Abstract
Extensive studies on CD4+ CD25+ regulatory T (Treg) cells suggest that they are important in regulating immune responses. However, mechanisms of peripheral Treg cell homeostasis are unknown. We found that stromal cells isolated from secondary lymphoid organs such as spleen and lymph nodes could support the survival of Treg cells. This was dependent on CD2 engagement and a direct interaction between Treg cells and stromal cells. In the presence of stromal cells, Bim, a pro-apoptotic factor, was partially decreased in Treg cells. This effect could be inhibited by anti-CD2 blocking antibodies, indicating that stimulation through CD2 on Treg cells regulates Bim expression, which may be relevant to Treg cell apoptosis. Therefore, Treg cell interactions with stromal cells through CD2 may be essential for Treg cell survival. Surprisingly, the expression of CD2 ligands on stromal cells was not detected. Hence, it is not clear how CD2 on Treg cells contributes to a direct interaction with the stromal cells and participates in survival support for Treg cells. Taken together, CD2 stimuli were mandatory for Treg cell survival with reduced Bim expression, but CD2 may not function as a direct receptor for molecules on stromal cells.
Keywords: Bim, CD2, homeostasis, regulatory T cells, stromal cells
Introduction
CD4+ CD25+ Foxp3+ regulatory T (Treg) cells were originally identified as regulators of tissue-specific autoimmunity.1–3 Now, it is widely accepted that Treg cells contribute to the regulation of various immune responses, including virus infections,4–6 parasite infections,7,8 and graft rejection.9,10 Despite extensive research on Treg cells, many issues regarding Treg cells, such as recall-antigen responses or peripheral homeostasis, are unclear.
Cytokines play a key role in lymphocyte homeostasis in the central and peripheral immune organs.11,12 Especially, interleukin-7 (IL-7) is an essential factor for T-cell homeostasis.13–16 However, Treg cells express lower levels of IL-7 receptor α chain (CD127) than other CD4+ T cells, suggesting that Treg cells depend less on IL-7 for homeostasis.17–19 Conversely, it has been reported that IL-7 contributes to Treg cell survival in vitro.20–22 Therefore, the importance of IL-7 for Treg cell homeostasis is still controversial. It is well accepted that other cytokines, such as transforming growth factor-β (TGF-β),23–25 IL-226–28 and CD28,29,30 a co-stimulatory molecule, play an important role in Treg cell homeostasis.
Similar to CD28, CD2 is an activating signal transducer for T cells.31 Interestingly, CD2 is a target gene for the transcription factor Foxp3,32 a master regulator of Treg cell development and function. CD2 signalling also induces Foxp3.33 Therefore, it is likely that CD2 also serves as a molecule for Treg cell development, function and homeostasis. Indeed, inducible Treg cell development and effector functions require CD2 signalling. Interestingly, the morbidity or risk of multiple sclerosis33 and rheumatoid arthritis34 correlated with polymorphisms of CD58, a ligand of human CD2. In addition, Treg cells from patients with multiple sclerosis had defective CD2 signalling,33 indicating that CD2 and its ligand are essential for Treg cell functions and may also contribute to Treg cell homeostasis that can prevent autoimmunity. However, the roles of CD2 in Treg cell homeostasis have not been elucidated.
To understand the behaviour of Treg cells in the periphery, including homeostasis, we attempted to establish allogeneic antigen-specific Treg cell lines or clones. We observed that Treg cells were well maintained on a layer of stromal cells, which were unexpected contaminants from the Treg cell purification. Hence, in this study, we explored the role of Treg cell–stromal cell interactions in Treg cell homeostasis.
Materials and methods
Mice
BALB/c mice and C57BL/6 mice were purchased from CLEA Japan, Inc. (Tokyo, Japan) or bred by ourselves. Female 6- to 8-week-old mice were used in this study. Mice were housed under specific pathogen-free conditions in our Laboratory Animal Research Centre and were handled according to the Guidelines for the Care and Use of Laboratory Animals, Dokkyo Medical University (protocol #0341).
Cell preparation and culture
Peripheral CD4+ T cells or CD4+ CD25+ regulatory T cells were purified as follows. A single cell suspension prepared from spleens and lymph nodes was treated by incubation in dishes coated with anti-CD45R (B220) monoclonal antibodies (mAbs) for 20–30 min at 37°. Using this procedure, many B cells and adhesive cells were removed. Then, to remove cells other than CD4+ cells, the cell suspension was treated with a mAb cocktail, including biotinylated mAbs specific for CD8a, CD11b, CD45R, CD49b and Ter-119, followed by a depletion procedure using a combination of streptavidin–conjugate MicroBeads (Miltenyi Biotec, Cologne, Germany) and the depletes program of the autoMACS System (Miltenyi Biotec). To obtain purified CD4+ cells or CD25+ cells, the cells were further stained with FITC-conjugated anti-CD4 or anti-CD25 mAbs. Then, CD4+ or CD25+ cells were purified using a combination of anti-FITC MicroBeads (Miltenyi Biotec) and the possels program of the autoMACS System (Miltenyi Biotec). The purity of CD4+ cells or CD4+ CD25+ cells sorted by the autoMACS system were > 99% or > 95%, respectively. For all experiments, except for those using purified Treg cells, one experiment is representative for one mouse. Cells were prepared from two mice as a source of purified Treg cells. Stromal cells were established from unexpected contaminants, which were obtained in the process of Treg cell enrichment (purity > 86%) from BALB/c peripheral lymphoid organs. The phenotype of stromal cells was analysed by flow cytometry or RT-PCR. Lymphocytes were cultured in complete RPMI (RPMI-1640 supplemented with 5% heat-inactivated fetal calf serum, 10 mm HEPES, 2 mm l-glutamine, 1 mm sodium pyruvate, 100 U/ml penicillin, 0·1 mg/ml streptomycin and 50 mm 2-mercaptoethanol). CHO-K1 cells and HEK293 cells were used to obtain CD48 transfectants and soluble CD2-Fc fusion proteins, respectively. In some cultures, cell culture inserts (BD Falcon, Franklin Lakes, NJ) were used to separate cultures.
RT-PCR and ELISA
Total RNA samples from stromal cells were prepared using a combination of QIAshredder and RNeasy Mini Kit (Qiagen, Hilden, Germany) and used according to the manufacturer's instructions. Then, cDNA was synthesized from RNA samples using a ReverTra Ace-α-kit (Toyobo Co., Ltd., Osaka, Japan) according to the manufacturer's protocol. PCR primers were designed using the primer3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3.cgi) and were synthesized by Sigma Genosys (Tokyo, Japan). Primers for RT-PCR were as follows: β-actin: (5′-TGTTACCAACTGGGACGACA-3′ and 5′-GGGGTGTTGAAGGTCTCAAA-3′); TGF-β1: (5′-TGGTGGCTGTCTTTTGCG-3′ and 5′-TCTCTGTGGAGCTGAAGCAA-3′); TGF-β2: (5′-GACCCCACATCTCCTGCTAA-3′ and 5′-TTCGATCTTGGGCGTATTTC-3′). Primer pairs for IL-7 (5′-ACATCATCTGAGTGCCACA-3′ and 5′-CTCTCAGTAGTCTCTTTAG-3′) were described by Chen et al.35 Primer pairs for Fsp1 (Atl1): (5′-ACAGTTGGGGTGGGTTTTCAG-3′ and 5′-GTCTAACTCAAAGGAATGGTCGT-3′) and CD248: (5′-CAACGGGCTGCTATGGATTG-3′ and 5′-GCAGAGGTAGCCATCGACAG-3′) were designed by primerbank (http://pga.mgh.harvard.edu/primerbank/index.html). The PCR was performed as follows: 50° for 2 min, 95° for 10 min, and then the indicated number of cycles of 95° for 15 seconds and 60° for 1 min. Taq DNA polymerase for PCR was purchased from Sigma. ELISA kits for murine TGF-β and for murine IL-2 were purchased from Biosource International, Inc., (Camarillo, CA) and Genzyme/Techne Corporation (Minneapolis, MN), respectively. ELISA was performed according to the manufacturer's instructions.
Flow cytometry and monoclonal antibodies
Single-cell suspensions were prepared and cells were stained with mAbs labelled with FITC, phycoerythrin (PE) and phycoerythrin–Cychrome 5 (PE-Cy5). Dye-conjugated mAbs used in this study were as follows: FITC-conjugated anti-CD25 mAb was purchased from Imgenex Corporation, San Diego, CA. The PE-conjugated anti-Bim mAb (clone Ham151-149) was purchased from two companies: AbD Serotec, Oxford, UK and Santa Cruz Biotechnology Inc., Santa Cruz, CA. Isotype controls, PE-conjugated rat IgG2a (for anti-Foxp3 mAb) and PE-conjugated Armenian hamster IgG (for anti-Bim mAb) were purchased from eBioscience Inc. (San Diego, CA) and Biolegend (San Diego, CA), respectively. The FITC-conjugated anti-CD48 mAb was purchased from AbD Serotec. All other dye-conjugated mAbs used in this study were purchased from eBioscience, Inc. For detection of CD2 ligands, cells were treated with 5 μg CD2-Fc before staining with a combination of biotin-SP-conjugated goat antibodies specific for anti-human IgG (Fcγ) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and streptavidin-PE (BD Biosciences, Franklin Lakes, NJ). As a control for CD2-Fc fusion protein, recombinant human IgG1 Fc (Sino Biological Inc., Beijing, China) was used. Culture supernatant from an anti-CD16/CD32 (FcγR) mAb producing hybridoma, clone 2.4G2, was prepared in our laboratory to block non-specific binding of antibodies to FcγR. Flow cytometry data acquisition and analysis were performed using FACSCalibur and cellquest software (Becton Dickinson, Franklin Lakes, NJ). The TGF-β-neutralizing mAb, clone 1D11, was purchased from R&D Systems, Inc. (Minneapolis, MN). Anti-CD2 blocking mAbs, clone 12-15 and RM2-5, were purchased from Novus Biologicals, LLC (Littleton, CO) and eBioscience, respectively. Isotype controls for anti-CD2 mAbs (rat IgG1 and IgG2b) were purchased from Biolegend, Inc. Blocking mAbs for CD29, CD51 and CD61 were purchased from BD Biosciences. The mAbs were added into the culture at a final concentration of 10 μg/ml.
Annexin V staining and carboxyfluorescein succinimidyl ester labelling
To detect apoptotic cells, PE-labelled annexin V (BD Biosciences) staining was performed. Cells stained with FITC-conjugated anti-CD25 mAb and PE-Cy5-conjugated anti-CD4 mAb were stained with PE-conjugated annexin V. For in vitro cell proliferation assays, lymphocytes from BALB/c mice were labelled with 5-(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) by incubating 1 × 107 cells/ml in PBS with 0·5 mm CFSE for 8 min at room temperature. The CFSE-labelled cells were used for further experiments.
cDNA cloning
The cDNAs for murine CD48 and the extracellular domain of murine CD2 were generated by RT-PCR. Total RNA was isolated from the spleen cells of BALB/c mice using an RNAeasy Plus Mini Kit (Qiagen). Then, the RNA was converted to cDNA using the total RNA and the ReverTra Ase α (Toyobo) with dT primer. To clone cDNAs, PCR amplifications were employed using the KOD-Plus-Neo (Toyobo) with primer pairs specific for full-sized murine CD48 (5′-ATGTGCTTCATAAAACAGGG-3′, 5′-CTTGTCAGGTTAACAGGATCC-3′)36 or the extracellular portion of murine CD2 (5′-CACAAGCTTAAGATGAAATGTAAATTCCTGG-3′, 5′-CATGTCGACTGGACAGTTAACAACTTCC-3′).37 The cloned cDNAs were sequenced with abi prism® 377-XL DNA sequencer (Applied Biosystems, Foster City, CA). A cDNA fragment for the human Fc portion was obtained from a construct containing soluble Fas-human Fc fusion protein, pcDNAI-sFas-hIgG, a kind gift from Dr Lixin Zheng (Laboratory of Immunology, NIAID, NIH, Bethesda, MD). The experimental design for using human-origin samples was approved by the Ethics Committee in Dokkyo Medical University (approval #23005).
Establishment of a stable CD48-expressing CHO-K1 clone, CHO-K1/Cd48
A CD48 expression vector, pcDNA3.1-Cd48, was constructed by the insertion of murine CD48 cDNA into a pcDNA3.1(+) expression vector (Invitrogen, Carlsbad, CA). The linearized pcDNA3.1-Cd48 was introduced into CHO-K1 cells, which were grown in Ham's F-12 supplemented with 10% fetal bovine serum to 80% confluency, using FuGENE 6 (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions. Two days after transfection, G418 (Enzo Life Sciences Ltd., Exeter, UK) was added to the culture at a final concentration of 600 μg/ml for drug selection. Ten days after the addition of G418, the G418-resistant cells were cloned by limiting dilution. The expression levels of CD48 on the G418-resistant CHO-K1 clones were assessed by flow cytometry.
Generation of a CD2-Fc fusion protein
The chimeric cDNA, which consisted of the extracellular portion of murine CD2 cDNA and human Fcγ cDNA, was inserted into the expression vector pcDNA3.1 to obtain CD2-Fc, a fusion protein of the extracellular domain of murine CD2 and human Fc. CD2-Fc was produced in HEK293 cells transfected with the CD2-Fc expression vector. HEK293 cells were grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum to 80% confluency before transfection. Transfection and drug-selection procedures were performed as for the establishment of CHO-K1/Cd48 cells. To obtain CD2-Fc fusion protein, CD2-Fc producing HEK293 cells were cultured in serum-free medium HyQ SFM4 HEK293 (Thermo Fisher Scientific Inc., Waltham, MA). CD2-Fc proteins were purified from the supernatant by a column operation using Protein G Sepharose 4 fast flow (GE Healthcare Bio-Sciences AB, Uppsala, Sweden).
Statistical analysis
The two-tailed paired Student's t-test was used to determine the statistical significance. Values of P < 0·05 were considered statistically significant.
Results
Stromal cells preferentially support functional CD4+ CD25+ regulatory T cells
To elucidate the nature of Treg cells, we attempted to generate Treg cell lines. We observed that Treg cells were well maintained when cultured with stromal cells, which had a fibroblast-like shape, and expressed a number of immune molecules on their surface (Table 1).
Table 1.
Summary of phenotypic analysis of stromal cells
| Expression levels1 | Molecules |
|---|---|
| Negative | CD2, CD11a, CD19, CD21, CD23, CD31, CD40, CD45(B220), CD482, CD69, CD102, CD122, DX-5, OX40L |
| Positive | |
| (+) | CD44, CD2483, Fsp13 |
| (++) | CD11b, CD51, CD61, CD71, CD98, podoplanin (gp38), Sca-1 |
| (+++) | CD29 |
| Inducible4 | MHC class II, CD54 |
(+), (++) and (+++) indicate the strength of immunostaining. Roughly, (+): <10-fold, (++): 10–100-fold, (+++): >100-fold, of intensity compared with control staining.
Assessed by flow cytometry (except CD248 and Fsp1).
Details are described in the text.
Assessed by RT-PCR.
Expression was detected after co-culture with lymphocytes.
As summarized in Table 2, numbers of CD4+ CD25− cells and CD4+ CD25+ cells cultured with stromal cells in the absence of exogenous cytokines were increased compared with cells cultured without stromal cells. The index of cell number of CD25+ cells was greater than that of CD25− cells, suggesting that the stromal cells preferentially support CD25+ Treg cells.
Table 2.
Index of cell number after co-culture1
| Index | |||||
|---|---|---|---|---|---|
| Cell fraction | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 5 |
| CD4+ CD25− cells | 1·6 | 3·0 | 0·4 | 1·7 | 9·1 |
| CD4+ CD25+ cells | 2·8 | 5·1 | 2·6 | 3·6 | 16·6 |
Exp, experiment.
T cells were cultured with stromal cells and the cell number index of CD4+ CD25− or CD4+ CD25+ cells was calculated.
Index of cell numbers was calculated compared with the control culture (without stromal cells).
This was further confirmed by the following experiments. Lymph node cells from BALB/c mice were cultured with or without stromal cells in the absence of exogenous cytokines. Five days after culture, cells were harvested and the expression levels of CD4, CD25 and Foxp3 were analysed. As shown in Fig. 1(a,b), even in the absence of exogenous IL-2, greater numbers of Foxp3-expressing CD4+ CD25+ cells were observed in the culture with stromal cells than in the control culture without stromal cells. Similar results were observed from co-cultures of purified CD4+ CD25+ cells with stromal cells. Compared with the control culture without stromal cells, more living cells were found in the co-culture with stromal cells, suggesting that Treg cells receive direct support from stromal cells (Fig. 1c).
Figure 1.
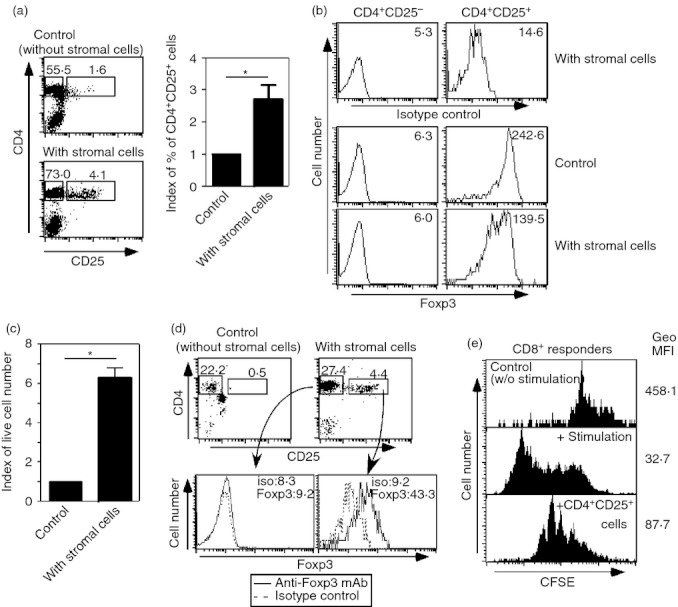
Stromal cells from peripheral lymphoid tissues support functional regulatory T (Treg) cells in vitro. (a, b) Lymph node cells were co-cultured with or without stromal cells in the absence of exogenous cytokines. Five days after culture, cells were harvested and expression levels of CD4, CD25 (a) and Foxp3 (b) were assessed. Index of proportion of CD4+ CD25+ cells are summarized by bar graphs (*P < 0·01, n = 8). (c) CD4+ CD25+ cells purified from lymph nodes and spleens were co-cultured with or without stromal cells in the absence of exogenous cytokines. Five days after culture, the numbers of live cells were counted. The index of live cell numbers was calculated. Cell numbers from control culture (without stromal cells) were assigned a value of one (*P < 0·01, n = 3). (d) CD4+ cells from spleen and lymph nodes were co-cultured with or without stromal cells. Fourteen days later, cells were harvested and expression levels of CD4, CD25 and Foxp3 were assessed. Expression levels of Foxp3 in CD4+ CD25+ T cells are depicted as histograms. (e) CD25+ cells were sorted from the co-culture with stromal cells depicted in (d). These CD25+ cells were added into mixed lymphocyte cultures (MLC), in which carboxyfluorescein succinimidyl ester (CFSE) -labelled responders were used. ‘Control’: CFSE-labelled cells were cultured without stimulators. ‘+ stimulation’: MLC where CFSE-labelled responders were used. ‘+ CD4+CD25+ cells’: MLC including sorted CD25+ cells. Geometric MFI (Geo MFI) in each panel is indicated. Five days after the MLC, the intensity of CFSE in CD8+ T cells was analysed. The data shown represent one of two separate experiments. Proportions of designated areas of dot-plots and MFI of histograms are indicated.
To assess the regulatory functions of CD4+ CD25+ cells from stromal cell co-culture, CD4+ T cells from a mixture of lymph node cells and splenocytes were cultured with the stromal cells for 14 days without exogenous cytokines and their suppressive activity against T-cell division was measured in a mixed lymphocyte culture. Fourteen days later, CD4+ CD25+ Foxp3+ cells were present in the culture with the stromal cells, and there were virtually no Treg cells in the control culture (Fig. 1d). Then, the CD25+ cells were isolated and were added into a mixed lymphocyte culture, in which CFSE labelled-responders were used. As the added CD4+ CD25+ cells were CFSE negative, cell division (as measured by CFSE intensity of CD8+ cells) was assessed to rule out the contamination of these cells in the analysis. The addition of CD25+ cells from co-culture with stromal cells inhibited CD8+ T-cell division in the mixed lymphocyte culture (Fig. 1e), indicating that CD4+ CD25+ Foxp3+ cells had regulatory functions. Taken together, these data indicate that stromal cells support functional Treg cell homeostasis in vitro.
Stromal cells inhibit Treg cell apoptosis but do not induce proliferation or Treg cell conversion
To investigate how stromal cells assist Treg cells, we first tested the possibility that stromal cells promote Treg cell proliferation. CFSE-labelled CD4+ cells were co-cultured with stromal cells. Five days later the CFSE intensity of CD4+ CD25+ cells was assessed. CD4+ CD25+ cell division was not observed in the co-culture with stromal cells (Fig. 2a).
Figure 2.
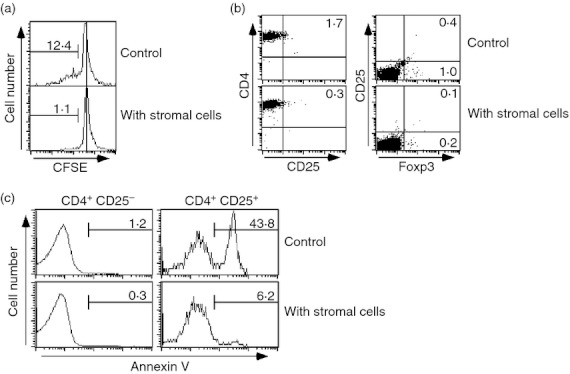
Stromal cells support regulatory T (Treg) cells by preventing cell death, but not by enhancement of proliferation or conversion from conventional T cells. (a) Carboxyfluorescein succinimidyl ester (CFSE) -labelled splenocytes or lymph node cells were co-cultured with or without stromal cells. Five days after culture, the CFSE intensity of CD4+ CD25+ cells was assessed. (b) CD4+ CD25− cells were sorted and co-cultured with stromal cells for 5 days. Then, expression levels of CD25 and Foxp3 were analysed. (c) CD4+ cells from lymph nodes and spleens were co-cultured with stromal cells. As a control culture, CD4+ cells were cultured alone. Two days later, apoptotic cells from the fractions of CD4+ CD25− and CD4+ CD25+ cells were assessed by annexin V staining. The data shown represent one of two (a and b) or three (c) experiments. Proportions of designated areas are indicated.
Next, we determined whether stromal cells promoted the conversion of CD4+ CD25− conventional T cells to CD4+ CD25+ Treg cells. CD4+ CD25− T cells were co-cultured with stromal cells. After 5 days of culture, we observed no cells with a Treg phenotype (CD4+ CD25+ Foxp3+), suggesting that the stromal cells did not promote conversion of conventional T cells to CD4+ CD25+ Foxp3+ Treg cells (Fig. 2b).
Finally, the effect of stromal cells on Treg cell survival and apoptosis was assessed. CD4+ cells isolated from spleens and lymph nodes were cultured with or without stromal cells in the absence of exogenous cytokines. Two days later, apoptotic cells were assessed by annexin V staining. Only a few apoptotic cells were observed in the CD25− conventional CD4+ T-cell fraction in either the control or stromal cell culture (Fig. 2c). In contrast, 43·8% of cells among the CD4+ CD25+ fraction were annexin V positive in the control culture without stromal cells. However, only 6·2% of the CD4+ CD25+ fraction were annexin V positive in the culture with the stromal cells (Fig. 2c). Collectively, these data indicate that the stromal cells prevented cell death or supported survival of Treg cell homeostasis in vitro.
Soluble factors, such as IL-7 and TGF-β, are not mandatory for stromal cell-induced survival of Treg cells
Recently, Simonetta et al.38 reported that IL-7 contributed to Treg cell homeostasis in the periphery, similar to conventional T cells. We observed IL-7 expression at the RNA level in the stromal cells used for co-culture (Fig. 3a). Stromal cells also produced TGF-β, which plays a key role in Treg cell homeostasis, similar to ST-2 cells, bone marrow-derived stromal cells39 (Fig. 3b,c). Importantly, although IL-7 expression in stromal cells and ST-2 cells was detected by RT-PCR, IL-7 protein was not detected by ELISA, suggesting that the stromal cells produced very low levels of IL-7 or that they did not produce IL-7 protein (data not shown). To examine the importance of these cytokines in stromal cell support for Treg cells, we used ST-2, which produce both IL-7 and TGF-β in greater quantities than the stromal cells used in the present system (Fig. 3a–c). The proportion of cells with a CD4+ CD25+ Foxp3+ Treg phenotype in the culture of lymph node cells with ST-2 cells was reduced compared with those in the culture with stromal cells (Fig. 3d). Furthermore, the addition of anti-TGF-β neutralizing mAb into the co-culture of lymph node cells with the stromal cells did not affect the composition of CD4+ CD25+ Foxp3+ cells (Fig. 3d). Taken together, these data suggest that IL-7 and TGF-β are unlikely to contribute towards the stromal cell support for Treg cells. It was previously reported that IL-7 and IL-2 support T-cell survival both in vivo and in vitro.11–21,22 Therefore, IL-2 production from stromal cells was assessed. As shown in Fig. 3(e), IL-2 was detected in culture supernatants from co-cultures of the stromal cells with CD4+ T cells at both day 2 and day 5. To examine the effects of these culture supernatants, including IL-2, on Treg cell support, culture supernatants from the stromal cell cultures or co-cultures of the stromal cells with CD4+ cells were supplemented at a final concentration of 50% in CD4+ T-cell cultures. Supernatant supplementation did not affect CD4+ CD25+ cells, suggesting that humoral factors in supernatant from stromal cells did not contribute to Treg cell survival (Fig. 3f). To confirm this possibility, peripheral lymphocytes (a mixture of lymph node cells and spleen cells) and stromal cells were co-cultured separately using a cell culture-insert system. In the control culture, 2·6% of cells had a Treg phenotype (CD4+ Foxp3+) (Fig. 3g). When lymphocytes were in contact with the stromal layer, 7·9% of cells had a CD4+ Foxp3+ phenotype. However, if direct interactions between lymphocytes and stromal cells were prevented using cell-culture inserts, the proportion of Treg cells was significantly decreased (3·5%) (Fig. 3g). The proportions of CD4+ CD25+ T cells in these cultures were consistent with those of CD4+ Foxp3+ T cells (Fig. 3g). In addition, neither interferon-α nor interferon-β was detected by ELISA in the supernatants from the stromal cell culture (data not shown). Taken together, these results suggested that direct interactions between stromal cells and Treg cells, rather than indirect mechanisms by soluble factors such as cytokines, are mandatory for Treg cell support by stromal cells.
Figure 3.
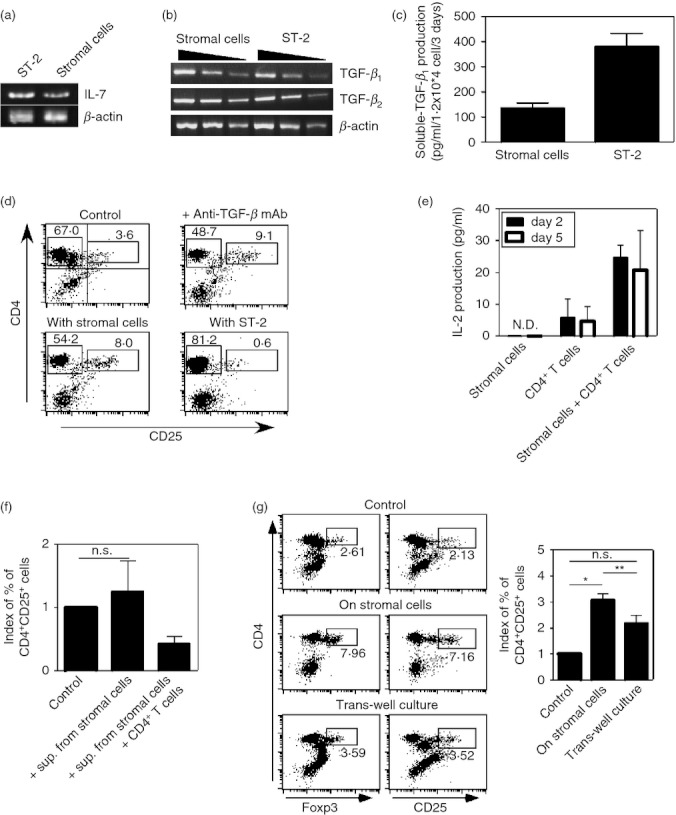
Soluble factors, such as interleukin-7 (IL-7) and transforming growth factor-β (TGF-β), do not contribute to stromal cell support for regulatory T (Treg) cells. (a) IL-7, or (b) TGF-β1 and TGF-β2 expression in stromal cells was assessed by RT-PCR. Total RNA prepared from stromal cells and ST-2 cells was used. (c) Culture supernatants of stromal cells and ST-2 cells (1·2 × 104 cells, for 3 days culture) were harvested. TGF-β1 concentrations in the supernatants were measured by ELISA. (d) Cells from lymph nodes were cultured with stromal cells or ST-2 cells for 5 days. Expression levels of CD4 and CD25 were assessed by flow cytometry. TGF-β neutralizing monoclonal antibody (mAb), clone 1D11, was added into the co-culture of lymphocytes and stromal cells. Proportions of CD4+ CD25+ cells and CD4+ CD25− cells are indicated. (e) Culture supernatants of stromal cells, CD4+ T cells, and CD4+ T cells with stromal cells (stromal cells: 5 × 103, CD4+ T cells: 5 × 105, for 5 days culture) were harvested. IL-2 concentrations in the supernatants were measured by ELISA. ND, not detected. (f) Supernatants from stromal cell culture or co-culture of stromal cells with CD4+ T cells were added into CD4+ T-cell culture at a final concentration of 50%. Five days later, proportions of CD4+CD25+ cells among the cultures were assessed. Indexes of proportions of CD4+ CD25+ cells are indicated. (NS indicates ‘not significant’, n = 4). (g) Lymphocytes, prepared from lymph nodes and spleens, and stromal cells were co-cultured in the same culture well. Lymphocytes were allowed to interact with the stromal cells directly (middle panel) or they were co-cultured separately using a cell culture insert system (bottom panels). As a control culture, lymphocytes were cultured alone (top panels). Five days after culture, expression levels of CD4, CD25 and Foxp3 were assessed. Indexes of proportions of CD4+ CD25+ cells in the cultures are summarized by bar graphs. (*P < 0·0001, **P < 0. 01, NS indicates ‘not significant’, n = 5) The data shown represent one of two (a–c) or three (e) experiments.
CD2-mediated direct interactions play a key role in stromal cell support for Treg cells
According to the above data, it is possible that molecule(s) contributing to the stromal cell–Treg cell direct interaction play a key role in Treg cell survival exerted by the stromal cells. To explore the critical molecule(s) contributed to Treg cell survival by the stromal cells, several function-blocking mAbs to cell surface molecules were added into the co-culture of CD4+ T cells and stromal cells. As stromal cells highly express CD51, CD61 and CD29 (Table 1), mAbs against these molecules were tested. The addition of these mAbs did not affect Treg cell numbers in the co-culture (Fig. 4a).
Figure 4.
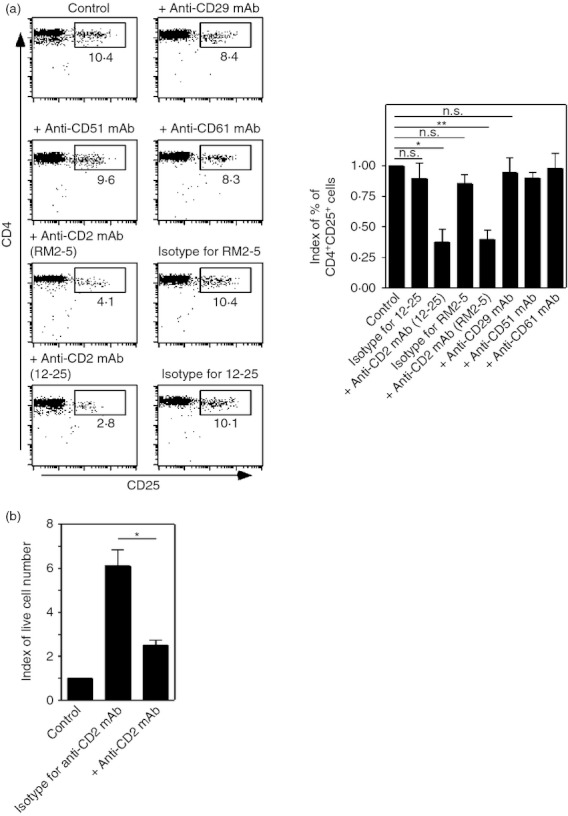
CD2 mediated-direct interactions between regulatory T (Treg) cells and stromal cells are critical for Treg cell survival. (a) CD4+ T cells from lymph nodes and spleens were co-cultured with the stromal cells. CD4+ T cells were co-cultured with stromal cells. The indicated monoclonal antibodies (mAbs) were added at the beginning of the cultures at a final concentration of 10 μg/ml. Five days later, cells were harvested and expression levels of CD4 and CD25 were assessed. The proportions of designated areas are indicated. (*P < 0·01, **P < 0·005, NS indicates ‘not significant’, n = 4) (b) CD4+ CD25+ cells purified from lymph nodes and spleens were co-cultured with or without stromal cells. Anti-CD2 mAb (clone 12-25) or isotype-matched mAbs were added to the co-cultures. Five days after culture, the numbers of living cells were counted. Indexes of live cell numbers were calculated. Cell numbers from controls (without stromal cells) were assigned a value of one. (*P < 0·01, n = 3).
Recently, it was shown that CD2 plays a critical role in Treg type 1 cell differentiation,40 and that CD2 engagement enhanced Treg cell functions.41 Hence, we examined the role of CD2 in Treg cell survival supported by stromal cells. Two individual anti-CD2 mAbs, RM2-5 and 12-25, and their isotype-matched control antibodies were used. The inhibition of CD2 engagement by anti-CD2 mAbs, not by their isotype-matched control antibodies, dramatically decreased the Treg cell fraction in the co-culture (Fig. 4a). This was further confirmed by an experiment using purified CD4+ CD25+ cells and stromal cells. As shown in Fig. 1(c), the number of living CD4+ CD25+ cells was greatly increased in the co-culture of purified CD4+ CD25+ cells with stromal cells. Whereas isotype-matched controls for anti-CD2 mAb did not affect the numbers of living cells in the co-culture (Fig. 1c and 4b), the addition of anti-CD2 mAb significantly decreased living cell numbers in the co-culture (Fig. 4b). Therefore, we focused on the role of CD2 in Treg cell survival supported by stromal cells.
Stromal cells support Treg cell survival by a CD2-mediated pathway
Our data indicated that stromal cells supported Treg cell survival by CD2. To confirm this, anti-CD2 blocking mAbs were added into the co-culture of CD4+ T cells and stromal cells. Annexin V+ cells among the CD4+ CD25+ fraction were greatly reduced by the presence of stromal cells (Fig. 2c). However, Treg cell survival supported by stromal cells was inhibited by the addition of anti-CD2 mAbs, indicating that CD2-mediated interactions between Treg cells and stromal cells are critical for Treg cell survival (Fig. 5; right histogram panels and summarized in the bar graph). In contrast, blockade of CD2 function did not affect conventional CD4+ CD25− cells in the same culture (Fig. 5 left histogram panels).
Figure 5.
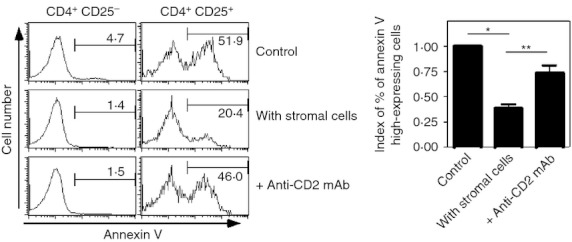
Addition of anti-CD2 monoclonal antibody (mAb) inhibits the survival of regulatory T (Treg) cells supported by stromal cells. CD4+ T cells were co-cultured with stromal cells. Anti-CD2 mAb (clone RM2-5, at a final concentration of 10 μg/ml) was added into the culture with the stromal cells (bottom panels). Two days later, cells were harvested and apoptotic cells were detected by annexin V staining. To discriminate between conventional T cells and Treg cells, CD4 and CD25 expression were assessed simultaneously. Proportions of designated areas are indicated. Indexes of proportions of annexin V high-expressing cells among CD4+ CD25+ cell fraction are summarized by bar graphs. (*P < 0·005, **P < 0. 02, n = 3).
Bim expression in Treg cells is partially decreased by the co-culture with stromal cells
Bim, a pro-apoptotic factor, is critical for the survival and homeostasis of conventional T cells42,43 and Treg cells.44 Therefore, we next examined whether the regulation of Bim expression contributed to CD2-mediated Treg cell survival by stromal cells, by measuring the expression levels of Bim in Treg cells. Peripheral CD4+ T cells from spleen and lymph nodes were co-cultured with or without stromal cells. Two days later, Bim expression from CD4+ CD25− or CD4+ CD25+ T cells was analysed by flow cytometry. While a small number of CD4+ CD25− T cells in the control culture expressed high levels of Bim, a large number of CD4+ CD25+ T cells that expressed high levels of Bim were observed in the same culture (26·3% versus 42·6%) (Fig. 6a). In the presence of stromal cells, the proportion of Bim high-expressing cells among CD4+ CD25+ T cells decreased to nearly 50–60% of the control value (summarized in Fig. 6a; bar graph). The partial reduction of Bim-expressing CD4+ CD25+ T cells by stromal cells was reversed by the addition of anti-CD2 mAb. In contrast, Bim expression in CD4+ CD25− T cells was not affected by stromal cells or anti-CD2 mAb (Fig. 6a). Using purified CD4+ CD25+ T cells instead of CD4+ T cells, similar results were observed (Fig. 6b). Taken together, these results suggest that Bim expression in Treg cells, but not in conventional T cells, is in part regulated by stromal cells via CD2.
Figure 6.
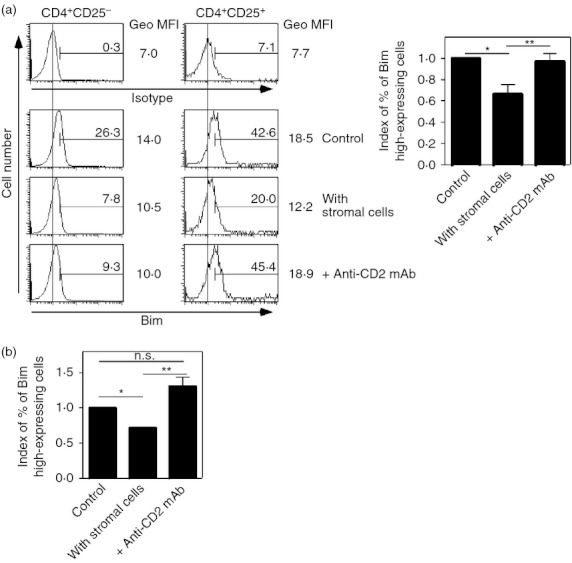
Bim expression in regulatory T (Treg) cells, but not conventional T cells, is decreased by co-culture with stromal cells. (a) CD4+ T cells were cultured with or without stromal cells for 2 days. Anti-CD2 monoclonal antibody (mAb) (clone 12-25, at a final concentration of 10 μg/ml) was added into a culture with stromal cells (bottom panels). After the culture, expression levels of Bim in CD4+ CD25− cells and CD4+ CD25+ cells were assessed. Proportions of designated areas and geometric MFI (GeoMFI) of histograms are indicated. Indexes of proportions of Bim high-expressing cells among the CD4+ CD25+ cell fractions are summarized by bar graphs. (*P < 0·01, **P < 0. 0005, n = 9) (b) CD4+ CD25+ cells were co-cultured with or without stromal cells. Anti-CD2 mAb was added into the co-culture. Five days after culture, the numbers of living cells were counted. Indexes of live cell numbers were calculated. Cell numbers from controls (without stromal cells) were assigned a value of one. (*P < 0·01, **P < 0·05, NS indicates ‘not significant’, n = 3).
Ligands for CD2 are not detected on stromal cells
Hence, it was suggested that Treg cells could interact with the stromal cells directly through CD2 and its ligand. CD48 is the principal ligand for murine CD2, and therefore its expression on stromal cells was assessed by flow cytometry. Surprisingly, CD48 was not detected on the stromal cells (Fig. 7a). As an antibody quality control, CHO-K1/Cd48 cells, a CD48 transfectant of CHO-K1 cells, was used (Fig. 7b) and indicated that CD48 was not expressed on stromal cells. This was further confirmed by RT-PCR (data not shown). Next, CD2 ligands other than CD48 expressed by stromal cells were analysed. For this, soluble CD2-Fc, constructed by the fusion of a CD2 extra-cellular portion to an Fc fragment from human IgG was used. We did not observe binding of CD2-Fc to the stromal cells (Fig. 7c), although CD2-Fc could bind to CHO-K1/Cd48 cells (Fig. 7d). The binding of CD2-Fc to CHO-K1/Cd48 cells was inhibited by the addition of anti-CD2 mAbs, indicating that the CD2-Fc binds to ligands for CD2 on these cells. Therefore, stromal cells do not express any known ligands for CD2.
Figure 7.
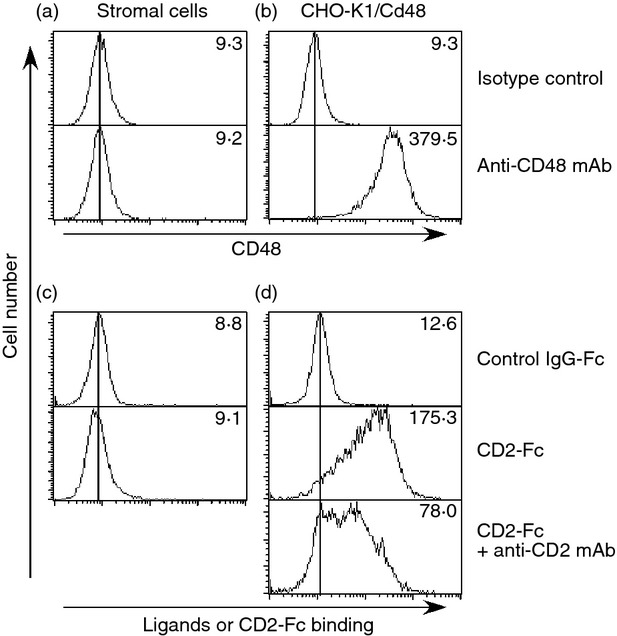
Stromal cells do not express CD2 ligands. Expression levels of CD48 (a) and ligands for CD2-Fc (c) on stromal cells were assessed. CHO-K1/Cd48 cells, CD48-transfectants, were used as quality controls for anti-CD48 monoclonal antibody (mAb) (b) and CD2-Fc fusion protein (d). To qualify the binding-specificity of CD2-Fc to its ligands, anti-CD2 mAb (clone RM2-5) was used (d, bottom panel). Results of isotype matched-controls are depicted in the upper panels of each figure. The data shown represent one of three experiments. MFI of histograms are indicated.
Discussion
In this study, we found that Treg cell apoptosis was preferentially prevented by co-culture with stromal cells derived from peripheral lymphoid organs. Importantly, similar results were obtained when using purified CD4+ CD25+ T cells, CD4+ cells or whole lymph node cells, indicating that stromal cell support for Treg cell survival was direct and independent of any other cell types.
Cytokines such as IL-7 play a key role in T-cell homeostasis,13–16 and many reports indicate that IL-7 is not mandatory for Treg cell homeostasis unlike conventional peripheral CD4+ T cells.17–19 Recently, Simonetta et al.38 reported that IL-7 plays a role in Treg cell homeostasis. Therefore, we first tested whether IL-7 and/or TGF-β contributed to Treg cell support by stromal cells. Stromal cells expressed both IL-7 and TGF-β. However, comparison of stromal cells with IL-7 and TGF-β high-expressing ST-2 stromal cells, or by the addition of anti-TGF-β neutralizing antibodies to the culture, demonstrated that these cytokines do not contribute to Treg cell survival. In addition, IL-2 was also detected in the culture supernatants from co-cultures of CD4+ cells with the stromal cells, but not the stromal cell culture, suggesting that the detected IL-2 was produced by CD4+ cells but not by the stromal cells. Co-culture of purified Treg cells revealed that in the absence of conventional CD4+ T cells, Treg cell survival was supported by stromal cells, suggesting that IL-2 from conventional CD4+ T cells does not contribute to Treg cell survival. This finding was confirmed by supernatant-transfer experiments and by cultures using a trans-well system to separate contact between T cells and stromal cells. Importantly, while proportions of CD4+ CD25+ T cells in the trans-well culture were not statistically significant compared with controls without stromal cells, trans-well experiments did not completely abolish survival support. Hence, humoral factors are not essential, but may contribute to Treg cell survival when co-cultured with stromal cells. This point should be resolved in future studies.
We then focused on the role of direct Treg cell–stromal cell interactions for Treg cell survival. Using blocking antibodies, we found that CD2 expression on Treg cells is important for their survival in this system. Although CD2 is expressed on virtually all T cells including conventional CD4+ T cells,45,46 stromal cells preferentially support Treg cell survival through CD2 signalling. This may explained because stromal cells partially inhibit the expression of Bim in Treg cells, which is a regulator of Treg cell death in the periphery.44 Treg cells, but not conventional T cells, express high levels of Bim in vitro without stromal cells and in the absence of exogenous cytokines. Therefore, stromal cells preferentially affect Treg cells, but not conventional T cells. As Bim expression in Treg cells correlates with the detection of apoptotic cells in the same culture conditions, the Treg cell death observed in this system may be controlled by Bim. The expression levels of other cell death regulatory factors, Fas and Bcl-2, in Treg cells were not affected by co-culture with stromal cells (data not shown). Whereas Bim function is controlled by Bcl-2,47 Bcl-2 levels in Treg cells were not affected by stromal cells, suggesting that CD2-mediated Bim regulation may not depend on Bcl-2. The pathway of CD2-mediated Bim regulation should be clarified in future studies.
As shown in Fig. 6(a), Bim high-expressing Treg cells in co-cultures with stromal cells decreased to approximately 60% of those in control cultures in a 2-day assay. However, at the same time-points, annexin-V-positive Treg cells comprised 40% of the control (Fig. 5). In this system, we could not strictly determine the threshold of Bim levels to elicit apoptosis. Hence, a discrepancy in the ratios of Bim high-expressing cells and annexin-V-positive cells was observed. Another possibility is the existence of a Bim-independent pathway of CD2-mediated regulation of Treg cell apoptosis. Further studies will be required to clarify these possibilities.
Stromal cells did not express any known ligands for CD2, including CD48, indicating that T cells, including both conventional and regulatory T cells, do not directly interact with stromal cells via CD2. Therefore, even though CD2 is expressed on conventional T cells, CD2-mediated interactions with stromal cells do not occur. Importantly, expression levels of CD2 on Treg cells are slightly lower than, or similar to those on conventional CD4+ CD25− T cells (data not shown), whereas CD48 is expressed on both types of cells at similar levels (data not shown). Hence, the number of CD2 and CD48 molecules on Treg cells may not explain the findings, indicating that the quality, rather than quantity, of CD2 signal in Treg cells is key for Bim regulation. How does CD2 on Treg cells act as a critical molecule for stromal cell-mediated Treg cell survival? CD2 on Treg cells may interact with CD48 on T cells to transduce signals, which up-regulate receptors for unidentified ligands on the stromal cells. The unidentified molecules contribute to direct Treg cell–stromal cell interactions and to reduce Bim expression in Treg cells. Alternatively, CD2 signalling in Treg cells induced by CD48 on T cells may modify signalling pathways, which regulate Bim expression, controlled by stromal cells via unknown molecules. Currently, we have no direct evidence to support these possibilities. However, one candidate molecule is glucocorticoid-induced tumour necrosis factor receptor (TNFR) -related protein (GITR), a member of the TNFR super-family, which is preferentially expressed on Treg cells.48,49 Signalling through 4-1BB, a member of the TNFR super-family, can down-regulate Bim levels in human umbilical cord blood Treg cells50 and in CD8+ T cells.51 Furthermore, extracellular signal regulated kinase (ERK) signalling is important for reduced Bim expression52 and GITR stimulation can enhance ERK signalling.53 Together this suggests that CD2 and GITR signals may co-operate to reduce Bim expression through the ERK pathway in Treg cells. Further studies are required to clarify this hypothesis.
Chougnet et al.44 reported that Treg cells expressing low levels of Bim accumulate in old mice and might cause age-related immune suppression. Interestingly, IL-2 induced aged Treg cells to proliferate. Therefore, if sufficient exogenous IL-2 is provided to a co-culture of Treg cells and stromal cells, they may proliferate in a similar manner to Treg cells found in aged mice, which express low levels of Bim. If this is true, the culture system described here may help to establish Treg cell lines. In addition, age-related immune suppression may be treated by modifying CD2 signals in Treg cells.
Another issue to be resolved is the real nature of the stromal cells supporting Treg cell survival. Phenotypic studies suggested that the stromal cells (gp38++, CD31−, IL-7 mRNA+) may be similar to the fibroblastic reticular cells in T-cell zones reported by Link et al.54 To determine the identity of these cells, precise studies such as analyses of cytokine production and histological location will be required. Additionally, we found stromal cells supporting Treg cells in long-term Treg cell cultures. To find stromal cells with the potential to support Treg cells, long-term Treg cell cultures are required. As other studies may not maintain Treg cells in vitro, this may explain why similar effects of stromal cells have not been previously reported by other groups. In the future, it is possible that these stromal cells can help to manipulate Treg cells in vitro.
Finally, the most important and interesting issue is whether these in vitro findings are reflected in vivo. The use of mice with a specific gene deficiency is an excellent way to determine the roles of molecules in vivo. However, CD2-deficient mice55 have not been reported to develop autoimmunity caused by a reduction of peripheral Treg cells as a result of increasing apoptosis. An explanation may be that since CD2 is critical for the full activation of all T cells, T cells in CD2-deficient mice may not have the potential to induce autoimmunity. Therefore, future studies should use mice deficient for CD2 expression only on Treg cells. We are currently trying to generate mice with CD2-deficient Treg cells using the Cre-loxP system, by crossing CD2-flox mice with Foxp3-Cre mice.
In conclusion, there are many unresolved questions regarding the CD2-mediated regulation of Treg cell survival in the periphery, and answers to these questions may enhance our ability to regulate immune responses.
Acknowledgments
We thank Ms Yoshie Nitta for secretarial assistance. We are also grateful to the Laboratory Animal Research Centre, Dokkyo Medical University for allowing us to use the facility and for their excellent help. The authors thank Ms Yasuko Nonaka for technical assistance. This work was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (24591454 to T.K.), a grant from Seki Minato Foundation (to H.K.), and a Dokkyo University School of Medicine Investigator-Initiated Research Grant (to Y. Kas.).
Glossary
- CFSE
5-(6)-carboxyfluorescein diacetate succinimidyl ester
- ERK
extracellular signal-regulated kinase
- GITR
glucocorticoid-induced tumour necrosis factor receptor
- IL-7
interleukin-7
- mAb
monoclonal antibody
- MLC
mixed lymphocyte culture
- PE-Cy5
phycoerythrin-Cychrome 5
- TGF
transforming growth factor
- TNFR
tumour necrosis factor receptor
- Treg
regulatory T
Disclosures
The authors have no financial conflict of interest.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 2.Asano M, Toda M, Sakaguchi N, et al. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003;81:331–71. doi: 10.1016/s0065-2776(03)81008-8. [DOI] [PubMed] [Google Scholar]
- 4.Suvas S, Kumaraguru U, Pack CD, et al. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suvas S, Azkur AK, Kim BS, et al. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–32. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 6.Dittmer U, He H, Messer RJ, et al. Functional impairment of CD8+ T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid Y, Piccirillo CA, Mendez S, et al. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 8.Hisaeda H, Maekawa Y, Iwakawa D, et al. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann P, Ermann J, Edinger M, et al. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingsley CI, Karim M, Bushell AR, et al. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–6. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 11.Alves NL, Arosa FA, van Lier RA. Common γ chain cytokines: dissidence in the details. Immunol Lett. 2007;108:113–20. doi: 10.1016/j.imlet.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the γc-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 13.von Freeden-Jeffry U, Vieira P, Lucian LA, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–26. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puel A, Ziegler SF, Buckley RH, et al. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nat Genet. 1998;20:394–7. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 15.Schluns KS, Kieper WC, Jameson SC, et al. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 16.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naïve T cells. Proc Natl Acad Sci USA. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cozzo C, Larkin J, III, Caton AJ. Cutting edge: self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:5678–82. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- 18.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harnaha J, Machen J, Wright M, et al. Interleukin-7 is a survival factor for CD4+ CD25+ T-cells and is expressed by diabetes-suppressive dendritic cells. Diabetes. 2006;55:158–70. [PubMed] [Google Scholar]
- 21.Pandiyan P, Lenardo MJ. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol Direct. 2008;3:6. doi: 10.1186/1745-6150-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonetta F, Chiali A, Cordier C, et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol. 2010;40:2528–38. doi: 10.1002/eji.201040531. [DOI] [PubMed] [Google Scholar]
- 23.Marie JC, Letterio JJ, Gavin M, et al. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity. 2006;25:441–5. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:445–71. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Bayer AL, Yu A, Adeegbe D, et al. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J Exp Med. 2005;201:769–77. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontenot JD, Rasmussen JP, Gavin MA, et al. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 28.Setoguchi R, Hori S, Takahashi T, et al. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 30.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–52. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 31.Beyers AD, Barclay AN, Law DA, et al. Activation of T lymphocytes via monoclonal antibodies against rat cell surface antigens with particular reference to CD2 antigen. Immunol Rev. 1989;111:59–77. doi: 10.1111/j.1600-065x.1989.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 32.Marson A, Kretschmer K, Frampton GM, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–5. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Jager PL, Baecher-Allan C, Maier LM, et al. The role of the CD58 locus in multiple sclerosis. Proc Natl Acd Sci USA. 2009;106:5264–9. doi: 10.1073/pnas.0813310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raychaudhuri S, Thomson BP, Remmers EF, et al. Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat Genet. 2009;41:1313–8. doi: 10.1038/ng.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Liu W, Ambrosino C, et al. Impaired generation of bone marrow B lymphocytes in mice deficient in C/EBPβ. Blood. 1997;90:156–64. [PubMed] [Google Scholar]
- 36.Wong YW, Williams AF, Kingsmore SF, et al. Structure, expression, and genetic linkage of the mouse BCM1 (OX45 or Blast-1) antigen. Evidence for genetic duplication giving rise to the BCM1 region on mouse chromosome 1 and the CD2/LFA3 region on mouse chromosome 3. J Exp Med. 1990;171:2115–30. doi: 10.1084/jem.171.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutschmann R, Karjalainen K. Mouse LFA-3 studied with chimeric soluble CD2 shows preferential expression on lymphoid cells. Eur J Immunol. 1991;21:1379–84. doi: 10.1002/eji.1830210608. [DOI] [PubMed] [Google Scholar]
- 38.Simonetta F, Gestermann N, Martinet KZ, et al. Interleukin-7 influences FOXP3+CD4+ regulatory T cells peripheral homeostasis. PLoS ONE. 2012;7:e36596. doi: 10.1371/journal.pone.0036596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunji Y, Sudo T, Suda J, et al. Support of early B-cell differentiation in mouse fetal liver by stromal cells and interleukin-7. Blood. 1991;77:2612–7. [PubMed] [Google Scholar]
- 40.Wakkach A, Cottrez F, Groux H. Differentiation of regulatory T cells 1 is induced by CD2 costimulation. J Immunol. 2001;167:3107–13. doi: 10.4049/jimmunol.167.6.3107. [DOI] [PubMed] [Google Scholar]
- 41.Magnani CF, Alberigo G, Baccgetta R, et al. Killing of myeloid APCs via HLA class I, CD2 and CD226 defines a novel mechanism of suppression by human Tr1 cells. Eur J Immunol. 2011;41:1652–62. doi: 10.1002/eji.201041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 43.Wojciechowski S, Tripathi P, Bourdeau T, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–75. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chougnet CA, Tripthi P, Lages CS, et al. A major role for Bim in regulatory T cell homeostasis. J Immunol. 2011;186:156–63. doi: 10.4049/jimmunol.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bierer BE, Burakoff SJ. T cell adhesion molecules. FASEB J. 1988;2:2584–90. doi: 10.1096/fasebj.2.10.2838364. [DOI] [PubMed] [Google Scholar]
- 46.Bierer BE, Sleckman BP, Ratnofsky SE, et al. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989;7:579–99. doi: 10.1146/annurev.iy.07.040189.003051. [DOI] [PubMed] [Google Scholar]
- 47.Bouillet P, O'Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol. 2009;9:514–9. doi: 10.1038/nri2570. [DOI] [PubMed] [Google Scholar]
- 48.McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu J, Yamazaki S, Takahashi T, et al. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 50.Hippen KL, Harker-Murray P, Porter SB, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847–57. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabbagh L, Pulle G, Liu Y, et al. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol. 2008;180:8093–101. doi: 10.4049/jimmunol.180.12.8093. [DOI] [PubMed] [Google Scholar]
- 52.Ewings KE, Wiggins CM, Cook SJ. Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels. Cell Cycle. 2007;6:2236–40. doi: 10.4161/cc.6.18.4728. [DOI] [PubMed] [Google Scholar]
- 53.Ronchetti S, Zollo O, Bruscoli S, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–22. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 54.Link A, Vogt TK, Favre S, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naïve T cells. Nat Immunol. 2007;8:1255–65. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 55.Killeen N, Stuart SG, Littman DR. Development and function of T cells in mice with a disrupted CD2 gene. EMBO J. 1992;11:4329–36. doi: 10.1002/j.1460-2075.1992.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


