Abstract
Background
The scope of prehospital (PH) interventions has expanded recently; not always with clear benefit. PH crystalloid resuscitation has been challenged, particularly in penetrating trauma. Optimal PH crystalloid resuscitation strategies remain unclear in blunt trauma as does the influence of PH hypotension. The objective was to characterize outcomes for PH crystalloid volume in patients with and without PH hypotension.
Methods
Data were obtained from a multicenter prospective study of blunt injured adults transported from the scene with ISS > 15. Subjects were divided into HIGH (>500cc) and LOW (≤500cc) PH crystalloid groups. Propensity adjusted regression determined the association of PH crystalloid group with mortality and acute coagulopathy (admission INR>1.5) in subjects with and without PH hypotension (SBP<90mmHg) after controlling for confounders.
Results
Of 1,216 subjects, 822 (68%) received HIGH PH crystalloid and 616 (51%) had PH hypotension. Initial base deficit and ISS were similar between HIGH and LOW crystalloid groups in subjects with and without PH hypotension. In subjects without PH hypotension, HIGH crystalloid was associated with an increase in the risk of mortality (HR 2.5; 95%CI 1.3 – 4.9, p<0.01) and acute coagulopathy (OR 2.2; 95%CI 1.01–4.9, p=0.04) but not in subjects with PH hypotension. HIGH crystalloid was associated with correction of PH hypotension on ED arrival (OR 2.02; 1.06–3.88, p=0.03). The mean corrected SBP in the ED was 104mmHg. Each 1mmHg increase in ED systolic blood pressure was associated with a 2% increase in survival in subjects with PH hypotension (OR 1.02; 1.01–1.03, p<0.01).
Conclusions
In severely injured blunt trauma patients, PH crystalloid >500cc was associated with worse outcome in patients without PH hypotension but not with PH hypotension. HIGH crystalloid was associated with corrected PH hypotension. This suggests PH resuscitation should be goal directed based on the presence or absence of PH hypotension.
Level of Evidence
II, Therapeutic Study
Keywords: Prehospital, Crystalloid, Resuscitation, Blunt trauma, Outcome
INTRODUCTION
Injury is unquestionably a time sensitive disease.(1) This renders prehospital (PH) trauma care a critical component of trauma systems. PH providers must balance initiation of life-saving interventions following severe injury with rapid transport to a trauma center. As emergency medical systems (EMS) have evolved, the scope of therapy has expanded substantially. This includes initiation of intravenous access and fluid resuscitation. Although beneficial to many, there is evidence that not all injured patients benefit from PH fluid. Several studies support the “scoop and run” approach with limited PH interventions and rapid transport improving outcomes in trauma patients.(2–4)
The value of PH crystalloid following injury has been questioned, as there is little evidence of benefit. In the setting of penetrating torso trauma, hypotensive resuscitation with limited or no crystalloid has proven effective.(5) Conversely, several groups have demonstrated no increase in the risk of mortality with the administration of PH fluid in multiple settings.(6–8) Further, PH resuscitation is essential to avoid secondary injury in traumatic brain injury.(9, 10)
The role of PH crystalloid resuscitation in the setting of blunt trauma remains unclear, and there is little to guide PH providers in these patients. Current guidelines for PH fluid resuscitation notes scarce evidence in this population and could only recommend small boluses to achieve a palpable radial pulse or coherent mental status.(11)
The objective of the current study was to characterize outcomes associated with PH crystalloid volume resuscitation in severely injured blunt trauma patients. As PH hypotension has been well documented as an ominous predictor in this population,(12, 13) we hypothesized that outcomes associated with PH crystalloid resuscitation would differ based on the presence or absence of PH hypotension.
METHODS
Data were obtained from the Inflammation and the Host Response to Injury Large Scale Collaborative Program (www.gluegrant.org), supported by the National Institute of General Medical Sciences (NIGMS), which is a multicenter prospective cohort study of blunt injured adults with hemorrhagic shock designed to characterize the genomic and proteomic response following injury.(14) Patients admitted to one of seven institutions over an eight year period (2003–2010) were included. Inclusion criteria for the overall cohort study included: blunt mechanism, presence of PH or emergency department hypotension (Systolic blood pressure [SBP] < 90 mmHg) or an elevated base deficit (> 6 meq/L), blood transfusion requirement within the first 12hrs, and any body region exclusive of the brain with an abbreviated injury score (AIS) ≥ 2, allowing exclusion of patients with isolated traumatic brain injury (TBI). Patients < 18 or > 90 years of age and those with cervical spinal cord injury were also excluded. Clinical data were entered and stored in TrialDb, a web-based data collection platform, by trained research nurses.(15) Integrity of the data was maintained through ongoing curation and external data review by an independent chart abstractor.
Standard operating procedures were developed and implemented across all institutional centers to minimize variation in post-injury care, including: early goal directed resuscitation, strict glycemic control, venous thromboembolism prophylaxis, appropriate low tidal volume ventilation, ventilator associated pneumonia management, and restrictive transfusion guidelines.(16–19) While patients were admitted to the ICU, multiple organ dysfunction scores for renal, hepatic, cardiovascular, metabolic, hematologic, respiratory, and neurological systems were determined daily.(20–23) The diagnosis of MOF required a maximum Marshall Multiple Organ Dysfunction score >5.
Inclusion criteria for the current analysis were (1) scene transport; (2) injury severity score (ISS) >15; (3) known volume of PH crystalloid (including zero); and (4) recorded PH SBP. PH crystalloid volume was defined as HIGH when more than 500mL was given. This cut off was utilized based on a practical unit of volume for PH providers and prior work.(7) Subjects were then dichotomized as receiving HIGH or LOW PH crystalloid. Subjects were further categorized as to the presence or absence of PH hypotension, defined as PH SBP < 90mmHg. Demographics, injury characteristics, resuscitation requirements, and outcomes were compared for HIGH vs. LOW PH crystalloid groups in univariate analysis within both the hypotensive and non-hypotensive cohorts.
A propensity score for receiving HIGH PH crystalloid was developed. A logistic regression model with the outcome of HIGH crystalloid was used with the covariates of PH time (total time from injury to hospital arrival), PH blood, PH SBP, ISS, and initial base deficit. This model designated a propensity score between 0 and 1 corresponding to the likelihood of receiving HIGH PH crystalloid based on the above factors. These factors were selected based on the limited PH variables in the database that would be available to PH providers and reasonably may influence the volume of PH crystalloid administration. ISS and initial base deficit, while not available to PH providers, were utilized as surrogates of injury severity that a PH provider would subjectively evaluate and use to guide resuscitation efforts as well. A receiver operating characteristic curve was developed with area under the curve (AUC) calculation to test the effectiveness of the propensity score. The AUC was 0.75, indicating a strong ability to determine the propensity to receive HIGH crystalloid. Table 1 demonstrates quartiles for the propensity score in HIGH and LOW groups (p<0.001). The propensity score was utilized as a covariate in outcome modeling to control for the propensity to receive HIGH PH crystalloid. The outcome models had c-statistics ranging from 0.89 to 0.98, demonstrating excellent model fit and discrimination. Collinearity diagnostics in the outcome models demonstrated the tolerance statistic was >0.10 and the variance inflation factor statistic <10 for all regression variables including the PH crystalloid propensity score, indicating low likelihood of multicollinearity.
Table 1.
Distribution of propensity scores to receive HIGH PH crystalloid
| Quartile | HIGH | LOW |
|---|---|---|
| 25% | 0.29 | 0.10 |
| 50% (median) | 0.45 | 0.20 |
| 75% | 0.57 | 0.35 |
The outcomes of interest included 30 day in-hospital mortality and acute traumatic coagulopathy (ATC), defined as admission INR of >1.5. Cox proportional hazards regression was utilized to determine the independent risk of mortality across HIGH and LOW PH crystalloid groups after controlling for confounders. Logistic regression was utilized to determine the independent risk of ATC across HIGH and LOW PH crystalloid groups. The outcome models were performed in patients with and without PH hypotension. Covariates used in the regression models included age, gender, PH packed red blood cell (PRBC) volume, PH heart rate, PH GCS, total PH time, ISS, admission base deficit, admission hemoglobin, admission INR, emergency department (ED) temperature, ED hypotension, vasopressor use, early surgery defined as laparotomy or thoracotomy within 48 hours, total 24 hour PRBC, fresh frozen plasma (FFP), platelet, and crystalloid volumes, treating trauma center, and propensity to receive HIGH crystalloid. Similar models were constructed to examine early mortality in the first 24 hours.
To further characterize the relationship between PH crystalloid volume and mortality for dose related effects, Cox-adjusted survival curves were constructed across PH crystalloid volume in five subgroups: 0mL, 1–500mL, 501–1000mL, 1001–2000mL, and >2000mL. Additionally, primary cause of death was compared between HIGH and LOW PH crystalloid groups in subjects with and without PH hypotension.
As PH hypotension is a significant predictor of adverse outcome,(12, 13) the relationship between PH hypotension and ED hypotension was explored. Logistic regression was used to determine the association of PH crystalloid group on correcting PH hypotension (i.e. SBP >90mmHg in the ED in subjects with PH hypotension). Further, the association of ED SBP and survival was determined in a logistic regression model utilizing age, sex, PH time, initial base deficit, and ISS as covariates to determine if there was any survival benefit of correcting PH hypotension.
Data analysis was conducted using SPSS version 19 (Chicago, IL). For univariate analyses Chi-square tests were used to compare categorical variables, and Mann-Whitney tests were used to compare continuous variables. Continuous data are presented as median (interquartile range [IQR]) or mean±standard deviation unless noted. To address missing data, multiple imputation was performed for HR, SBP, GCS, PH time, ISS, and base deficit. Multiple imputation using a fully conditional specification model based on available demographics, prehospital physiology, and PH time with a successive impute and predict algorithm for each variable was performed using a single imputation step to develop a complete dataset. A p value of ≤ 0.05 was considered significant. The institutional review board of each participating center approved the study.
RESULTS
Of the 2,007 subjects enrolled in the prospective cohort, 791 were excluded (90% due to transfer status or ISS < 15), leaving 1,216 (61%) subjects for analysis. The median age was 41 (26–54) and median ISS 34 (25–43). There were 616 (51%) subjects with PH hypotension, and 822 (69%) subjects received more than 500mL of PH crystalloid. The incidence of TBI was similar between those receiving HIGH and LOW PH crystalloid (27% vs. 24%, p=0.26)
In subjects without PH hypotension, there was no significant difference between HIGH and LOW PH crystalloid groups in demographics, ISS, or initial base deficit (p>0.05). Subjects that received HIGH PH crystalloid had longer PH times, higher admission INR, and 24 hour resuscitation requirements. Subjects in the HIGH PH crystalloid group had a higher rate of multiple organ failure (MOF) and acute respiratory distress syndrome (ARDS). There was no difference is 24 hour or 30 day mortality in univariate analysis (Table 2).
Table 2.
Demographics, injury characteristics, and outcomes in subjects without PH hypotension
| HIGH | LOW | p value | |
|---|---|---|---|
| N (%) | 342 (59) | 241 (41) | - |
| Age (years) | 41 (26 – 55) | 43 (29 – 54) | 0.23 |
| Gender (% male) | 68 | 64 | 0.33 |
| PH SBP low (mmHg) | 110±21 | 116±22 | <0.01 |
| PH time (mins) | 72 (48 – 90) | 48 (36 – 66) | <0.01 |
| Initial BD | −8.4±4 | −8.4±5 | 0.41 |
| Initial INR | 1.5±0.7 | 1.2±0.3 | <0.01 |
| ISS | 41 (34 – 50) | 34 (27 – 43) | 0.10 |
| 24hr PRBC (units) | 6.8 (3.5 – 11.4) | 4.7 (3.3 – 14.2) | <0.01 |
| 24hr FFP (units) | 4 (1.0 – 8.1) | 1.2 (0 – 3.7) | <0.01 |
| 24hr PLT (6 pack) | 0 (0 – 1.1) | 0 (0 – 0.7) | <0.01 |
| 24hr Crystalloid (L) | 13.6 (10.1 – 18.8) | 10.5 (6.9 – 14.2) | <0.01 |
| 30 day mortality (%) | 17 | 12 | 0.09 |
| 24 hour mortality (%) | 6 | 7 | 0.86 |
| ATC (%) | 27 | 7 | <0.01 |
| MOF (%) | 35 | 25 | 0.01 |
| ARDS (%) | 27 | 19 | 0.03 |
PH, prehospital; SBP, systolic blood pressure; BD, base deficit; PRBC, packed red blood cells; FFP, fresh frozen plasma; PLT, platelets; ATC, acute traumatic coagulopathy
Within the PH hypotension cohort there was no difference between HIGH and LOW groups in demographics, ISS, or initial base deficit (p>0.05). Subjects that received HIGH PH crystalloid had longer PH times, higher admission INR, and 24 resuscitation requirements. There was no difference in MOF, ARDS, or mortality between crystalloid groups in univariate analysis. (Table 3).
Table 3.
Demographics, injury characteristics, and outcomes in subjects with PH hypotension
| HIGH | LOW | p value | |
|---|---|---|---|
| N (%) | 480 (80) | 123 (20) | - |
| Age (years) | 40 (25 – 52) | 40 (29 – 54) | 0.28 |
| Gender (% male) | 66 | 76 | 66 |
| PH SBP low (mmHg) | 66±25 | 70±19 | 0.07 |
| PH time (mins) | 66 (48 – 90) | 42 (30 – 66) | <0.01 |
| Initial BD | −8.8±5 | −9.5±6 | 0.10 |
| Initial INR | 1.6±0.9 | 1.3±0.6 | <0.01 |
| ISS | 41 (31 – 50) | 41 (29 – 50) | 0.53 |
| 24hr PRBC (units) | 8.2 (4.7 – 15.1) | 7 (3.3 – 14.2) | 0.02 |
| 24hr FFP (units) | 4 (1.3 – 9.1) | 2.6 (0 – 6.7) | <0.01 |
| 24hr PLT (6 pack) | 0.7 (0 – 1.7) | 0 (0 – 1.1) | 0.06 |
| 24hr Crystalloid (L) | 14.1 (10.5 – 19.1) | 10.7 (7.1 – 17.8) | <0.01 |
| 30 day mortality | 18 | 19 | 0.90 |
| 24 hour mortality | 8 | 7 | 0.58 |
| ATC (%) | 33 | 8 | <0.01 |
| MOF (%) | 31 | 38 | 0.16 |
| ARDS (%) | 29 | 23 | 0.18 |
PH, prehospital; SBP, systolic blood pressure; BD, base deficit; PRBC, packed red blood cells; FFP, fresh frozen plasma; PLT, platelets; ATC, acute traumatic coagulopathy
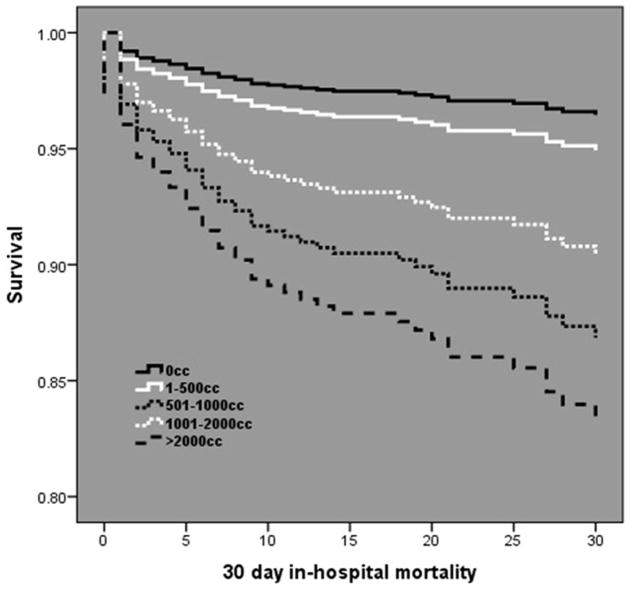
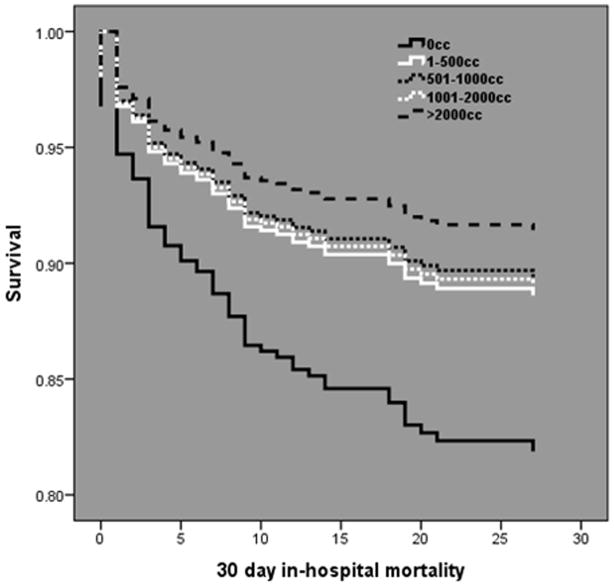
Cox regression analysis demonstrated in subjects without PH hypotension, HIGH PH crystalloid was independently associated with a more than two fold increase in 30 day in-hospital mortality (HR 2.45; 95%CI 1.25 – 4.83, p=0.01). In subjects with PH hypotension, PH crystalloid volume was not associated with mortality (Fig. 1). When stratified by volume subgroup, mortality was directly related with increasing PH crystalloid volume in subjects without PH hypotension, with the lowest mortality occurring in those receiving no PH crystalloid (Fig. 2). Conversely, mortality was inversely related with increasing PH crystalloid volume in subjects with PH hypotension, with the lowest mortality occurring in those receiving >2000mL of PH crystalloid (Fig. 3).
Figure 1.
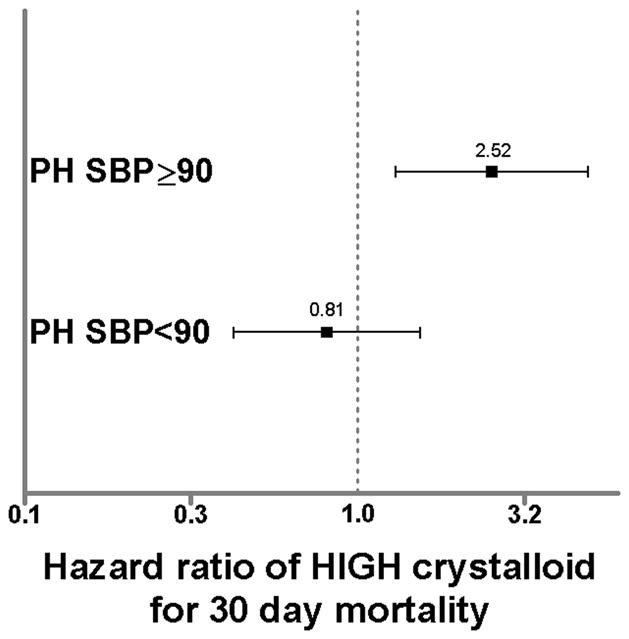
Hazard ratios from Cox regression for 30 day mortality in subjects without PH hypotension (PH SBP≥90) and with PH hypotension (PH SBP<90). Hazard ratios represent HIGH relative LOW PH crystalloid group. Bars represent 95% confidence intervals. Bars that do not cross 1.0 are considered significant (p<0.05).
Figure 2.
Cox-adjusted survival curve analysis in subjects without PH hypotension over 30 days post injury. A direct dose related relationship exists between mortality and PH crystalloid volume.
Figure 3.
Cox-adjusted survival curve analysis in subjects with PH hypotension over 30 days post injury. An inverse dose related relationship exists between mortality and PH crystalloid volume.
Logistic regression showed HIGH PH crystalloid was an independent predictor of ATC in subjects without PH hypotension (OR 2.21; 95%CI 1.01 – 4.86, p=0.04). HIGH PH crystalloid demonstrated a trend as a predictor of ATC in subjects with PH hypotension, but did not reach statistical significance (OR 2.55; 95%CI 0.88 – 7.39, p=0.08).
Analysis of 24 hour mortality demonstrated no association with PH crystalloid volume in subjects with PH hypotension (OR 1.40; 9%CI 0.33 – 6.03, p=0.65). For subjects without PH hypotension, HIGH PH crystalloid demonstrated a trend towards increased 24 hour mortality, but did not reach statistical significance (OR 3.68; 95%CI 0.78 – 17.24, p=0.10).
When comparing the primary cause of death across PH crystalloid groups, the largest differences in subjects without PH hypotension were seen for brain death (16% HIGH vs. 6% LOW) and MOF (16% HIGH vs. 9% LOW). The largest difference in subjects with PH hypotension was TBI (2% HIGH vs. 13% LOW). Due to small numbers in each cause of death, none of the comparisons reached statistical significance.
When exploring the effect of correcting PH hypotension, HIGH PH crystalloid was independently associated with absence of hypotension (SBP > 90mmHg) in the ED in subjects with PH hypotension (OR 2.02; 95%CI 1.06 – 3.88, p=0.03). The mean ED SBP in subjects with corrected PH hypotension was 104±15mmHg. Mortality in subjects with corrected PH hypotension was significantly lower than subjects with uncorrected PH hypotension on arrival to the ED (13% vs. 21%, p=0.04). In subjects with PH hypotension, each 1mmHg increase in arrival ED SBP was independently associated with a 2% increase in overall survival (OR 1.02; 95%CI 1.01 – 1.03, p<0.01).
DISCUSSION
Prehospital fluid resuscitation has evolved substantially. Accumulating evidence demonstrates conflicting conclusions regarding the impact PH fluids have on outcome. Bickell and colleagues randomized patients with penetrating torso injury to delayed versus immediate PH fluid resuscitation, finding reduced mortality in the delayed group.(5) The authors postulated that delayed resuscitation reduced bleeding propagated by increasing the blood pressure, a theory supported by animal data.(24–26)
It is not clear that these findings apply equally in severe blunt trauma and several authors have documented no worsening of outcomes with PH fluid administration and some benefit in the setting of head injury.(6–11, 27) Prior studies have been limited by several factors. Findings may not reflect the capabilities of current PH providers. Further, many studies did not control for factors known to influence outcomes in trauma patients. Finally, PH times in prior studies are short and bias the results towards not observing an effect from PH fluids.
The results of this analysis demonstrate in patients without PH hypotension, a PH crystalloid volume >500mL was associated with a two fold increase in mortality after controlling for confounders. This effect exhibited a dose response relationship, with increasing volume of PH crystalloid associated with increasing mortality. Sampalis and colleagues also demonstrated that PH crystalloid resuscitation was associated with an increase in the risk of death after controlling for confounders.(28) The authors found the mortality risk was greater with longer PH times; however they did not control for PH SBP. Haut and colleagues found that PH intravenous access was associated with increased mortality.(29) This study was limited, however; due to the inability to differentiate intravenous access only versus fluid resuscitation and what volume of PH fluid was administered.
Furthermore, HIGH PH crystalloid was independently associated with a two fold increase in the risk for ATC. In non-survivors, MOF was the cause of death nearly twice as often in the HIGH group when compared with the LOW group. Additionally, there were no differences in early mortality between the groups, but an increase in the risk of mortality at 30 days in patients without PH hypotension receiving higher PH crystalloid volume, suggesting the sequelae of higher crystalloid resuscitation develops after the first 24 hours.
Previous work has also demonstrated that large volume crystalloid infusion is associated with systemic acquired coagulopathy and hemodilution.(30, 31) Brackenridge et al reported large volume crystalloid resuscitation was associated with MOF at 28 days.(32) More recent data has demonstrated higher volumes of PH crystalloid were associated with hyperfibrinolysis on thromboelastography at admission.(33) The authors noted each additional liter of PH crystalloid increased the odds of developing hyperfibrinolysis by 15%. This would be consistent with the dose response relationship seen in the current data. The downstream effects of coagulopathy, MOF, and hyperfibrinolysis may drive the associated increase in 30day mortality, particularly since there was no increase in 24hour mortality.
Conversely, in patients with PH hypotension, PH crystalloid volume was not a predictor of mortality. PH crystalloid volume demonstrated an inverse dose response relationship with mortality. The risk for ATC was increased in the HIGH group, although this was not significant following adjustment.
A number of studies have shown no effect on overall survival by PH fluid administration. Turner and colleagues randomized patients to standard PH fluid therapy versus no PH fluid therapy, finding no difference at 6 months.(6) Kaweski et al reported similar mortality rates for patients receiving standard fluids versus no fluids when stratified by ISS and hypotension.(34) The authors also noted that PH hypotension was associated higher mortality; however PH resuscitation did not reduce the rate. Smith et al examined patients categorized by presenting SBP.(35) The authors found that PH crystalloid did not alter SBP and a third of deaths were felt to be surgically correctable. They concluded the risks of delay with PH resuscitation outweigh the benefits. A more recent study found that intravenous access did not prolong scene time and patients receiving PH crystalloid had higher survival but this did not reach significance.(27) Dula and colleagues studied hypotensive patients receiving no PH crystalloid or >500mL matched for ISS.(7) Although the authors demonstrated PH crystalloid increased SBP, no difference in mortality was seen.
HIGH crystalloid was associated with correction of hypotension on arrival to the ED in the current study. This is consistent with prior reports of patients receiving at least 500mL of PH crystalloid.(7) Increasing SBP in the ED was associated with increasing survival in those with PH hypotension. It should be noted that correction of PH hypotension in the HIGH PH crystalloid group did not result in return to “normotension”. There is evidence that limited resuscitation to a SBP of 100mmHg results is similar mortality as a target SBP of 70mmHg.(8) Patients received just enough crystalloid to correct hypotension, avoiding “popping the clot”. Further, it is well established in patients with TBI, PH resuscitation should keep SBP >90mmHg to maintain cerebral perfusion,(11) which may have played a role in the higher TBI deaths in the LOW crystalloid group. While there was no direct decrease in mortality associated with HIGH PH crystalloid in the PH hypotension cohort, this data suggests patients may derive some benefit of PH crystalloid resuscitation to correct PH hypotension.
This analysis has several limitations. The current study is a secondary analysis of a prospective cohort and was not designed to address these specific questions. PH variables were limited and there may be factors related to administration of PH fluids and outcomes that were not analyzed for this study. Further, PH care was not standardized between centers. There is no data on why individual patients received higher or lower PH crystalloid. There was also no information on the type of crystalloid administered, precluding analysis of this potential confounder. The propensity score performed well with the limited data; however it does not account for all variation in PH crystalloid administration. For these reasons, HIGH PH crystalloid may be viewed as a marker of injury; however we would then expect a deleterious effect irrespective of PH hypotension which was not seen.
This study demonstrates in severely injured blunt trauma patients, PH crystalloid volume greater than 500mL is associated with an increased risk of mortality and coagulopathy in the absence of PH hypotension. Mortality and coagulopathy were not associated with PH crystalloid volume in patients with PH hypotension. Higher volume PH crystalloid was, however, associated with correction of PH hypotension, and improving SBP on hospital admission in turn associated with increasing survival. These findings suggest PH crystalloid resuscitation should be goal directed based on the presence or absence of PH hypotension. Presently crystalloid remains the mainstay of PH fluid resuscitation and further prospective study is warranted to determine optimal PH crystalloid resuscitation strategies in the setting of blunt trauma.
Acknowledgments
Funding/Support: NIH NIGMS U54 GM062119-1 and NIH NIGMS K23GM093032-1.
Footnotes
This paper was presented as an oral presentation at the annual meeting of the American Association for the Surgery of Trauma in Kauai, Hawaii, September 11th–15th, 2012.
Author Contributions: J.B.B. and J.L.S. designed the study, performed the literature search, data collection and analysis. All authors contributed to data interpretation, manuscript preparation, and critical revision of the manuscript.
References
- 1.Sampalis JS, Denis R, Lavoie A, et al. Trauma Care Regionalization: A Process-Outcome Evaluation. The Journal of Trauma and Acute Care Surgery. 1999;46:565–581. doi: 10.1097/00005373-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Seamon MJ, Fisher CA, Gaughan J, et al. Prehospital Procedures Before Emergency Department Thoracotomy: “Scoop and Run” Saves Lives. The Journal of Trauma and Acute Care Surgery. 2007;63:113–120. doi: 10.1097/TA.0b013e31806842a1. [DOI] [PubMed] [Google Scholar]
- 3.Krausz MM, Bar-Ziv M, Rabinovici R, et al. “Scoop and Run” or Stabilize Hemorrhagic Shock With Normal Saline or Small-Volume Hypertonic Saline? The Journal of Trauma and Acute Care Surgery. 1992;33:6–10. doi: 10.1097/00005373-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Demetriades DCL, Cornwell E, et al. Paramedic vs Private Transportation of Trauma Patients: Effect on Outcome. Archives of Surgery. 1996;131:133–138. doi: 10.1001/archsurg.1996.01430140023007. [DOI] [PubMed] [Google Scholar]
- 5.Bickell WH, Wall MJ, Pepe PE, et al. Immediate versus Delayed Fluid Resuscitation for Hypotensive Patients with Penetrating Torso Injuries. New England Journal of Medicine. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 6.Turner J, Nicholl J, Webber L, et al. A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma. Health technology assessment (Winchester, England) 2000;4:1–57. [PubMed] [Google Scholar]
- 7.Dula DJ, Wood GC, Rejmer AR, et al. Use of prehospital fluids in hypotensive blunt trauma patients. Prehospital emergency care : official journal of the National Association of EMS Physicians and the National Association of State EMS Directors. 2002;6:417–420. doi: 10.1080/10903120290938058. [DOI] [PubMed] [Google Scholar]
- 8.Dutton RP, Mackenzie CF, Scalea TM. Hypotensive Resuscitation during Active Hemorrhage: Impact on In-Hospital Mortality. The Journal of Trauma and Acute Care Surgery. 2002;52:1141–1146. doi: 10.1097/00005373-200206000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Wald SL, Shackford SR. The Effect of Secondary Insults on Mortality and Long-Term Disability After Severe Head Injury in A Rural Region Without A Trauma System. The Journal of Trauma and Acute Care Surgery. 1993;34:377–382. doi: 10.1097/00005373-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Shackford SR. Prehospital Fluid Resuscitation of Known or Suspected Traumatic Brain Injury. The Journal of Trauma and Acute Care Surgery. 2011;70:S32–S33. doi: 10.1097/TA.1090b1013e31821a35858. [DOI] [PubMed] [Google Scholar]
- 11.Cotton BA, Jerome R, Collier BR, et al. Guidelines for Prehospital Fluid Resuscitation in the Injured Patient. The Journal of Trauma and Acute Care Surgery. 2009;67:389–402. doi: 10.1097/TA.0b013e3181a8b26f. [DOI] [PubMed] [Google Scholar]
- 12.Franklin GA, Boaz PW, Spain DA, et al. Prehospital Hypotension as a Valid Indicator of Trauma Team Activation. The Journal of Trauma and Acute Care Surgery. 2000;48:1034–1039. doi: 10.1097/00005373-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bilello JF, Davis JW, Lemaster D, et al. Prehospital Hypotension in Blunt Trauma: Identifying the “Crump Factor”. The Journal of Trauma and Acute Care Surgery. 2011;70:1038–1042. doi: 10.1097/TA.0b013e31819638d0. [DOI] [PubMed] [Google Scholar]
- 14.Maier RV, Bankey P, McKinley B, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: Patient-Oriented Research Core-Standard Operating Procedures for Clinical Care: Foreward. The Journal of Trauma and Acute Care Surgery. 2005;59:762–763. [PubMed] [Google Scholar]
- 15.Brandt CA, Deshpande AM, Lu C, et al. TrialDB: A web-based Clinical Study Data Management System. AMIA Annual Symposium proceedings / AMIA Symposium AMIA Symposium; 2003. p. 794. [PMC free article] [PubMed] [Google Scholar]
- 16.Harbrecht BG, Minei JP, Shapiro MB, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: Patient-Oriented Research Core-Standard Operating Procedures for Clinical Care: VI. BLOOD GLUCOSE CONTROL IN THE CRITICALLY ILL TRAUMA PATIENT. The Journal of Trauma and Acute Care Surgery. 2007;63:703–708. doi: 10.1097/TA.0b013e31811eadea. [DOI] [PubMed] [Google Scholar]
- 17.Nathens AB, Johnson JL, Minei JP, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: Patient-Oriented Research Core-Standard Operating Procedures for Clinical Care: I. Guidelines for Mechanical Ventilation of the Trauma Patient. The Journal of Trauma and Acute Care Surgery. 2005;59:764–769. [PubMed] [Google Scholar]
- 18.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Reponse to Injury, a Large-Scale Collaborative Project: Patient-Oriented Research Core-Standard Operating Procedures for Clinical Care: II. Guidelines for Prevention, Diagnosis and Treatment of Ventilator-Associated Pneumonia (VAP) in the Trauma Patient. The Journal of Trauma and Acute Care Surgery. 2006;60:1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. [DOI] [PubMed] [Google Scholar]
- 19.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: Patient-Oriented Research Core-Standard Operating Procedures for Clinical Care: III. Guidelines for Shock Resuscitation. The Journal of Trauma and Acute Care Surgery. 2006;61:82–89. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 20.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 21.Carrico CJ, Meakins JL, Marshall JC, et al. Multiple-organ-failure syndrome. Archives of surgery (Chicago, Ill : 1960) 1986;121:196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 22.Marshall JC. Organ dysfunction as an outcome measure in clinical trials. The European journal of surgery Supplement : = Acta chirurgica Supplement. 1999:62–67. doi: 10.1080/11024159950188583. [DOI] [PubMed] [Google Scholar]
- 23.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Critical care medicine. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Krausz MM, Hirsh M. Bolus versus Continuous Fluid Resuscitation and Splenectomy for Treatment of Uncontrolled Hemorrhagic Shock after Massive Splenic Injury. The Journal of Trauma and Acute Care Surgery. 2003;55:62–68. doi: 10.1097/01.TA.0000074110.77122.46. [DOI] [PubMed] [Google Scholar]
- 25.Capono A, Safar P, Tisherman S, et al. Treatment of Uncontrolled Hemorrhagic Shock: Improved Outcome With Fluid Restriction. The Journal of Trauma and Acute Care Surgery. 1993;35:984. [Google Scholar]
- 26.Stern SA, Kowalenko T, Younger J, et al. Comparison of the effects of bolus vs. slow infusion of 7. 5% NaCl/6% dextran-70 in a model of near-lethal uncontrolled hemorrhage. Shock (Augusta, Ga) 2000;14:616–622. doi: 10.1097/00024382-200014060-00008. [DOI] [PubMed] [Google Scholar]
- 27.Eckstein M, Chan L, Schneir A, et al. Effect of Prehospital Advanced Life Support on Outcomes of Major Trauma Patients. The Journal of Trauma and Acute Care Surgery. 2000;48:643–648. doi: 10.1097/00005373-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Sampalis JS, Tamim H, Denis R, et al. Ineffectiveness of On-Site Intravenous Lines: Is Prehospital Time the Culprit? The Journal of Trauma and Acute Care Surgery. 1997;43:608–617. doi: 10.1097/00005373-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Haut ER, Kalish BT, Cotton BA, et al. Prehospital intravenous fluid administration is associated with higher mortality in trauma patients: a National Trauma Data Bank analysis. Annals of surgery. 2011;253:371–377. doi: 10.1097/SLA.0b013e318207c24f. [DOI] [PubMed] [Google Scholar]
- 30.Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: its pathophysiology and treatment in the injured patient. World journal of surgery. 2007;31:1055–1064. doi: 10.1007/s00268-006-0653-9. [DOI] [PubMed] [Google Scholar]
- 31.Cohen MJ, West M. Acute Traumatic Coagulopathy: From Endogenous Acute Coagulopathy to Systemic Acquired Coagulopathy and Back. The Journal of Trauma and Acute Care Surgery. 2011;70:S47–S49. doi: 10.1097/TA.0b013e31821a5c24. [DOI] [PubMed] [Google Scholar]
- 32.Brakenridge SC, Phelan HA, Henley SS, et al. Early blood product and crystalloid volume resuscitation: risk association with multiple organ dysfunction after severe blunt traumatic injury. The Journal of trauma. 2011;71:299–305. doi: 10.1097/TA.0b013e318224d328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotton BA, Harvin JA, Kostousouv V, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73:365–370. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 34.KAWESKI SM, SISE MJ, VIRGILIO RW. The Effect of Prehospital Fluids on Survival in Trauma Patients. The Journal of Trauma and Acute Care Surgery. 1990;30:1215–1219. doi: 10.1097/00005373-199010000-00005. [DOI] [PubMed] [Google Scholar]
- 35.SMITH JP, BODAI BI, HILL AS, et al. Prehospital Stabilization of Critically Injured Patients: A Failed Concept. The Journal of Trauma and Acute Care Surgery. 1985;25:65–70. doi: 10.1097/00005373-198501000-00011. [DOI] [PubMed] [Google Scholar]