Abstract
The ability of T cells to activate antimicrobial pathways in infected macrophages is essential to host defence against many intracellular pathogens. Here, we compared the ability of two T-cell-mediated mechanisms to trigger antimicrobial responses against Mycobacterium tuberculosis in humans, CD40 activation and the release of interferon-γ (IFN-γ). Given that IFN-γ activates a vitamin D-dependent antimicrobial response, we focused on induction of the key components of this pathway. We show that activation of human monocytes via CD40 ligand (CD40L) and IFN-γ, alone, and in combination, induces the CYP27b1-hydroxylase, responsible for the conversion of 25-hydroxyvitamin D (25D) to the bioactive 1,25-dihydroxyvitamin D (1,25D), and the vitamin D receptor (VDR). The activation of the vitamin D pathway by CD40L and IFN-γ results in up-regulated expression of the antimicrobial peptides, cathelicidin and DEFB4, as well as induction of autophagy. Finally, activation of monocytes via CD40L and IFN-γ results in an antimicrobial activity against intracellular M. tuberculosis. Our data suggest that at least two parallel T-cell-mediated mechanisms, CD40L and IFN-γ, activate the vitamin D-dependent antimicrobial pathway and trigger antimicrobial activity against intracellular M. tuberculosis, thereby contributing to human host defence against intracellular infection.
Keywords: antimicrobial peptides, autophagy, Mycobacterium tuberculosis, T cells, vitamin D
Introduction
Mycobacterium tuberculosis, the causative agent of tuberculosis, infects approximately 8·8 million people and causes 1·4 million deaths worldwide per year. The discovery that T cells via the secretion of interferon-γ (IFN-γ) induce an antimicrobial pathway in human monocytes/macrophages that is dependent on the availability of serum 25-hydroxyvitamin D (25D)2 identified one mechanism of human host defence against tuberculosis. Interferon-γ induced the CYP27b1-hydroxlase, resulting in the increased conversion of 25D to 1,25-dihydroxyvitamin D (1,25D), which triggered activation of the vitamin D receptor (VDR).2 Activation of the VDR subsequently induced cathelicidin and DEFB4 antimicrobial peptides, autophagy and phagosome maturation, resulting in effective antimicrobial activity against intracellular M. tuberculosis infection.2 However, M. tuberculosis can also inhibit IFN-γ activation pathways in human monocytes/macrophages.2,3 In addition to the ability of T cells to release IFN-γ, the induction of CD40 ligand (CD40L) and its subsequent interaction with CD40 on monocytes/macrophages provides a parallel mechanism by which T cells directly activate infected monocytes.4,5 In this regard CD40L, which is mainly expressed on, but can also be secreted by activated T cells,4,6 is a potent inducer of the T helper type 1 promoting cytokine interleukin-12 (IL-12) in CD40 expressing monocytes.7,8 The clinical importance of the CD40-dependent activation of human monocytes/macrophages is underscored by the fact that patients with genetic deficiencies affecting the CD40-dependent IL-12 induction by T cells and patients with complete defects in CD40 or CD40L show increased susceptibility to mycobacterial infections, including tuberculosis.9–13 However, in a previous report, activation of human monocytes infected with M. tuberculosis via CD40 did not result in an antimicrobial response.14 In this study, we investigated whether activation of CD40 on infected monocytes via CD40L contributes to the host response against intracellular M. tuberculosis infection.
Material and methods
Reagents
Recombinant human soluble CD40L (sCD40L) was purchased from Peprotech (Rocky Hill, NJ) and recombinant IFN-γ (rIFN-γ) was purchased from BD Biosciences (San Jose CA). Soluble CD40L was used at a concentration of 5 μg/ml and rIFN-γ was used at a concentration of 10 ng/ml. Endotoxin levels of CD40L and IFN-γ were below 0·1 ng/μg as certified by the manufacturer and below the detection limit as confirmed by us using the Limulus amoebocyte lysate assay (Lonza, Basel, Switzerland). The M. tuberculosis H37Rv Whole Cell Lysate (M. tuberculosis sonicate) was from BEI Resources (Manassas, VA). Tuberculin purified protein derivative (PPD) was from Statens Serum Institute (Copenhagen, Denmark). Monoclonal antibody against CD40L (clone 24–31) and the matched IgG1 isotype control were purchased from BioLegend (San Diego, CA) and used for the blocking experiments at a concentration of 10 μg/ml. For FACS analyses the following monoclonal antibodies were used: peridinin chlorophyll protein-conjugated CD14 (clone TÜK4, Miltenyi Biotec, Bergisch Gladbach, Germany), allophycocyanin-conjugated CD3 (clone BW264/56, Miltenyi Biotec), allophycocyanin-conjugated CD40 (clone 5C3, BD Biosciences), FITC-conjugated CD40L (clone TRAP1, BD Biosciences), LC3 (clone 4E12, MBL Int., Woburn, MA) followed by an FITC-conjugated anti-mouse (clone A85-1, BD Biosciences) as well as the appropriate isotype controls.
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Boards of the University of California at Los Angeles, CA, USA and the local Ethic Committee (Ethikkommission) of the University of Cologne, Germany. All donors provided written informed consent for the collection of peripheral blood and subsequent analysis.
Serum collection and 25D quantification
Serum was collected and circulating concentrations of 25D were determined by radioimmunoassay as previously described.15
Monocyte culture
Whole blood from healthy donors was obtained with informed consent. Peripheral blood mononuclear cell (PBMCs) were isolated by Ficoll-Paque (GE Healthcare, Little Chalfont, UK). Monocytes were isolated by adherence to plastic for 2 hr as described elsewhere2 or via MACS® cell separation (Miltenyi Biotec) according to the manufacturer's instructions. Monocytes were cultured in 10% human serum (25D level ≥ 98 nm). We observed differences in the kinetics of CD40L and IFN-γ-mediated gene expression of cathelicidin and DEFB4 in several donors. Therefore gene expression was analysed in monocytes incubated for 18 and/or 22 hr on 24-well plates.
PCR
Messenger RNA was isolated from the cells using an RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer's recommended protocol and cDNA was prepared and mRNA levels were assessed and calculated with quantitative PCR as previously described.15 Primer sequences for human cathelicidin, DEFB4, CYP27B1, VDR and h36B4 were previously reported.2,15
Infection of monocytes, treatment with CD40L and IFN-γ and quantification of mycobacterial growth
Infection and treatment of human monocytes and quantification of intracellular mycobacterial growth was performed as previously described.15,27 In brief, monocytes, found to be > 80–90% CD14+, were infected for 4 hr or overnight with single cell suspensions of M. tuberculosis at a multiplicity of infection (MOI) of five in a six-well plate. Extracellular bacteria were removed by vigorous washing. Adherent cells were detached by treatment with EDTA and plated at a concentration of 5 × 105 cells/ml in a 24-well plate. The efficiency of infection, as quantified by auramine rhodamine stain, was in a range of 20–35%. Infected cells were treated with IFN-γ and/or CD40L, which were present throughout the 6-day culture period in 10% vitamin D-sufficient human serum. To determine the number of viable intracellular bacilli, infected cells were lysed with 0·3% saponin. Lysates of infected cells were re-suspended vigorously, transferred into screw-capped vials and sonicated in a pre-heated (37°) water bath sonicator for 10 min. Aliquots of the lysates were diluted 10-fold in 7H9-medium. Four dilutions of each sample were plated in duplicates on 7H11 agar plates and incubated at 37° for 21 days. At all time-points an aliquot of unlysed, infected cells was harvested and counted. This allowed an exact quantification of cells as well as the determination of cellular viability by trypan blue exclusion. Recovery of cells was > 70% in all experiments, with cell viability regularly exceeding 90% of total cells.
Flow cytometry and saponin-resistant LC3-II staining
Staining for surface molecules CD3, CD40L (on T cells), CD14 and CD40 (on monocytes) was performed with monoclonal antibodies. Saponin-resistant intracellular LC3-II staining was performed as described previously.16 Briefly, cells were permeabilized by washing with 0·05% saponin in PBS. Intracellular staining with anti-LC3 monoclonal antibody followed by the FITC-conjugated secondary antibody was performed in the same buffer. Flow cytometry was conducted on a FACSCalibur (BD Biosciences) and data were analysed using FlowJo software (Tree Star, Ashland, OR).
PBMC assays
Whole blood from bacillus Calmette–Guérin-vaccinated donors was obtained with informed consent. The PBMCs were isolated by Ficoll–Paque and were stimulated with 10 μg/ml M. tuberculosis sonicate in the absence or presence of CD40L blocking antibody or the corresponding IgG1 isotype, and cultured in 10% human serum (25D level ≥ 98 nm) for 24 hr.
T-cell clone assays
Two T-cell clones (RDJ and CB) were generated using PPD and M. tuberculosis sonicate, respectively, by limiting dilution as described previously.39 Before use in monocyte stimulation experiments the clones were activated via plate-bound anti-CD3 (clone OKT3, Miltenyi) for 6 hr, then fixed for 15 min with 1% paraformaldehyde in PBS at 4°, washed twice, incubated overnight in PBS at 4° and washed again with PBS before use. Activated, fixed T cells were added for stimulation to monocytes in a ratio of 1 : 2 and co-cultured in 10% human serum (25D level ≥ 98 nm) for 19 hr in the absence or presence of the CD40L blocking antibody or of the corresponding IgG1 isotype.
Statistics
P-values were calculated using two-tailed Student's t-tests.
Results
CD40L is mainly expressed by activated T cells and is biologically active as a membrane-bound form as well as a secreted trimer.4,6 We investigated the ability of CD40L trimer to induce the vitamin D-dependent antimicrobial pathway2,15 in primary human monocytes, which express CD40, the receptor for CD40L (ref. 5 and Supplementary material, Fig. S1). Monocytes were activated with recombinant sCD40L and rIFN-γ alone or in combination. The induction of the 1-α-hydroxylase CYP27b1, responsible for conversion of 25D to the bioactive 1,25D, and the VDR gene expression were measured at 18 and 22 hr. The sCD40L and rIFN-γ increased CYP27B1 gene expression by 5·7-fold and 4·1-fold, as well as VDR gene expression by 3·6-fold and 4·3-fold in human monocytes, respectively (Fig. 1a,b, P ≤ 0·05). The combined treatment of sCD40L and rIFN-γ resulted in a 27-fold induction of CYP27B1 and a 7·8-fold induction of VDR gene expression (Fig. 1a,b, both P < 0·05). Based on these findings we next investigated the expression of the downstream effector molecules cathelicidin and DEFB4. The sCD40L stimulation of monocytes cultured in 10% 25D-sufficient human serum resulted in the up-regulation of cathelicidin gene expression by 1·8-fold and of DEFB4 gene expression by 2·4-fold (Fig. 1c,d, both P < 0·05). Consistent with our previous findings,2 rIFN-γ by itself induced cathelicidin gene expression to increase by 3·7-fold and DEFB4 by 4·7-fold (Fig. 1c,d, both P < 0·05). Furthermore, simultaneous treatment with sCD40L and rIFN-γ induced the up-regulation of cathelicidin gene expression by 4·8-fold and DEFB4 by 7·6-fold (Fig. 1c,d, both P < 0·05).
Figure 1.

CD40 ligand (CD40L) induces key genes of the vitamin D antimicrobial pathway. Primary human monocytes were stimulated with soluble CD40L (sCD40L) and/or recombinant interferon-γ (rIFN-γ) for 18 and/or 22 hr in 10% vitamin D-sufficient human serum. (a) CYP27B1, (b) vitamin D receptor (VDR), (c) cathelicidin, and (d) DEFB4 gene expression was assessed by quantitative PCR (mean fold change ± SEM, n = 4). *P ≤ 0·05.
A second key event in the vitamin D antimicrobial pathway is the induction of autophagy, which is required to overcome the phagosome maturation block in M. tuberculosis-infected monocytes/macrophages.2 We tested whether sCD40L alone or in combination with IFN-γ induces autophagy in monocytes by measuring intracellular saponin-resistant LC3-II by FACS.16 Increase in LC3-II is associated with the induction of autophagy. Consistent with previous findings we found that sCD40L17 and IFN-γ2 alone increased the percentage of LC3-II+ monocytes compared with media control (32% and 41%, respectively, versus 19%, Fig. 2, P < 0·05). The combination of sCD40L and IFN-γ resulted in 33% LC3-II+ cells (Fig. 2, P < 0·05). Together, these findings show that at least two T-cell signals can activate key components of the vitamin D antimicrobial pathway, the induction of antimicrobial peptides and autophagy.
Figure 2.

CD40 ligand (CD40L) induces autophagy. Primary human monocytes were stimulated with soluble CD40L (sCD40L) and/or recombinant interferon-γ (rIFN-γ) for 19 hr in 10% vitamin D-sufficient human serum. Autophagy was measured by saponin-resistant LC3-II staining. Histogram plots from one representative donor and the summary of four donors are shown (% LC3-II+ cells ±SEM). *P < 0·05.
Given that CD40L not only functions as a secreted trimer, but also in its membrane-bound form on activated T cells, we next investigated if T cells through surface expression of CD40L activate autophagy in human monocytes by two different approaches. First, we stimulated PBMC cultures from bacillus Calmette–Guérin-vaccinated individuals with M. tuberculosis sonicate for 24 hr and measured saponin-resistant LC3-II in monocytes. Monocytes in these cultures were identified by CD14 co-staining. Monocyte LC3-II was significantly enhanced in M. tuberculosis-stimulated cultures compared with untreated cultures (24% versus 3%, Fig. 3a, P < 0·05). Given that multiple factors in this system can increase autophagy in the monocytes, e.g. direct activation of Toll-like receptor 2/1 (TLR2/1) on monocytes by lipoproteins or T-cell secretion of IFN-γ, we added monoclonal blocking antibody against CD40L to these cultures to investigate if CD40/CD40L interaction contributes to the induction of autophagy. Addition of anti-CD40L blocking antibody, but not the IgG1 isotype, inhibited autophagy induction by approximately 60% (Fig. 3a, P < 0·05). Second, we generated two T-cell clones (RDJ and CB) using tuberculin PPD and M. tuberculosis sonicate, respectively, by limiting dilution. Both T-cell clones expressed CD40L when activated for 6 hr by plate-bound anti-CD3 (data not shown). Paraformaldehyde-fixed, CD3-activated T cells were used to activate human monocytes. The T-cell clones enhanced autophagy in monocytes as measured by an increase of saponin-resistant LC3-II compared with media control (33% versus 23%, Fig. 3b, P < 0·05). In these experiments, addition of anti-CD40L blocking antibody, but not the IgG1 isotype, inhibited autophagy induction by almost 100% (Fig. 3b, P < 0·05).
Figure 3.
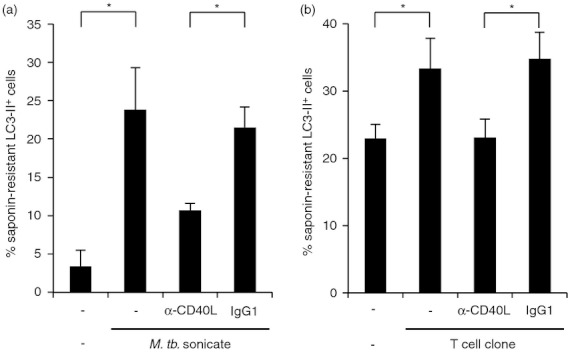
CD40 ligand (CD40L) expressed on T cells promotes autophagy. (a) Peripheral blood mononuclear cells (PBMCs) from bacillus Calmette–Guérin-vaccinated individuals were incubated with Mycobacterium tuberculosis sonicate in 10% vitamin D-sufficient human serum for 24 hr and analysed for saponin-resistant LC3-II by FACS. CD14-positive monocytes were identified by CD14 co-staining (% LC3-II+ monocytes ±SEM, n = 3). (b) T-cell clones (RDJ and CB) were activated for 6 hr via plate-bound anti-CD3, fixed with paraformaldehyde and co-incubated for 19 hr with primary human monocytes in 10% vitamin D-sufficient human serum. Monocyte saponin-resistant LC3-II was measured by FACS. T cells and monocytes were distinguished by CD3 co-staining (% LC3-II+ monocytes ±SEM, n = 5; data are summarized from five different monocytes donors, of which one donor was stimulated with T-cell clone RDJ only, two were stimulated with T-cell clone CB only, and two were stimulated with both T-cell clones. In the last of these treatments, the data from both T-cell clone stimulations were averaged before inclusion in the figure) *P ≤ 0·05.
Because CD40L and IFN-γ up-regulated the antimicrobial peptides cathelicidin and DEFB4, as well as autophagy, we hypothesized that both CD40L and IFN-γ activate an antimicrobial activity against M. tuberculosis. Hence, primary human monocytes were infected with the virulent M. tuberculosis strain H37Rv and the infected cells were activated with sCD40L and/or rIFN-γ. Treatment with sCD40L, rIFN-γ, as well as the combination of sCD40L and rIFN-γ, resulted in a comparable growth restriction of intracellular M. tuberculosis as determined in colony-forming unit assays, showing an antimicrobial activity in the range of 60–70% (Fig. 4 P ≤ 0·05).
Figure 4.

CD40 ligand (CD40L) induced antimicrobial activity against Mycobacterium tuberculosis. Primary human monocytes were infected with M. tuberculosis H37Rv and cultured with medium, soluble CD40 L (sCD40L) and/or recombinant IFN-γ (rIFN-γ) in 10% vitamin D-sufficient human serum. Viable bacteria were quantified by colony-forming unit assay on day 6 (mean ± SEM, n = 5). *P ≤ 0·05.
Discussion
In this study we show that two T-cell-derived stimuli, IFN-γ and CD40L, activate antimicrobial activity in human monocytes resulting in growth inhibition of intracellular M. tuberculosis. In addition, both IFN-γ and CD40L acted in parallel to induce key components of the vitamin D-dependent antimicrobial pathway, suggesting a common mechanism of antimicrobial response. The ability of CD40L and IFN-γ to trigger key components of the vitamin D-dependent antimicrobial pathway was demonstrated according to the induction of CYP27B1, VDR, and the downstream effector molecules cathelicidin and DEFB4, as well as autophagy, all of which are required for effective antimicrobial activity against M. tuberculosis. We have previously shown that the TLR2/1L and/or IFN-γ-mediated up-regulation of CYP27b1 results in enhanced intracellular conversion of 25D to 1,25D.2,15 Subsequently, 1,25D triggered the VDR, resulting in the induction of genes encoding for cathelicidin and DEFB4 antimicrobial peptides, which both contain vitamin D response elements in their promoters.2,15,18 In addition, induction of cathelicidin and DEFB4 was dependent on CYP27b1 and VDR function. Activation of monocytes/macrophages also induced 25D-dependent autophagy, important to overcome the ability of M. tuberculosis to block phagolysosomal maturation2,19 and facilitate the delivery of the antimicrobial peptides to the intracellular compartments containing the pathogen.2,20,21 Moreover, antimicrobial activity of macrophages was dependent on serum concentration of 25D.2 Finally, specific gene knockdown of cathelicidin and DEFB4 abolished vitamin D-dependent antimicrobial activity against M. tuberculosis.18,22
In the context of infection, T-cell secretion of IFN-γ has the advantage of locally activating a larger number of monocytes/macrophages in a contact-independent fashion. In contrast, T-cell expression of CD40L provides a contact-dependent mechanism to activate an antimicrobial response in monocytes. The CD40L–CD40 triggered antimicrobial pathway thereby allows the acquired immune system to specifically target infected cells during antigen-specific interactions between T cells and antigen-presenting cells. In addition, CD40 activation provides a mechanism by which M. tuberculosis-specific T cells not secreting IFN-γ, e.g. T helper type 17 and type 22 cells,23 but expressing CD40L can trigger an antimicrobial response.
Of relevance, CD40L and IFN-γ induced antimicrobial activity against M. tuberculosis in experiments, in which sera containing adequate levels of vitamin D were employed. To our knowledge, our study, in which we used serum containing sufficient levels of vitamin D, is the first report demonstrating that the CD40L–CD40 interaction induces a direct antimicrobial activity against virulent M. tuberculosis in human monocytes/macrophages. Previously, Larkin et al., using serum with an unknown level of vitamin D, found that CD40L did not inhibit virulent M. tuberculosis in human monocytes.14 The fact that the vitamin D antimicrobial pathway was triggered by CD40 activation may suggest that this pathway is linked to the antimicrobial response, as has been shown in detail in the case of TLR2/1L and IFN-γ activation.2,15,18,19,21,24 The induction of cathelicidin and DEFB4 has also been demonstrated to be necessary and sufficient for the induction of antimicrobial activity against M. tuberculosis by our laboratory and at least three other groups.2,15,18,19,21,22,25,26 It remains to be determined whether vitamin D levels are critical for the observed CD40L-induced antimicrobial activity. Moreover, the vitamin D pathway is only one antimicrobial pathway and it is likely that other antimicrobial pathways are involved in antimicrobial mechanisms against M. tuberculosis in human monocytes and macrophages.
Although there was variation in the magnitude by which CD40L, IFN-γ and CD40L plus IFN-γ induced vitamin D antimicrobial pathway genes, the level of mycobacterial growth inhibition was in the range of 60–70% in all cases. Similarly, IFN-γ, TLR2/1 or 1,25D-mediated growth inhibition of M. tuberculosis in human monocytes did not exceed 60%.2,15,27 These observations might reflect that there is a quantitative limit on the number of organisms that can be killed by an individual monocyte, as has been shown in other in vitro systems.28 In fact, CD40L and IFN-γ, even though synergizing in the induction of the antimicrobial peptides cathelicidin and DEFB4, did not synergize in the induction of autophagy. It is possible that the regulation of additional genes may be critical for antimycobacterial host defence pathways or that M. tuberculosis infection of monocytes partially inhibits CD40 activation pathways, as has been demonstrated for IFN-γ activation.2,29
Although both TLR2/1 ligand and IFN-γ induction of the vitamin D antimicrobial pathway involve different receptors, activation converges on the induction of IL-15, triggering a key step in the activation of the vitamin D antimicrobial pathway.2,30 Interleukin-15 is also induced by CD40L on the surface of dendritic cells31 with the IL-15/IL15Rα chain complex able to activate IL-15Rβ/IL-15Rγ chain expressing target cells in trans.32 The CD40L induced pro-inflammatory cytokine response is inhibited by 1,25D, so that the generation of 1,25D at the site of infection might provide a negative feedback mechanism that prevents excessive inflammation.33 This may be supported by the ability of CD40L-expressing T cells to promote CYP27b1 expression and function in dendritic cells resulting in the conversion of 25D to 1,25D, and the subsequent regulation of the balance between regulatory and inflammatory T-cell responses.34 In addition to its direct effect on activating the vitamin D-dependent antimicrobial pathway, CD40 activation can contribute indirectly to the host response through induction of the IL-12 and consequently the Th1 cytokine IFN-γ.2,30
The key role for CD40 activation in host defence against M. tuberculosis is reflected by the increased susceptibility of individuals with genetic mutations in the CD40L–CD40 axis to mycobacterial infections.35,36 Although the role of CD40L in mouse models of tuberculosis is less clear,37,38 the antimicrobial pathways that kill M. tuberculosis in mouse versus human macrophages are different.2,27 CD40L might also contribute to disease pathology in human mycobacterial infection, given its ability to provide T-cell help for CD40-expressing B cells, resulting in B-cell proliferation and isotype class switching.4
In summary, the present data provide insight into how CD40 activation contributes to host defence against tuberculosis in humans, demonstrating that CD40 triggers an antimicrobial activity against the pathogen in infected cells. In addition, the demonstration that two parallel T-cell-mediated pathways, i.e. IFN-γ and CD40L, converge on a common vitamin D-dependent antimicrobial mechanism, further suggests the relevance of this pathway for host defence. The existence of the parallel IFN-γ and CD40L mechanisms by which T cells trigger antimicrobial responses supports a pivotal role for the acquired immune system in activating monocytes and macrophages in the host defence against tuberculosis in humans.
Acknowledgments
We thank Barry Bloom for insightful scientific discussions. We thank Bettina Hussak and Julia Steiger for excellent technical assistance. Current address of M.F.: Department of Dermatology, University of Cologne, Germany. The following reagent was obtained through BEI Resources, NIAID, NIH: Mycobacterium tuberculosis, Strain H37Rv, Whole Cell Lysate, NR-14822. This work was funded by National Institutes of Health grants NIH P50 – AR063020, RO1 AI022553, AR040312, AI047868, AI073539, by the Deutsche Forschungsgemeinschaft FA849/1 and SFB829, the European Skin Research Foundation, and the Ministry of Innovation, Science, Research and Technology of the German State of North Rhine-Westphalia.
Disclosures
The authors declare that they have no competing interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Monocytes express CD40. (A) Freshly isolated primary human monocytes were stained for CD40 surface expression and analysed by FACS (grey shaded area: CD40 antibody, black line: isotype control). (B) Primary human monocytes were stimulated with soluble CD40 ligand and interleukin-1β secretion into cell culture supernatants was measured by Cytokine Bead Array (CBA) after 20 hr (n = 5).
References
- 1.World Health Organization. 2011/2012 Tuberculosis Global Facts. http://www.who.int/tb/publications/2011/factsheet_tb_2011.pdf. 2012. [accessed 01 February 2013]
- 2.Fabri M, Stenger S, Shin DM, et al. Vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:104ra2. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fortune SM, Solache A, Jaeger A, Hill PJ, Belisle JT, Bloom BR, Rubin EJ, Ernst JD. Mycobacterium tuberculosis inhibits macrophage responses to IFN-γ through myeloid differentiation factor 88-dependent and -independent mechanisms. J Immunol. 2004;172:6272–80. doi: 10.4049/jimmunol.172.10.6272. [DOI] [PubMed] [Google Scholar]
- 4.Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help) J Exp Med. 1992;175:1091–101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–74. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graf D, Muller S, Korthauer U, van Kooten C, Weise C, Kroczek RA. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur J Immunol. 1995;25:1749–54. doi: 10.1002/eji.1830250639. [DOI] [PubMed] [Google Scholar]
- 7.Shu U, Kiniwa M, Wu CY, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40–CD40 ligand interaction. Eur J Immunol. 1995;25:1125–8. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi PS, Bleharski JR, Uyemura K, et al. A role for CD40–CD40 ligand interactions in the generation of type 1 cytokine responses in human leprosy. J Immunol. 2000;165:1506–12. doi: 10.4049/jimmunol.165.3.1506. [DOI] [PubMed] [Google Scholar]
- 9.Filipe-Santos O, Bustamante J, Haverkamp MH, et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J Exp Med. 2006;203:1745–59. doi: 10.1084/jem.20060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen RC, Armitage RJ, Conley ME, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259:990–3. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 11.Aruffo A, Farrington M, Hollenbaugh D, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- 12.DiSanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541–3. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 13.Korthauer U, Graf D, Mages HW, et al. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:539–41. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 14.Larkin R, Benjamin CD, Hsu YM, Li Q, Zukowski L, Silver RF. CD40 ligand (CD154) does not contribute to lymphocyte-mediated inhibition of virulent Mycobacterium tuberculosis within human monocytes. Infect Immun. 2002;70:4716–20. doi: 10.1128/IAI.70.8.4716-4720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 16.Eng KE, Panas MD, Karlsson Hedestam GB, McInerney GM. A novel quantitative flow cytometry-based assay for autophagy. Autophagy. 2010;6:634–41. doi: 10.4161/auto.6.5.12112. [DOI] [PubMed] [Google Scholar]
- 17.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116:2366–77. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu PT, Schenk M, Walker VP, et al. Convergence of IL-1β and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS ONE. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin DM, Yuk JM, Lee HM, et al. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signaling. Cell Microbiol. 2010;12:1648–65. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci U S A. 2007;104:6031–6. doi: 10.1073/pnas.0700036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–43. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Qiao D, Fu X, Lao S, Zhang X, Wu C. Identification of Mycobacterium tuberculosis-specific Th1, Th17 and Th22 cells using the expression of CD40L in tuberculous pleurisy. PLoS ONE. 2011;6:e20165. doi: 10.1371/journal.pone.0020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martineau AR, Nhamoyebonde S, Oni T, et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci U S A. 2011;108:19013–7. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 26.Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012;8:e1002689. doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thoma-Uszynski S, Stenger S, Takeuchi O, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–7. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka Y, Kiyotaki C, Tanowitz H, Bloom BR. Reconstitution of a variant macrophage cell line defective in oxygen metabolism with a H2O2-generating system. Proc Natl Acad Sci USA. 1982;79:2584–8. doi: 10.1073/pnas.79.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ting LM, Kim AC, Cattamanchi A, Ernst JD. Mycobacterium tuberculosis inhibits IFN-γ transcriptional responses without inhibiting activation of STAT1. J Immunol. 1999;163:3898–906. [PubMed] [Google Scholar]
- 30.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–20. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuniyoshi JS, Kuniyoshi CJ, Lim AM, Wang FY, Bade ER, Lau R, Thomas EK, Weber JS. Dendritic cell secretion of IL-15 is induced by recombinant huCD40LT and augments the stimulation of antigen-specific cytolytic T cells. Cell Immunol. 1999;193:48–58. doi: 10.1006/cimm.1999.1469. [DOI] [PubMed] [Google Scholar]
- 32.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 33.Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1α,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45:190–7. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Jeffery LE, Wood AM, Qureshi OS, et al. Availability of 25-hydroxyvitamin D3 to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189:5155–64. doi: 10.4049/jimmunol.1200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Jong R, Altare F, Haagen IA, et al. Severe mycobacterial and salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–8. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 36.Zhang SY, Boisson-Dupuis S, Chapgier A, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-α/β, IFN-γ, and IFN-λ in host defense. Immunol Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- 37.Campos-Neto A, Ovendale P, Bement T, Koppi TA, Fanslow WC, Rossi MA, Alderson MR. CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol. 1998;160:2037–41. [PubMed] [Google Scholar]
- 38.Lazarevic V, Myers AJ, Scanga CA, Flynn JL. CD40, but not CD40L, is required for the optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity. 2003;19:823–35. doi: 10.1016/s1074-7613(03)00324-8. [DOI] [PubMed] [Google Scholar]
- 39.Salgame P, Abrams JS, Clayberger C, Goldstein H, Convit J, Modlin RL, Bloom BR. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


