Abstract
Background
Cancer patients are at increased risk for depression compared with individuals with no cancer diagnosis, yet few interventions target depressed cancer patients.
Methods
Efficacy of psychotherapeutic and pharmacologic interventions for depression in cancer patients who met an entry threshold for depressive symptoms was examined by meta-analysis. Five electronic databases were systematically reviewed to identify randomized controlled trials meeting the selection criteria. Effect sizes were calculated using Hedges’ g and were pooled to compare pre- and postrandomization depressive symptoms with a random effects model. Subgroup analyses tested moderators of effect sizes, such as comparison of different intervention modalities, with a mixed effects model. All statistical tests were two-sided.
Results
Ten randomized controlled trials (six psychotherapeutic and four pharmacologic studies) met the selection criteria; 1362 participants with mixed cancer types and stages had been randomly assigned to treatment groups. One outlier trial was removed from analyses. The random effects model showed interventions to be superior to control conditions on reducing depressive symptoms postintervention (Hedges’ g = 0.43, 95% confidence interval = 0.30 to 0.56, P < .001). In the four psychotherapeutic trials with follow-up assessment, interventions were more effective than control conditions up to 12–18 months after patients were randomly assigned to treatment groups (P < .001). Although each approach was more effective than the control conditions in improving depressive symptoms (P < .001), subgroup analyses showed that cognitive behavioral therapy appeared more effective than problem-solving therapy (P = .01), but not more effective than pharmacologic intervention (P = .07).
Conclusions
Our findings suggest that psychological and pharmacologic approaches can be targeted productively toward cancer patients with elevated depressive symptoms. Research is needed to maximize effectiveness, accessibility, and integration into clinical care of interventions for depressed cancer patients.
By the year 2005, nearly 500 unique studies, 63% of which involved randomized designs, constituted the knowledge base on the efficacy of psychosocial interventions for individuals diagnosed with cancer (1). This literature often targets multiple outcomes, such as quality of life, distress, physical symptoms, and fatigue (1). Dramatically fewer trials assess depression as a primary outcome, although it is one of the most disabling psychological conditions experienced by cancer patients. Still fewer trials target the subgroup of individuals most in need of an intervention: those who have elevated depressive symptoms or are clinically depressed. The primary goal of this meta-analysis is to examine randomized controlled trials (RCTs) testing the efficacy of psychotherapeutic and pharmacologic interventions for depressive symptoms in individuals who are diagnosed with cancer and meet an entry threshold for elevated depressive symptoms.
Risk for depression among the population after a cancer diagnosis is higher compared with the population with no diagnosis (2), and prevalence of major depression diagnosed via standardized interview is estimated to be approximately 16% (3). Depression not only represents a psychological burden for adults with cancer but also carries negative health and behavioral consequences, including nonadherence to medical regimens (4), increased emergency and inpatient service use (5), delayed return to work (6), and possibly elevated rates of suicidal ideation or suicide (7–10) and mortality (10–17). In light of the pervasive impact of depression, it is essential to investigate whether published interventions are effective in reducing depression among cancer patients and to determine which approaches are most promising.
Reviewers of the literature on interventions designed to improve quality of life, including depression, in individuals diagnosed with cancer have drawn inconsistent conclusions regarding the efficacy of interventions (18–30). However, most interventions include participants regardless of their baseline standing on the outcome of interest. For example, one review of 60 psychosocial interventions for depressive symptoms or anxiety in cancer patients revealed that only 5% of studies restricted eligibility to patients meeting a specified threshold for distress (24). Individuals with low depressive symptoms at baseline might not be likely to benefit from interventions (29,31,32), in that their outcome assessment contains little room for improvement (although prevention of future depressive symptoms and benefit on other outcomes are possible). Combining trials that do and do not use an eligibility criterion for depression may underestimate intervention efficacy for individuals in most need of an intervention. Specifically selecting trials for cancer patients with elevated depressive symptoms or diagnosed depression, Rodin et al. (33) conducted a systematic review of 11 interventions. They found “limited evidence for the effectiveness of pharmacological and psychosocial interventions in the treatment of cancer patients with depressive disorders” (33).
To our knowledge, no previously published review has included a quantitative analysis of the efficacy of psychosocial and pharmacologic approaches for individuals diagnosed with cancer who meet entry criteria for elevated depressive symptoms. Our review identifies existing interventions that can be productively directed toward depressed cancer patients, illuminates limitations in the current literature, and proposes directions for research and clinical practice. In addition to estimating the efficacy of extant interventions and the duration of the effects through a meta-analysis, we examined whether efficacy varies as a function of therapeutic approach (ie, specific psychotherapy and pharmacotherapy). We also considered other potential moderators including characteristics of the samples, cancer, interventions, and trial quality by pooling relevant data from the trials that met the eligibility criteria.
Methods
Literature Search Strategy
MEDLINE, PsycINFO, CINAHL, EMBASE, and Cochrane Library databases were systematically searched using appropriate controlled-vocabulary terms specific to each database (Supplementary methods, available online) and keyword searching for terms indicating depression and frequently used assessments of depressive symptoms (such as depression, depressive disorder, Beck Depression Inventory); cancer (including neoplasm, cancer, leukemia); and intervention trials (including random, placebo, treatment). The searches were inclusive of studies published in English from each database’s inception through October 31, 2011. We also examined the references of the articles identified through the searches and relevant reviews and meta-analyses. We did not include three of the studies included in the systematic review by Rodin et al. (33) because two studies (34,35) did not include an eligibility criterion for depressive symptoms and one (36) was not a randomized trial.
Article Selection Strategy
Four pairs of raters (S. Hart, M. Hoyt, M. Diefenbach, D. Anderson, K. Kilbourn, L. Craft, J. Steel, A. Stanton) reviewed unique entries from database searches. Each rater reviewed the abstract independently, and articles were obtained when the pairs agreed that the article required full-text examination; discrepancies were resolved by a third rater. Rater pairs conducted full-text review of the resulting articles for the following eligibility criteria: 1) adult participants (18 years or older) with a cancer diagnosis at the time of study entry; 2) inclusion criterion of elevated depressive symptoms at the time of study entry, as defined by each author, including specified threshold on a questionnaire or interview assessment; 3) random assignment to one or more interventions vs a usual care, placebo, attention control, or waiting-list control condition; and 4) depressive symptoms assessed as an outcome. Any RCT that included a psychosocial/behavioral intervention such as cognitive behavioral therapy (CBT), physical activity, and/or pharmacologic component qualified for inclusion, including collaborative care and complementary approaches such as mind–body approaches.
Five trials employing mixed eligibility thresholds in which presence of depressive symptoms was not required [eg, depressive symptoms and/or smoking and/or problem drinking (37); depressive symptoms and/or anxiety (38,39) and/or fatigue (40); depressive symptoms and pain (41)] did not qualify for inclusion. Also, eight trials that compared two interventions in the absence of a control condition were not included (42–49).
Article Review Strategy
Using an online coding program designed for this project, the aforementioned four pairs of raters independently coded selected trials. Data regarding characteristics of the sample, intervention, and instrumentation were extracted, as were continuous data from assessments of depressive symptoms (percentage of participants meeting author-defined criteria for categorical “responder” or “remitted” were not extracted), to calculate effect sizes. The program produced a list of discrepancies, which were then systematically resolved by consensus, and final data were entered for each study. Studies were assessed for quality using a modified 14-item version of the PEDro scale (50), which was designed to identify studies that are generalizable, internally valid, and interpretable. The modified coding scheme was identical to the 11-item scale (50, http://www.pedro.org.au/english/downloads/pedro-scale/), with three additional items. Two items assessed quality of treatment fidelity (use of a treatment manual, monitoring of treatment implementation), as recommended by the Treatment Fidelity Workgroup of the National Institutes of Health Behavior Change Consortium (51). The third item assessed provision of loss to follow-up information, included as an important quality indicator in other reviews of interventions for cancer patients (26). When published articles did not present sufficient data to calculate the effect sizes, we contacted authors for the required information.
Statistical Analysis
Comprehensive Meta-Analysis (version 2.2.055, Biostat, Englewood, NJ) was used to calculate effect sizes and analyze data (52). Hedges’ g was calculated for each trial and the overall pool of trials and was used as a measure of the effect size. Hedges’ g is calculated on the basis of the standardized mean difference effect size, which uses the pooled within-groups SD but corrects for bias from small sample sizes and therefore is considered to be more accurate than the standardized effect size typically measured by Cohen’s d (53). Hedges’ g is a conservative estimate of effect size, which typically is interpreted by Cohen’s d (54) guidelines (small effect = 0.20, medium effect = 0.50, large effect = 0.80). A positive effect size indicates that the intervention was superior to the control condition in reducing depressive symptoms, whereas a negative effect size indicates that the control condition outperformed the intervention. For example, a Hedges’ g of 0.50 would indicate that the intervention condition produced a reduction in depressive symptoms of half a standard deviation more than did the control condition.
When outcome data for more than one measure of depressive symptoms were reported in a given study (a violation of outcome independence), we used the mean of the effect sizes (55). A variety of approaches exist to examine multiple assessments of the same construct, including multivariable approaches (56). We used the commonly employed method of combining effect sizes for multiple outcomes, which tends to yield a more conservative effect size estimate than do multivariable approaches (55,57). This was applicable for four studies (58–61). Two separate effect sizes were calculated for trials containing two intervention groups (59,62,63), in light of the observation that the two active treatments differed substantially in approach or content, such as two different medications or CBT vs social support.
Individual effect sizes were pooled in the Comprehensive Meta-Analysis program and were calculated separately for prerandomization compared with postintervention outcomes. The assessment closest in time to completion of the intervention was used for calculation of posttreatment effect size. Effect sizes were also calculated for prerandomization to longer-term follow-up assessment, when follow-up assessments were reported. Because none of the studies reported the correlation between baseline and postintervention outcomes, we used a fixed correlation of 0.90 for within-group comparison. This approach has a small chance of overestimating effect size and has been used in earlier psychological intervention research examining improvement rates (64).
Variability was assumed to be caused by both sampling error and random differences; therefore, the data were fit to a random effects model, which provides more conservative estimates than a fixed effects model (65). A random effects estimate assumes additional variance beyond the set of studies and facilitates generalizability of results. We examined the distribution of effect sizes to identify any extreme values for possible elimination from analyses (66).
We identified several a priorimoderators, of which the primary one involved a comparison of specific psychotherapeutic and pharmacologic approaches. Others included PEDro trial quality criteria, sample and cancer characteristics (ie, sociodemographics, cancer type, cancer stage, time elapsed since diagnosis), questionnaire vs interview assessment approach, and intervention characteristics, such as length, group vs individual, and type of control group. Examination of any moderator required that it must be represented in a minimum of two trials, have sufficient variability across studies (note that the majority of PEDro criteria showed little variability across studies), and contain sufficient descriptive data for meaningful analysis. The subgroup analyses used a mixed effects model, which pools studies within subgroups with a random effects model, but tested for statistically significant between-groups differences with a fixed effects model. A Bonferroni correction was used to control for inflation of Type I error when multiple between-groups comparisons were conducted. To test homogeneity of variance of the different effect sizes, we used the Q test statistic (66), which indicates whether the variability in effect sizes is within the range that would be expected if all studies shared a common population effect size. We also examined the I 2 statistic, which ranges between 0% and 100% and indicates the proportion of the total variance accounted for by heterogeneity (larger percentages reflect greater heterogeneity). We calculated 95% confidence intervals (CI) around I 2 (67), using the noncentral χ 2-based approach within the heterogi module for Stata (68). For continuous moderators, such as mean age, we used meta-regression techniques in the Comprehensive Meta-Analysis program, using the method of moments estimator.
To evaluate sources of bias, we created and examined a funnel plot, which graphically displays the measure of study effect size against sample size. A funnel plot displaying absent publication bias should result in a symmetrical inverted funnel-shaped graph. We also used Duval and Tweedie’s (69) trim and fill procedure, which “trims” off the asymmetric outlying part of the funnel plot, the remainder of which is used to estimate the true center of the plot. The procedure calculates the number of missing studies and provides an estimate of what the effect size would have been without bias, on the basis of “filling in” or imputing missing studies. Egger’s test of the intercept, a linear regression method, was used to further capture the bias in the funnel plot (70). The fail-safe N also was calculated to determine the number of non–statistically significant trials missing from the analysis that would, if included, reduce the observed effect size to a non–statistically significant level.
ResultsSelection of RCTs
A search of the five databases identified 7,770 unique studies, of which the rater pairs conducted full-text review of 350 articles (Figure 1). Fourteen unique trials and one report of a follow-up analysis met all inclusion criteria. Five reports included sufficient data to calculate effect size (58–60,63,71), and five authors provided additional data that included pre- and all postintervention means, SDs, and sample sizes separated based on the treatment arm (61,62,72–75). Therefore, 10 unique trials (six psychotherapeutic and four pharmacologic) that included a total of 1362 participants with mixed cancer types and stages who had been randomly assigned to treatment groups were included in the meta-analysis (Figure 1). For the remaining four articles, including two publications from one trial, the authors were unable to provide essential data (76–79).
Figure 1.
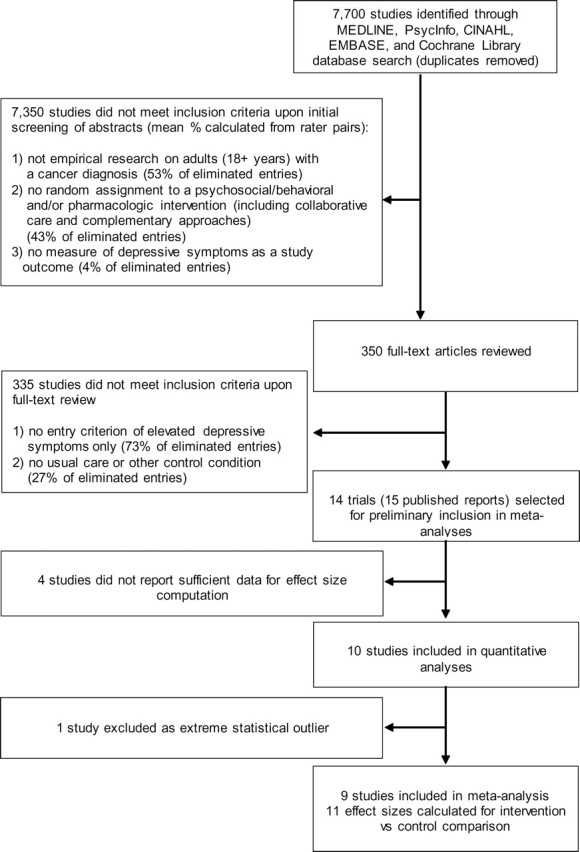
Flow chart of study identification by systematic literature review.
Description of the Sample of RCTs
Table 1 provides demographic and cancer-related data for the 10 trials from which patient data were included in the meta-analysis. Of the 10 trials, seven enrolled outpatients with mixed cancer types and stages. The time elapsed after cancer diagnosis ranged widely from 6 or fewer weeks to 7 years.
Table 1.
Patients and cancer characteristics from studies included in the meta-analysis
| Author, publication date (reference) | No. of individuals invited to participate | No. of participants randomly assigned | No. of participants postintervention* | Mean age†, y | No. of female participants (%)‡ | Education§ | No. of white participants (%)‡ | Cancer type | Stage | Inpatient or outpatient | Point in medical treatment|| | Mean time after diagnosis | Funding source(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Psychotherapeutic trials | |||||||||||||
| Dwight-Johnson et al., 2005 (72) | 81 | 55 | 30 | 47 | 55 (100.0) | 84% less than high school | 0 (0.0)¶ | Breast or cervical | Mixed | Outpatient | NR | ≥12 wk | National Cancer Institute (No. R21 CA93390-01); UCLA/National Institute of Mental Health Faculty Scholars Program (NIMH grant No. K-12-MH00990-01–01) |
| Ell et al., 2008 and 2011 (73,75) | 571 | 472 | 258 | 49% ≥ 50 | 399 (84.5) | 64% less than high school | 21 (4.5)# | Mixed | Mixed | Outpatient | Mixed | >12 wk | National Cancer Institute, Office of Cancer Survivorship (No. R01-CA105269) |
| Evans and Connis, 1995 (59) | 78 | 78 | 72 | 54 | 25 (34.7)** | Mean = 11 y | 43 (59.7)** | Mixed | Stage II | Outpatient | Active treatment | 12 wk | VA Health Services Research and Development Program (investigator initiated research IIR, No. 90.045) |
| Nezu et al., 2003 (63) | 150 | 150 | 132 | 47 | 89 (67.4)**,†† | Mean =15 y | 102 (77.2)**,†† | Mixed | Non-metastatic | NR | Active treatment | ≤6 mo | National Cancer Institute (No. R01-CA61313) |
| Savard et al., 2006 (61) | 59‡‡ | 45 | 37 | 51 | 45 (100.0) | 30% high school or less | 45 (100.0) | Breast | Metastatic | Outpatient | Mixed | 78 wk | Canadian Breast Cancer Research Initiative (No. 010436); Canadian Institutes of Health Research |
| Strong et al., 2008 (74) | 334 | 200 | 196 | 57 | 141 (70.5) | NR | NR | Mixed | Mixed | Outpatient | Mixed | 66 wk | Cancer Research UK |
| Pharmacologic trials | |||||||||||||
| Costa et al., 1985 (58) | NR | 73 | 51 | 52 | 73 (100.0) | NR | NR | Mixed | Mixed | 96% inpatient | Active treatment | NR | None declared |
| Fisch et al., 2003 (71) | NR | 163 | 83 | 60 | 81 (49.7)†† | NR | 146 (89.6)†† | Mixed | Advanced, incurable | Outpatient | Mixed | NR | Mary Margaret Walther Program for Cancer Care Research, Indianapolis, IN; Eli Lilly Company, Indianapolis, IN |
| Musselman et al., 2006 (62) | NR | 35 | 21 | 54 | 35 (100.0) | 23% high school or less | 29 (82.9) | Breast | Mixed | Outpatient | Mixed | Last cancer treatment within past 5 y | GlaxoSmithKline, King of Prussia, PA; Emory University Hospital General Clinical Research Center; National Institutes of Health (No. MO1-RR00039) |
| Razavi et al., 1996 (60) | 103 | 91 | 69 | 53 | 73 (80.2)†† | NR | NR | Mixed | Mixed | NR | NR | 6 wk to 7 y | Lilly France and Lilly Benelux |
* Immediate postintervention assessment point or assessment point closest in time to intervention completion. The following studies used data imputation (including last observation carried forward) or multilevel modeling, so the number of participants used in our meta-analysis corresponds to the number of participants at the baseline assessment: Costa et al. (58), Dwight-Johnson et al. (72), and Musselman et al. (62). NR = not reported.
† Other statistics related to age are reported in the table if the mean age was not available.
‡ Percentages are based on number of participants randomly assigned, with exceptions noted.
§ When the percentage is displayed, the remainder of the sample had higher education than the descriptor displayed.
|| Mixed point in medical treatment describes any combination of pretreatment, active treatment, or posttreatment.
¶ All participants were Latina.
# Data provided directly from authors of studies.
** Percentage based on number of participants postintervention.
†† Absolute numbers were extrapolated from reported percentages.
‡‡ Of the 59 individuals invited to participate, 45 were randomly assigned to treatment arms, seven declined to participate, and seven were not present for clinical interview.
Across the six psychotherapeutic trials, 1273 eligible adult cancer patients were invited to take part, of whom 1000 (78%) were randomly assigned to treatment arms and 725 (72.5%) completed the postintervention assessment (Table 1). Most participants were women (76%; not weighted by sample size) and on average were 51 years of age. The education level and racial/ethnic composition of the included patients varied widely.
Only one of the four pharmacologic trials reported the number of adults invited to participate (Table 1). A total of 362 participants were randomly assigned to treatment arms, and 224 (62%) completed the postintervention assessment. Participants were mostly women (83%) and had an average age of 55 years. These reports did not provide sufficient data on education level, and only two studies reported data on race/ethnicity (>80% of patients were white).
Table 2 displays recruitment and intervention characteristics. Five of the six psychotherapeutic trials used systematic screening, which included screening of consecutive potentially eligible patients attending clinic to identify participants. Four trials evaluated a form of problem-solving therapy (PST, three of these within a collaborative care or multimodal approach that included antidepressant medication as an option), and two trials administered CBT. All studies except one used an individual intervention delivery approach (group delivery in 59), which ranged from 4 to 10 sessions, with the exception that participants in the study by Ell et al. (73,75) had access to up to 1 year of collaborative care. Interventionists were trained psychologists or psychology graduate students, social workers, nurses, oncologists, or psychiatrists. Usual care, enhanced usual care (eg, usual care plus written educational or resource information), or a waiting list for treatment constituted the control conditions.
Table 2.
Recruitment and intervention characteristics of studies included in the meta-analysis
| Author, publication date (reference) | Recruitment method | Intervention type | Delivery format | Intervention length | Total time or daily dose per intervention protocol* | Type of therapist | Control intervention |
|---|---|---|---|---|---|---|---|
| Psychotherapeutic trials | |||||||
| Dwight-Johnson et al., 2005 (72) | Systematic screening | PST or medication in collaborative care | Individual | 8 sessions PST or 8 wk medication | NR | Social worker and oncologist/psychiatrist | Usual care |
| Ell et al., 2008; 2011 (73,75) | Systematic screening | PST and/or medication in collaborative care | Individual | Access up to 12 mo | NR | Social worker and psychiatrist | Enhanced usual care |
| Evans and Connis, 1995 (59) | Systematic screening | CBT, social support | Group | 8 sessions | 480 min | Social worker | Offered crisis consultation and individual therapy (only 2 participants elected a single crisis consultation) |
| Nezu et al., 2003 (63) | Nonsystematic screening | PST, PST with significant other | Individual | 10 sessions | 900 min | Psychology graduate students and social workers | Wait-list |
| Savard et al., 2006 (61) | Systematic screening | CBT | Individual | 8 sessions | 480–720 min | Psychologist | Wait-list |
| Strong et al., 2008 (74) | Systematic screening | Education, problem solving, and medication | Individual | 10 sessions | 450 min | Nurse | Usual care |
| Pharmacologic trials | |||||||
| Costa et al., 1985 (58) | NR | Mianserin | Individual | 4 wk | 60 mg | NR | Placebo pill |
| Fisch et al., 2003 (71) | Systematic screening | Fluoxetine | Individual, by mail delivery | 12 wk | 20 mg | NR | Placebo pill |
| Musselman et al., 2006 (62) | NR | Desipramine or paroxetine | Individual | 6 wk | 125–200 mg desipramine; 20–40 mg paroxetine | NR | Placebo pill |
| Razavi et al., 1996 (60) | NR | Fluoxetine | NR | 5 wk (first wk was placebo) | 20 mg | NR | Placebo pill |
* Medication dosage is daily goal dose after phase-in period; in some instances, goal dosages were modified according to therapeutic effect. CBT = cognitive behavioral therapy; NR = not reported; PST = problem-solving therapy.
The goal of the four pharmacologic trials was to evaluate the efficacy of various antidepressant medications against a pill placebo (Table 2). Only one trial reported that screening was systematic (71). Pharmacologic interventions ranged in duration from 4 to 12 weeks.
Table 3 summarizes how depression was defined and assessed. Studies most often employed validated questionnaire and/or interview assessments to assess both eligibility and outcomes. Postintervention assessments ranged from 8 weeks to 12 months after random assignment to treatment arms in psychotherapeutic trials and 28 days to 12 weeks in pharmacologic trials. Of the 10 trials, only four psychotherapeutic trials included a follow-up assessment, which ranged from 6 to 24 months. Baseline levels of depressive symptoms typically ranged from mild to moderate for psychotherapeutic and pharmacologic trials, depending on the outcome measure used.
Table 3.
Depression assessments and baseline depression severity
| Author, publication date (reference) | Screening criterion | Timing of assessments | Report type | Assessment instrument | Mean depression score at baseline assessment (SD) |
|---|---|---|---|---|---|
| Psychotherapeutic trials | |||||
| Dwight-Johnson et al., 2005 (72) | From PHQ-9/PRIME-MD, major depressive disorder, dysthymia, or persistent (baseline and 1 mo later) depressive symptoms | Baseline, 4 mo, 8 mo | Self-report | PHQ-9 | PST = 12.60 (7.00); control = 13.40 (7.20) |
| Ell et al., 2008; 2011 (73,75) | On PHQ-9, one of two cardinal symptoms of depression for more than half the days to nearly every day and score ≥10 on PHQ-9 and/or two DSM-IV questions for dysthymia | Baseline, 12 mo, 18 mo, 24 mo* | Self-report | PHQ-9 | PST = 13.17 (4.50); control = 12.79 (4.40) |
| Evans and Connis, 1995 (59) | ≥16 on CES-D | Baseline, 8 wk, 6 mo | Self-report | CES-D | CBT = 27.20 (8.80); social support = 27.90 (8.40); control = 29.00 (7.00) |
| Self-report | SCL-90-R Depression | CBT = 1.80 (0.50); social support = 1.40 (0.70); control = 1.30 (0.70) | |||
| Nezu et al., 2003 (63) | ≥14 on HAM-D and T-score ≥63 on Global Severity Index of Brief Symptom Inventory | Baseline, 10 wk† | Clinician-rated | HAM-D | PST = 20.40 (4.21); PST with significant other = 21.28 (3.66); control = 21.23 (3.33) |
| Savard et al., 2006 (61) | ≥7 on HADS or ≥15 on BDI | Baseline, 8 wk‡ | Self-report | HADS | CBT = 9.42 (2.43); control = 8.87 (2.60) |
| Self-report | BDI | CBT = 21.13 (5.41); control = 20.40 (5.76) | |||
| Clinician-rated | HAM-D | CBT = 14.21 (4.26); control = 14.40 (4.52) | |||
| Strong et al., 2008 (74) | ≥15 on HADS and SCID major depressive disorder of ≥1 mo and ≥1.75 on SCL-20 Depression | Baseline, 3 mo, 6 mo, 12 mo | Self-report | SCL-20 Depression | PST/education = 2.40 (0.45); control = 2.34 (0.45) |
| Pharmacologic trials | |||||
| Costa et al., 1985 (58) | 1) Depression diagnosis according to Stewart et al. (1965) and Kathol and Petty (1981) criteria; 2) depression succeeding or paralleling development of cancer; 3) ≥41 ZSRDS; and 4) ≥16 HAM-D (first 17 items) | Baseline, 28 days§ | Clinician-rated | CGI-S | Mianserin = 3.33 (1.19); control = 3.32 (1.09) |
| Clinician-rated | HAM-D | Mianserin = 20.60 (3.62); control = 20.80 (3.85) | |||
| Self-report | ZSRDS | Mianserin = 50.10 (6.31); control = 51.20 (6.56) | |||
| Fisch et al., 2003 (71) | ≥2 on Two-Question Screening Survey (author-developed to assess depressed mood and anhedonia) | Baseline, 12 wk|| | Self-report | Brief ZSRDS | Fluoxetine = 24.44 (6.56); control = 23.09 (5.91) |
| Musselman et al., 2006 (62) | Clinical diagnosis of major depressive disorder for ≥1 mo assessed by DSM-III-R criteria and ≥14 HAM-D (1st 17 items) | Baseline, 6 wk | Clinician-rated | HAM-D¶ | Desipramine = 23.00 (6.16); paroxetine = 21.00 (5.66); control = 23.91 (4.99) |
| Razavi et al., 1996 (60) | HADS ≥13 before and after 1 wk on placebo; DSM-III-R major depressive disorder or adjustment disorder with depressed mood or mixed features | Baseline, 5 wk | Self-report | HADS | Fluoxetine = 22.70 (6.00); control = 23.50 (5.50) |
| Clinician-rated | MADRS | Fluoxetine = 26.10 (7.10); control = 25.20 (7.70) | |||
| Self-report | SCL-90-R Depression | Fluoxetine = 1.60 (0.70); control = 1.60 (0.80) | |||
* Assessments were also conducted at 6 months, which was before completion of the intervention. BDI = Beck Depression Inventory; CBT = cognitive behavioral therapy; CES-D = Center for Epidemiologic Studies Depression Scale; CGI-S = Clinical Global Impression-Severity; DSM = Diagnostic and Statistical Manual; HADS = Hospital Anxiety and Depression Scale; HAM-D = Hamilton Rating Scale for Depression (also abbreviated as HRSD); MADRS = Montgomery-Asberg Depression Rating Scale; PHQ-9 = Patient Health Questionnaire; PRIME-MD = Primary Care Evaluation of Mental Disorders; PST = problem-solving therapy; SCID = Structured Clinical Interview for DSM Disorders; SCL = Symptom Checklist; ZSRDS = Zung Self-Rating Depression Scale.
† Assessments were also conducted at 6 and 12 months, but participants in the waitlist control were no longer assessed.
‡ Assessments were also conducted at 20 and 32 weeks, but participants in the treatment and wait-list groups were combined for analysis.
§ Assessments were also conducted at days 7, 14, and 21 after study enrollment, which was before completion of the intervention.
|| Assessments were also conducted at three other posttreatment points before 12 weeks.
¶ The CGI-S was also used to assess depression in this trial; however, data were not available from the author.
Table 4 displays coding of the PEDro criteria, including quality of randomization procedures, methods of blinding, reporting of data, data analysis, treatment fidelity, and adequacy of follow-up. Trials met 9–12 of the 14 quality criteria. One trial (63) did not meet the allocation concealment criterion. Two trials (62,71) did not have groups similar at baseline on prognostic indicators, such as cancer stage. As is standard in these trials, no psychotherapeutic trial blinded treatment allocation for subjects or therapists. Two trials did not report blinding treatment allocation for assessors (59,60). Three studies had measures of key outcomes on more than 85% of participants (59,63,74). Five trials did not report having a treatment manual (58–60,62,71). Two trials did not report monitoring of treatment implementation (59,60).
Table 4.
Summary of quality of the studies included in the meta-analysis
| Modified Physiotherapy Evidence Database (PEDro) criterion | Author, publication date (reference)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Psychotherapeutic trials | Pharmacologic trials | |||||||||
| Dwight-Johnson et al., 2005 (72) | Ell et al., 2008; 2011 (73,75) | Evans & Connis, 1995 (59) | Nezu et al., 2003 (63) | Savard et al., 2006 (61) | Strong et al., 2008 (74) | Costa et al., 1985 (58) | Fisch et al., 2003 (71) | Musselman et al., 2006 (62) | Razavi et al., 1996 (60) | |
| Eligibility criteria were specified | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Subjects were randomly assigned to treatment groups | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Allocation was concealed | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| The groups were similar at baseline regarding most important prognostic indicators | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No† | No | Yes |
| All participants were blinded to treatment | No | No | No | No | No | No | Yes | Yes | Yes | Yes |
| There was blinding of all therapists who administered the therapy‡ | No | No | No | No | No | No | Yes | Yes | Yes | Yes |
| All assessors who measured at least one key outcome were blinded to treatment information | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to treatment groups | No | No | Yes | Yes | No | Yes | No | No | No | No |
| All participants for whom outcome measures were available received the treatment or control intervention as allocated or, when this was not done, data for at least one key outcome was analyzed by “intention to treat” (including imputation) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The results of between-group statistical comparisons were reported for at least one key outcome | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The study provided both point measures and measures of variability for at least one key outcome§ | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The study had an adequate treatment fidelity protocol, including manualized treatment|| | Yes | Yes | No | Yes | Yes | Yes | No | No | No | No |
| The study had an adequate treatment fidelity protocol, including monitoring of treatment implementation | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Loss to follow-up information was provided | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total no. of above criteria met | 11 | 11 | 9 | 11 | 11 | 12 | 12 | 11 | 11 | 10 |
* “Yes” indicates that the criterion was evidenced in the article, whereas “No” indicates that the criterion is not evidenced, not applicable, not coded, or could not be determined in the article.
† Baseline group differences were controlled statistically.
‡ This criterion typically is not applicable to psychotherapeutic and other behavioral interventions.
§ Data were provided in the article or obtained directly from the authors.
|| Data were not coded for pharmacologic interventions.
Effect of Intervention Compared With Control Conditions
All 10 studies (with 13 comparisons of active treatment against the control condition after taking into account two intervention groups in three trials) reported posttest intervention effects against a control group. As displayed in Table 5, the random effects model showed that intervention conditions produced statistically significant reductions in depressive symptoms compared with control conditions [Hedges’ g = 1.01, 95% CI = 0.48 to 1.54, P < .001]. Heterogeneity was very high (I 2 = 93.4%, 95% CI = 90.0 to 95.0; Q = 181.1, degrees of freedom [df] = 12, P < .001). Examination of the effect sizes for outliers indicated that the two comparisons by Nezu et al. (63) produced very large effects for PST (Hedges’ g = 3.57, 95% CI = 2.90 to 4.23, P < .001) and PST with involvement of a significant other (Hedges’ g = 4.32, 95% CI = 3.55 to 5.08, P < .001), both of which were dramatically larger than those of other studies (Table 5). A common recommendation for handling outliers is to remove them from the effect size distribution (66); eliminating that trial changed the overall effect size considerably, and the random effects model revealed a smaller effect. This model indicated that active treatments produced statistically significant reductions in depressive symptoms compared with control conditions; heterogeneity was not statistically significant (Hedges’ g = 0.43, 95% CI = 0.30 to 0.56, P < 0.001; I 2 = 0%, 95% CI = 0.0 to 60.0; Q = 8.67, df = 10, P = .56) (Table 5). Consequently, that trial was removed from further analyses. Figure 2 displays the forest plot for the remaining nine trials. As noted earlier, four studies used more than one outcome measure, and two studies had more than one intervention group (one of which also reported multiple outcomes). Table 5 shows that using only the lowest reported effect size for each of these studies did not substantially influence the statistically significant effect on depressive symptoms (Hedges’ g = 0.36, 95% CI = 0.22 to 0.49, P < .001), as was true when the analysis was limited to the highest reported effect size (Hedges’ g = 0.50, 95% CI = 0.31 to 0.70, P < .001).
Table 5.
. Effects of intervention compared with control conditions and subgroup meta-analyses
| Study or subgroup | Hedges’ g* (95% CI) | P |
|---|---|---|
| Effect of intervention vs control conditions | ||
| All included trials (58–63,71–75)† | 1.01 (0.48 to 1.54) | <.001 |
| Included trials (58–62,71–75)‡; outlier trial (63) removed§ | 0.43 (0.30 to 0.56) | <.001 |
| Included trials (58–62,71–75) using lowest reported effect size | 0.36 (0.22 to 0.49) | <.001 |
| Included trials (58–62,71–75) using highest reported effect size | 0.50 (0.31 to 0.70) | <.001 |
| Effect of intervention compared with control conditions postintervention and at follow-up assessments | ||
| Included trials with 6–8 mos postrandomization follow-up assessments (59,72,74) | ||
| Postintervention | 0.54 (0.27 to 0.82) | <.001 |
| Follow-up | 0.56 (0.34 to 0.78) | <.001 |
| Included trials with 12–18 mos postrandomization follow-up assessments (73–75) | ||
| Postintervention | 0.34 (0.16 to 0.53) | <.001 |
| Follow-up | 0.37 (0.18 to 0.55) | <.001 |
| Included trial with 24 mos postrandomization follow-up assessments (75) | ||
| Postintervention | 0.26 (0.02 to 0.51) | .040 |
| Follow-up | 0.19 (-0.08 to 0.46) | .173 |
| Subgroup analyses | ||
| Effect of CBT vs PST vs pharmacologic interventions | ||
| CBT(59,61) | 0.83 (0.48 to 1.18) | <.001 |
| PST (72–75) | 0.33 (0.15 to 0.50) | <.001 |
| Pharmacologic (58,60,62,71) | 0.44 (0.19 to 0.68) | <.001 |
| Effect of intervention in psychotherapeutic trials meeting PEDro criterion: Whether at least 85% of study participants had postintervention data available for analysis | ||
| Criterion met (59,74) | 0.58 (0.34 to 0.81) | <.001 |
| Criterion not met (61,72,73) | 0.31 (0.10 to 0.52) | .004 |
* Hedges’ g was calculated on the basis of the standardized mean difference effect size which uses the pooled within-groups SD. Subgroup analyses used a mixed effects model, which pools studies within subgroups with a random effects model, but tested for statistically significant between-groups differences with a fixed effects model. All P values are two-tailed. CBT = cognitive behavioral therapy; PST = problem-solving therapy.
† Heterogeneity: I 2 = 93.4%, 95% CI = 90.0 to 95.0; Q = 181.1, degrees of freedom (df) = 12, P < .001.
‡ Heterogeneity: I 2 = 0%, 95% CI = 0.0 to 60.0; Q = 8.67, df = 10, P = .56.
§ Comparisons from Nezu et al. (63) included examination of effects of PST (Hedges’ g = 3.57, 95% CI = 2.90 to 4.23, P < .001) and PST with involvement of a significant other (Hedges’ g = 4.32, 95% CI = 3.55 to 5.08, P < .001).
Figure 2.
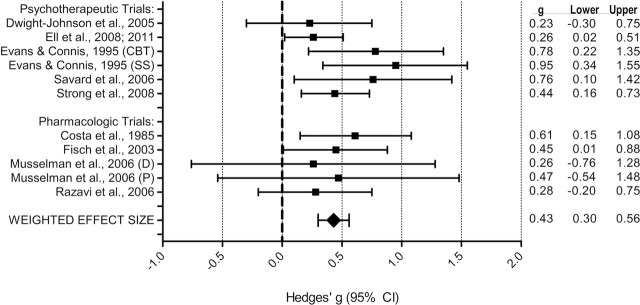
. Forest plot of effect sizes (Hedges’ g, designated g in the figure) for trials included in the meta-analysis (58–62,72–75). The corresponding 95% CI (designated “Lower” and “Upper” and indicated graphically by whisker bars) are also given. Effect sizes for the trials containing two intervention groups are displayed separately (59,62). CBT = cognitive behavioral therapy; CI = confidence interval; D = desipramine; P = paroxetine; SS = social support.
Postintervention Compared With Follow-up Assessments
Four trials (all psychotherapeutic) provided information on duration of the intervention effects (59,72–75). Three trials (59,72,74), provided follow-up data 6–8 months postrandomization, and Strong et al. (74) also included 12-month follow-up. The fourth trial (73,75) had follow-up at 18 and 24 months after random assignment to treatment arms. Because two trials had more than one follow-up and Ell et al. (73,75) conducted a longer follow-up, we performed analyses in three ways (Table 5).
First, the three trials with follow-up at 6–8 months (59,72,74) produced statistically significantly greater reductions in depressive symptoms than did the control conditions postintervention (Hedges’ g = 0.54, 95% CI = 0.27 to 0.82, P < .001) and at 6–8 months (Hedges’ g = 0.56, 95% CI = 0.34 to 0.78, P < .001). Second, the two trials that provided data at 12–18 months (73–75) demonstrated statistically significantly greater reductions in depressive symptoms favoring intervention vs control conditions (Hedges’ g = 0.34, 95% CI = 0.16 to 0.53, P < .001 at postintervention; Hedges’ g = 0.37, 95% CI = 0.18 to 0.55, P < .001 at 12–18 months). For the trial that had 24-month follow-up (75), the intervention was effective postintervention (Hedges’ g = 0.26, 95% CI = 0.02 to 0.51, P = .04), but not at 24 months after random assignment (Hedges’ g = 0.19, 95% CI = 20.08 to 0.46, P = .17).
Subgroup Analyses
Effects of CBT vs PST vs pharmacologic interventions were assessed. For the psychotherapeutic studies, we created subgroups on the basis of the theoretical approach employed: CBT (59,61) vs PST (delivered within a collaborative care model or a multimodal intervention approach) (72–75). We then performed a subgroup comparison for CBT vs PST vs pharmacologic interventions (Table 5). CBT, PST, and pharmacologic interventions were more effective than control conditions in reducing depressive symptoms, as indicated by statistically significant effect sizes for each comparison (Hedges’ g = 0.83, 95% CI = 0.48 to 1.18, P < .001; Hedges’ g = 0.33, 95% CI = 0.15 to 0.50, P <.001; Hedges’ g = 0.44, 95% CI = 0.19 to 0.68, P < .001, respectively). The difference among the three groups was statistically significant (P = .04). Post hoc analyses revealed a statistically significant difference between CBT and PST (P = .01), but the difference was not statistically significant between CBT and pharmacologic interventions (P = .07) or between PST and pharmacologic interventions (P = .48). The difference between CBT vs PST remained statistically significant after a Bonferroni correction for multiple tests (P = .02).
The only PEDro criterion that had sufficient variability for analysis was whether at least 85% of study participants had postintervention outcome data available for analysis (Table 4). Of the psychotherapeutic studies, two met this criterion (59,74) and demonstrated a statistically significant reduction in depressive symptoms for active compared with control conditions (Hedges’ g = 0.58, 95% CI = 0.34 to 0.81, P < .001) (Table 5). The three studies that did not meet this criterion (61,72,73) also produced a statistically significant improvement in depressive symptoms (Hedges’ g = 0.31, 95% CI = 0.10 to 0.52, P = .004) (Table 5). The two effect sizes were similar (P = .10). Note that none of the pharmacologic trials met this criterion.
None of the continuous variables examined through metaregression were statistically significantly associated with the observed overall effect size. Specifically, age (P = .91), percentage of female participants (P = .13), the number of psychotherapeutic sessions (P = .98), and the number of weeks on medication (P = .93) were not statistically significantly associated with the effect of active treatment on depressive symptoms as determined by the method of moments estimator (Supplementary Table 1, available online).
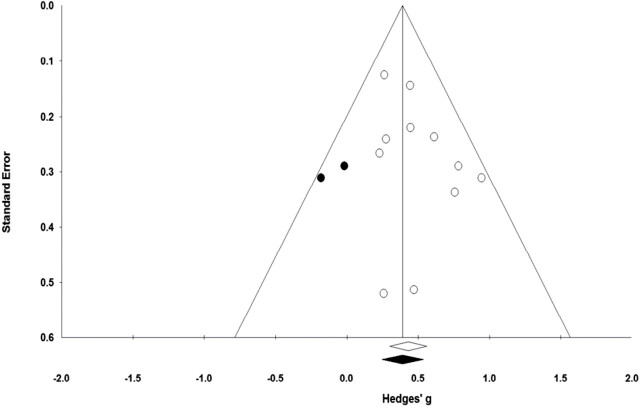
Publication Bias
The funnel plot (Figure 3) shows a generally even distribution of effect sizes by standard errors, but the right side of the plot was populated by an additional two studies, compared with the left side. The slight skewness on the right side was confirmed by the fact that Duval and Tweedie’s (69) trim and fill procedure imputed two studies on the left side of the plot, which indicates slight publication bias. As shown in Figure 3, the two studies that were imputed using the procedures described in the statistical analysis section slightly reduced the overall effect size (Hedges’ g = 0.39). However, the 95% CI of 0.24 to 0.54 for this effect size did not include 0. Egger’s test of the intercept was not statistically significant (P = .16) for bias in the funnel plot. The fail-safe N (the number of unpublished studies reporting statistically nonsignificant results needed to reduce the observed effect to statistical nonsignificance) of 106 confirms the relative stability of the observed effect size.
Figure 3.
. Funnel plot of standard error by Hedges’ g for trials included in the meta-analysis. The open circles represent observed studies and the filled circles represent imputed studies. The open diamond represents the observed effect size. The closed diamond represents the imputed effect size from Duval and Tweedie’s (69) trim and fill procedure. Egger’s test of the intercept indicated bias in the funnel plots was not statistically significant (P = .16).
Discussion
This meta-analysis of RCTs reveals that the set of five psychotherapeutic (after removal of one outlier) and four pharmacologic interventions were reliably superior in reducing depressive symptoms relative to control conditions for adults diagnosed with cancer who met an eligibility threshold for elevated depressive symptoms. The statistically significant effect size across trials was moderate and indicated that post-treatment, depressive symptoms were slightly less than a half SD lower for adults in intervention groups compared with those who were assigned to control treatment. In the four psychotherapeutic trials that included a longer-term follow-up (ie, 6–24 months postrandomization; note that the interventions were of variable length), the follow-up effect sizes remained statistically significant up to 12–18 months after randomization (P < .001), indicating sustained effects.
The effect size obtained in this meta-analysis is somewhat lower than that found in a meta-analysis of interventions for adults who met an entry criterion for depression but were not selected for diagnosis of a medical disorder (80) and is comparable to that of a meta-analysis (81) of 15 RCTs of psychological interventions in patients who had elevated depressive symptoms and had been diagnosed with one of 10 different medical disorders, including four oncology samples (d = .42 after two outliers with very large effect sizes were removed). Previous meta-analyses (23,27,29) have documented the efficacy of psychotherapeutic interventions for cancer patients unselected at study entry for depression, particularly for individuals with relatively high baseline depressive symptoms (31,32,82). For example, Schneider et al. (31) conducted a meta-analysis of 27 psychosocial interventions (12 RCTs; 15 single-group designs) for cancer patients to examine whether preintervention depressive symptoms, as assessed by the Hospital Anxiety and Depression Scale (83), moderated intervention efficacy. Effects on HADS depressive symptoms were negligible when baseline depressive symptoms were low and pronounced when symptoms were relatively high. This finding did not vary by study design or intervention type, setting, or dose, and it held both immediately after treatment and 2–7 months later, although it weakened at longer follow-up. Baseline depressive symptoms explained 51.5% of the variance in treatment efficacy on depressive symptoms. Randomized and single-group interventions that specifically selected participants for elevated distress produced larger effect sizes than did nonselective interventions, but this factor was not a unique moderator of outcomes after statistical control for baseline depressive symptoms.
Our findings advance this literature by demonstrating that psychological and pharmacologic approaches, evaluated in RCTs, can be targeted productively toward cancer patients in need of intervention by virtue of clinical depression or elevated depressive symptoms. Whether tailoring or targeting of interventions to be responsive to specific characteristics of depression in the context of cancer, such as depression history or perceived cancer-related losses, produces more potent effects requires study.
In the current meta-analysis, heterogeneity of effect sizes was not marked across trials, and subgroup analyses suggest that effects did not vary systematically as a function of trial attrition rate, participant age and sex, or intervention length. However, a comparison of CBT, PST, and pharmacologic approaches revealed that CBT produced statistically significantly larger effects than did PST and a somewhat but statistically nonsignificantly larger effect than pharmacologic interventions; PST and pharmacologic interventions were similar. It is important to note that these comparisons are not equivalent to comparing the different interventions directly within an RCT. Thus, the finding of the superiority of CBT must be interpreted cautiously and within the context of the specific trials (Tables 1–4). In addition to their distinct intervention content, the CBT trials also differ from the other approaches in that the CBT trials on average included somewhat fewer participants, arguably employed less stringent eligibility criteria for depressive symptoms, and had less elapsed time from baseline to postintervention assessment compared with PST (but not with pharmacologic trials). In addition, CBT was the sole therapeutic approach of those trials, whereas PST was administered within a collaborative care (72,73,75) or multimodal approach (74), which included a pharmacologic option and did not invariably include PST (72,73,75). Furthermore, although interventionists received systematic training in PST, they did not necessarily have a background in delivering psychological treatments (74), whereas interventionists in the CBT trials were experienced in that modality. These last two points also suggest the importance of continued research on the effectiveness of psychosocial interventions delivered within a specialized psycho-oncology context vs an approach imported from integrated primary care (84–86).
It is also important to note that the outlier removed from analysis was a PST trial that yielded very large effect sizes (63). We could not definitively identify why this trial yielded extremely large effects. However, it differed from the other RCTs in that it was conducted by founders of the treatment approach, provided intensive training of therapists on the manualized protocol, involved weekly supervision provided by the founders, used recruitment of interested patients rather than systematic screening of a clinic or other population, and required as an eligibility criterion that a significant other be willing to participate in the intervention, all of which may have contributed to the outlying large effect sizes. The advantage of CBT compared with other approaches to treat depression in the general population has been suggested in other reviews (87,88); however, randomized trials are required to test its advantage over other treatments in depressed cancer patients. Although head-to-head comparisons of intervention approaches are accruing, none of the 10 trials identified in our literature search compared the same two interventions (42–45,47–49,59,62,63). In the most relevant trial (47), a form of PST was compared with behavioral activation treatment in breast cancer patients with major depression. Both interventions were effective in decreasing depressive symptoms, with gains maintained or augmented throughout 12 months.
Several limitations of this meta-analysis and the current literature are apparent. First, three trials could not be included in the analysis because of incomplete data, and it is possible that some trials were missed, even in this comprehensive search. The finding that more than 100 trials—each demonstrating no statistically significant reduction in depression for the intervention (vs control condition)—would be needed to nullify the statistically significant effect of intervention, however, suggests the effect’s stability. Nonetheless, the observation that only ten trials were available for analysis highlights the paucity of interventions targeted to cancer patients with elevated depressive symptoms and points to the importance of conducting additional RCTs. Second, differences among trials were pronounced on some features, including sample size, participants’ education level, gender, race/ethnicity, cancer stage, and time elapsed after cancer diagnosis, interventionist type and expertise, intervention length, and entry criteria for and operationalization of depression. Although statistically significant heterogeneity in effect sizes was not evident once the one outlier was removed, a larger pool of trials might yield important moderators of intervention effects. Third, relatively few intervention approaches were represented in this review; approaches receiving current attention in cancer populations, such as physical activity interventions, require study for their impact on depression. Finally, generalizability of the findings to other groups (eg, adolescents/young adults) awaits additional research.
Limitations in the individual trials also suggest that additional research is essential. Specifically, five trials relied on questionnaire assessment of depressive symptoms rather than interview-based assessment for depressive disorder, medication protocols were no longer than 12 weeks in duration, attrition from the point of random assignment to the postintervention assessment exceeded 33% in four of 10 trials (62,71–73), and six trials did not include follow-up assessment beyond the postintervention point.
The current meta-analysis revealed reliable positive effects of psychotherapeutic and pharmacologic interventions, with some advantage for CBT, for adults diagnosed with cancer who had comorbid elevated depressive symptoms or a depression diagnosis. These data are consonant with findings from the literature on depression in the general adult population showing that bona-fide psychotherapies and second-generation antidepressants are effective at reducing depression in the short term (89). Our findings inform two crucial clinical questions, the first of which regards the advisability of screening for depression in cancer patients and subsequent provision of interventions specifically targeted toward individuals with elevated symptoms. A recent meta-analysis (31) suggests that preintervention assessment of distress may be an efficient way to identify those who are most likely to benefit from therapeutic intervention. Further, the National Comprehensive Cancer Network guidelines (90) and an Institute of Medicine report (91) emphasize the importance of screening cancer patients for distress. Reviews indicate that screening for depression has not yet become a standard part of oncology care (92,93), however, and controversy exists regarding the utility of psychosocial screening in oncology settings (94–97). Our findings demonstrate that when assessment is performed with psychometrically sound questionnaires or interviews and intervention is targeted toward cancer patients meeting criteria for elevated depressive symptoms, reliable improvement in depression occurs, at least within a research context. The available evidence suggests that patients who demonstrate elevated symptoms of depression should be referred for treatment. However, two caveats are important. First, because screening itself was not randomized in these trials, we can conclude that only the combination of depression assessment and intervention (not screening by itself) is effective. Indeed, authors of a recent review reported that no RCTs have examined whether screening for depression in cancer patients improves depression outcomes and outweighs the potential harms of such screening (98). Second, dissemination research on the effectiveness of interventions for depressed cancer patients in clinical settings is lacking.
In light of the efficacy of existing interventions demonstrated in this meta-analysis, a second question is whether additional interventions require development and investigation for individuals with cancer who are depressed. Burgeoning awareness of the importance of depression in individuals with cancer is evidenced by on-going trials of interventions targeted at this group (99,100). We would argue that such intervention development and evaluation in RCTs remains essential for several reasons. First, conclusions regarding intervention efficacy are limited by the small number of existing trials and their lack of efficacy assessment beyond 3 months for pharmacologic trials and 24 months after randomization for psychotherapeutic trials. Second, examination of important moderators of efficacy was limited by the small number of available trials. For example, insufficient data were available to examine whether interventions are differentially effective for depressed individuals with advanced cancers vs early-stage disease. A particularly interesting question is whether interventions require tailoring to individuals who have a premorbid history of depression (101) and to individuals who develop depression at particular points in the cancer trajectory (eg, at diagnosis and medical treatment, after treatment completion, at cancer recurrence; 102,103). Third, the obtained moderate effect size leaves room for improvement in the power of interventions to ameliorate depression. Finally, interventions that are maximally accessible to diverse groups require study. Most interventions in the present review were delivered in person in individual sessions, which requires substantial resources on the part of both recipients and professionals. In light of the psychological, behavioral, and economic toll of depression, as well as its health burden on adults with cancer, intensive attention to the development and dissemination of maximally effective, efficient, and accessible interventions for heterogeneous cancer patients is warranted.
Footnotes
Funding
This work was supported in part by grants from the National Cancer Institute of the National Institutes of Health (grant number 1R01CA133081 to ALS and K Weihs, 5K07CA118576-02 to JLS, and 5K07CA134936-03 to LLC).
Notes
The study sponsor (National Cancer Institute) played no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the systematic review and meta-analysis; or the decision to submit the systematic review and meta-analysis for publication. The authors wish to acknowledge the Society of Behavioral Medicine and its Evidence-Based Behavioral Medicine Committee, which organized the authorship group, including committee member Dr. Joost Dekker (VU University Medical Center, Amsterdam, The Netherlands), who offered suggestions regarding the conduct of the review. Society of Behavioral Medicine, however, has not commissioned, supervised, sanctioned, approved, overseen, reviewed, or exercised editorial control over the publication’s content. Accordingly, any views of the authors set forth herein are solely those of the authors and not of the Society of Behavioral Medicine. The following individuals offered suggestions regarding conceptualization and analysis: Drs Suzanne Miller (Fox Chase Cancer Center, Philadelphia, PA), Paul Jacobsen (Moffitt Cancer Center, Tampa, FL), and Karen Mustian (University of Rochester Medical Center, Rochester, NY). Dr Jennifer Duffecy (Northwestern University, Chicago, IL) maintained and provided technical support for the electronic database. Mr Tenbroeck Smith of the American Cancer Society supported a web site for sharing of articles. Several authors of the articles reviewed herein provided data and responded to questions regarding their trials. The authors express their gratitude to these organizations and individuals.
Affiliations of authors: Department of Psychology, Ryerson University, Toronto, ON (SLH); Department of Psychology, Hunter College, City University of New York, New York, NY (MAH); Departments of Urology and Oncological Sciences, Mount Sinai School of Medicine, New York, NY (MD); Department of Psychology, The Ohio State University, Columbus, OH (DRA); Department of Psychology, University of Colorado Denver, Denver, CO (KMK); Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL (LLC, DCM, BS); Departments of Surgery and Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA (JLS); Department of Clinical Psychology and EMGO Institute of Health and Care Research, VU University, Amsterdam, the Netherlands (PC); Galter Health Sciences Library, Northwestern University, Chicago, IL (MB); Departments of Psychology and Psychiatry/Biobehavioral Sciences and Division of Cancer Prevention and Control Research, University of California Los Angeles, Los Angeles, CA (ALS).
References
- 1. Moyer A, Sohl SJ, Knapp-Oliver SK, et al. Characteristics and methodological quality of 25 years of research investigating psychosocial interventions for cancer patients Cancer Treat Rev 2009;35 5):475 484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polsky D, Doshi JA, Marcus S, et al. Long-term risk for depressive symptoms after a medical diagnosis Arch Intern Med 2005;165 11):1260 1266 [DOI] [PubMed] [Google Scholar]
- 3. Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies Lancet Oncol 2011;12 2):160 174 [DOI] [PubMed] [Google Scholar]
- 4. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence Arch Intern Med 2000;160 14):2101 2107 [DOI] [PubMed] [Google Scholar]
- 5. Himelhoch S, Weller WE, Wu AW, et al. Chronic medical illness, depression, and use of acute medical services among Medicare beneficiaries Med Care 2004;42 6):512 521 [DOI] [PubMed] [Google Scholar]
- 6. Steiner J, Cavender T, Nowels C, et al. The impact of physical and psychosocial factors on work characteristics after cancer Psycho-oncology 2008;17 2):138 147 [DOI] [PubMed] [Google Scholar]
- 7. Miller M, Mogun H, Azrael D, et al. Cancer and the risk of suicide in older Americans J Clin Oncol 2008;26 29): 4720 4724 [DOI] [PubMed] [Google Scholar]
- 8. Misono S, Weiss NS, Fann JR, et al. Incidence of suicide in persons with cancer J Clin Oncol 2008;26 29):4731 4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walker J, Waters RA, Murray G, et al. Better off dead: suicidal thoughts in cancer patients J Clin Oncol 2008;26 29):4725 4730 [DOI] [PubMed] [Google Scholar]
- 10. Fang F, Fall K, Mittleman MA, et al. Suicide and cardiovascular death after a cancer diagnosis N Engl J Med 2012;366 14):1310 1318 [DOI] [PubMed] [Google Scholar]
- 11. Brown KW, Levy AR, Rosberger Z, et al. Psychological distress and cancer survival: a follow-up 10 years after diagnosis Psychosom Med 2003;65 4):636 643 [DOI] [PubMed] [Google Scholar]
- 12. Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer J Am Geriatr Soc 2004;52 1):106 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mykletun A, Bjerkeset O, Dewey M, et al. Anxiety, depression, and cause-specific mortality: the HUNT study Psychosom Med 2007;69 4):323 331 [DOI] [PubMed] [Google Scholar]
- 14. Onitilo AA, Nietert PJ, Egede LE. Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site Gen Hosp Psychiatry 2006;28 5):396 402 [DOI] [PubMed] [Google Scholar]
- 15. Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis Psychol Med 2010;40 11):1797 1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Satin JR, Linden W, Phillips MF. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis Cancer 2009;115 22):5349 5361 [DOI] [PubMed] [Google Scholar]
- 17. Steel JL, Geller DA, Gamblin C, et al. Depression, immunity, and survival in patients with hepatobiliary carcinoma J Clin Oncol 2007;25 17):2397 2405 [DOI] [PubMed] [Google Scholar]
- 18. Akechi T, Okuyama T, Onishi J, et al. Psychotherapy for depression among incurable cancer patients (Review) Cochrane Database Syst Rev 2008;16 2):Art. No.CD0055372008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andersen BL. Biobehavioral outcomes following psychological interventions for cancer patients J Consult Clin Psychol 2002;70 3):590 610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andrykowski MA, Manne SL. Are psychological interventions effective and accepted by cancer patients? I. Standards and levels of evidence Ann Behav Med 2006;32 2):93 97 [DOI] [PubMed] [Google Scholar]
- 21. Barsevick AM, Sweeney C, Haney E, et al. A systematic qualitative analysis of psychoeducational interventions for depression in patients with cancer Oncol Nurs Forum 2002;29 1):73 84 [DOI] [PubMed] [Google Scholar]
- 22. Coyne JC, Lepore SJ, Palmer SC. Efficacy of psychosocial interventions in cancer care: evidence is weaker than it first looks Ann Behav Med 2006;32 2):104 110 [DOI] [PubMed] [Google Scholar]
- 23. Devine EC, Westlake SK. The effects of psychoeducational care provided to adults with cancer: meta-analysis of 116 studies Oncol Nurs Forum 1995;22 9):1369 1381 [PubMed] [Google Scholar]
- 24. Jacobsen PB, Donovan KA, Swaine ZN, et al. Management of anxiety and depression in adult cancer patients: toward an evidence-based approach. In: Chang AE, Ganz PA, Hayes DF. et al. , eds. Oncology: An Evidence-Based Approach Philadelphia, PA: Springer; 2006:1552 1579 [Google Scholar]
- 25. Meyer TJ, Mark MM. Effects of psychosocial interventions with adult cancer patients: a meta-analysis of randomized experiments Health Psychol 1995;14 2):101 108 [DOI] [PubMed] [Google Scholar]
- 26. Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: overview and recommendations for future research J Natl Cancer Inst 2002;94 8):558 584 [DOI] [PubMed] [Google Scholar]
- 27. Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses Int J Psychiatry Med 2006;36 1):3 34 [DOI] [PubMed] [Google Scholar]
- 28. Rehse B, Pukrop R. Effects of psychosocial interventions on quality of life in adult cancer patients: meta-analysis of 37 published controlled outcome studies Patient Educ Couns 2003;50 2):179 186 [DOI] [PubMed] [Google Scholar]
- 29. Sheard T, Maguire P. The effect of psychological interventions on anxiety and depression in cancer patients: results of two meta-analyses Br J Cancer 1999;80 11):1770 1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams S, Dale J. The effectiveness of treatment for depression/depressive symptoms in adults with cancer: a systematic review Br J Cancer 2006;94 3):372 390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider S, Moyer A, Knapp-Oliver S, et al. Pre-intervention distress moderates the efficacy of psychosocial treatment for cancer patients: a meta-analysis J Behav Med 2010;33 1):1 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heron-Speirs HA, Harvey ST, Baken DM. Moderators of psycho-oncology therapy effectiveness: addressing design variable confounds in meta-analysis Clin Psychol Sci Pract 2012;19 1):49 71 [Google Scholar]
- 33. Rodin G, Lloyd N, Katz M, et al. The treatment of depression in cancer patients: a systematic review Support Care Cancer 2007;15 2):123 136 [DOI] [PubMed] [Google Scholar]
- 34. McQuellon RP, Wells M, Hoffman S, et al. Reducing distress in cancer patients with an orientation program Psycho-oncology 1998;7 3):207 217 [DOI] [PubMed] [Google Scholar]
- 35. Kissane DW, Bloch S, Smith GC, et al. Cognitive-existential group psychotherapy for women with primary breast cancer: a randomized controlled trial Psycho-oncology 2003;12 6):532 546 [DOI] [PubMed] [Google Scholar]
- 36. Sharpe M, Strong V, Allen K, et al. Management of major depression in outpatients attending a cancer centre: a preliminary evaluation of a multicomponent cancer nurse-delivered intervention Br J Cancer 2004;90 2):310 313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duffy SA, Ronis DL, Valenstein M, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients Cancer Epidemiol Biomarkers Prev 2006;15 11):2203 2208 [DOI] [PubMed] [Google Scholar]
- 38. Wilkinson SM, Love SB, Westcombe AM, et al. Effectiveness of aromatherapy massage in the management of anxiety and depression in patients with cancer: a multicenter randomized controlled trial J Clin Oncol 2007;25 5):532 539 [DOI] [PubMed] [Google Scholar]
- 39. Goerling U, Foerg A, Sander S, et al. The impact of short-term psycho-oncological interventions on the psychological outcome of cancer patients of a surgical-oncology department—a randomized controlled study Eur J Cancer 2011;47 13):2009 2014 [DOI] [PubMed] [Google Scholar]
- 40. Stockler MR, O'Connell R, Nowak AK, et al. Effect of sertraline on symptoms and survival in patients with advanced cancer, but without major depression: a placebo-controlled double-blind randomised trial Lancet Oncol 2007;8 7):603 612 [DOI] [PubMed] [Google Scholar]
- 41. Kroenke K, Theobald D, Wu J, et al. Effect of telecare management on pain and depression in patients with cancer: a randomized trial JAMA 2010;304 2):163 171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holland JC, Morrow GR, Schmale A, et al. A randomized clinical trial of alprazolam versus progressive muscle relaxation in cancer patients with anxiety and depressive symptoms J Clin Oncol 1991;9 6):1004 1011 [DOI] [PubMed] [Google Scholar]
- 43. Holland JC, Romano SJ, Heiligenstein JH, et al. A controlled trial of fluoxetine and desipramine in depressed women with advanced cancer Psycho-oncology 1998;7 4):291 300 [DOI] [PubMed] [Google Scholar]
- 44. Moorey S, Greer S, Bliss J, et al. A comparison of adjuvant psychological therapy and supportive counseling in patients with cancer Psycho-oncology 1998;7 3):218 228 [DOI] [PubMed] [Google Scholar]
- 45. Pezzella G, Moslinger-Gehmayr R, Contu A. Treatment of depression in patients with breast cancer: a comparison between paroxetine and amitriptyline Breast Cancer Res Treat 2001;70 1):1 10 [DOI] [PubMed] [Google Scholar]
- 46. Razavi D, Kormoss N, Collard A, et al. Comparative study of the efficacy and safety of trazodone versus clorazepate in the treatment of adjustment disorders in cancer patients: a pilot study J Int Med Res 1999;27 6):264 272 [DOI] [PubMed] [Google Scholar]
- 47. Hopko DR, Armento MEA, Robertson SMC, et al. Brief behavioral activation and problem-solving therapy for depressed breast cancer patients: randomized trial J Consult Clin Psychol 2011;79 6):834 849 [DOI] [PubMed] [Google Scholar]
- 48. Rodríguez Vega B, Palao A, Torres G, et al. Combined therapy versus usual care for the treatment of depression in oncologic patients: a randomized controlled trial Psycho-oncology 2011;20 9):943 952 [DOI] [PubMed] [Google Scholar]
- 49. Feng Y, Wang X, Li S, et al. Clinical research of acupuncture on malignant tumor patients for improving depression and sleep quality J Tradit Chin Med 2011;31 3):199 202 [DOI] [PubMed] [Google Scholar]
- 50. Centre for Evidence-Based PhysiotherapyPEDro ScaleSydney, Australia: Centre for Evidence-Based Physiotherapy; 2009. http://www.pedro.org.au Accessed April 20, 2012. [Google Scholar]
- 51. Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium Health Psychol 2004;23 5):443 451 [DOI] [PubMed] [Google Scholar]
- 52. Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis Version 2 Englewood, NJ: Biostat; 2005. [Google Scholar]
- 53. Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators J Educ Stat 1981;6 2):107 128 [Google Scholar]
- 54. Cohen J. Statistical Power Analysis for the Behavioral Sciences 2nd ed Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 55. Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV. The Handbook of Research Synthesis New York, NY: Russell Sage Foundation; 1994:231 244 [Google Scholar]
- 56. Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods 2nd ed Newbury Park, CA: Sage; 2002. [Google Scholar]
- 57.Hunter JE, Schmidt FL. Thousand Oaks, CA: Sage Publications; 2004. Methods of Meta-Analysis: Correcting Error and Bias in Research Findings. 2nd ed. [Google Scholar]
- 58. Costa D, Mogos I, Toma T. Efficacy and safety of mianserin in the treatment of depression of women with cancer Acta Psychiatr Scand 1985;72suppl 320:85 92 [DOI] [PubMed] [Google Scholar]
- 59. Evans RL, Connis RT. Comparison of brief group therapies for depressed cancer patients receiving radiation treatment Public Health Rep 1995;110 3):306 311 [PMC free article] [PubMed] [Google Scholar]
- 60. Razavi D, Allilaire JF, Smith M, et al. The effect of fluoxetine on anxiety and depression symptoms in cancer patients Acta Psychiatr Scand 1996;94 3):205 210 [DOI] [PubMed] [Google Scholar]
- 61. Savard J, Simard S, Giguere I, et al. Randomized clinical trial on cognitive therapy for depression in women with metastatic breast cancer: psychological and immunological effects Palliat Support Care 2006;4 3):219 237 [DOI] [PubMed] [Google Scholar]
- 62. Musselman DL, Somerset WI, Guo Y, et al. A double-blind, multicenter, parallel-group study of paroxetine, desipramine, or placebo in breast cancer patients (stages I, II, III, and IV) with major depression J Clin Psychiatry 2006;67 2):288 296 [DOI] [PubMed] [Google Scholar]
- 63. Nezu AM, Nezu CM, Felgoise SH, et al. Project Genesis: assessing the efficacy of problem-solving therapy for distressed adult cancer patients J Consult Clin Psychol 2003;71 6):1036 1048 [DOI] [PubMed] [Google Scholar]
- 64. Hesser H, Weise C, Rief W, Andersson G. The effect of waiting: a meta-analysis of wait-list control groups in trials for tinnitus distress J Psychosom Res 2011;70 4):378 384 [DOI] [PubMed] [Google Scholar]
- 65. Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis Psychol Methods 1998;3 4):486 504 [Google Scholar]
- 66. Lipsey MW, Wilson DB.Practical Meta-AnalysisApplied Social Research Methods Series (vol. 49). Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 67. Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses BMJ 2007;335 7626):914 916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Orsini N, Higgins J, Bottai M, et al. Heterogi: Stata module to quantify heterogeneity in a meta-analysis, revised 2006-01-25 [computer program]. Statistical Software Components S449201. Boston, MA: Boston College Department of Economics; 2005. http://EconPapers.repec.org/software/bocbocode/s449201.htmAccessed April 20, 2012 [Google Scholar]
- 69. Duval SJ, Tweedie RL. Trim and fill: a simple funnel plot-based method of testing and adjusting for publication bias in meta-analysis Biometrics 2000;56 2):455 463 [DOI] [PubMed] [Google Scholar]
- 70. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test Br Med J 1997;315 7109):629 634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fisch MJ, Loehrer PJ, Kristeller J, et al. Fluoxetine versus placebo in advanced cancer outpatients: a double-blinded trial of the Hoosier Oncology Group J Clin Oncol 2003;21 10):1937 1943 [DOI] [PubMed] [Google Scholar]
- 72. Dwight-Johnson M, Ell K, Lee P-J. Can collaborative care address the needs of low-income Latinas with comorbid depression and cancer? Results from a randomized pilot study Psychosomatics 2005;46 3):224 232 [DOI] [PubMed] [Google Scholar]
- 73. Ell K, Xie B, Quon B, et al. Randomized controlled trial of collaborative care management of depression among low-income patients with cancer J Clin Oncol 2008;26 10):4488 4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial Lancet 2008;372 9632):40 48 [DOI] [PubMed] [Google Scholar]
- 75. Ell K, Xie B, Kapetanovic S, et al. One-year follow-up of collaborative depression care for low-income, predominantly Hispanic patients with cancer Psychiatr Serv 2011;62 2):162 170 [DOI] [PubMed] [Google Scholar]
- 76. van Heeringen K, Zivkov M. Pharmacological treatment of depression in cancer patients. A placebo-controlled study of mianserin Br J Psychiatry 1996;169 4):440 443 [DOI] [PubMed] [Google Scholar]
- 77. Greer S, Moorey S, Baruch JD, et al. Adjuvant psychological therapy for patients with cancer: a prospective randomised trial Br Med J 1992;304 6828):675 680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Moorey S, Greer S, Watson M, et al. Adjuvant psychological therapy for patients with cancer: outcome at one year Psycho-oncology 1994;3 1):39 46 [Google Scholar]
- 79. Navari RM, Brenner MC, Wilson MN. Treatment of depressive symptoms in patients with early stage breast cancer undergoing adjuvant therapy Breast Cancer Res Treat 2008;112 1):197 201 [DOI] [PubMed] [Google Scholar]
- 80. Cuijpers P, van Straten A, Warmerdam L, et al. Characteristics of effective psychological treatments of depression: a meta-regression analysis Psychother Res 2008;18 2):225 236 [DOI] [PubMed] [Google Scholar]
- 81. van Straten A, Geraedts A, Verdonch-de Leeuw I, et al. Psychological treatment of depressive symptoms in patients with medical disorders: a meta-analysis J Psychosom Res 2010;69 1):23 32 [DOI] [PubMed] [Google Scholar]
- 82. Beltman MW, Voshaar RCO, Speckens AE. Cognitive-behavioural therapy for depression in people with a somatic disease: meta-analysis of randomised controlled trials Br J Psychiatry 2010;197 1):11 19 [DOI] [PubMed] [Google Scholar]
- 83. Zigmond AS, Snaith RP. The hospital anxiety and depression scale Acta Psychiatr Scand 1983;67 6):361 370 [DOI] [PubMed] [Google Scholar]
- 84. Bower P, Gilbody S, Richards D, et al. Collaborative care for depression in primary care: making sense of a complex intervention: systematic review and meta-regression Br J Psychiatry 2006;189 6):484 493 [DOI] [PubMed] [Google Scholar]
- 85. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes Arch Intern Med 2006;166 21):2314 2321 [DOI] [PubMed] [Google Scholar]
- 86. Ell K, Aranda MP, Xie B, et al. Collaborative depression treatment in older and younger adults with physical illness: pooled comparative analysis of three randomized clinical trials Am J Geriatr Psychiatry 2010;18 6):520 530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tolin DF. Is cognitive-behavioral therapy more effective than other therapies? A meta-analytic review Clin Psychol Rev 2010;30 6):710 720 [DOI] [PubMed] [Google Scholar]
- 88. Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses Clin Psychol Rev 2006;26 1):17 31 [DOI] [PubMed] [Google Scholar]
- 89. Speilmans GI, Berman MI, Usital AN. Psychotherapy versus second-generation antidepressants in the treatment of depression: a meta analysis J Nerv Ment Dis 2011;199 3):142 149 [DOI] [PubMed] [Google Scholar]
- 90. National Comprehensive Cancer NetworkNCCN Clinical Practice Guidelines in Oncology—Distress Management (Version 1) [Electronic data type]. 2007. http://www.nccn.org/professionals/physician_gls/PDF/distress.pdf Accessed April 20, 2012.
- 91. Institute of Medicine Cancer Care for the Whole Patient: Meeting Psychosocial Needs. Board on Health Care Services. Washington, DC: The National Academic Press; 2007. [Google Scholar]
- 92. Carlson LE, Bultz BD. Cancer distress screening: needs, methods and models J Psychosom Res 2003;55 5):403 409 [DOI] [PubMed] [Google Scholar]
- 93. Kruijver IP, Garssen B, Visser AP, et al. Signalising psychosocial problems in cancer care: the structural use of a short psychosocial checklist during medical or nursing visits Patient Educ Couns 2006;62 2):163 177 [DOI] [PubMed] [Google Scholar]
- 94. Velikova G. Patient benefits from psychosocial care: screening for distress and models of care J Clin Oncol 2010;28 33):4871 4873 [DOI] [PubMed] [Google Scholar]
- 95. Carlson LE, Groff SL, Maciejewski O, et al. Screening for distress in lung and breast cancer outpatients: a randomized controlled trial J Clin Oncol 2010;28 33):4884 4891 [DOI] [PubMed] [Google Scholar]
- 96. Palmer S, van Scheppingen C, Coyne JC. Clinical trial did not demonstrate benefits of screening patients with cancer for distress J Clin Oncol 2011;29 10):e277 e278 [DOI] [PubMed] [Google Scholar]
- 97. van Scheppingen C, Schroevers MJ, Smink A, et al. Does screening for distress efficiently uncover meetable unmet needs in cancer patients? Psycho-oncology 2011;20 6):655 663 [DOI] [PubMed] [Google Scholar]
- 98. Meijer A, Roseman M, Milette K, et al. Depression screening and patient outcomes in cancer: a systematic review PLoS One 2011;6 11):e2718110.1371/journal.pone.00278181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Walker J, Cassidy J, Sharpe M, et al. The second Symptom Management Research Trial in Oncology (SMaRT Oncology-2): a randomised trial to determine the effectiveness and cost-effectiveness of adding a complex intervention for major depressive disorder to usual care for cancer patients Trials 2009;10 18). 10.1186/1745-6215-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Walker J, Cassidy J, Sharpe M, et al. The third Symptom Management Research Trial in Oncology (SMaRT Oncology-3): a randomised trial to determine the efficacy of adding a complex intervention for major depressive disorder (Depression Care for People with Lung Cancer) to usual care, compared to usual care alone in patients with lung cancer Trials 2009;10 92). 10.1186/1745-6215-10-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zautra AJ, Davis MC, Reich JW, et al. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression J Consult Clin Psychol 2008;76 3):408 421 [DOI] [PubMed] [Google Scholar]
- 102. Dunn LB, Cooper BA, Neuhaus J, et al. Identification of distinct depressive symptoms trajectories in women following surgery for breast cancer Health Psychol 2011;30 6):683 692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Henselmans I, Helgeson VS, Seltman H, et al. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis Health Psychol 2010;29 2):160 168 [DOI] [PubMed] [Google Scholar]