mϕs, a TGR5 agonist could inhibit tumour necrosis factor-α production. These results indicate that TGR5 signalling may have the potential to modulate immune responses in inflammatory bowel disease.
Keywords: bile acid, Crohn's disease, intestinal macrophage, TGR5, tumour necrosis factor α
Introduction
The immune system is intricately constituted to maintain homeostasis and avoid excessive or deficient responses against foreign antigens, which causes immunological disorders. Recently, macrophages (Mϕs), major components of innate immunity, were circumstantially analysed for their characteristics. The study showed that Mϕs have several subpopulations1,2 that were classified broadly into two categories: an inflammatory phenotype that is stimulated to produce pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α) or an anti-inflammatory phenotype that induces immune tolerance by producing interleukin-10 (IL-10).
Macrophages have been also classified according to their anatomical locations. Intestinal Mϕs play a role in the maintenance of immunological homeostasis by regulation of some inflammatory cytokines against foreign antigens such as commensal bacteria, even if phagocytic and bactericidal activity remains.2,3 We previously reported that mouse intestinal Mϕs produce IL-10 in response to bacterial stimulation, which prevents an excess immune response.4 Furthermore, we identified that there are two subsets of resident mouse intestinal Mϕs, and monocyte chemoattractant protein-1 is important for the composition of IL-10-producing intestinal Mϕs.5 Denning et al.6 also reported that intestinal Mϕs can induce regulatory T cells.
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis, is caused by inadequate immunological responses against luminal antigens, which results in chronic inflammation in some intestinal regions.7 In IBD, the homeostatic function of intestinal Mϕs is disrupted and abnormal immune responses of intestinal Mϕs contribute to the development of chronic inflammation. In fact, it has been reported that Mϕs expressing the innate-immune receptor CD14 infiltrate the intestinal mucosa of patients with IBD.8–10 TREM-1+ Mϕs in the lamina propria (LP) of patients with IBD mediate chronic inflammation.11 We also demonstrated that intestinal CD68+ Mϕs in patients with CD produce most of the IL-18 that contributes to the promotion of the T helper type 1 immune response.12 Recently, we have identified unique CD33+ CD14+ CD68+ intestinal Mϕs that strongly contribute to CD pathogenesis by their pro-inflammatory cytokine production and antigen presentation function that cooperates with T cells and mucosal natural killer cells.10,13,14 Therefore, conditioning of innate immune cells to an immunoregulatory phenotype may become a novel therapeutic strategy. We previously reported that bile acids (BAs) and synthetic retinoic acid receptor agonists can induce IL-12-hypoproducing dendritic cells (DCs).15,16
Bile acids play important roles not only in lipid metabolism, but also in signal transduction via several receptors. TGR5, a G-protein coupled receptor, was identified by an exhaustive search of the GenBank DNA database.17,18 TGR5 gene expression covers a broad area to varying degrees,17–19 including the hepatobiliary system such as liver sinusoidal endothelial cells,20 gallbladder epithelial cells21 and elsewhere. TGR5 is considered to be closely associated with various biological functions. In metabolism, BAs promote energy expenditure in brown adipose tissue via activated thyroid hormone,22 and they increase intestinal glucagon-like peptide-1 secretion for glucose homeostatic control.23 In the immune system, BAs are classically known to inhibit inflammatory cytokine production.24,25 In terms of the immunological function of TGR5, it has recently become evident that CD14+ monocytes show high TGR5 expression16,17 and TGR5 expresses on some Mϕs, such as Kupffer cells.26 A TGR5 agonist inhibits an atherosclerosis reduced by Mϕ inflammation.27 These results suggest that TGR5 can regulate innate immunity.
There are only a few reports detailing the relationships between TGR5 and gut immunity. In an experimental mouse colitis model, TGR5 regulates intestinal inflammation by a reduction in inflammatory cytokines.28 However, there is no report of a survey of human intestinal samples in terms of gut immunological regulation by TGR5. Recently, we have found that Mϕs differentiated by macrophage colony-stimulating factor (M-CSF) and interferon-γ (IFN-γ) (Mγ-Mϕs) show a similar phenotype to that of intestinal CD14+ Mϕs, which evoke inflammation in patients with IBD, in terms of cytokine production including TNF-α, IL-6 and IL-23, and the expression pattern of cell surface markers.10 We hypothesized that Mγ-Mϕs mimic LP CD14+ Mϕs expressing TGR5, and TGR5 signalling can suppress the production of pro-inflammatory cytokines by inflammatory Mϕs. In the present study, we investigated TGR5 expression in several innate immune cell populations, including Mγ-Mϕs differentiated in vitro and the intestinal CD14+ Mϕs of patients with CD, and whether a TGR5 agonist can suppress pro-inflammatory cytokine production by these cells.
Materials and methods
Heat-treated bacterial antigen
Gram-positive Enterococcus faecalis (ATCC29212) was cultured in brain–heart infusion medium. Bacteria were harvested and washed twice with ice-cold PBS (Wako Chemicals, Osaka, Japan). Bacterial suspensions were heated at 80° for 30 min, washed, resuspended in PBS and stored at − 80°. Complete killing was confirmed by 24 hr incubation at 37° on solid growth medium.
In vitro monocyte differentiation
Peripheral blood mononuclear cells were isolated from heparinized peripheral blood samples by density gradient centrifugation using Lymphoprep (Nycomed Pharma, Oslo, Norway). Cells were aspirated from the gradient interface, washed in PBS, and resuspended at 1 × 106 cells/ml in RPMI-1640 medium (Sigma-Aldrich, St Louis, MO) containing 10% heat-inactivated fetal bovine serum (BioSource, Camarillo, CA), 100 U/ml penicillin and 100 mg/ml streptomycin (Invitrogen, La Jolla, CA). Monocytes were purified using a magnetic cell separation system (MACS; Miltenyi Biotec, Auburn, AL) with anti-human CD14 microbeads (Miltenyi Biotec). Monocytes were seeded in dishes at a density of 1 × 106 cells/2 ml culture medium. Based on a review of monocyte differentiation,29 some cultures contained 50 ng/ml M-CSF (R&D Systems, Minneapolis, MN) for M-CSF-differentiated macrophages (M-Mϕs), 50 ng/ml M-CSF and 100 ng/ml IFN-γ (R&D) for Mγ-Mϕs, 50 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) (R&D) for GM-CSF-differentiated macrophages (GM-Mϕs) or 20 ng/ml GM-CSF and 20 ng/ml IL-4 (R&D) for DCs. After 1, 3 and 6 days, cells were collected. We defined stimulated monocytes for 6 days as completely differentiated. Some samples were used for real-time quantitative PCR (RT-qPCR), immunofluorescence and Western blot analyses.
Peripheral blood samples were collected from healthy donors after obtaining written informed consent. All experiments were approved by the Institutional Review Board of the Keio University School of Medicine.
RT-qPCR analyses
Total RNA was isolated from monocytes using an RNeasy Micro Kit (Qiagen, Washington DC) and reverse transcribed using a Primescript RT Reagent Kit (Takara Bio Inc., Otsu, Japan) according to the manufacturer's instructions. RT-qPCR was performed using a TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) with gene-specific primers for TGR5 (Hs01937849_s1) and β-actin (Hs99999903_m1) as an internal control. The PCR amplifications were performed using a DNA Engine Opticon 2 system or CFX96 Real-Time System (Bio-Rad, Hercules, CA), and data were analysed with Opticon Monitor software (MJ Research, Waltham, MA).
TGR5 immunofluorescence
Cells were fixed with 4% paraformaldehyde and blocked in Protein Block serum-free Ready-To-Use (Dako, Glostup, Denmark) for 1 hr at room temperature. Then, cells were incubated with a rabbit anti-TGR5 antibody (Abcam, Cambridge, MA) for 2 hr, followed by an Alexa Fluor 568-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR) for 1 hr, while protected from light. Nuclei were counterstained with Hoechst 33342 trihydrochloride, trihydrate (Invitrogen). The fluorescence images obtained were analysed by Lumina Vision software (Mitani, Tokyo, Japan).
Stimuli for differentiated monocytes
Differentiated monocytes described above were seeded in 96-well culture dishes at a density of 2 × 105 cells/200 μl culture medium. Then, cells were stimulated with the following: heat-killed E. faecalis (multiplicity of infection = 100), lipopolysaccharide (LPS; Sigma), BAs; chenodeoxycholic acid (CDCA; Sigma), deoxycholic acid (DCA; Sigma), lithocholic acid (LCA; Sigma), cholic acid (CA; Nacalai Tesque, Kyoto, Japan) or ursodeoxycholic acid (UDCA; Tokyo Chemical Industry, Tokyo, Japan), a 3-(2-chlorophenyl)-N-(4-chlorophenyl)-N,5-dimethylisoxazole-4-carboxamide as a TGR5 agonist (Bio Vision, Mountain View, CA) which was derived by Evans et al.30 (Fig. 1), a p38 mitogen-activated protein kinase inhibitor (Merck Millipore, Tokyo, Japan), a toll-like receptor 4 inhibitor (Imgenex, San Diego, CA) or 8-bromoadenosine 3′, 5′-cyclic monophosphate as a cAMP reagent (Sigma). After 1 day of stimulation, supernatants were collected for cytokine assays, and the remaining cells were used for viability assays.
Figure 1.
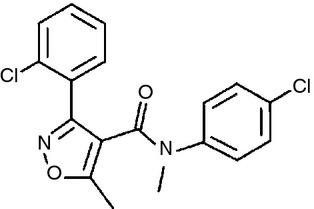
Chemical structure of the 3-(2-chlorophenyl)-N-(4-chlorophenyl)-N, 5-dimethylisoxazole-4-carboxamide as the TGR5 agonist we used in this study. This compound precursor was discovered by a high-throughput screening of the GlaxoSmithKline Pharmaceuticals (Collegeville, PA) compound library using a luciferase assay, the production of which was caused by cAMP. In the process of exploring substitution derivative analysis, this TGR5 agonist was found out as a high affinity for TGR5 (pEC50 = 6·8) of U2-OS cells.
Flow cytometry
For labelling cell surface markers, monoclonal antibodies against CD14, CD33, CD205, CD206, CD209, CD80, CD86, CD1a, CD40, PD-L1, PD-L2, CD16, CD32 and CD64 were purchased from BD Pharmingen (San Jose, CA). Monoclonal antibody against TGR5 was purchased from R&D. The fluorescence intensity of labelled cell surface markers was assessed using an FACS Calibur flow cytometer (BD), and the data were analysed using CellQuest (BD) or FlowJo (TreeStar Inc., Ashland, OR) software. Dead cells were excluded from analyses by propidium iodide staining.
Analysis of cytokine production
Production levels of TNF-α were assessed using a Human Inflammation Cytometric Bead Array Kit (BD) with the FACS Calibur, according to the manufacturer's instructions.
cAMP production assay
Mγ-Mϕs (1 × 105 cells) were treated with the TGR5 agonist (10 μm) for 5 min in the presence of 1 mm 3-isobutyl-1-methylxanthine (Sigma). The amount of cAMP was determined with a cAMP-Screen System (Applied Biosystems).
Western blotting
Mγ-Mϕs stimulated by 10 ng/ml LPS and 10 μm TGR5 agonist were used for intracellular signalling protein detection. Cells (1 × 105 cells/well of a 96-well plate) were washed with ice-cold PBS containing 0·1% protease inhibitor cocktail (Sigma), and then lysed in 15 μl RIPA buffer (Thermo Fisher Scientific, Waltham, MA) containing 0·5% protease inhibitor cocktail (Sigma) and 1 mM Na3VO4. After centrifugation, supernatants mixed with lithium dodecyl sulphate sample buffer and reducing agents (Invitrogen) were electrophoresed on a Mini PROTEAN TGX gel (Bio-Rad). Separated proteins were transferred by an iBlot Dry Blotting System (Invitrogen) according to the manufacturer's instructions. The membrane was immersed in Starting Block T20 (TBS) Blocking Buffer (Thermo Fisher Scientific) for 1 hr. After blocking, primary antibodies were added to the membranes, followed by incubation at 4° overnight. After incubation with a horseradish peroxidase-conjugated anti-rabbit secondary antibody (Cell Signaling, Danvers, MA), blots were visualized using an Amersham ECL Prime western Blotting Detection Reagent (GE Healthcare, Buckinghamshire, UK) and analysed using an ImageQuant LAS 4000 mini (GE Healthcare). The antibodies used were anti-phospho-c-Fos, anti-c-Fos and anti-β-actin (all Cell Signaling). Immunoblotted membranes were repeatedly used after treatment with a Ten-min Western Blot Re-Probe Kit (Jacksun Easy Biotech, Bronx, NY).
Tissue samples
Normal intestinal mucosa was obtained from macroscopically and microscopically unaffected areas in patients with colon cancer as non-IBD samples. Intestinal IBD mucosa was obtained from surgically resected specimens taken from patients with CD and diagnosed based on clinical, radiographic, endoscopic and histological findings, according to established criteria. All experiments were approved by the Institutional Review Board of Keio University School of Medicine and Yokohama Municipal Citizen's Hospital, Inagi Municipal Hospital, and written informed consent was obtained from all patients.
Analysis of lamina propria mononuclear cells
Lamina propria mononuclear cells (LPMCs) were isolated from intestinal specimens using a technique described elsewhere,31 with some modification. Briefly, dissected mucosa was incubated in calcium and magnesium-free Hanks' balanced salt solution (HBSS) (Sigma) containing 2·5% heat-inactivated fetal bovine serum and 1 mm dithiothreitol (Sigma) to remove mucus. The mucosa was then incubated twice in HBSS containing 1 mm EDTA (Sigma) for 45 min at 37°. Tissues were collected and incubated in HBSS containing 1 mg/ml collagenase type 3 and 0·1 mg/ml DNase I (Worthington Biochemical, Lakewood, CO) for 60 min at 37°. Cells were pelleted and resuspended in a 40% Percoll solution (GE Healthcare), and then layered onto 60% Percoll before centrifugation for 20 min at room temperature. Viable LPMCs were recovered from the 40–60% layer interface. CD14+, CD3+, CD56+ and CD14− CD3− CD56− subsets of LPMCs were purified using MACS. Cells were used for RT-qPCR analysis of TGR5 and the cytokine production assay.
Cell viability assay
PrestoBlue Cell Viability Reagent (Invitrogen), diluted at 1 : 10 with medium, was added to differentiated monocytes (2 × 105 cells/100 μl diluted solution) in a 96-well plate. Samples were then incubated for 30 min at 37°. PrestoBlue is reduced from blue resazurin to red redorufin in the presence of viable cells. Fluorescence (excitation, 570 nm; emission, 600 nm) was measured with a Benchmark plus (Bio-Rad).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software version 4.0 (GraphPad Software Inc., San Diego, CA). Kruskal–Wallis one-way analysis of variance and the Bonferroni post-hoc test were used for examining statistical differences among more than three groups, and the Student's t-test was used for two groups. A value of P < 0·05 was considered statistically significant.
Results
Expression of TGR5 in Mγ-Mϕs compared with other peripheral blood monocytes
To investigate the expression of TGR5 among several phenotypes of innate immune cells, we first used RT-qPCR to analyse the TGR5 mRNA level in several types of mononuclear cell lineages differentiated from peripheral blood. The results showed that TGR5 mRNA was down-regulated in almost all Mϕs and DCs differentiated in vitro, except for Mγ-Mϕs. On day 1, Mγ-Mϕs maintained their TGR5 mRNA level at the same level as that of peripheral CD14+ monocytes (Fig. 2a). We confirmed that maintenance of the TGR5 mRNA level occurred in an IFN-γ concentration-dependent manner (Fig. 2b). The TGR5 mRNA level of Mγ-Mϕs was conserved on day 6 (Fig. 2c). In addition, when IFN-γ was added to differentiated M-Mϕs on day 6, IFN-γ did not affect the TGR5 mRNA level (Fig. 2d). These findings suggest that IFN-γ stimulation at the initial phase of differentiation is important to maintain the TGR5 mRNA level in Mϕs. We also analysed TGR5 expression by immunofluorescence and flow cytometry. Mγ-Mϕs expressed more TGR5 proteins than M-Mϕs (Fig. 2e,f). The morphology and expression of cell surface markers in Mγ-Mϕs and M-Mϕs were shown in the Supplementary material, Fig. S1.
Figure 2.
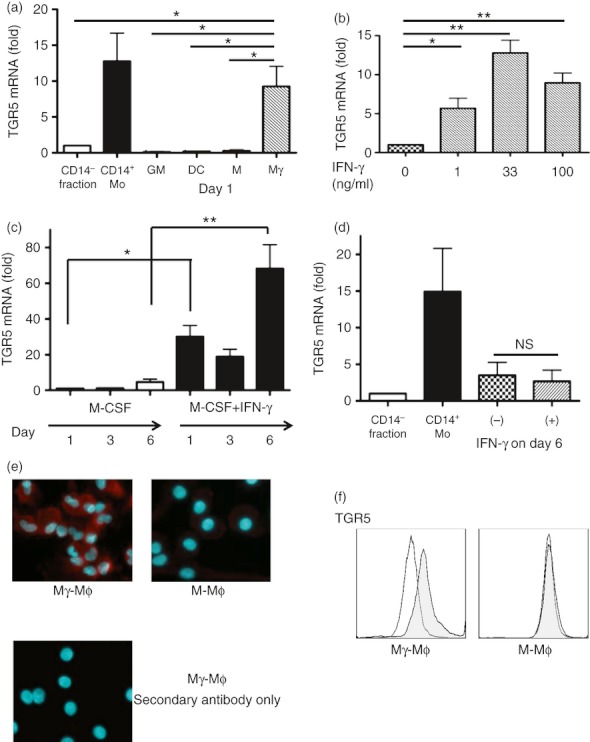
Macrophages differentiated with macrophage colony-stimulating factor and interferon-γ (Mγ-Mϕs) differentiated from peripheral blood CD14+ monocytes maintain TGR5 expression. (a) The mRNA level of TGR5 in the CD14− fraction of peripheral blood mononuclear cells (PBMCs), CD14+ monocytes and Mϕs differentiated with macrophage colony-stimulating factor alone (M-Mϕs), dendritic cells (DCs), Mϕs differentiated with granulocyte–macrophage colony-stimulating factor and interferon-γ (GM-Mϕs) and Mγ-Mϕs on day 1 was measured by real-time quantitative PCR. The mRNA level of TGR5 was normalized to that of β-actin, and is shown as the fold change based on the mRNA levels in the CD14− fraction. Results are shown as the means ± SEM from three independent experiments. Statistical analysis was performed by Kruskal–Wallis one-way analysis of variance (anova)and the Bonferroni post-hoc test for multiple comparisons. *P < 0·05, Mo: monocytes, GM: GM-Mϕs, DC: dendritic cells, M: M-Mϕs, Mγ: Mγ-Mϕs. (b) The mRNA level of TGR5 in differentiated Mγ-Mϕs for each interferon-γ concentration (0, 1, 33 and 100 ng/ml) on day 1. The mRNA level of TGR5 was normalized to that of β-actin, and is shown as the fold change based on the mRNA levels in Mϕs without interferon-γ stimulation. Results are shown as the means ± SEM from five independent experiments. Statistical analysis was performed by Kruskal–Wallis one-way anova and the Bonferroni post-hoc test for multiple comparisons. *P < 0·05, **P < 0·01. (c) The mRNA level of TGR5 in differentiated M-Mϕs and Mγ-Mϕs on day 1, 3 and 6. The mRNA level of TGR5 was normalized to that of β-actin, and is shown as the fold change based on the mRNA level in M-Mϕs on day 1. Results are shown as the means ± SEM from five independent experiments. Statistical analysis was performed by Kruskal–Wallis one-way anova and the Bonferroni post-hoc test for multiple comparisons. *P < 0·05, **P < 0·01. (d) The mRNA level of TGR5 in the CD14− fraction of PBMCs, CD14+ monocytes (Mo) and differentiated M-Mϕs on day 7, which were stimulated with or without interferon-γ on day 6. The mRNA level of TGR5 was normalized to that of β-actin, and is shown as the fold change based on the mRNA levels in CD14− fraction of PBMCs. Results are shown as the means ± SEM from four independent experiments. Statistical analysis was performed by Kruskal–Wallis one-way anova and the Bonferroni post-hoc test for multiple comparisons. (e) Fluorescence imaging of M-Mϕs and Mγ-Mϕs on day 6 of differentiation are shown. Mγ-Mϕs stained with the secondary antibody only was shown as the negative control. Red: TGR5, Blue: nuclei. (f) Flow cytometry analysis for TGR5 expression of differentiated M-Mϕs and Mγ-Mϕs (on day 6). The shaded histogram shows the profiles of the TGR5 antibody staining and the open histogram shows staining with the isotype control.
A TGR5 agonist suppresses bacterially induced reduction of TNF-α in Mγ-Mϕs
To demonstrate the anti-inflammatory function of TGR5 signalling in Mγ-Mϕs, we analysed the inhibitory effect of a TGR5 agonist on TNF-α production in Mγ-Mϕs stimulated with commensal bacterial antigen. As expected, the TGR5 agonist significantly suppressed TNF-α production in a concentration-dependent manner (Fig. 3a). This function was not affected by DMSO (see Supplementary material, Fig. S2). The TGR5 agonist did not act in other types of Mϕs and DCs, which did not express TGR5 (see Supplementary material, Fig. S3a–c). To confirm that this suppressive function caused an effect on TNF-α produced via the toll-like receptor 4 pathway correctly, we used LPS instead of E. faecalis for stimulation of Mγ-Mϕs. We also found that the TGR5 agonist suppressed LPS-induced TNF-α production (Fig. 3b), but it did not affect anti-inflammatory cytokine, IL-10 production (see Supplementary material, Fig. S4). This TGR5 agonist did not change the cell surface marker on the Mγ-Mϕs (see Supplementary material, Fig. S5). With regard to peripheral CD14+ monocytes, which express TGR5, the TGR5 agonist suppressed TNF-α production as well (see Supplementary material, Fig. S6). Furthermore, we analysed whether BAs could suppress TNF-α production in the same manner as that of the TGR5 agonist. As shown in Fig. 3(c), DCA and LCA, which are considered natural TGR5 ligands, suppressed TNF-α production. The viability of Mγ-Mϕs treated with the TGR5 agonist and BAs was not significantly altered (Fig. 3d,e). To compare with a TGR5 agonist, we used a toll-like receptor 4 inhibitor and a p38 mitogen-activated protein kinase inhibitor for the evaluation of TNF-α inhibition. The inhibitory effect of TGR5 agonist was comparable with that of p38 mitogen-activated protein kinase inhibitor on TNF-α production at 10 mm concentration (Fig. 3f). It suggests that a TGR5 agonist has an anti-inflammatory effect that compares favourably with other types of anti-inflammatory reagents.
Figure 3.
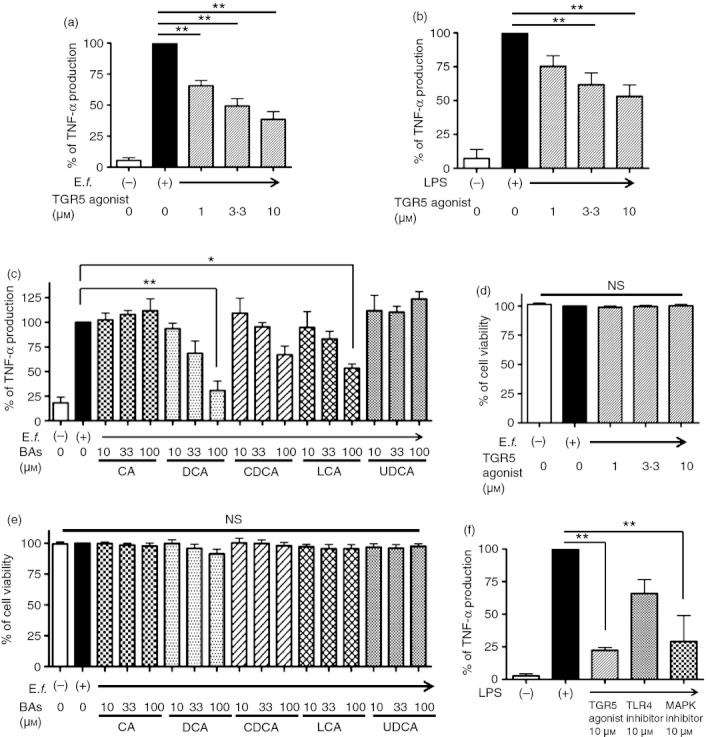
A TGR5 agonist and bile acids (BAs) suppress tumour necrosis factor-α (TNF-α) production from macrophages differentiated with macrophage colony-stimulating factor (M-CSF) and interferon-γ (IFNγ) (Mγ-Mϕs) stimulated by Enterococcus faecalis or lipopolysaccharide (LPS). (a) The Mγ-Mϕs were differentiated from CD14+ monocytes with M-CSF and IFNγ; 50 and 100 ng/ml, respectively). After 6 days in culture, TNF-α levels in culture supernatants were analysed after 24 hr of stimulation with E. faecalis (multiplicity of infection = 100) with or without the TGR5 agonist (1, 3·3 and 10 μm). Results are shown as the means ± SEM from seven independent experiments as relative percentages of the TNF-α levels in Mγ-Mϕs stimulated with E. faecalis only. Statistical analysis was performed by Kruskal–Wallis one-way analysis of variance (anova) and the Bonferroni post-hoc test for multiple comparisons. **P < 0·01. (b) Similar TNF-α analysis to that in (a), stimulated with LPS (100 ng/ml) with or without the TGR5 agonist. Results are shown as the means ± SEM from five independent experiments as relative percentages of the TNF-α levels in Mγ-Mϕs stimulated with LPS only. Statistical analysis was performed by Kruskal–Wallis one-way anova and the Bonferroni post-hoc test for multiple comparisons. **P < 0·01. (c) Several BAs inhibited TNF-α production, which was promoted by E. faecalis (multiplicity of infection = 100) after 24 hr of stimulation, from Mγ-Mϕs on day 6. Results are shown as the means ± SEM from three independent experiments as relative percentages of the TNF-α levels in Mγ-Mϕs stimulated with E. faecalis only. Statistical analysis was performed by Kruskal–Wallis one-way anova and the Bonferroni post-hoc test for multiple comparisons. *P < 0·05, **P < 0·01. CA, cholic acid; DCA, deoxycholic acid; CDCA, chenodeoxycholic acid; LCA, lithocholic acid; UDCA, ursodeoxycholic acid; (d, e) Cell viability of peripheral blood mononuclear cell-derived Mγ-Mϕs. Mγ-Mϕs were differentiated from monocytes by 6 days of culture with M-CSF and IFNγ (control). Other stimuli were added on day 6; TGR5 agonist (1, 3·3 and 10 μm) in (d), and BAs including CA, DCA, CDCA, LCA and UDCA in (e). Cell viability was measured by PrestoBlue Cell Viability Reagent. Results are shown as the means ± SEM from each three independent experiments as relative percentages of the viability of Mγ-Mϕs stimulated with E. faecalis only. Statistical analysis was performed by Kruskal–Wallis one-way anova. (f) TNFα levels in Mγ-Mϕs culture supernatants were analysed after 24 hr stimulation with LPS (100 ng/ml) with or without the inflammatory agents as follows: TGR5 agonist, toll-like receptor (TLR) 4 inhibitor and p38 mitogen-activated protein kinase (MAPK) inhibitor. Results are shown as the means ± SEM from three independent experiments as relative percentages of TNFα levels in Mγ-Mϕs stimulated with LPS only. Statistical analysis was performed by Kruskal–Wallis one-way anova and the Bonferroni post-hoc test for multiple comparisons. **P < 0·01.
cAMP, a second messenger of TGR5, also suppresses TNF-α production in Mγ-Mϕs
We next investigated whether cAMP, a downstream molecule of TGR5, could suppress TNF-α production in Mγ-Mϕs. As shown in Fig. 4(a, b), cAMP also suppressed TNF-α production in a similar way to the suppression by the TGR5 agonist in Mγ-Mϕs stimulated with E. faecalis or LPS. The viability of Mγ-Mϕs modified by cAMP was not significantly altered (Fig. 4c). Consistent with previous reports,16–18,23,27,28 the TGR5 agonist also up-regulated cAMP in Mγ-Mϕs (Fig. 4d) and induced phosphorylation of c-Fos (Fig. 4e) after LPS stimulation.
Figure 4.
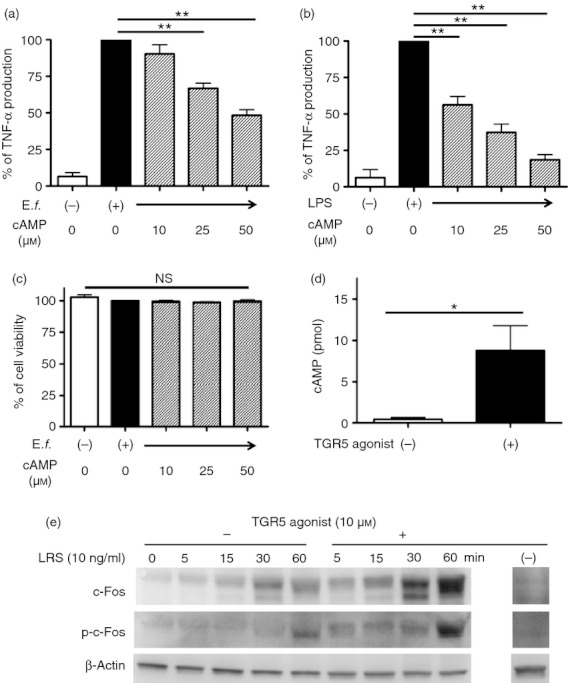
cAMP related to TGR5 as a second messenger suppresses tumour necrosis factor-α (TNF-α) production from macrophages differentiated with macrophage colony-stimulating factor (M-CSF) and interferon-γ (IFNγ) (Mγ-Mϕs) stimulated by Enterococcus faecalis or lipopolysaccharide (LPS). (a) Differentiated Mγ-Mϕs stimulated by E. faecalis (100 multiplicity of infection) on day 6 were treated with cAMP (10, 25 and 50 μm). TNF-α levels in culture supernatants were analysed after 24 hr of stimulation. Results are shown as the means ± SEM from four independent experiments as relative percentages of the TNF-α levels in Mγ-Mϕs stimulated with E. faecalis only. Statistical analysis was performed by Kruskal–Wallis one-way analysis of variance (anova) and the Bonferroni post-hoc test for multiple comparisons. **P < 0·01. (b) Similar TNF-α analysis as in (a) stimulated with LPS (100 ng/ml) with or without cAMP. Results are shown as the means ± SEM from six independent experiments as relative percentages of the TNF-α levels in Mγ-Mϕs stimulated with LPS only. Statistical analysis was performed by Kruskal–Wallis one-way anova and the Bonferroni post-hoc test for multiple comparisons. **P < 0·01. (c) Cell viability of Mγ-Mϕs modified by cAMP. Mγ-Mϕs were differentiated from monocytes by 6 days of culture with M-CSF and IFNγ (control). cAMP (10, 25 and 50 μm) was added on day 6. Cell viability was measured by PrestoBlue Cell Viability Reagent. Results are shown as the means ± SEM from each three independent experiments as relative percentages of the viability of Mγ-Mϕs stimulated with E. faecalis only. Statistical analysis was performed by Kruskal–Wallis one-way anova. (d) cAMP production in Mγ-Mϕs stimulated by the TGR5 agonist. Results are shown as the means ± SEM from five independent experiments. Statistical analysis was performed by the Student's t-test. *P < 0·05. (e) On day 6, Mγ-Mϕs were treated with the TGR5 agonist (10 μm) or control medium for 120 min and then stimulated by LPS (10 ng/ml) or control medium. Western blotting of c-Fos, p-c-Fos and β-actin at 0, 5, 15, 30 and 60 min is shown.
Intestinal CD14+ Mϕs from CD patients express TGR5
We previously reported that intestinal CD14+ Mϕs contribute to the pathogenesis of CD by the production of a large amount of pro-inflammatory cytokines in response to bacterial stimulation.10 We investigated whether TGR5 expression in the intestinal CD14+ Mϕs of CD patients was the same as that in in vitro Mγ-Mϕs that mimic CD14+ Mϕs. The TGR5 mRNA level in LPMCs obtained from inflamed CD mucosa, which was distinguished by TNF-α production (see Supplementary material, Fig. S7), exhibited significantly up-regulated TGR5 mRNA, compared with that in the LPMCs of patients without IBD (Fig. 5a). Moreover, isolated CD14+ Mϕs highly expressed TGR5 mRNA, compared with CD14+ Mϕ-depleted LPMCs (Fig. 5b). These findings revealed that intestinal CD14+ Mϕs are the cells that express TGR5 among LPMCs. Importantly, the TGR5 agonist suppressed bacterially stimulated TNF-α production in CD LPMCs (Fig. 6a) and isolated CD14+ Mϕs (Fig. 6b).
Figure 5.
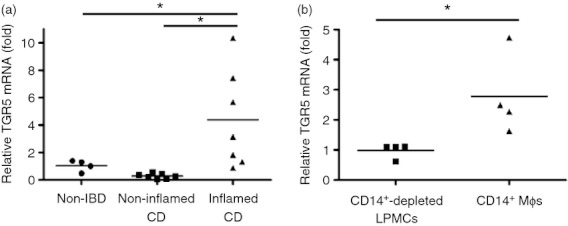
Lamina propria mononuclear cells (LPMCs) from patients with Crohn's disease CD express TGR5 similarly to that in macrophages differentiated with macrophage colony-stimulating factor (M-CSF) and interferon-γ (IFNγ) (Mγ-Mϕs). (a) The mRNA level of TGR5 in LPMCs of non-irritable bowel disease (non-IBD), non-inflamed Crohn's disease (CD) and inflamed CD patients was measured by real-time quantitative PCR. The mRNA level of TGR5 was normalized to that of β-actin and is shown as the fold change based on the non-IBD, which is set to 1. Results are shown as the means ± SEM from four non-IBD, seven non-inflamed CD and seven inflamed CD samples. Statistical analysis was performed by Kruskal–Wallis one-way analysis of variance and the Bonferroni post-hoc test for multiple comparisons. *P < 0·05. (b) The mRNA level of TGR5 in CD14+-depleted LPMCs and CD14+ intestinal Mϕs from CD patients was measured by real-time quantitative PCR. The mRNA level of TGR5 was normalized to that of β-actin, and is shown as the fold change based on the mRNA levels in CD whole LPMCs before sorting. Results are shown as the means ± SEM from four CD14+-depleted LPMCs and four CD14+ intestinal Mϕs samples. Statistical analysis was performed by Kruskal–Wallis one-way analysis of variance and the Bonferroni post-hoc test for multiple comparisons. *P < 0·05.
Figure 6.
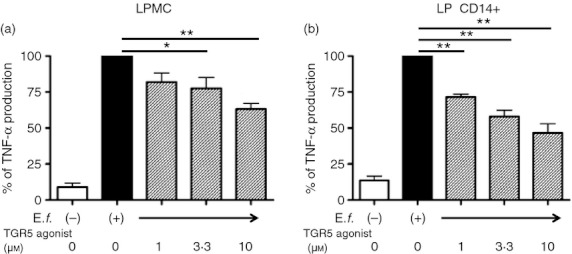
The TGR5 agonist suppresses tumour necrosis factor-α (TNF-α) production from the lamina propria mononuclear cells (LPMCs) and CD14+ intestinal macrophages (Mϕs) of patients with Crohn's disease (CD) in the same manner as that of macrophages differentiated with macrophage colony-stimulating factor (M-CSF) and interferon-γ (IFNγ) (Mγ-Mϕs) stimulated by Enterococcus faecalis. (a) TNF-α levels in CD LPMC culture supernatants were analysed after 24 hr stimulation with E. faecalis (multiplicity of infection = 100) with or without the TGR5 agonist (1, 3·3 and 10 μm). Results are shown as the means ± SEM from five independent experiments as relative percentages of TNF-α levels in LPMCs stimulated with E. faecalis only. Statistical analysis was performed by Kruskal–Wallis one-way analysis of variance and the Bonferroni post-hoc test for multiple comparisons. *P < 0·05, **P < 0·01. (b) CD14+ intestinal Mϕs of CD patients were analysed as described in (a). Results are shown as the means ± SEM from three independent experiments as relative percentages of TNF-α levels in CD14+ intestinal Mϕs stimulated with E. faecalis only. Statistical analysis was performed by Kruskal–Wallis one way analysis of variance and the Bonferroni post-hoc test for multiple comparisons. **P < 0·01.
Discussion
In this study, we demonstrated that TGR5, which is a G-protein coupled receptor for BAs, is expressed in both the Mγ-Mϕs and intestinal CD14+ Mϕs of patients with CD, which produce large amounts of TNF-α in response to bacterial stimuli, and that a TGR5 agonist can suppress such a response. It has been reported that TGR5 is expressed in human peripheral monocytes.16,17 Although transcriptional regulation of TGR5 has not been identified, TGR5 expression was conserved only in Mγ-Mϕs. In contrast, its expression was down-regulated in other fully differentiated mononuclear cell lineages. Interestingly, an effect of IFN-γ was only observed when IFN-γ was added at the initial phase of differentiation. These results suggest that IFN-γ may contribute to conserved expression of TGR5 in monocytes.
Recently, it has been suggested that TGR5 plays a role in immunological regulation via cAMP, a second messenger with an immunoregulatory function.16,17,27,28 Based on the inhibiting effect of inhibitor of nuclear factor-κBα (IκBα), Wang et al.32 reported an inhibitory mechanism of NF-κB, in which TGR5 enhances the cytosolic protein β-arrestin 2 that inhibits phosphorylation of IκBα by bacterial antigen stimulation, resulting in suppression of TNF-α production. Pols et al.27 also reported that TGR5 induces a decrease of phospho-IκBα (p-IκBα). Based on these results, we also examined whether TGR5 inhibits phosphorylation of IκBα, but the TGR5 agonist did not affect the level of p-IκBα in Mγ-Mϕs (data not shown). In other studies, Koga et al.33 demonstrated that cAMP induced by prostaglandin E2 suppresses TNF-α transcription by up-regulation of c-Fos that binds to nuclear factor-κB p65 protein, and this complex cannot bind to the TNF promoter in DCs or RAW264.7 cells. In addition, c-Fos is stabilized by phosphorylation from Iκ kinase β via the toll-like receptor 4 pathway that is triggered by innate immune responses against bacterial antigen.33 This inhibition of the TNF-α promoter binding effect is not associated with IκBα. We hypothesized that in mononuclear cell lineages, TGR5 has an immunoregulatory function by inducing cAMP, which is similar to the mechanism of prostaglandin E2. In our experiments, the concentration of cAMP in Mγ-Mϕs was increased by treatment with the TGR5 agonist that induced phosphorylation of c-Fos (p-c-Fos). These findings suggest that the TGR5 agonist may suppress TNF-α transcription via p-c-Fos in Mγ-Mϕs.
Several BAs, including both primary and secondary BAs, have been reported as natural ligands of TGR5. Of these BAs, LCA activates TGR5 with an EC50 of ∼ 600 nm, suggesting that LCA is a physiological ligand for TGR5, whereas UDCA has low affinity.17,24,34 Consistent with previous observations, our study indicated that DCA and LCA had an anti-inflammatory potential (Fig. 3c). In our study, the TGR5 agonist and BAs did not affect the viability of Mϕs (Fig. 3d,e). Although the detailed mechanism is not clarified on the Mγ-Mϕs, Sato et al.35 investigated the binding affinity of BAs to TGR5 from the aspect of chemical structures by luciferase assay methods on TGR5-transfected Chinese hamster ovary (CHO) cells. They revealed that TGR5 were highly bound to LCA, DCA, CDCA, CA and UDCA in this order. Their binding affinity to TGR5 was affected by the absence or existence of hydroxylation in positions 6, 7 and 12 of the steroid ring, which was constituted of bile acids. LCA and DCA do not have a hydroxylation at 7 of the steroid ring, but others have one. It is possible that the binding affinity to TGR5 on the Mγ-Mϕs depends on the molecular structure of BAs. Both DCA and LCA have fewer hydroxyl groups, suggesting that a hydrophobic structure facilitates binding to TGR5.
Importantly, the TGR5 agonist suppressed TNF-α production by intestinal CD14+ Mϕs that play a central role in the pathogenesis of CD. Modification and conditioning of innate immune cells have been considered as therapeutic strategies for treating chronic inflammatory disorders such as CD. Previously, we reported that tetomilast (OPC-6535), which inhibits phosphodiesterase-4, suppresses production of pro-inflammatory cytokines in human Mϕs.36 Retinoic acid receptors and TGR5 affect the differentiation process of DCs and induce an IL-12-hypoproducing DC phenotype.15,16 Here, we demonstrated that a TGR5 agonist suppressed TNF-α production by the inflammatory phenotype of Mϕs. Although a precise mechanism remains unclear, Cipriani et al.28 reported that TGR5 is associated with regulation of experimental colitis in a mouse model by not only inflammatory cytokine suppression in immune cells, but also by maintaining the epithelial architecture as shown by an abnormal distribution of the epithelial tight junction protein zonulin 1 on the colon in dextran sulphate sodium-induced colitis in TGR5-deficient mice. These results suggest that TGR5 is involved in intestinal homeostasis by regulation of the immune cell response and maintenance of the epithelial barrier function.
In conclusion, our results clearly demonstrate the expression profiles of TGR5 in several mononuclear cell types and the potential of a TGR5 agonist as an anti-inflammatory molecule in innate immune cells. Modification of TGR5 signalling may have a potential to regulate immune reaction in chronic inflammatory disorders such as CD.
Acknowledgments
We thank Drs Naoyuki Kobayashi and Junichi Saito (Inagi Municipal Hospital) for providing surgical specimens. We also thank Drs Jun Miyoshi, Yohei Mikami and Shinta Mizuno (Keio University) for their scientific suggestions. This work was supported in part by a Grant-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan Society for the Promotion of Science, and Keio University Medical Fund.
Glossary
- BA
bile acid
- CA
cholic acid
- CD
Crohn's disease
- CDCA
chenodeoxycholic acid
- CHO, Chinese hamster ovary; DC
dendritic cell
- DCA
deoxycholic acid
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- GM-Mϕ
macrophage derived by GM-CSF
- HBSS
Hanks' Balanced Salt solution
- IBD
inflammatory bowel disease
- IFN
interferon
- IκBα
inhibitor of nuclear factor-κBα
- IL
interleukin
- LCA
lithocholic acid
- LP
lamina propria
- LPMC
lamina propria mononuclear cell
- LPS
lipopolysaccharide
- MACS
magnetic cell separation system
- M-CSF
macrophage colony-stimulating factor
- Mϕ
macrophage
- M-Mϕ
macrophage derived by M-CSF
- Mγ-Mϕ
derived by M-CSF and IFN-γ
- MOI
multiplicity of infection
- p-c-Fos
phospho-c-Fos
- p-IκBα
phospho-IκBα
- RT-qPCR
real time-quantitative polymerase chain reaction
- TNF
tumour necrosis factor
- UDCA
ursodeoxycholic acid
Disclosure
The authors declare no conflict of interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Morphology and expression of cell surface markers in macrophages differentiated with macrophage colony-stimulating factor and interferon-γ (Mγ-Mφs) and macrophages differentiated with macrophage colony-stimulating factor only (M-Mφs).
Figure S2. The tumour necrosis factor-α assay is not influenced by solvents.
Figure S3. The TGR5 agonist cannot inhibit tumour necrosis factor-α production from macrophages differentiated with macrophage colony-stimulating factor alone (M-Mφs), dendritic cells (DCs) and macrophages differentiated with granulocyte–macrophage colony-stimulating factor (GM-Mφs.
Figure S4. The TGR5 agonist cannot inhibit interleukin-10 production from macrophages differentiated with macrophage colony-stimulating factor and interferon-γ (Mγ-Mφs).
Figure S5. Surface phenotypes on the macrophages differentiated with macrophage colony-stimulating factor and interferon-γ (Mγ-Mφs) modified with or without the TGR5 agonist.
Figure S6. Tumour necrosis factor-α levels in peripheral CD14+ monocytes culture supernatants were analysed after 24 hr stimulation with lipopolysaccharide (LPS; 100 ng/ml) with or without the TGR5 agonist (1, 3·3 and 10 μm).
Figure S7. Inflamed Crohn's disease lamina propria mononuclear cells (CD LPMCs) had higher tumour necrosis factor-α level than non-inflamed.
Figure S8. Effects of the TGR5 agonist on tumour necrosis factor-α production from several subsets of Crohn's disease lamina propria mononuclear cells (CD LPMCs) stimulated with Enterococcus faecalis.
References
- 1.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–44. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 3.Smythies LE, Sellers M, Clements RH, Mosteller-barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamada N, Hisamatsu T, Okamoto S, et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–8. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 5.Takada Y, Hisamatsu T, Kamada N, et al. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol. 2010;184:2671–6. doi: 10.4049/jimmunol.0804012. [DOI] [PubMed] [Google Scholar]
- 6.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 7.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogler G, Andus T, Aschenbrenner E, Vogl D, Falk W, Schölmerich J, Gross V. Alterations of the phenotype of colonic macrophages in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:893–9. doi: 10.1097/00042737-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Rogler G, Hausmann M, Spöttl T, et al. T-cell co-stimulatory molecules are upregulated on intestinal macrophages from inflammatory bowel disease mucosa. Eur J Gastroenterol Hepatol. 1999;11:1105–11. doi: 10.1097/00042737-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14+ intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J Clin Invest. 2008;118:2269–80. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenk M, Bouchon A, Seibold F, Mueller C. TREM-1-expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007;117:3097–106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanai T, Watanabe M, Okazawa A, et al. Interleukin 18 is a potent proliferetive factor for intestinal mucosal lymphocytes in Crohn's disease. Gastroenterology. 2000;119:1514–23. doi: 10.1053/gast.2000.20260. [DOI] [PubMed] [Google Scholar]
- 13.Kamada N, Hisamatsu T, Honda H, et al. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J Immunol. 2009;183:1724–31. doi: 10.4049/jimmunol.0804369. [DOI] [PubMed] [Google Scholar]
- 14.Takayama T, Kamada N, Chinen H, et al. Imbalance of NKp44+ NKp46– and NKp44– NKp46+ natural killer cells in the intestinal mucosa of patients with Crohn's disease. Gastroenterology. 2010;139:882–92. doi: 10.1053/j.gastro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 15.Wada Y, Hisamatsu T, Kamada N, Okamoto S, Hibi T. Retinoic acid contributes to the induction of IL-12-hypoproducing dendritic cells. Inflamm Bowel Dis. 2009;15:1548–56. doi: 10.1002/ibd.20934. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa R, Takayama T, Yoneno K, et al. Bile acids induce monocyte differentiation toward interleukin-12 hypo-producing dendritic cells via a TGR5-dependent pathway. Immunology. 2012;136:153–62. doi: 10.1111/j.1365-2567.2012.03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 18.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–9. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama T, Tanaka K, Suzuki J, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191:197–205. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- 20.Keitel V, Reinehr R, Gatsios P, Rupprecht C, Görg B, Selbach O, Häussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 21.Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Häussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50:861–70. doi: 10.1002/hep.23032. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 23.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calmus Y, Guechot J, Podevin P, Bonnefis MT, Giboudeau J, Poupon R. Differential effects of chenodeoxycholic and ursodeoxycholic acids on interleukin 1, interleukin 6 and tumor necrosis factor-α production by monocytes. Hepatology. 1992;16:719–23. doi: 10.1002/hep.1840160317. [DOI] [PubMed] [Google Scholar]
- 25.Greve JW, Gouma DJ, Buurman WA. Bile acids inhibit endotoxin-induced release of tumor necrosis factor by monocytes: an in vitro study. Hepatology. 1989;10:454–8. doi: 10.1002/hep.1840100409. [DOI] [PubMed] [Google Scholar]
- 26.Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 27.Pols TW, Nomura M, Harach T, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747–57. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipriani S, Mencarelli A, Chini MG, et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS ONE. 2011;6:e25637. doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akagawa KS. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Int J Hematol. 2002;76:27–34. doi: 10.1007/BF02982715. [DOI] [PubMed] [Google Scholar]
- 30.Evans KA, Budzik BW, Ross S, et al. Discovery of 3-aryl-4-isoxazolecarboxamides as TGR5 receptor agonists. J Med Chem. 2009;52:7962–5. doi: 10.1021/jm901434t. [DOI] [PubMed] [Google Scholar]
- 31.Kanai T, Watanabe M, Okazawa A, et al. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn's disease. Gastroenterology. 2001;121:875–88. doi: 10.1053/gast.2001.28021. [DOI] [PubMed] [Google Scholar]
- 32.Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-Protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54:1421–32. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koga K, Takaesu G, Yoshida R, Nakaya M, Kobayashi T, Kinjyo I, Yoshimura A. Cyclic adenosine monophosphate suppresses the transcription of proinflammatory cytokines via the phosphorylated c-Fos protein. Immunity. 2009;30:372–83. doi: 10.1016/j.immuni.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30:570–80. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Sato H, Macchiarulo A, Thomas C, et al. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure–activity relationships, and molecular modeling studies. J Med Chem. 2008;51:1831–41. doi: 10.1021/jm7015864. [DOI] [PubMed] [Google Scholar]
- 36.Ichikawa H, Okamoto S, Kamada N, et al. Tetomilast suppressed production of proinflammatory cytokines from human monocytes and ameliorated chronic colitis in IL-10-deficient mice. Inflamm Bowel Dis. 2008;14:1483–90. doi: 10.1002/ibd.20524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


