Abstract
Background
B-type natriuretic peptide (BNP), a key cardiac hormone in cardiorenal homeostasis, is produced as a 108 amino acid pro-hormone proBNP1-108. proBNP1-108 is converted to a biologically active peptide BNP1-32 and an inactive NT-proBNP1-76. The widely accepted model is that the normal heart releases a proteolytically processed BNP1-32 and NT-proBNP, while the diseased heart secretes high amounts of unprocessed/glycosylated proBNP1-108 or inappropriately processed BNPs. In contrast, circulating proBNP1-108 has recently been identified in normal subjects, indicating that the normal heart also secretes unprocessed proBNP1-108. However, the mechanism of proBNP1-108 secretion from normal heart remains elusive. Our goal is to determine the molecular mechanisms underlying proBNP1-108 intracellular trafficking and secretion from normal heart.
Methods
We expressed pre-proBNP in cardiomyocytes, and determined the subcellular localization, dominant intracellular and extracellular forms of BNP.
Results
Intracellular immunoreactive BNPs accumulated in the Golgi apparatus, which were distributed throughout the cytoplasm as secretory vesicles. The predominant intracellular form of BNP was non-glycosylated proBNP1-108, rather than BNP1-32. Glycosylated proBNP1-108, but not non-glycosylated proBNP1-108, was detected as the major extracellular form in the culture supernatants of pre-proBNP-expressing cell lines or primary human cardiomyocytes. Ablation of O-glycosylation of proBNP1-108 at T71 residue, near the convertase recognition site, reduced the extracellular proBNP1-108 and increased extracellular BNP1-32.
Conclusions
Intracellular proBNP trafficking occurs through a conventional Golgi-ER pathway. Glycosylation of proBNP1-108 controls the stability and processing of extracellular proBNP1-108. Our data establish a new B-type natriuretic peptide secretion model where the normal cardiac cells secrete glycosylated proBNP1-108.
Introduction
B-type natriuretic peptide (BNP) is a key regulatory hormone that increases sodium excretion, lowers blood pressure, suppresses the renin-angiotensinaldosterone system, and inhibits cardiomyocyte hypertrophy and proliferation of cardiac fibroblasts 1-3. Human BNP is produced as a 108 amino acid pro-hormone proBNP1-108 (proBNP), which is converted to a biologically active peptide BNP1-32 (BNP32) and an inactive NT-proBNP through proteolytic cleavage 4, 5. A cardiac serine protease, corin, and a ubiquitous serine protease, furin, are currently proposed as possible convertases 6-8. Low concentrations of BNP32 and NT-proBNP have been detected as circulating forms of BNP in normal subjects, while increased concentrations of unprocessed proBNP1-108 circulate in CHF patients 9-14. These observations have established the concept that the normal heart releases a proteolytically processed BNP32 and NT-proBNP, while diseased heart secretes a high amount of inactive, inappropriately processed proBNP1-108. It should be noted, however, that detection of proBNP in normal subjects has been problematic because commonly used detection assays are directed against BNP32 or NT-proBNP that in part cross-react with proBNP 9. Recently, Giuliani et al. used a novel proBNP detection assay, which utilizes an antibody specific to the junction between NT-proBNP and BNP32 but not to the NT-proBNP or BNP32 itself, and detected proBNP in 50 adults without clinical evidence of cardiovascular disease 12. We used the same proBNP detection assay for 1,939 subjects and demonstrated circulating proBNP in all normal humans 15. These observations indicate the release of unprocessed proBNP from the normal heart. The identification of circulating proBNP in plasma samples of normal subjects changes the current model of proBNP processing in the heart before release into circulation.
Elucidation of proBNP intracellular trafficking, secretion and maturation/processing is vital to our understanding of how the active form of BNP is processed in normal subjects, and to explain why high concentrations of immunoreactive (ir) BNPs in CHF patients have such impaired biological activity. However, the precise mechanisms underlying proBNP trafficking, maturation and secretion remain to be determined. Therefore, to study these mechanisms, we used BNP domain mutants and determined the molecular mechanisms underlying the secretion of proBNP.
Methods
Cell culture and plasmids
HEK 293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% calf serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. A murine atrial cardiomyocyte cell line, HL-116, was kindly provided by Dr. William C. Claycomb (Louisiana State University Medical Center) and cultured in Claycomb's medium with 10% FBS, 100 μM norepinephrine, and 4 mM L-glutamine on 0.02% gelatin/fibronectin-coated flasks or plates. Normal human cardiomyocytes (48 yo female, Caucasian, ventricle-derived) were purchased from Promocell (Heidelberg, Germany), and maintained under manufacture's guidelines. The corin-expressing plasmid 6 was kindly provided by Dr. Qingyu Wu (Cleveland Clinic).
Lentiviral vector production
HIV-based lentiviral vectors were generated by three plasmid transfection in 293T cells (see Supplementary Materials that accompany the online version of this paper for more details).
Transfection, Immunoblotting, Immuno-staining and Immunoprecipitation
FuGene6 (Roche) was used for transfection. Antibodies used in this study, including anti-BNP32 or anti-proBNP antibodies, are summarized in Table 1. Detailed protocols are described in Supplementary Materials that accompany the online version of this paper.
Table 1.
Antibodies used in this study.
| Name | Company | Species | Detection | BNP Epitope |
|---|---|---|---|---|
| mAb 24C5 | Abcam | mouse | BNP32 proBNP108 | BNP11-22 |
| mAb 50E1 | Abcam | mouse | proBNP108 NT-proBNP | proBNP77-108 |
| mAb 15F11 | Abcam | mouse | proBNP108 NT-proBNP | proBNP13-27 |
| Polyclonal | Abcam | rabbit | BNP32 proBNP108 | BNP1-32 |
| Polyclonal | Phoenix | rabbit | BNP32 proBNP108 | BNP1-32 |
| Polyclonal | Phoenix | rabbit | proBNP108 NT-proBNP | proBNP22-46 |
| Polyclonal | Abcam | rabbit | corin spacer | |
| Polyclonal | Cell Signaling | rabbit | EEA1 | |
| Polyclonal | Cell Signaling | rabbit | AIF | |
| mAb | Sigma | mouse | β-actin | |
| mAb | Abcam | mouse | giantin | |
| mAb | Sigma | mouse | α-actinin |
mAb, monoclonal antibody
Results
Supranuclear localization of unlabeled BNP in murine cardiomyocyte cells
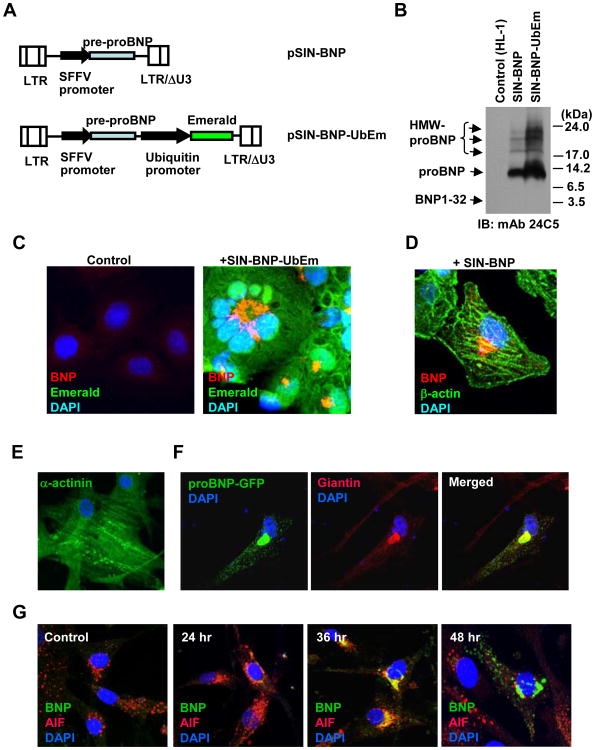
We first examined the intracellular trafficking of BNP using green fluorescent protein (GFP)-tagged constructs. Our data demonstrated that the signal peptide controls the supranuclear accumulation of intracellular BNP, and that the supranuclear BNP-GFP signals co-localized frequently with Golgi marker signals (Supplementary Fig. S1). Since GFP-tagging could cause an artifact due to artificial protein structures, we evaluated the intracellular trafficking of unlabeled BNP using constructs expressing pre-proBNP1-108. Due to the low transfection efficiency of murine cardiomyocyte HL-1 cells, we introduced the pre-proBNP1-108 expression cassette through lentiviral vector transduction. We generated a lentiviral vector pSIN-BNP-UbEm, which expresses the wildtype pre-proBNP protein as well as a modified GFP Emerald independently, and a vector pSIN-BNP, which encodes a wild-type pre-proBNP alone (Fig. 1A). After infection of HL-1 cells with the SIN-BNP-UbEm vector, over 90% of treated cells became Emerald GFP-positive, indicating efficient lentiviral transduction of HL-1 cells. When BNP expression was verified by an anti-BNP32 MAb 24C5, we found 11 kDa proBNP signals as a dominant intracellular form in HL-1 cells expressing pre-proBNP (Fig. 1B). We also observed glycosylated, high molecular weight proBNP, but failed to detect a BNP32 signal in the cells. After verifying expression of the pre-proBNP construct, we examined the intracellular localization of irBNPs in the cells. When the pre-proBNP-expressing HL-1 cells were analyzed by immunostaining with an anti-proBNP MAb 15F11, clear supranuclear localization of irBNPs (red) was found in the cells which showed diffuse cytoplasmic Emerald GFP signals (Fig. 1C). Using anti-BNP32 polyclonal antibodies, we also observed regular supranuclear irBNPs as well as discrete cytoplasmic body signals (red) in SIN-BNP-infected HL-1 cells (Fig. 1D), confirming the results observed with GFP-tagged BNP proteins.
Fig. 1. Supranuclear localization of wildtype B-type natriuretic peptide in mouse cardiomyocytes.
A. Schematic representation of lentiviral vectors used in this study. pSIN-BNP encodes human pre-proBNP. pSIN-BNP-UbEm has human pre-proBNP and additional Emerald GFP expression cassettes. SFFV, spleen focus forming virus promoter; LTR, long terminal repeat. B. Vector-transduced HL-1 cell lysates were harvested, and analyzed by Western blotting for pre-proBNP expression with anti-BNP32 MAb 24C5. High molecular weight (HMW) proBNP and non-glycosylated proBNP were indicated. IB, immunoblotting; mAb, monoclonal antibody. C. Untransduced (Control) and SIN-BNP-UbEm-transduced HL-1 cells were fixed, and immunoreactive BNPs were detected by immunostaining with a BNP32 MAb 50E1 (Red). D. Detection of supranuclear accumulation of immunoreactive BNPs in human pre-proBNP-expressing HL-1 cells by anti-BNP32 rabbit polyclonal antibodies (Abcam). Cells were counter-stained with anti-β-Actin antibody (green) and DAPI (blue). E. Detection of α-actinin in primary human cardiomyocytes (HCMC). HCMC were fixed and stained with anti-α-actinin antibody (green) and DAPI (blue). F. HCMC were transfected with a plasmid expressing proBNP-GFP. Three days after transfection, cells were fixed and immuno-stained for the Golgi apparatus marker Giantin. Localization of GFP-fused BNP signals (green) and Golgi apparatus signals (red) were analyzed by confocal microscopy. G. B-type natriuretic peptide localization time-series were analyzed after transduction of HCMC with SIN-proBNP vector. Cells were fixed after the time course of 24-h, 36-h, and 48-h, and immunoreactive BNPs were detected by anti-BNP32 MAb 50E1 (green). Cells were counterstained with AIF (red) and DAPI (blue).
BNP trafficking through the Golgi apparatus in human cardiomyocytes
We next assessed the subcellular localization of proBNP in primary human cardiomyocytes, which were stained positive for α-actinin, a cardiomyocyte-specific marker (Fig. 1E). When the GFP-tagged full-length pre-proBNP construct was transfected, proBNP-GFP signals were observed in the supranuclear region, which was also positive for a Golgi protein giantin, and in cytoplasmic bodies (Fig. 1F). When human cardiomyocytes were infected with the pre-proBNP-expressing lentiviral vector SIN-BNP and periodically observed for subcellular BNP localization, strong supranuclear signals of irBNPs were observed at 36 h after vector infection, while prominent cytoplasmic body signals were detected at 48 h after vector infection (Fig. 1G).
Expression of pre-proBNP leads to efficient secretion of glycosylated proBNP
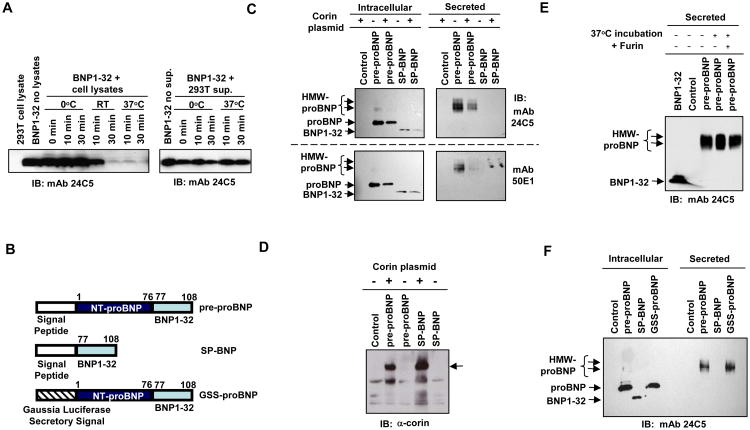
We first examined the stability of BNP32 in 293T cell lysates and supernatants. Cell lysates prepared in RIPA buffer containing protease inhibitor cocktail (Clontech) were mixed with synthesized BNP32 and incubated at 0, 22 or 37°C for 0, 10 or 30 min, and then analyzed by immunoblotting with anti-BNP32 monoclonal antibody 24C5. BNP32 was rapid degraded at 37 °C in 293T lysates, even in the presence of protease inhibitors, but not at 0°C (Fig. 2A, left panel). When synthesized BNP32 was incubated with 293T culture supernatant, BNP32 was stable even at 37°C (Fig. 2A, right panel). To avoid degradation of proBNP and BNP32 during sample collection, we processed BNP-expressing cells and supernatants at 0°C in the following experiments.
Fig. 2. Secretion of HMW proBNP in 293T cells.
A. Stability of synthesized BNP32 in the presence of 293T cell lysates (left panel) or culture supernatants. BNP32 was incubated at the indicated temperatures for 0, 10 and 30 min. B. Schematic illustration of two B-type natriuretic peptide-expression constructs, pre-proBNP and SP-BNP, and the GSS-proBNP construct, in which the normal pre-proBNP secretory signal sequence was replaced with a sequence encoding 17 amino acids of gaussia luciferase secretory signal (MGVKVLFALICIAVAEA). C. Pre-proBNP- or SP-BNP-expressing constructs were co-transfected with the corin plasmid, and cell lysates and filtered culture supernatants were analyzed for the molecular forms of immunoreactive BNPs with anti-BNP32 monoclonal antibodies 24C5 and 50E1. D. Corin expression was confirmed by anti-corin antibody. Arrow indicates the Corin-specific signals. E. Stability of secreted HMW proBNP in the presence or absence of furin. The 293T supernatant containing HMW proBNP was incubated at 37 °C for 30 min in the presence or absence of furin and 1 × furin buffer. Immunoreactive BNPs were dtected by immunoblotting with monoclonal antibody 24C5. F. 293T cells were transfected with plasmids encoding pre-proBNP, SP-BNP or GSS-proBNP, and intracellular and extracellular immunoreactive BNPs were detected by the BNP32 antibody 24C5. HMW, high molecular weight; IB, immunoblotting; mAb, monoclonal antibody.
We then analyzed the molecular forms of BNP in pre-proBNP expressing cells and in the supernatants. We generated mammalian expression plasmids which encoded full-length human pre-proBNP or a pre-proBNP mutant with a deletion in the NT-proBNP region (SP-BNP) (Fig. 2B). 293T cells were transfected with the pre-proBNP-expression construct, and intracellular forms of irBNPs were determined by three antibodies; polyclonal anti-proBNP and monoclonal anti-BNP32 antibodies 24C5 and 50E1. Non-glycosylated proBNP (11 kDa) was identified as the predominant intracellular form of BNP (Fig. 2C). Similar results were observed with anti-BNP32 polyclonal antibodies (not shown). Over-expression of corin did not enhance the processing of non-glycosylated 11 kDa proBNP (Fig. 2C and 2D). Although furin is a ubiquitously expressed protease, we did not see the proteolytically cleaved, mature BNP32 signal in cell lysates (Fig. 2C). When the secreted forms of BNP in the culture supernatants were examined, we found high molecular weight (HMW) forms (17 ∼ 22 kDa) of proBNP, but not non-glycosylated proBNP, in the supernatants (Fig. 2C). Incubation of the HMW forms of proBNP with furin did not lead to any cleaved form or reduction of HMW proBNP (Fig. 2E). These data demonstrated that glycosylated proBNP is efficiently secreted from pre-proBNP-expressing cells and resistant to furin cleavage.
Transfection of the SP-BNP construct resulted in expression of intracellular BNP32, and additional corin expression did not affect the mobility of the SP-BNP signal (Fig. 2C). No irBNP was detected in the supernatants of SP-BNP-expressing cells. When we analyzed the concentrations of irBNPs in the culture supernatants by using BNP32 ELISA (Phoenix), we found 6.2 ng/ml and 106 ng/ml of irBNPs (means of four independent experiments) in the SP-BNP- and wildtype pre-proBNP-transfected supernatants, respectively, suggesting the critical role of NT-proBNP region in efficient BNP secretion.
Replacement of the BNP signal peptide with a potent secretory signal does not support secretion of non-glycosylated proBNP
No detection of extracellular non-glycosylated proBNP could be due to a weak secretory signal activity of the BNP signal peptide sequence, we tested whether introduction of the potent secretory signal from Gaussia luciferase 17 could accelerate secretion of non-glycosylated proBNP1-108 or BNP1-32. GSS-proBNP, a pre-proBNP mutant with the Gaussia luciferase secretory signal (Fig. 2B) demonstrated the same phenotype as the wildtype pre-proBNP; efficient secretion of HMW proBNP but not detectable non-glycosylated proBNP in the supernatants (Fig. 2F). We concluded that the lack of detectable non-glycosylated proBNP in the supernatant is not due to weak secretory signal activity of pre-proBNP signal peptide.
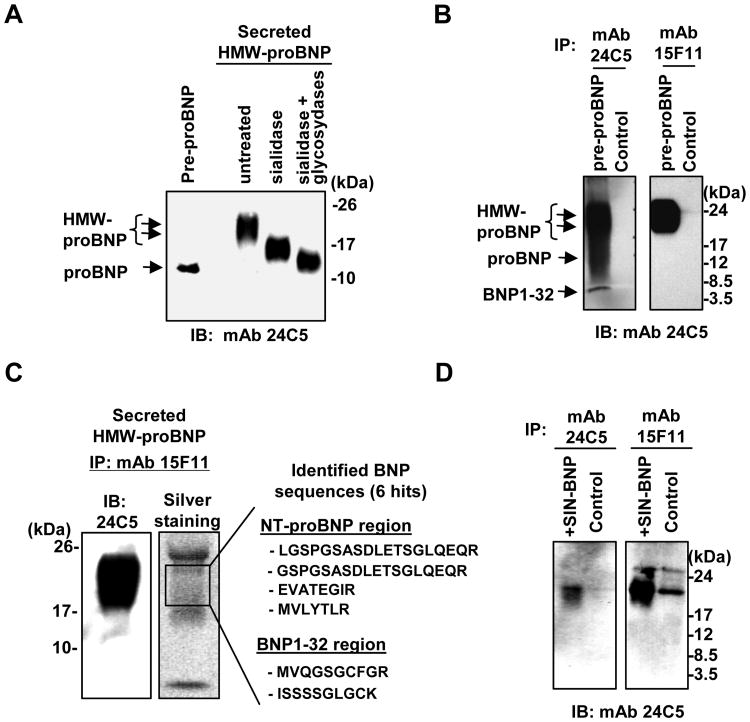
Secreted, high molecular weight BNPs are O-glycosylated proBNP
We further examined the identity of immunoreactive, HMW proBNP. Treatment of secreted HMW proBNP with a mixture of O-link deglycosylation enzymes (O-Glycosidase, B-Galactosidase, Glucosaminidase, PNGase F) and sialidase reduced the molecular weights of immunoreactive proBNP to 11∼13 kDa species. Since there is no N-linked glycosylation site in pre-proBNP, our data indicate that secreted HMW proBNP is O-glycosylated (Fig. 3A). When irBNPs were enriched by immunoprecipitation with two anti-BNP32 polyclonal antibodies, glycosylated and non-glycosylated proBNP were observed in pre-proBNP-expressing 293T cell lysates. In contrast, these antibodies could pull-down glycosylated proBNP, but not non-glycosylated proBNP, from the supernatants (Supplemental Fig. S2). When the MAb 24C5 was used to enrich irBNPs in the pre-proBNP-expressing 293T supernatants, glycosylated proBNP and BNP32, but not non-glycosylated proBNP, were efficiently pulled down (Fig. 3B). The anti-proBNP MAb 15F11 also enriched the HMW, glycosylated proBNP in the supernatants, but failed to pull-down non-glycosylated proBNP, verifying the secretion of glycosylated proBNP from pre-proBNP-expressing 293T cells (Fig. 3B). In addition, when immunoprecipitation-enriched HMW BNP (18 to 22 kDa) were analyzed by tandem mass spectrometry, we found 6 proBNP-derived peptides (Fig. 3C, 4 peptides from NT-proBNP region and 2 peptides from BNP1-32 region), further demonstrating the secreted, immunoreactive HMW BNPs are of proBNP.
Fig. 3. Primary human cardiomyocytes secrete HMW proBNP.
A. Deglycosylation of high molecular weight (HMW) BNP in pre-proBNP-expressing 293T supernatants by sialidase and O-glycosidases reduced the molecular weights. Non-glycosylated proBNP was used as a control. Immunoreactive BNPs were detected by HRP-conjugated antibody 24C5. B. Anti-BNP32 and proBNP monoclonal antibodies, 24C5 and 15F11, were used to pull-down immunoreactive BNPs in pre-proBNP-expressing 293T supernatants. C. 15F11-enriched proteins were separated by SDS-PAGE and probed for immunoreactive BNPs (left) or visualized by silver-staining (middle). The 18-22 kDa proteins were sequenced by tandem-MS, and identified pre-proBNP-derived peptides were listed (right). D. Supernatants of primary human cardiomyocytes (control) and human cardiomyocytes transduced with the pre-proBNP-expressing lentiviral vector SIN-BNP were analyzed by immuno-precipitation with antibodies 24C5 and 15F11. B-type natriuretic peptide signals were detected by HRP-conjugated anti-BNP32 antibody 24C5. IB, immunoblotting; IP, immuno-precipitation; mAb, monoclonal antibody.
Human cardiomyocytes secrete glycosylated proBNP as a dominant form
We examined the extracellular forms of BNP using primary human cardiomyocytes. To enrich low concentrations of endogenous BNP expression in the culture supernatants of primary human cardiomyocytes, we used antibodies 24C5 and 15F11 for immunoprecipitation. As a positive control, we used the culture supernatants from primary human cardiomyocytes which were transduced with the pre-proBNP-expressing lentiviral vector, SIN-BNP. Immunoprecipitation with the antibody 24C5 detected the glycosylated proBNP in the SIN-BNP-transduced cardiomyocyte supernatant (Fig. 3D, left panel). A faint signal for the glycosylated proBNP was also seen in the untreated sample (Fig. 3D, left panel). When the proBNP MAb 15F11 was used for immunoprecipitation, we could detect glycosylated proBNP, but not non-glycosylated proBNP, in the supernatants harvested from vector-infected and untreated cardiomyocytes (Fig. 3D, right panel). Extracellular BNP32 was undetectable in this assay. These observations demonstrate that human cardiomyocytes also secrete glycosylated proBNP.
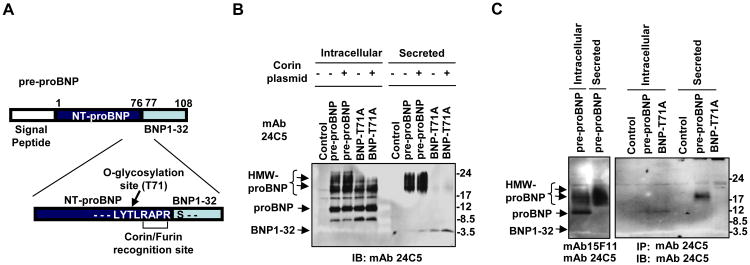
Glycosylation of proBNP at T71 residue controls the levels of extracellular proBNP
Semenov et al. previously reported that prevention of O-glycosylation of proBNP at T71 amino acid residue resulted in enhanced proBNP processing 18. Because the dominant extracellular form of BNP was unprocessed and glycosylated (Fig. 2 and 3), we hypothesized that the glycosylation at the T71 residue also plays the key role in the secretion of glycosylated proBNP. We generated an expression plasmid encoding a pre-proBNP with a T71A amino acid substitution by extension PCR (Fig. 4A). Transfection of 293T cells with the T71A mutant resulted in intracellular accumulation of non-glycosylated proBNP, which was comparable to the wildtype pre-proBNP construct (Fig. 4B). Ablation of T71 glycosylation resulted in reduced abundance of glycosylated forms of proBNP in the cells (Fig. 4B). Although other glycosylation sites in the NT-proBNP region remained intact, notably reduced concentrations of glycosylated proBNP were observed in the supernatants of cells transfected with the T71A mutation (Fig. 4B). When compared with the wildtype pre-proBNP construct, the T71A mutant showed increased levels of BNP32 in the supernatants (Fig. 4B). These observations suggest two possibilities; the essential role of T71 glycosylation in efficient proBNP secretion, or efficient secretion but rapid degradation of the T71A mutant. To address these possibilities, we analyzed the concentrations of extracellular irBNPs. The ELISA assay for irBNPs (Phoenix) detected comparable levels of BNPs in the supernatants producing wildtype pre-proBNP or T71A mutant (wildtype 122 ng/ml, T71A 112 ng/ml). These data suggest that the secretion efficiency of irBNPs was similar between wildtype pre-proBNP and the T71A mutant, whereas stability of extracellular proBNP is impaired by ablation of T71A glycosylation.
Fig. 4. Ablation of T71 glycosylation reduces extracellular proBNP.
A. Illustration of proBNP showing amino acid sequence around the corin/furin recognition site in conjunction with O-glycosylation site at residue T71. B. 293T cells were transfected with a plasmid expressing either wild-type pre-proBNP or pre-proBNP-T71A in the presence or absence of a corin expression plasmid. Cell lysates and supernatants were used for Western blotting with HRP-conjugated anti-BNP32 MAb 24C5. C. HL-1 cardiomyocyte cells were transfected with plasmids expressing pre-proBNP or T71A mutant, and the cell lysates and supernatants were analyzed by IP with the antibody 24C5 for immunoreactive BNPs. IB, immunoblotting; mAb, monoclonal antibody.
Despite the increased BNP32 in the supernatant, BNP32 was not detectable in the cell lysates of T71A mutant-expressing cells. The ubiquitous endogenous convertase furin or over-expressed corin did not result in accumulation of intracellular BNP32 (Fig. 4B). These observations suggest that neither furin nor corin cleave intracellular proBNP even when the T71 glycosylation was ablated. Given the detection of BNP32 in supernatants, but not in the producer cells, it is plausible that cleavage of proBNP with T71A mutant occurred at the late stage of BNP secretion or immediately after secretion. In addition, we noted the increased concentration of an undefined, 8 kDa form of intracellular BNP in the T71A mutant-transfected cells. It is possible that the lack of glycosylation of proBNP at T71 exposed a cryptic protease-recognition site for some other cellular protease, resulting in an accelerated cleavage of proBNP in the middle of NT-proBNP region.
We also examined the molecular forms of intracellular and extracellular BNP with or without T71A mutation in a cardiomyocyte cell line. When pre-proBNP-expressing HL-1 cells were used for IP with proBNP MAb 15F11, we could detect non-glycosylated proBNP and glycosylated proBNP in the cell lysates and the culture supernatant, respectively (Fig. 4C, left panel). Similarly, IP with the anti-BNP32 antibody 24C5 detected non-glycosylated proBNP in cell lysates and glycosylated proBNP in the supernatant of wild-type pre-proBNP-expressing HL-1 cells (Fig. 4C, left panel). Although non-glycosylated proBNP was detected in the pre-proBNP-T71A-transfected cell lysates, secretion of glycosylated proBNP was not observed with the T71A mutant. Extracellular BNP32 was below the detectable concentration, indicating that glycosylation of pre-proBNP at residue T71 also controlled the concentrations of extracellular proBNP in cardiomyocyte cultures.
Discussion
Our study of intracellular BNP trafficking demonstrated that synthesized proBNP first accumulates in the Golgi apparatus for post-translational modification and then trafficked to cell surface as cytoplasmic secretory vesicles. Although many proteins are known to be transported in Golgi-independent manner through non-conventional secretory pathways (for the review see 19), our results demonstrated that proBNP1-108 trafficking is through the conventional ER-Golgi secretory pathway.
We demonstrate detection of O-glycosylated proBNP as the dominant extracellular form of irBNPs. This observation confirms the recent findings of O-glycosylated proBNP secretion in pre-proBNP-transfected cell lines 18, 20. Our multiple attempts failed to detect non-glycosylated form of extracellular proBNP. Moreover, the T71A mutant, which ablates one of the O-glycosylation sites near the proBNP cleavage site (71TLRAPR↓S, with T71 glycosylation site and RXXR↓S protease recognition/cleavage sequence), resulted in severe reduction in the extracellular proBNP. These data demonstrate that the glycosylation at the T71 residue is essential to stabilize extracellular proBNP. Intriguingly, a similar finding was reported for the secretion of fibroblast growth factor 23 (FGF23) 21. The O-glycosylation of FGF23 precursor protein at the threonine residue adjacent to the convertase cleavage site (171TPIPRRHTR↓S, with T171 O-glycosylation site and RXXR↓S convertase cleavage sequence) was essential to obtain high levels of extracellular FGF23 precursor protein 21. Similar to the O-glycosylation of proBNP at residue T71, where the glycosylation controls cleavage of proBNP (Fig. 4 and 18), the glycosylation of FGF23 at T171 residue also blocks FGF23 processing by a convertase 21. These observations suggest the existence of a universal, glycosylation-controlled mechanism, which stabilizes extracellular pro-hormone or cytokine precursors and prevents premature hormone activation by a convertase processing. Indeed, through screening of the human protein data base, we found several secretory proteins which have similar T(or S)-X(n)-RXXR↓ sequences, including endothelin 1 (ET1) pre-prohormone (preproET1; 40TPSPPWRLRRSKR↓C) and von Willebrand factor (VWF) preprotein (768SHRSKR↓S). It is possible that O-glycosylations near the convertase recognition sites also play a role in stabilization of extracellular preproET1 or VWF preproteins.
A ubiquitous cellular protease Furin has been proposed as a BNP convertase 7, 22. Semenov et al. reported that deglycosylation of proBNP or disruption of the O-glycosylation at T71 were necessary for furin to process proBNP, suggesting that glycosylation of proBNP at T71 masks the furin recognition site 18. We also found that extracellular, glycosylated proBNP was resistant to furin cleavage. We therefore hypothesized that intracellular non-glycosylated proBNP, but not glycosylated proBNP, would be efficiently processed by endogenous furin in BNP producing cells. However, repeated attempts failed to detect intracellular BNP32 in the 293T overexpression system, where non-glycosylated proBNP was a dominant intracellular form. Similarly, the T71A mutant, which should be more accessible for furin recognition, did not show increased intracellular BNP32 accumulation. Instead, we found increased alternatively processed form of BNP (∼8 kDa, Fig. 4B) in the T71A mutant-expressing cells. These observations suggest that intracellular proBNP is inherently resistant to intracellular furin cleavage. It is plausible that synthesized proBNP is compartmentalized in subcellular localizations where intracellular furin cannot easily access. In addition to furin, ANP convertase corin is also proposed as a BNP convertase 6. However, overexpression of corin in proBNP-expressing cells showed little effects on the concentrations of intracellular or extracellular BNP32. We did not find proBNP processing into BNP32 in HL-1 cells, which express endogenous corin 23. These observations suggest that corin may not process intracellular proBNP and proBNP is released as pro-hormone.
Detection of unprocessed or glycosylated proBNP in CHF patients 12, 13, 24 has established the concept that plasma proBNP is due to a defect in cardiac proBNP processing with the spillover of unprocessed proBNP into the plasma 9, 25, 26. Our observations of circulating proBNP in normal subjects 15 and the efficient secretion of glycosylated proBNP upon expression of pre-proBNP provide a novel model of BNP secretion, where glycosylated proBNP is a dominant form of BNP released from the normal heart. Since glycosylated proBNP shows a biological activity 6- to 8-fold less than that of BNP32 in vitro 13, it is possible that a subset of glycosylated proBNP function without proteolytic processing in vivo. However, since previous studies have demonstrated circulating NT-proBNP and BNP32 in human circulation, it is likely that glycosylated proBNP is processed into BNP32 in the circulation or on the target cells. This newer model provides an interesting possibility of no difference in BNP secretory process between diseased and normal hearts. It is plausible that CHF patients may have defects in processing the circulating proBNP. In this context, further understanding of the BNP maturation mechanism is critical.
The issues remain to be determined include (i) where the proBNP processing occurs after secretion, (ii) what protease works as a convertase for the secreted proBNP, (iii) how the BNP convertase can access to the recognition site in proBNP that is likely masked by glycosylation, and (iv) whether CHF patients have any defect in the processing steps of secreted proBNP. As for the problem of glycosylation masking the convertase recognition site, the study by Seferian et al. may provide an important clue 27. The authors have demonstrated that the central region (residues 28–56), but not the C-terminal region (residues 61-76), of circulating NT-proBNP in human blood is glycosylated. Given that proBNP is secreted as a fully glycosylated form, their observation may suggest the presence of de-glycosylation process before convertase recognition. Since a circulating glycosidase is known to play a role in serum vitamin D3-binding protein deglycosylation 28, it is conceivable that a glycosidase in the circulation, or on the target cells, may remove the O-glycosylation at T71 of proBNP, which facilitates the proteolytic processing of proBNP for local BNP32 activation. Indeed, we have recently shown that non-glycosylated proBNP can be processed in plasma in vitro 8. Further studies on the molecular forms of BNPs in healthy and diseased hearts and the precise mechanism of glycosylated proBNP processing in vivo would provide critical information for the diagnostic and therapeutic BNP applications.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by RO1 HL098502-01A1 (to A. C. and Y. I.), by Mayo Foundation, Marriott Individualized Medicine Award, Bernard and Edith Waterman Pilot Grant (to Y. I.) and NIH grants RO1 HL098502 (to Y.I. and A.C.) and RO1 HL36634 and PO1 HL76611 (to J. C. B.).
Footnotes
Disclosures: The authors have no conflict of interests.
References
- 1.Yamamoto K, Burnett JC, Jr, Redfield MM. Effect of endogenous natriuretic peptide system on ventricular and coronary function in failing heart. Am J Physiol. 1997;273:H2406–14. doi: 10.1152/ajpheart.1997.273.5.H2406. [DOI] [PubMed] [Google Scholar]
- 2.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Nat Acad Sci U S A. 2000;97:4239–44. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimasa T, Uehira M, et al. Molecular cloning of the complementary DNA and gene that encode mouse brain natriuretic peptide and generation of transgenic mice that overexpress the brain natriuretic peptide gene. J Clin Invest. 1994;93:1911–21. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki E, Hirata Y, Kohmoto O, Sugimoto T, Hayakawa H, Matsuoka H, et al. Cellular mechanisms for synthesis and secretion of atrial natriuretic peptide and brain natriuretic peptide in cultured rat atrial cells. Circ Res. 1992;71:1039–48. doi: 10.1161/01.res.71.5.1039. [DOI] [PubMed] [Google Scholar]
- 5.Minamino N, Kangawa K, Matsuo H. Isolation and identification of a high molecular weight brain natriuretic peptide in porcine cardiac atrium. Biochem Biophys Res Commun. 1988;157:402–9. doi: 10.1016/s0006-291x(88)80061-5. [DOI] [PubMed] [Google Scholar]
- 6.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Nat Acad Sci U S A. 2000;97:8525–9. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinhelper ME. Structure, expression, and genomic mapping of the mouse natriuretic peptide type-B gene. Circ Res. 1993;72:984–92. doi: 10.1161/01.res.72.5.984. [DOI] [PubMed] [Google Scholar]
- 8.Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, et al. Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–7. doi: 10.1373/clinchem.2010.153908. [DOI] [PubMed] [Google Scholar]
- 9.Heublein DM, Huntley BK, Boerrigter G, Cataliotti A, Sandberg SM, Redfield MM, Burnett JC., Jr Immunoreactivity and guanosine 3′,5′-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension. 2007;49:1114–9. doi: 10.1161/HYPERTENSIONAHA.106.081083. [DOI] [PubMed] [Google Scholar]
- 10.Yandle TG, Richards AM, Gilbert A, Fisher S, Holmes S, Espiner EA. Assay of brain natriuretic peptide (BNP) in human plasma: evidence for high molecular weight BNP as a major plasma component in heart failure. J Clin Endocrinol Metab. 1993;76:832–8. doi: 10.1210/jcem.76.4.8473392. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu H, Masuta K, Aono K, Asada H, Sasakura K, Tamaki M, et al. Molecular forms of human brain natriuretic peptide in plasma. Clin Chim Acta. 2002;316:129–35. doi: 10.1016/s0009-8981(01)00745-8. [DOI] [PubMed] [Google Scholar]
- 12.Giuliani I, Rieunier F, Larue C, Delagneau JF, Granier C, Pau B, et al. Assay for measurement of intact B-type natriuretic peptide prohormone in blood. Clin Chem. 2006;52:1054–61. doi: 10.1373/clinchem.2005.061770. [DOI] [PubMed] [Google Scholar]
- 13.Liang F, O'Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, et al. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49:1071–8. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 14.Schellenberger U, O'Rear J, Guzzetta A, Jue RA, Protter AA, Pollitt NS. The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch Biochem Biophys. 2006;451:160–6. doi: 10.1016/j.abb.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Macheret F, Boerrigter G, McKie PM, Costello-Boerrigter L, Lahr B, Heublein D, et al. Pro-B-type natriuretic peptide1-108 circulates in the general community: plasma determinants and detection of left ventricular dysfunction. J Am Coll Cordiol. 2011;57:1386–95. doi: 10.1016/j.jacc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Nat Acad Sci U S A. 1998;95:2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen B, Deng Y, Guan J, Yan W, Wang Y, Tan W, Gao J. Signal peptide replacements enhance expression and secretion of hepatitis C virus envelope glycoproteins. Acta biochim biophys Sin. 43:96–102. doi: 10.1093/abbs/gmq117. [DOI] [PubMed] [Google Scholar]
- 18.Semenov AG, Postnikov AB, Tamm NN, Seferian KR, Karpova NS, Bloshchitsyna MN, et al. Processing of pro-brain natriuretic peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clin Chem. 2009;55:489–98. doi: 10.1373/clinchem.2008.113373. [DOI] [PubMed] [Google Scholar]
- 19.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–55. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J, Pristera N, Wang W, Zhang X, Wu Q. Effect of sialylated O-glycans in pro-brain natriuretic peptide stability. Clin Chem. 2010;56:959–66. doi: 10.1373/clinchem.2009.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato K, Jeanneau C, Tarp MA, Benet-Pages A, Lorenz-Depiereux B, Bennett EP, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–7. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 22.Sawada Y, Suda M, Yokoyama H, Kanda T, Sakamaki T, Tanaka S, et al. Stretch-induced hypertrophic growth of cardiocytes and processing of brain-type natriuretic peptide are controlled by proprotein-processing endoprotease furin. J Biol Chem. 1997;272:20545–54. doi: 10.1074/jbc.272.33.20545. [DOI] [PubMed] [Google Scholar]
- 23.Gladysheva IP, Robinson BR, Houng AK, Kovats T, King SM. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J Mol Cell Cardiol. 2008;44:131–42. doi: 10.1016/j.yjmcc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Hammerer-Lercher A, Halfinger B, Sarg B, Mair J, Puschendorf B, Griesmacher A, et al. Analysis of circulating forms of proBNP and NT-proBNP in patients with severe heart failure. Clin Chem. 2008;54:858–65. doi: 10.1373/clinchem.2007.090266. [DOI] [PubMed] [Google Scholar]
- 25.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Nat Acad Sci U S A. 2005;102:17442–7. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seferian KR, Tamm NN, Semenov AG, Mukharyamova KS, Tolstaya AA, Koshkina EV, et al. The brain natriuretic peptide (BNP) precursor is the major immunoreactive form of BNP in patients with heart failure. Clin Chem. 2007;53:866–73. doi: 10.1373/clinchem.2006.076141. [DOI] [PubMed] [Google Scholar]
- 27.Seferian KR, Tamm NN, Semenov AG, Tolstaya AA, Koshkina EV, Krasnoselsky MI, et al. Immunodetection of glycosylated NT-proBNP circulating in human blood. Clin Chem. 2008;54:866–73. doi: 10.1373/clinchem.2007.100040. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto N, Naraparaju VR, Moore M, Brent LH. Deglycosylation of serum vitamin D3-binding protein by alpha-N-acetylgalactosaminidase detected in the plasma of patients with systemic lupus erythematosus. Clin immunol immunopathol. 1997;82:290–8. doi: 10.1006/clin.1996.4320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.