Abstract
Plasmacytoid dendritic cells (pDC) in mesenteric lymph nodes (MLN) may be important regulators of both inflammatory and non-inflammatory mucosal immune responses but human studies are rare. Here we compare pDC from human MLN and peripheral blood (PB) by phenotype and function. MLN from patients with or without inflammatory bowel disease (IBD) undergoing colon surgery and PB from patients with IBD and from controls were used to isolate mononuclear cells. The pDC were analysed by flow cytometry for the expression of CD40, CD80, CD83, CD86, CCR6, CCR7, CX3CR1, CD103 and HLA-DR. Purified pDC from MLN and PB were stimulated with staphylococcus enterotoxin B (SEB), CpG-A, interleukin-3 (IL-3), SEB + IL-3, CpG-A + IL-3 or left unstimulated, and cultured alone or with purified allogeneic CD4+ CD45RA+ HLA-DR- T cells. Subsequently, concentrations of IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17, interferon-α (IFN-α), IFN-γ and tumour necrosis factor-α (TNF-α) in culture supernatants were determined by multiplex bead array. The PB pDC from IBD patients exhibited an activated and matured phenotype whereas MLN pDC and control PB pDC were less activated. CpG-A and CpG-A + IL-3-stimulated MLN pDC secreted less IL-6 and TNF-α compared with PB pDC from controls. Compared with co-cultures of naive CD4 T cells with PB pDC, co-cultures with MLN pDC contained more IL-2, IL-10 and IFN-γ when stimulated with SEB and SEB + IL-3, and less IFN-α when stimulated with CpG-A. MLN pDC differ phenotypically from PB pDC and their pattern of cytokine secretion and may contribute to specific outcomes of mucosal immune reactions.
Keywords: cytokines, inflammation, inflammatory bowel disease, plasmacytoid dendritic cells, T cells
Introduction
Dendritic cells (DC) initiate and regulate various immune responses, particularly by the presentation of antigen and the stimulation of T and B cells.1 They comprise a heterogeneous population of leucocytes that can be distinguished by their phenotype, location and function.2
Human DC consist of two main subsets, myeloid DC (mDC) and plasmacytoid DC (pDC).3 Whereas mDC are CD11c+ DC-SIGN+ cells, pDC express CD123, BDCA-2 and BDCA-4 but no CD11c.4,5 Plasmacytoid DC are characterized by their production of large amounts of IFN-α (IFN-α) after viral infection leading to the activation of natural killer cells, natural killer T cells, B cells, mDC, monocytes,6 and to T-cell polarization.7 The pDC secrete a cytokine pattern distinct from that of mDC and respond to different stimuli according to their specific Toll-like receptor 7 (TLR7) and TLR9 expression.3 Cytokines produced by pDC may induce different types of T-cell polarization:2 for example, low levels of interleukin-12 (IL-12), activation of pDC with IL-34 or pDC maturation by CD154 favour T helper type 2 reactions whereas virus-activated pDC represent the interferon-α (IFN-α) -mediated activation of the innate immune system and promote T helper type 1 immune responses.8
Mucosal pDC and mDC have been investigated in only a few human studies, although accumulation of activated DC at the sites of inflammation may contribute to the pathogenesis of human inflammatory bowel disease (IBD).6,9–12 Reduced proportions of immature pDC have been found in the peripheral blood (PB) of patients with IBD, suggesting their recruitment to inflamed mucosal tissues.13,14 Additionally, mesenteric lymph node (MLN) pDC express the chemokine receptor CCR6, which could explain musosal accumulation at inflamed tissue sites as a consequence of up-regulated mucosal addressines such as CCL20 or mucosal addressin cell adhesion molecule-1.15,16 Most mucosal DC that enter the MLN originate from the lamina propria17 and therefore might play a role in the regulation of immunity and tolerance.3,18,19
Oral tolerance is characterized by the antigen-specific suppression of systemic cellular and humoral immune responses to an orally administered antigen20 and may result from a variety of effects including clonal anergy, clonal deletion or active regulation.21 Recent data from murine models suggest that one central mechanism might be the transforming growth factor-β-dependent and retinoic acid-dependent induction of immunosuppressive T regulatory cells by a specific DC subpopulation in MLN, i.e., CD11c+ CD103+ mDC originating from the lamina propria.22–24 This population has been shown to induce CCR9-expressing and α4β7 integrin-expressing T cells, respectively.25 Of note, tolerogenic functions of CD11c+ CD103+ mDC in MLN have recently been described in humans also.25,26
Recent data from murine models indicate a possible role of pDC in oral tolerance apart from that of mDC.27–30 In humans, IL-10-producing Foxp-3+ CD4+ CD25+ regulatory T cells can be induced by TLR9-activated pDC obtained from PB.31 The MLN compartment appears to be crucial for the observed dysregulation of T helper type 1/type 17 responses in IBD32 but a precise characterization of human MLN pDC is missing.
In this study, we compared pDC from MLN and PB. However, MLN from controls can rarely be obtained, and pDC in the PB of patients with IBD are too few for most functional assays. Because we did not detect differences in the phenotype of pDC in MLN from patients with or without IBD we decided to compare MLN pDC with pDC in PB from IBD patients and controls, respectively. In addition, cytokine secretion of purified pDC isolated from MLN and from PB of controls was determined as well as the induction of effector T cells by pDC from MLN and PB in co-culture with allogeneic naive T cells. We hypothesized that MLN pDC and PB pDC subsets would differ due to distinct compartments of origin and inflammatory conditions, and that such differences would affect the induction of cytokine-secreting T effector cells.
Materials and methods
Patients and samples
Mesenteric lymph nodes not required for diagnostic assessment were obtained from patients undergoing colon surgery (six women, six men; age 54 ± 18 years; Crohn's disease n = 1, ulcerative colitis n = 5, diverticulitis n = 4, diverticulosis n = 2). Peripheral blood was obtained from patients with IBD (seven women, three men; age 40 ± 9 years) and healthy controls (six women, four men; age 36 ± 10 years). Buffy coats from blood of healthy donors (seven women, three men; age 49 ± 14 years) were received from the DRK-Blutspendedienst Ost (Berlin, Germany). The study was conducted with the understanding and the consent of each participant and was approved by the local ethics committee.
Isolation of mononuclear cells from human PB and MLN
The MLN were cut free from surrounding tissue and suspended in RPMI containing l-glutamine, 10% fetal calf serum and 100 U/ml penicillin, 100 μg/ml streptomycin (Biochrom, Berlin, Germany). Single lymph nodes were injected with 1 ml collagenase D (4000 Mandl units; Worthington, Lakewood, NJ), incubated for 30 min at 37° and passed through a 70-μm cell strainer. After washing twice, MLN mononuclear cells were isolated by Ficoll density gradient centrifugation. The PB mononuclear cells were isolated by Ficoll density gradient centrifugation.
Isolation of pDC from PB and MLN mononuclear cells
At least 4 × 108 mononuclear cells were used for pDC purification using a magnetic cell separation technique (Miltenyi Biotech, Bergisch-Gladbach, Germany). In brief, non-DC were depleted using the lineage cocktail 1 (containing FITC-conjugated antibodies against CD3, CD14, CD16, CD19, CD20 and CD56; BD Pharmingen, Heidelberg, Germany) and anti-FITC-conjugated microbeads (Miltenyi Biotec). Thereafter, pDC were enriched using an anti-BDCA-4 monoclonal antibody (mAb; Miltenyi Biotec). The purity of the isolated pDC was determined by flow cytometry with anti-BDCA-2 mAb (Miltenyi Biotec) and anti-CD123 mAb (BD Biosciences, Heidelberg, Germany) and was always > 95% (data not shown).
Purification of naive T cells
After mononuclear cell isolation by Ficoll density gradient centrifugation CD4+ CD45RA+ HLA-DR– naive T cells were isolated by magnetic cell separation technique from human buffy coats using the ‘Naive CD4+ T cell Isolation Kit II’ (Miltenyi Biotec) and following the manufacturer's instructions. The purity of the isolated naive T-cell population was determined with anti-CD4 mAb, anti-CD45RA mAb and anti-HLA-DR mAb (all from BD Biosciences) by flow cytometry and was always > 99% (data not shown).
Cell culture
Purified pDC from MLN and PB were used for cell cultures and co-culture experiments with naive T cells. The pDC from MLN and PB were suspended in RPMI-1640 with l-glutamine (Gibco, Darmstadt, Germany) containing 100 U/ml penicillin, 100 μg/ml streptomycin (Biochrom) and 10% fetal calf serum (Linaris, Bettingen, Germany), and stimulated with staphylococcus enterotoxin B (SEB; 1 ng/ml, Sigma, Taufkirchen, Germany), CpG-A (1 μm; InvivoGen, San Diego, CA), IL-3 (10 ng/ml; Peprotech, Hamburg, Germany), SEB + IL-3, CpG-A + IL-3, or left unstimulated, and cultured alone for 2 days or with purified allogeneic CD4+ CD45RA+ HLA-DR– T cells in a ratio of 1 : 10 for 3 and 7 days at 37° and 5% CO2. Supernatants were collected and stored at −80° until further analysis.
Cell staining and flow cytometry
Cells were stained in PBS containing 0·5% BSA (PAA, Pasching, Austria), 0·1% NaN3 (Merck, Darmstadt, Germany) and 0·1% Beriglobin (ZLB Behring, Hattersheim, Germany). The following mAb were used: anti-CD4 (clone SK3), anti-CD25 (clone 2A3), anti-IFN-γ (clone B27), anti-IL-4 (clone MP4-25D2), anti-IL-10 (clone JES3-19F1), anti-CD40 (clone 5C3), anti-CD86 (clone FUN-1), anti-CD103 (clone Ber-ACT8), anti-CCR6 (clone 11A9), anti-CCR7 (clone 3D12) and anti-HLA-DR (clone L243) all from BD Biosciences; anti-CD19 (clone HIB19), anti-CD14 (clone 61D3), anti-CD80 (clone 2D10) and anti-CD83 (clone HB15e) (eBioscience, San Diego, CA); anti-BDCA-1 (clone AD5-8E7), anti-BDCA-2 (clone AC144) and anti-BDCA-3 (clone AD5-14H12) were from Miltenyi Biotec; anti-CX3CR1 (clone 2A9-1) was purchased from Biolegend (Fell, Germany). At least 250 000 mononuclear cells were analysed on a FACSCalibur (BD Biosciences) and data were analysed with the flowjo software (Tree Star, Ashland, OR).
Determination of cytokine concentrations in culture supernatants
Interleukin-2, IL-4, IL-6, IL-10, tumour necrosis factor-α (TNF-α) and IFN-γ were quantified by human T helper type 1/type 2 cytokine cytometric bead array kit (BD, Heidelberg, Germany) according to the manufacturer's instructions and analysed with the FCAP-Array software (BD). Interleukin-1β, IL-8, IL-12, IL-17 and IFN-α were quantified using a human FlowCytomix Kit (eBioscience) according to the manufacturer's instructions and analysed by the flow cytomix pro software (Bender, San Diego, CA).
Statistical analysis
Selected data are presented as box plots which show medians, 25th and 75th centiles, and minimum and maximum values. Non-parametric statistical tests were applied. The Mann–Whitney U-test was used to analyse differences between unpaired data, P-values < 0·05 were considered significant.
Results
In this study, we compared human pDC from MLN and PB by phenotype and function. As we found no differences in MLN from patients with IBD (Crohn's disease, ulcerative colitis) and without IBD (diverticulitis and diverticulosis) we decided to pool these data. To allow a comparison between PB and MLN, pooled MLN data were separated into those with IBD and non-IBD origin whereby non-IBD data in the figures are depicted as marked dot plots. As MLN from non-IBD patients to some extent show signs of inflammation they were not considered as controls.
Increased proportion of pDC in MLN compared with PB
Within mononuclear cells from MLN, the median frequency of pDC (gated as living CD14– CD19– PI– BDCA-2+ CD11c– cells, Fig. 1a) was about 0·7% in comparison with median pDC frequencies of about 0·2% in peripheral mononuclear cells from patients with and without IBD and of < 0·1% in peripheral mononuclear cells from controls (Fig. 1b), respectively. Separated data from non-IBD and IBD are integrated in Fig. 1 although no differences in the pDC frequencies from patients with and without IBD were found.
Figure 1.
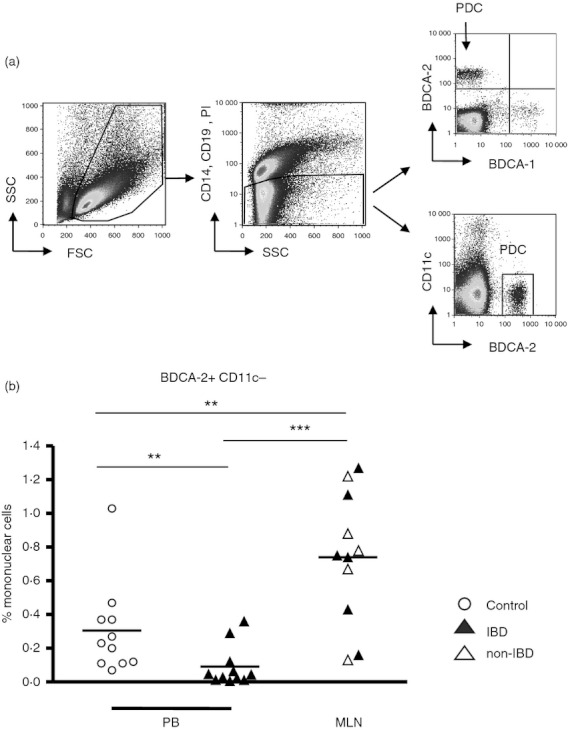
Plasmacytoid dendritic cells (pDC) in mononuclear cells from human mesenteric lymph nodes (MLN) and peripheral blood (PB). (a) Mononuclear cells isolated from MLN or PB were analysed by flow cytometry. After exclusion of CD14+, CD19+ and dead cells, pDC were defined as CD11c– and BDCA-2+ cells. (b) Proportions of pDC within mononuclear cells from MLN of patients with and without inflammatory bowel disease (IBD; n = 12, six non-IBD, six IBD) and PB (n = 10, all from IBD patients) as well as from control PB (n = 10). **P < 0·01, ***P < 0·001; Mann–Whitney U-test.
MLN pDC are phenotypically similar to PB pDC from healthy controls
We first compared the phenotype of pDC in MLN from patients with or without IBD but found no differences (Fig. 2). The co-stimulatory molecules CD80 and CD86 were expressed more frequently on PB pDC from IBD patients compared with MLN pDC and PB pDC from controls. CD83 was more frequently expressed by MLN pDC than by PB pDC from IBD patients (Fig. 2). In contrast, CD40 was less frequent on MLN pDC compared with PB pDC from either healthy controls or IBD patients (Fig. 2). The proportions of pDC expressing CD80, CD86, CD103 and CX3CR1 in PB from controls and in MLN did not differ (Fig. 2) whereas the integrin CD103 and fractalkine receptor CX3CR1 were expressed more frequently on PB pDC of IBD patients compared with MLN pDC and PB pDC of controls (Fig. 2). The CC chemokine receptor CCR6 was expressed less frequently on MLN pDC in comparison to PB pDC from controls whereas CCR7 expression was not different between MLN and PB from controls or patients with IBD (Fig. 2). As expected, the proportions of pDC in MLN and PB expressing HLA-DR were nearly 100% (Fig. 2).
Figure 2.
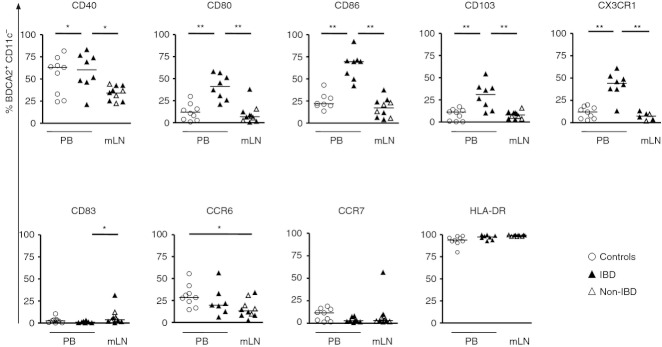
Expression of homing receptors, activation and maturation markers from mesenteric lymph node plasmacytoid dendritic cells (MLN pDC) of patients with and without inflammatory bowel disease (IBD; n = 10, six non-IBD, four IBD), from peripheral blood (PB) pDC (n = 8 to n = 10, all from IBD patients) and from PB of controls (n = 10). The pDC were identified as BDCA-2+ CD11c– cells and the proportions expressing CD40, CD80, CD83, CD86, HLA-DR, CCR6, CCR7, CD103, DC-SIGN and CX3CR1, were determined by flow cytometry. *P < 0·05, **P < 0·01, ***P < 0·001; Mann–Whitney U-test.
Cytokine secretion of pDC from MLN and PB
To investigate the potential of pDC from MLN and PB to produce cytokines, we cultured the cells in the presence or absence of CpG-A (which binds to TLR9 and induces cytokine secretion in pDC) alone or in combination with IL-3 (as the cells are known to express the IL-3 receptor, CD123) (Fig. 3).
Figure 3.
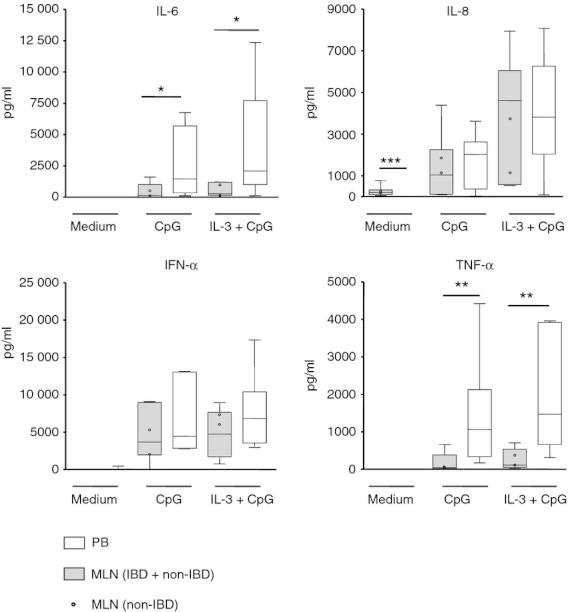
CpG-stimulated cytokine secretion of cultured plasmacytoid dendritic cells (pDC) from mesenteric lymph nodes (MLN) and peripheral blood (PB). Purified pDC from MLN of patients with and without inflammatory bowel disease (IBD; n = 8, two non-IBD, six IBD) and control PB (n = 8) were stimulated with CpG, interleukin-3 (IL-3), IL-3 + CpG, or left unstimulated and cultured for 2 days. The cytokine secretion in the supernatants was determined by cytometric bead array. (*P < 0·05, **P < 0·01, ***P < 0·001; Mann–Whitney U test).
Upon stimulation with CpG-A + IL-3, MLN pDC from IBD patients or non-IBD patients secreted less IL-6 and TNF-α than PB pDC whereas no differences were observed for IL-8 and IFN-α (Fig. 3). In unstimulated cells, MLN pDC secreted small but higher amounts of IL-8 compared with PB pDC (Fig. 3). Due to the very low levels, however, this might be of questionable biological relevance. No IL-1β, IL-2, IL-4, IL-10, IL-12p70, IL-17 or IFN-γ was detected in cultures of stimulated or unstimulated pDC from MLN or PB.
Distinct induction of cytokine secretion in naive T cells by pDC from MLN and PB
We were further interested in the cytokine secretion of naive CD4+ T cells in the presence of pDC (Fig. 4). As before, we first used CpG-A and IL-3 as stimuli, which both should mainly affect pDC as TLR9 is exclusively expressed by pDC and B cells in humans and naive T cells do not express CD123. Plasmacytoid DC from MLN or PB were cultured with allogeneic naive CD4+ CD45RA+ HLA-DR– T cells, stimulated with CpG-A or IL-3 or both, and cytokine concentrations were determined after 3 and 7 days. Control cells remained unstimulated. As before, we did not observe differences regarding the cytokine secretion of MLN pDC from patients with or without IBD (Fig. 4).
Figure 4.
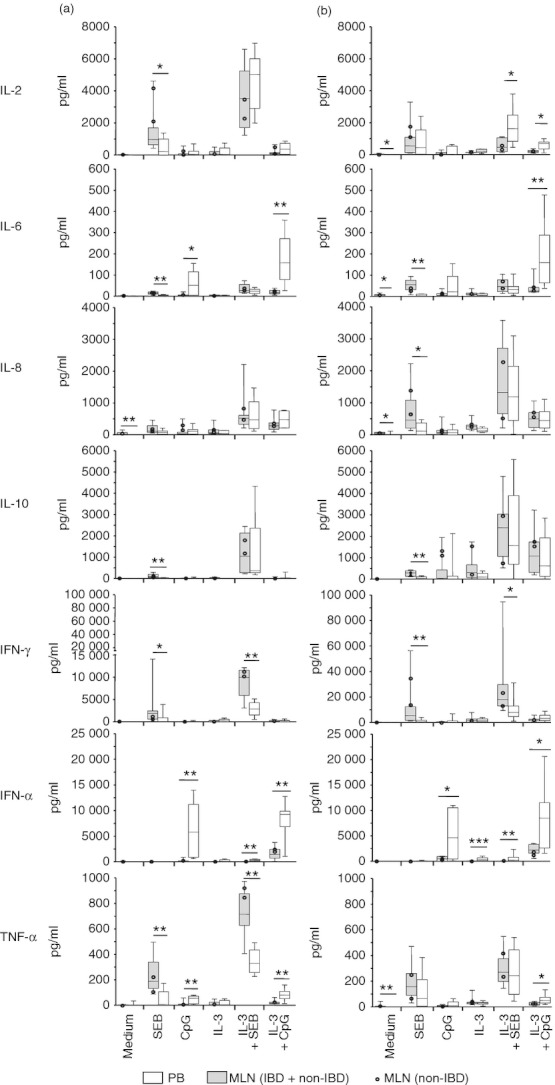
Cytokine secretion in co-cultures of naive allogeneic T cells with plasmacytoid dendritic cells (pDC) from mesenteric lymph nodes (MLN) and peripheral blood (PB). Purified pDC from MLN of patients with and without inflammatory bowel disease (IBD; n = 8, two non-IBD, six IBD), as well as from control PB (n = 8) were co-cultured with CD4+ CD45RA+ HLA-DR– naive allogeneic T cells for 3 days (a) and 7 days (b) in the presence of staphylococcus enterotoxin B (SEB), CpG, interleukin-3 (IL-3), IL-3 + SEB, or IL-3 + CpG-A, or left unstimulated. Cytokine concentrations in culture supernatants were determined by cytometric bead array. *P < 0·05, **P < 0·01, ***P < 0·001; Mann–Whitney U-test.
After 3 days of co-culture with allogeneic naive CD4+ CD45RA+ HLA-DR– T cells, cultures containing mLN pDC produced less TNF-α, IFN-α and IL-6 when stimulated with CpG-A or CpG-A + IL-3 than cultures with PB pDC (Fig. 4). After 7 days of co-culture, cultures containing mLN pDC produced less IFN-α in the presence of CpG-A, and less IFN-α, TNF-α, IL-2 and IL-6 when stimulated with CpG-A + IL-3 than cultures with PB pDC (Fig. 4). In the presence of IL-3, co-cultures with MLN pDC contained less IFN-α than those with PB pDC.
In another experimental setting, we used SEB (which bridges MHC-expressing cells with T cells and leads to a polyclonal T-cell activation)33 alone or in combination with IL-3 as stimuli. Here, we observed after 3 days of co-culture with allogeneic naive CD4+ CD45RA+ HLA-DR– T cells, that cultures containing mLN pDC produced more IFN-γ, TNF-α, IL-2, IL-6 and IL-10 when stimulated with SEB compared with PB pDC (Fig. 4). When stimulated with IL-3 + SEB, co-cultures containing mLN pDC produced more IFN-γ and TNF-α but less IFN-α than co-cultures containing PB pDC (Fig. 4). After 7 days, cultures containing mLN pDC produced more IFN-γ, IL-6, IL-8 and IL-10 when stimulated with SEB than cultures with PB pDC (Fig. 4). When stimulated with IL-3 + SEB, co-cultures containing MLN pDC produced less IFN-α and IL-2 than co-cultures containing PB pDC (Fig. 4). In comparison with co-cultures of PB pDC, unstimulated co-cultures with MLN pDC contained more IL-2, IL-6, IL-8 and TNF-α. No IL-1β, IL-4, IL-12p70 or IL-17 was detected in co-cultures with pDC from both MLN and PB irrespective of stimulus.
Discussion
In this study, we investigated whether MLN pDC from patients with or without IBD phenotypically and functionally differ from PB pDC from patients with IBD or from controls. In contrast to PB pDC from patients with IBD, MLN pDC from IBD patients or non-IBD patients irrespective of the underlying disease, showed a rather inactivated and immature phenotype similar to that of PB pDC from controls. In pDC cultures, we found that pDC from both MLN and PB secrete similar IFN-α and IL-8 levels in response to CpG-A and CpG-A + IL-3 indicating their antiviral and plasma cell-inducing activity.34,35 In contrast to PB pDC, MLN pDC secrete less IL-6 and TNF-α in response to CpG-A and CpG-A + IL-3, suggesting a diminished pro-inflammatory function of MLN pDC compared with their counterparts in PB. Further, comparison of supernatant cytokine secretion in cultures of isolated pDC and pDC co-cultured with naive T cells revealed that the pDC are the main source of IL-6, IL-8, TNF-α and IFN-α.
Immunogenic or tolerogenic effects elicited by pDC are dependent on their maturation and activation status.36 Immature pDC or pDC localized in certain anatomical compartments exert tolerogenic functions although the exact mechanisms remain to be explored.3 Immature human pDC enriched from human PB can induce anergy in CD4+ antigen-specific T-cell clones37 and pDC also contribute to peripheral tolerance by the induction the of IL-10-producing regulatory T cells that suppress the generation of T effector cells.38 Additionally, systemic depletion of pDC can also prevent oral tolerance.28 In the PB of patients with IBD, increased numbers of DC expressing CD83 and CD86 have been found and matured DC generated from peripheral blood monocytes of IBD patients show raised immune-stimulating abilities when compared with those of healthy volunteers.39
Activated DC found at the sites of inflammation in murine models of colitis might contribute to the pathogenesis,11 and there is increasing evidence that mucosal DC accumulated within inflamed tissue might also play a role in human IBD.16,40 Regarding DC in the peripheral blood of IBD patients, conflicting results have been reported. Increased proportions of HLA-DR+ DC expressing CD40 and CD86 within blood mononuclear cells of IBD patients were found by Vuckovic et al.14 In peripheral mononuclear cells of patients with active IBD, Baumgart et al.13 found reduced proportions of pDC and mDC that display an immature phenotype, suggesting enhanced recruitment to inflamed mucosal tissues. Our study confirms that pDC proportions in the PB from IBD patients are reduced compared with pDC in PB from controls but display an activated and matured phenotype.
Recently, it has been shown that CD103+ and CX3CR1+ lamina propria mDC are functionally different subsets that exert important immune regulatory functions.41–43 The functional relevance of CD103-expressing and CX3CR1-expressing pDC is currently not clear and remains to be explored in further studies. The reduced proportions of pDC expressing CCR6 found in MLN compared with PB pDC are compatible with the hypothesis that these cells may preferably accumulate at inflamed mucosal sites, probably via up-regulated CCL20 or mucosal addressin cell adhesion molecule-144 interacting with their receptors CCR6 and α4β7 integrin, respectively.45 However, lamina propria pDC were not investigated in our study. Although pDC from MLN were phenotypically rather similar to those from control PB, these populations differ functionally in their development of cytokine-producing T effector cells.
Resting pDC have been found to prime human naive CD4+ T cells into IL-10-producing regulatory T type 1 cells2 and other studies suggest that pDC have an intrinsic ability to prime naive T cells to produce IL-10, regardless of their maturation stages and activation signals.4,46 Induction of IL-10- and IFN-γ-producing T cells from naive T cells by TLR7/8-stimulated, monocyte-derived DC has also been described in humans47 and IL-10 and IFN-γ-producing T regulatory cells with immune suppressive functions could be induced by CpG-activated pDC obtained from PB of controls.31 Furthermore, the recently discovered Notch pathway appears to be of importance in pDC-induced T helper type 1 cell differentiation that is characterized by production of both IL-10 and IFN-γ.48 Although not fully characterized, IL-10 and IFN-γ double producers can be detected in human and murine chronic infections, and they are assumed to limit collateral damage caused by exaggerated inflammation.49 Interestingly, inducible co-stimulator-ligand-dependent IL-10 secretion by T cells is promoted by pDC.50 So our findings are compatible with the induction of IL-10- and IFN-γ-producing T effector cells by MLN pDC, which might represent a mechanism of anti-inflammatory self control.
CpG-stimulated MLN pDC but not PB pDC co-cultured with naive T cells revealed an up to 10-fold reduction of IFN-α in culture supernatants compared with cultured MLN and PB pDC, respectively. One explanation for this surprising observation might be enhanced consumption of IFN-α during naive T-cell differentiation by MLN pDC, which cannot be excluded but seems unlikely in the absence of supporting differences in our co-culture experiments. On the other hand, IFN-α production might be down-regulated in MLN pDC by rendered TLR7 or TLR927 signalling, which involves the interferon regulatory factors 5 and 7.51 (Over)production of IFN-α by pDC has been shown to be critical for disease progression in human psoriasis and systemic lupus erythematosus52 and inhibition of IFN-α in a xenograft model of murine psoriasis could inhibit the T-cell-dependent disease progression.53 Lipid mediators such as prostaglandin E2 have also been shown to inhibit the IFN-α secretion by human pDC.54
In summary, MLN pDC are rather immature and non-activated, similar in phenotype to control PB pDC. Functional analyses revealed the development of both IL-10-producing and IFN-γ-producing T effector cells by pDC from MLN but not from PB. Surprisingly, CpG-stimulated co-cultures of naive T cells with MLN pDC contained reduced IFN-α in the supernatants compared with such co-cultures with PB pDC. Our findings support a function skewed towards tolerance rather than inflammation of MLN pDC in line with other tolerogenic responses typical for mucosal immune reactions.
Acknowledgments
Ulrike Dethlefs, Sylvia Münchow and Yvonne Dell are gratefully acknowledged for technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft, Grant SFB633.
Glossary
- DC
dendritic cells
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- mAb
monoclonal antibody
- mDC
myeloid dendritic cells
- MLN
mesenteric lymph nodes
- PB
peripheral blood
- pDC
plasmacytoid dendritic cells
- SEB
staphylococcus enterotoxin B
- TLR
Toll-like receptor
- TNF-α
tumour necrosis factor-α
Disclosures
The authors have no conflict of interest or financial interest.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Ueno H, Klechevsky E, Morita R, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–42. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 4.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 5.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 6.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 7.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 8.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon α/β-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–26. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell SJ, Rigby R, English N, Mann SD, Knight SC, Kamm MA, Stagg AJ. Migration and maturation of human colonic dendritic cells. J Immunol. 2001;166:4958–67. doi: 10.4049/jimmunol.166.8.4958. [DOI] [PubMed] [Google Scholar]
- 10.Haidinger M, Poglitsch M, Geyeregger R, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–31. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 11.Silva MA. Intestinal dendritic cells and epithelial barrier dysfunction in Crohn's disease. Inflamm Bowel Dis. 2009;15:436–53. doi: 10.1002/ibd.20660. [DOI] [PubMed] [Google Scholar]
- 12.te Velde AA, van Kooyk Y, Braat H, Hommes DW, Dellemijn TA, Slors JF, van Deventer SJ, Vyth-Dreese FA. Increased expression of DC-SIGN+ IL-12+ IL-18+ and CD83+ IL-12– IL-18– dendritic cell populations in the colonic mucosa of patients with Crohn's disease. Eur J Immunol. 2003;33:143–51. doi: 10.1002/immu.200390017. [DOI] [PubMed] [Google Scholar]
- 13.Baumgart DC, Metzke D, Schmitz J, Scheffold A, Sturm A, Wiedenmann B, Dignass AU. Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut. 2005;54:228–36. doi: 10.1136/gut.2004.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vuckovic S, Florin TH, Khalil D, Zhang MF, Patel K, Hamilton I, Hart DN. CD40 and CD86 upregulation with divergent CMRF44 expression on blood dendritic cells in inflammatory bowel diseases. Am J Gastroenterol. 2001;96:2946–56. doi: 10.1111/j.1572-0241.2001.04686.x. [DOI] [PubMed] [Google Scholar]
- 15.Cook DN, Prosser DM, Forster R, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 16.Kaser A, Ludwiczek O, Holzmann S, et al. Increased expression of CCL20 in human inflammatory bowel disease. J Clin Immunol. 2004;24:74–85. doi: 10.1023/B:JOCI.0000018066.46279.6b. [DOI] [PubMed] [Google Scholar]
- 17.Turnbull EL, Yrlid U, Jenkins CD, Macpherson GG. Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo. J Immunol. 2005;174:1374–84. doi: 10.4049/jimmunol.174.3.1374. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 19.Rimoldi M, Chieppa M, Salucci V, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–14. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 20.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 22.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Invest. 2009;119:2441–50. doi: 10.1172/JCI39134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaensson E, Uronen-Hansson H, Pabst O, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 27.Dubois B, Joubert G, Gomez de Aguero M, Gouanvic M, Goubier A, Kaiserlian D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology. 2009;137:1019–28. doi: 10.1053/j.gastro.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 28.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilsborough J, George TC, Norment A, Viney JL. Mucosal CD8α+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 2003;108:481–92. doi: 10.1046/j.1365-2567.2003.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson E, Bilsborough JM, Viney JL. Regulation of mucosal dendritic cell function by receptor activator of NF-κB (RANK)/RANK ligand interactions: impact on tolerance induction. J Immunol. 2002;169:3606–12. doi: 10.4049/jimmunol.169.7.3606. [DOI] [PubMed] [Google Scholar]
- 31.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+ CD25+ regulatory T cells. J Immunol. 2004;173:4433–42. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 32.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–45. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 33.Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008;225:226–43. doi: 10.1111/j.1600-065X.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 34.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–62. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–70. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 36.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuwana M, Kaburaki J, Wright TM, Kawakami Y, Ikeda Y. Induction of antigen-specific human CD4+ T cell anergy by peripheral blood DC2 precursors. Eur J Immunol. 2001;31:2547–57. doi: 10.1002/1521-4141(200109)31:9<2547::aid-immu2547>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 38.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikeda Y, Akbar F, Matsui H, Onji M. Characterization of antigen-presenting dendritic cells in the peripheral blood and colonic mucosa of patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 2001;13:841–50. doi: 10.1097/00042737-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 40.de Baey A, Mende I, Baretton G, Greiner A, Hartl WH, Baeuerle PA, Diepolder HM. A subset of human dendritic cells in the T cell area of mucosa-associated lymphoid tissue with a high potential to produce TNF-α. J Immunol. 2003;170:5089–94. doi: 10.4049/jimmunol.170.10.5089. [DOI] [PubMed] [Google Scholar]
- 41.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varol C, Vallon-Eberhard A, Elinav E, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–12. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 43.Bogunovic M, Ginhoux F, Helft J, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–25. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, α4β7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–63. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skoberne M, Somersan S, Almodovar W, Truong T, Petrova K, Henson PM, Bhardwaj N. The apoptotic-cell receptor CR3, but not αvβ5, is a regulator of human dendritic-cell immunostimulatory function. Blood. 2006;108:947–55. doi: 10.1182/blood-2005-12-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lombardi V, Van Overtvelt L, Horiot S, Moingeon P. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of IL-10, IFN-γ, and IL-17A by naive CD4+ T cells. J Immunol. 2009;182:3372–9. doi: 10.4049/jimmunol.0801969. [DOI] [PubMed] [Google Scholar]
- 48.Rutz S, Janke M, Kassner N, Hohnstein T, Krueger M, Scheffold A. Notch regulates IL-10 production by T helper 1 cells. Proc Natl Acad Sci USA. 2008;105:3497–502. doi: 10.1073/pnas.0712102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–43. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ito T, Yang M, Wang YH, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–15. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Izaguirre A, Barnes BJ, Amrute S, et al. Comparative analysis of IRF and IFN-α expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol. 2003;74:1125–38. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 52.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 53.Nestle FO, Conrad C, Tun-Kyi A, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J Exp Med. 2005;202:135–43. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fabricius D, Neubauer M, Mandel B, Schutz C, Viardot A, Vollmer A, Jahrsdorfer B, Debatin KM. Prostaglandin E2 inhibits IFN-α secretion and Th1 costimulation by human plasmacytoid dendritic cells via E-prostanoid 2 and E-prostanoid 4 receptor engagement. J Immunol. 2010;184:677–84. doi: 10.4049/jimmunol.0902028. [DOI] [PubMed] [Google Scholar]


