Abstract
Obstructive sleep apnea (OSA) is characterized by episodes of repeated airway obstruction resulting in cessation (apnea) or reduction (hypopnea) in airflow during sleep. These events lead to intermittent hypoxia and hypercapnia, sleep fragmentation, and changes in intrathoracic pressure, and are associated with a marked surge in sympathetic activity and an abrupt increase in blood pressure. Blood pressure remains elevated during wakefulness despite the absence of obstructive events resulting in a high prevalence of hypertension in patients with OSA. There is substantial evidence that suggests that chronic intermittent hypoxia (CIH) leads to sustained sympathoexcitation during the day and changes in vasculature resulting in hypertension in patients with OSA. Mechanisms of sympathoexcitation include augmentation of peripheral chemoreflex sensitivity and a direct effect on central sites of sympathetic regulation. Interestingly, the vascular changes that occur with CIH have been ascribed to the same molecules that have been implicated in the augmented sympathetic tone in CIH. This review will discuss the hypothesized molecular mechanisms involved in the development of hypertension with CIH, will build a conceptual model for the development of hypertension following CIH, and will propose a systems biology approach in further elucidating the relationship between CIH and the development of hypertension.
Keywords: obstructive sleep apnea, hypertension, chronic intermittent hypoxia, sympathoexcitation, systems biology
I. INTRODUCTION
Obstructive sleep apnea (OSA) is a disease of disordered breathing during sleep. An estimated 40 million Americans suffer from OSA with a 3–7% prevalence in men and a 2–5% prevalence in women.1 Patients with OSA have pharyngeal closure while asleep due to a loss of tone of the upper airway muscles. As a result, they frequently stop breathing in sleep. Complete pharyngeal closure results in an absence of airflow (apnea), while partial closure results in a decrease in airflow (hypopnea) during breathing. Apneas and hypopneas can result in significant oxygen desaturation (hypoxia) and significant increase in arterial carbon dioxide (hypercapnia). Recurrent events of apneas and hypopneas lasting from a few seconds to almost a minute can occur throughout the sleep duration. These events are terminated by an arousal from sleep associated with tremendous increases in intrathoracic pressure that results in airway opening. Thus, these episodes are associated with chronic intermittent hypoxia (CIH) and hypercapnia, increase in intrathoracic pressure, and sleep fragmentation. Each of these episodes is associated with a marked surge in sympathetic activity and abrupt increase in blood pressure.2 Severity of OSA is defined by the sum of apnea and hypopneas per hour of sleep (apnea hypopnea index, or AHI). Normally there are less than five apneas and hypopneas per hour of sleep. Mild OSA is defined as an AHI of 5–15, moderate as an AHI of 15–30, and severe as an AHI of greater than 30 events per hour of sleep.3 Obesity and certain craniofacial features are notable predisposing factors for OSA. OSA occurs in obese patients during sleep as a result of increased fat deposition surrounding the upper airway resulting in a smaller airway lumen and increased collapsibility. The volume of adipose tissue is related to the presence and degree of OSA.4 Treatment of OSA is most often with continuous positive airway pressure (CPAP). Positive pressure is applied noninvasively through an airtight mask over the nose, or nose and mouth, during both inspiration and expiration. CPAP acts as a mechanical splint preventing recurrent obstruction of the airway during sleep, and is effective in the treatment of OSA.
II. HYPERTENSION AND OSA
The cardiovascular consequences of OSA are substantial, including hypertension, cardiac arrhythmias, congestive heart failure, and stroke,5 and increasing severity of OSA has been associated with an increased risk for the development of hypertension and stroke.6,7 Hypertension is highly prevalent in patient with OSA, with an incidence ranging from 30% to 60%.8 Hypertension in patients with OSA is characterized by an absence of expected normal nocturnal decline in systolic and diastolic pressures.9 Furthermore, blood pressure remains elevated during wakefulness in spite of the absence of obstructive events, and hypoxemia and is often difficult to control with drug therapy. Conversely, OSA is also prevalent in patients with hypertension, with approximately 30% of hypertensive individuals having OSA. The prevalence of OSA may be greater than 80% in middle-aged adults with drug-resistant hypertension.10
Hypertension associated with sleep apnea could potentially be linked to sleep fragmentation, changes in intrathoracic pressure, or to CIH. CIH has been implicated in the development of hypertension through the activation of the sympathetic nervous system and through altered vascular structure and function.2, 11
III. CIH and The activation of the sympathetic nervous system
Several animal and human studies have shown that CIH plays a major role in the development of hypertension through the activation of the sympathetic nervous system. For example, work in dogs12 and rats13 has shown that CIH alone can induce a persistent hypertension due to elevated sympathetic tone,14, 15 and that renal sympathectomy or adrenal medullectomy results in normalization of hypertension seen in rats exposed to CIH.14 Elevated sympathetic tone in patients with sleep apnea is seen at night and persists during the day,16, 17 and nasal CPAP therapy reduces the increased muscle sympathetic nerve activity in patients with OSA.18 The causes of this sympathoexcitation after withdrawal of the chemical stimuli remain uncertain, but evidence indicates that CIH leads to sympathoexcitation by two mechanisms, namely, (i) augmentation of peripheral chemoreflex sensitivity and (ii) direct effects on central sites of sympathetic regulation.
IV. THE CAROTID BODY AND ITS CONTRIBUTION TO THE ENHANCED SYMPATHETIC RESPONSES INDUCED BY CIH
In order to maintain a normal internal milieu, central brain stem neurons obtain sensory input about the level of arterial oxygen, carbon dioxide, and acid-base balance from peripheral and central chemoreceptors. One important peripheral chemoreceptor is the carotid body. The carotid body senses changes in arterial PO2, PCO2, and pH, releases neurotransmitters, and through afferent sensory projections to brain stem neurons controlling respiration and sympathetic outflow, causes changes in ventilation or sympathetic output.19 Glomus cells in the carotid body act as oxygen sensors, and are in close apposition to the carotid sinus nerve terminals whose soma are in the petrosal ganglion. Second-order neurons then project to the nucleus tractus solitarius in the brain stem that then send projections to the hypothalamic paraventricular nucleus and brain stem sympathoexcitatory sites including the C1 region of the rostral ventrolateral medulla (RVLM). Progressive hypoxia enhances peripheral hypoxic chemosensitivity, manifesting as an exponential increase in carotid sinus nerve activity.19 Several lines of evidence suggest that peripheral hypoxic chemosensitivity is augmented by exposure to CIH in various species including rats, cats, and in mice. Increase in carotid sinus nerve activity, increased sympathetic vasoconstrictor outflow, and enhanced chemoreflex-induced sympathoexcitation during subsequent acute hypoxia exposure have been reported in rats exposed to 14 days of cyclic hypoxia consisting of 20s of hypoxia every 5 min, 8 h per day.20 Intermittent hypoxia also increases the slope and intercept of sympathetic nerve activity in rats in response to acute hypoxia.21 In cats exposed to intermittent hypoxia 8 h per day for four days, there is an increase in baseline chemosensory discharge and the responses to acute mild and severe hypoxia.22 Carotid body responses to acute hypoxia are also augmented in mice exposed to 10 days of CIH (15 s of hypoxia followed by 5 min of normoxia, nine episodes per hour, 8 h per day).23 CIH induces a long-lasting activation of baseline carotid body activity and has been termed sensory long-term facilitation (sLTF). sLTF is unique to the stimulus of CIH on the carotid body, since comparative cumulative duration of chronic sustained hypoxia does not elicit such a response.24
V. MOLECULAR MECHANISMS UNDERLYING CIH-INDUCED CAROTID BODY CHEMOSENSORY POTENTIATION
A. Reactive Oxygen Species
Chronic intermittent hypoxia episodes cycle between progressive hypoxia followed by progressive reoxygenation. These hypoxia-reoxygenation cycles result in the accumulation of reactive oxygen species (ROS) and oxidative stress. Several human studies have demonstrated the generation of ROS in OSA,25,26 and have shown that therapy with nasal CPAP therapy reduces these oxidative stress markers.25 The NADPH oxidase (NOX) family of enzymes share the capacity to transport electrons across the plasma membrane and generate ROS.27 Animal studies have demonstrated that ROS is generated within the glomus cell of carotid body following CIH24 through the activation of NOX, specifically NOX2.28 NOX2 not only generates cytosolic ROS, but decreases activity of complex I of the mitochondrial electron transport chain, causing release of mitochondrial ROS.29 sLTF of the carotid body following CIH is prevented by either pretreatment with superoxide anion scavenger or inhibitors of NOX and in NOX2-deficient mice suggesting that the generation of ROS is necessary for the sLTF of the carotid body following CIH exposure.28,30 In addition ROS is necessary for the augmented responses of the carotid body to hypoxia following CIH.31
B. Hypoxia-Inducible Factor (HIF) and ROS
The hypoxia-inducible factor (HIF) family of transcription factors regulate the expression of various genes under condition of reduced oxygen availability. HIF-1 is composed of an oxygen-regulated HIF-1α subunit and a constitutively expressed HIF-1β subunit. Under normoxic conditions, HIF-1α is hydroxylated by prolyl hydroxylase domain proteins and hydroxylated HIF-1α is targeted for proteosomal degradation. Under hypoxic conditions, hydroxylation is inhibited and HIF-1α accumulates, dimerizes with HIF-1β, binds to hypoxia response elements, and activates the transcription of hundreds of target genes.32 HIF-2, a heterodimer composed of HIF-1β and HIF-2α (a paralogue of HIF-1α that is also regulated by oxygen-dependent hydroxylation), also mediates hypoxic responses, but is not as ubiquitously present in all tissues as HIF-1α.32 Recent evidence suggests the CIH upregulates HIF-1α but downregulates HIF-2α.33 Additionally, it has been shown that HIF-1 α heterozygous mice deficient in HIF-1α do not show sLTF, enhanced carotid body responses to hypoxia, and elevated ROS levels following CIH exposure, unlike wild-type littermates, suggesting that HIF-1α is necessary for the generation of ROS. On the other hand, in cell cultures exposed to intermittent hypoxia, HIF-1α accumulation has been shown to be due to increased generation of ROS by NADPH oxidase.34 Thus, it is likely that CIH initially activates NADPH oxidase, resulting in the generation of ROS that triggers activation of HIF-1α. Activation of HIF-1α promotes persistent increase in ROS through the transcriptional upregulation of pro-oxidants such as NOX.35 Recent evidence also suggests that in addition to upregulation of prooxidants such as NOX, there is downregulation of antioxidants such as superoxide dismutase-2 (SOD-2) with exposure to CIH.33, 36 It has been hypothesized that the downregulation of antioxidants is as a consequence of the downregulation of HIF-2α. Several lines of evidence support this hypothesis. HIF-2α regulates the transcription of several antioxidants including SOD-2.2,37 Overexpression of a transcriptionally active HIF-2α plasmid prevents intermittent hypoxia-induced downregulation of SOD-2 activity in PC12 cell cultures.3,36 Intermittent hypoxia induces downregulation of HIF-2α through calcium signaling. Calcium ions activate several downstream effector molecules including calpains that are proteases that mediate HIF degradation. ALLM, a potent inhibitor of calpains, rescues intermittent hypoxia-induced HIF-2α degradation, restores Sod-2 activity, and prevents elevation of ROS.36
C. Cellular Targets Of ROS In The Carotid Body And Their Role In CIH-Induced Hypertension
Vasoconstrictor peptides such as endothelin 1 (ET-1) are expressed in the glomus cells and blood vessels of the carotid body.38 ET-1 acts at two receptors, i.e., the endothelin A (ETA) receptor and the endothelin B (ETB) receptor. Functional studies with ETA receptor antagonists suggest that ET-1 causes chemoexcitation of the carotid body at the ETA receptor. Chronic hypoxia for 14 days increases expression of the ETA receptor and of preproendothelin, the precursor of ET-1, in the carotid body. Furthermore, increases in chemoreceptor activity within the carotid body parallels the increases in ET-1 and ETA expression.39 In cats exposed to CIH for four days, expression of ET-1 was increased tenfold in the carotid bodies and ETA/ ETB receptor antagonist bosentan inhibited the CIH-induced increase in basal and hypoxic chemosensory responses of these carotid bodies.22 More recently, Pawar et al. showed that administration of manganese tetrakis methyl porphyrin pentachloride (MnTMPyP), a scavenger of free radicals, prevents the augmented sensory responses and increase in ROS and ET-1 levels and ETA receptor mRNA following 10 days of CIH.31 These findings suggest that ROS-mediated increase in ET-1 levels and upregulation of ETA receptors are involved in the augmented hypoxic chemosensitivity of the carotid body following CIH exposure.
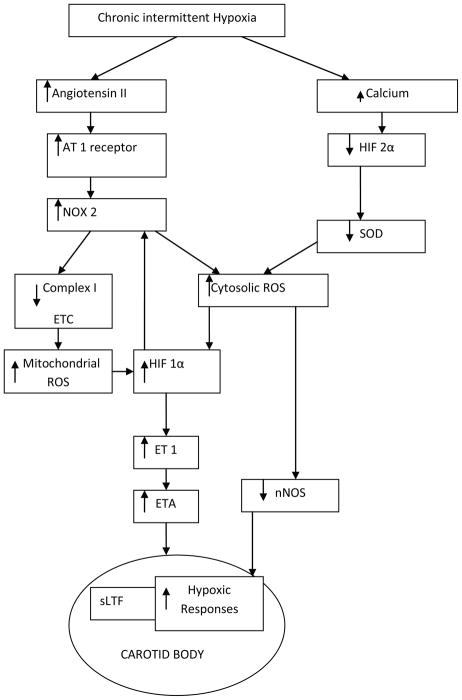
In addition to vasoconstrictor peptides such as ET-1, the renin angiotensin system has also been implicated in the enhancement of peripheral chemosensitivity. Similar to ET-1, Lam and Leung et al. have shown that angiotensin II (Ang II) enhances carotid body chemoreceptor activity.40 Recent evidence suggests a direct role for Ang II in enhancing chemosensitivity within the carotid body and not as a consequence of altered arterial pressure or blood flow. In an in vitro carotid body preparation, carotid sinus nerve activity is increased by Ang II.40 Angiotensinogen, after cleavage by angiotensin-converting enzyme forms Ang II. Angiotensinogen protein and mRNA have been found to be present in glomus cells. Similar to ET-1, chronic hypoxia upregulates the transcriptional and posttranscriptional expression of Ang II type 1 (AT1) receptors in the carotid body.41 CIH also increases carotid body AT1 receptor expression and, additionally, CIH-induced increase in ROS production is prevented by AT1 receptor blockade, suggesting that AngII signaling plays a role in CIH-mediated ROS production.42 Although ET-1 and Ang II may enhance chemoreceptor function, the constitutive isoforms of nitric oxide synthase (NOS), both endothelial NOS (eNOS) and neuronal NOS (nNOS), may cause inhibition of the chemoreceptor function. Similar to ET-1, eNOS and nNOS are present in the carotid body. Recent evidence suggests that both eNOS and nNOS modulate carotid chemoreceptor activity, 43 and that CIH causes a decrease in carotid body nNOS expression.42 Figure 1 describes a conceptual model of the molecular mechanisms involved in the sLTF and increased hypoxic chemosensitivity of the carotid body following CIH.
FIGURE 1.
Molecular mechanism of sensory long-term facilitation (sLTF) and increased hypoxic chemosensitivity of the carotid body following CIH. Chronic intermittent hypoxia leads to an increase in AT1 receptor expression which in turn results in NOX mediated increases in cytosolic and mitochondrial ROS. ROS activates HIF-1 α and induces sLTF in the carotid body through Endothelin 1. (AT1—angiotensin II type 1 receptor; NOX—NADPH oxidase; HIF— hypoxia inducible factor; SOD—super oxide dismutase; ETC—electron transport chain; ROS—reactive oxygen species; ET-1—endothelin 1; ETA—endothelin A receptor; nNOS—neuronal nitric oxide synthase).
VI. CENTRAL SITES OF SYMPATHETIC ACTIVATION
Similar to enhancement in the hypoxic chemosensitivity of the peripheral chemoreceptors, sites within the central sympathetic network may also show adaptations to chronic intermittent hypoxia, resulting in an increase in sympathetic output. Outflow from postganglionic sympathetic nerves are modulated by input from preganglionic neurons in the spinal cord, which in turn receive inputs from sympathetic premotor neurons in the central nervous system, including the rostral ventrolateral medulla (RVLM), the medullary raphe, the A5 area of the pons, and the paraventricular nucleus of the hypothalamus (PVN). In addition, these premotor neurons receive input from a number of central nervous system locations including neurons within the circumventricular organs (CVOs) in the laminal terminalis. All of these regions may be involved in sympathoexcitation, and many of these regions are hypoxia sensitive.44
VII. CELLULAR MECHANISMS OF CIH-INDUCED CHANGES IN SYMPATHETIC NERVOUS SYSTEM
A. CIH Activates Transcription Factors within Brain Regions Involved in Autonomic Control
FosB and Delta FosB are two proteins encoded by the Fos family of activator protein-1 (AP-1) complex transcription factors. In contrast to all other members of the Fos family, Delta FosB is unique in that it is extremely stable and has a very long half-life, and is induced in specific brain regions by repeated exposure to different stimuli. Once induced, it remains in these tissues for a prolonged period of time. Delta FosB has therefore been implicated in neuronal plasticity and adaptation.45 In a recent study examining the effect of a paradigm of CIH that resulted in the development of hypertension within one week in rats, it was found that staining for Delta FosB was increased in autonomic nuclei including in the CVO, subfornicular organ, median preoptic nucleus, nucleus of the solitary tract, A5, and RVLM. These findings suggest that AP-1 transcriptional regulation of central autonomic nuclei may play a role in adaptation that results in chronically elevated sympathetic nerve activity following CIH.46
B. Role of Renin-Angiotensin System in Central Sympathetic Responses to CIH
Although the kidney is the only organ that stores renin, components of the renin angiotensin system have been found in the brain, and angiotenin II (AngII) acts as a neurotransmitter involved in the regulation of sympathetic activity.47 Through the stimulation of AT1 receptors, AngII promotes sympathetic outflow and modulates the baroreflex. 48 AngII-containing neurons are sympathoexcitatory in the PVN, RVLM, and CVO.49 Interestingly, reactive oxygen species appear to be the key mediators of the action of AngII in the regulation of blood pressure in the central nervous system.50 AngII-induced pressor responses in the RVLM are mediated by AT1-dependent increase in NOX-derived ROS production.51 A recent study revealed augmented basal- and chemoreflex-stimulated lumbar sympathetic output following exposure to CIH was ameliorated in the presence of losartan (AT1 receptor blocker). Although the authors examined AT1 receptor and NOX subunit expression in the carotid bodies, they could not rule out that the sympathoexcitatory effects of CIH could also arise from the oxygen sensing neurons in the RVLM and that the effects of losartan was from the blockade of AT1 receptors within the central sympathetic neural network.42
C. Role of ET-1 in CIH-induced Increases in Sympathetic Responses
In addition to its role in enhancing peripheral chemosensitivity to CIH, ET-1 appears to also play a role in the central sympathetic responses to CIH. Rats exposed to three weeks of CIH showed significantly greater sympathetic responses following intracerebroventricularly administered ET-1 and a greater expression of ETA receptor protein in the subfornical organs than sham-exposed animals.52
D. Role of nNOS in the Central Sympathetic Output Following CIH
The PVN contains neurons that express nNOS, and neuronal activity in the PVN is regulated by NO.53 Direct administration of NO or a NO donor into the PVN decreases sympathetic nerve activity and lowers blood pressure, while inhibition of NO synthesis in the PVN results in sympathoexcitation.54 Thirty-five days of CIH resulted in the development of hypertension and a suppression of NO production in the PVN.55
E. Role of HO-1 in the Responses of Hypoxic Sensitivity of Sympathetic Activity to Chronic and Chronic Intermittent Hypoxia
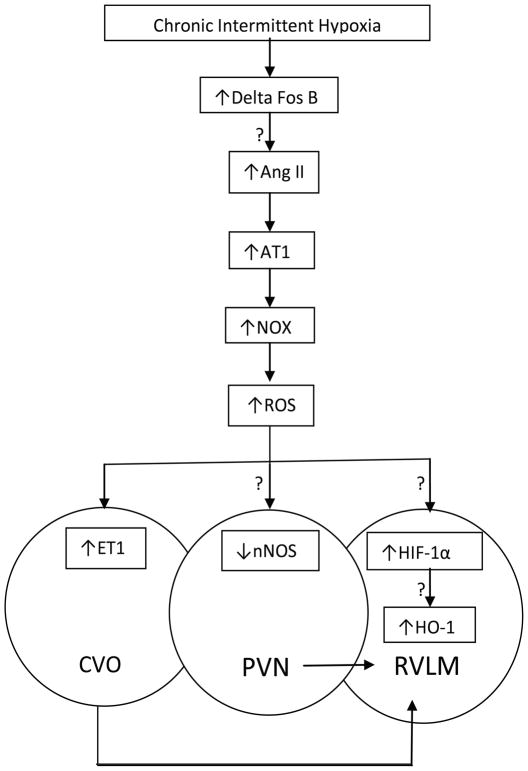
The C1 sympathoexcitatory neurons in the RVLM are hypoxia chemosensitive, and are excited by local hypoxia.56 The mechanism of hypoxic chemosensitivity of the C1 sympathoexcitatory neurons involves a heme-type oxygen-sensing protein, heme oxygenase (HO). HO catabolizes heme into biliverdin, iron, and carbon monoxide, and the likely cellular signals regulating the excitability of the chemosensitive cells are CO and/or biliverdin. Our recent findings have shown that HO is essential for the oxygen sensitivity of this brain stem chemosensitive region, since blocking HO blocks the excitatory response of neurons within this region to hypoxia.57 We have also shown that HO-1, which is an enzyme that is activated by both HIF-1α and AP-1, is necessary for maintaining the hypoxia chemosensitivity of this region during chronic hypoxia. 58 Preliminary data from our lab also suggest that HO-1 is necessary for maintenance of hypoxic sensitivity of sympathetic activity during 14 days of CIH. Since HO-1 is induced by HIF-1α, these findings suggest that HO-1 may be downstream of the actions of HIF-1α in the activation of the sympathetic nervous system following CIH. Figure 2 shows a conceptual model of the putative molecular mechanisms by which CIH could induce hypertension.
FIGURE 2.
Putative molecular mechanisms by which CIH induces increases in central sympathetic activation; “?” is used to designate hypothesized mechanism that has not been proven yet. Chronic intermittent hypoxia activates transcription factors such as Delta Fos B within brain regions involved in autonomic control. Additionally CIH induces a pressor response in the RVLM mediated by an AT-1 dependent increase in NOX-derived ROS production. ET1 mediated increases in ETA expression in the CVO, reduction in nNOS in the PVN and HIF-1 mediated increases in HO-1 all play a role in central sympathoexcitation following CIH. (AT1—angiotensin II type 1 receptor; NOX— NADPH oxidase; HIF—hypoxia-inducible factor; HO-1—heme oxygenase-1; ROS—reactive oxygen species; ET-1— endothelin 1; ETA—endothelin A receptor; nNOS—neuronal nitric oxide synthase; CVO—circumventricular organ; PVN—paraventricular nucleus; RVLM—rostralventrolateral medulla).
VIII. CIH AND VASCULAR CHANGES: MOLECULAR MECHANISMS AND RELATIONSHIP TO HYPERTENSION
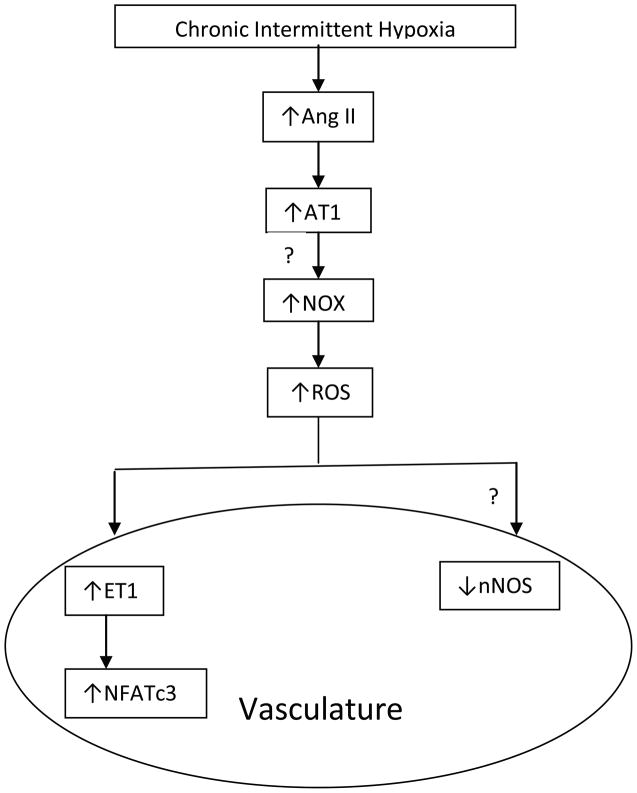
Endothelial dysfunction has been shown to be present following CIH in animals59 and in patients with OSA, and improves with nasal CPAP therapy. 60 Interestingly, the same molecules involved in the responses of the carotid body and central sympathetic pathways to CIH appear to be involved in the vascular changes that are seen with CIH. Studies in humans suggest that there is decreased bioavailability of NO in patients with OSA secondary to intermittent hypoxia, and that nasal CPAP therapy increases NO bioavailability.60–63 Additionally, plasma levels of AngII are elevated in patients with OSA.64 ET-1 plasma concentrations increase in rats exposed to 15 days of CIH,65 and patients with OSA have elevated plasma levels of ET-1.66 Pretreatment with a superoxide dismutase (SOD) mimetic tempol in rats prevented CIH-induced hypertension and ROS generation and the increase in plasma concentrations of ET-1 induced by CIH.67 These findings link CIH to ROS generation, ET-1, and hypertension. ET-1 in turn activates nuclear factor of activated T cells (NFAT). The NFAT family consists of four members of which NFATc3 has been implicated in vascular development and maintenance of the contractility of vascular smooth muscles.68, 69 A recent study demonstrated that CIH increases NFATc3 transcriptional activity in the aorta and mesenteric arteries and CIH-induced hypertension is attenuated by an NFAT inhibitor (cyclosporine-A) and that NFAc3 knockout mice do not develop hypertension following CIH. These findings suggest that ET-1 may act through NFATc3 in the induction of hypertension following CIH. Thus, vasoactive molecules such as endothelin, nitric oxide, and the renin-angiotensin system by acting on the vasculature enhance the role that sympathetic activation plays in the development of hypertension following CIH. Figure 3 shows the putative molecular mechanisms by which CIH could act on the vasculature with resultant hypertension.
FIGURE 3.
Putative molecular mechanisms by which CIH induces changes in vascular tone, resulting in the development of hypertension; “?” is used to designate hypothesized mechanism that has not been proven yet. Angiotensin II, ET1 and nNOS also play a role in CIH induced vascular changes resulting in hypertension. NFATc3, a nuclear factor involved in maintenance of vascular smooth muscle reactivity appears to be downstream of ET1 and appears to be important in the vascular changes and hypertension induced by CIH. (AT1—angiotensinII type 1 receptor; NOX—NADPH oxidase; ROS—reactive oxygen species; ET-1—endothelin 1; ETA—endothelin A receptor; nNOS— neuronal nitric oxide synthase; NFATc3—nuclear activator of T cells).
IX. SYSTEMS BIOLOGY APPROACHES TO HYPOXIA
Significant effort has been invested in understanding the neural mechanisms controlling respiratory rhythm generation through computational modeling, 70–75 And, in addition, in understanding the interplay between major physiological parameters in the context of respiration through more integrated computational models of the upper airway to simulate anatomic and physiologic manipulations of respiratory mechanics in sleep apnea76 and control-theoretic approaches.77–79 However, in recent years it is becoming ever more evident that it is important in gaining a deeper and more fundamental understanding of the signaling and transcriptional implications of hypoxia-induced alterations, the eventual hope being the integration of physiological, neuronal, and cellular-level information in developing a unified, predictive model of the link between hypertension and CIH. Comparative gene expression profiling offers the possibility of elucidating the emergent cellular responses to varying levels of hypoxic stress. This final section will aim at opening a discussion along two directions, namely, global expression profiling for deciphering the transcriptional and signaling details of the response, and systems biology models aimed at elucidating the interactions leading to said dynamic responses.
Global transcriptional analyses have been performed in cell cultures, model organisms, and mammals, shedding light on a complex response. Seta and Millhorn80 evaluated the oxygen-sensing capabilities of clonal cell lines in response to hypoxia. Using focused cDNA libraries along with microarray analyses, they studied the molecular and cellular basis of oxygen chemosensititvity and the regulation mechanisms of O2-responsive genes. The choice of the particular cell type (PC12 cell line) was based on its resemblance to carotid body type I cells. The purpose of the study was, specifically, to identify the implications of absence or presence of extracellular Ca2+. Van der Meer and coworkers81 examined changes in gene expression in zebra fish exposed to hypoxia in an attempt to shed light on the evolution of hypoxia tolerance as an adaptive response in vertebrates. Fish were exposed to a gradual decrease in oxygen over a period of four days, from 80–90% to 10% oxygen saturation, and were kept at this level for 21 days, with a control group maintained at the 80–90% level. Branchial arches on gill coves (an aquatic respiratory organ) were dissected and homogenized, and mRNA was analyzed. Following the analysis of the high-throughput mRNA data, a variety of hypoxia-induced gene expression changes were identified, and possibly contributed to a multitude of alternative adaptation mechanisms. Aiming at evaluating changes at the cellular level within the carotid body chemoreceptor, Ganforina and coworkers82 exposed female mice to normoxic and hypoxic conditions for 24 h, and the adrenal medulla and carotid bodies were subsequently dissected and mRNA was quantified. The study once again aimed at evaluating hypoxia-regulated genomescale changes in chemoreceptor cells. This was among the first transcriptional profiling studies of mouse carotid body response to physiologically sustained hypoxia, and led to the postulation of putative functional interactions among carotid body hypoxia regulated genes. This becomes a critically important component as we move toward a systems biology model of hypoxia. Of particular importance are kinetic models, at the cellular and molecular level, elucidating the implications of hypoxia-induced activation of critical transcription factors.
Qutub and Popel83 explored a critical component of the oxygen-sensing mechanism, namely, the switchlike changes in HIF-1 expression in response to gradual decreases in oxygen concentration. HIF-1 dynamics are particularly critical since it is estimated to regulate the expression of over 200 genes.84 An ordinary differential equation model describing the kinetics of 17 compounds was proposed to contribute toward a better understanding of the hypoxic response at the molecular level. Along the same line of thought, Zhang and coworkers85 proposed a systems biology approach to elucidate the implications of Nrf2-mediated responses. The interesting aspect of this work is that it begins to integrate principles of control theory and feedback regulation to assess the homeostatic control role of Nrf2-mediated regulation. Once again, gene and transcription factor dynamics are integrated in the context of a unified cellular level network model.
Availability of network structures composed of elements (genes, proteins) that are functionally related allows for the evaluation of alternative relationships in interpreting observed phenotypes. In that respect, a key property of HIF signaling has been extensively studied experimentally, and offers a great test bed for systems biology approaches. HIF plays a central role as a master regulator of oxygen-sensitive gene expression. However, evidence suggests an exponentially increased sensitivity as oxygen concentration drops.86 This “switchlike” behavior has been approximated computationally through alternative systems biology approaches. Starting with a basic interaction map of hypoxia-dependent genes, Kohn and coworkers87 demonstrated the possibility of a core subsystem that, in the absence of a feedback mechanism, can exhibit a HIF activity switchlike dependence on oxygen levels. By accounting for HIF-1 synthesis, the model can integrate growth factors and regulation of hypoxia responsive element (HRE)–dependent genes within a unique unifying framework. More recently, Yu and coworkers88 explored a more complex 23 molecular species network, further decomposed, by exploring tools from metabolic network analysis, into extreme pathways in an attempt to characterize activation/ deactivation of subpathways responsible for the observed switchlike behavior of HIF dependence. The value of the last two representative papers is that they demonstrate (i) the need for, and benefits from, integration of network information from large-scale genome-wide studies and advanced computational methods, and (ii) the possibility of generating mechanistic-based hypotheses able to interpret observed complex phenotypes in the context of implication of hypoxia.
Clearly, more work is needed to advance the state of the art lining physiology (outcome) as described in the early part of this review and cellular-level mechanisms (processes) driving those. Systems biology tools can definitely enable the rationalization, and more importantly close the loop, between outcome (hypertension) and processes (oxygen sensing). Initial efforts in this direction, albeit in a different context, are beginning to bear results.89
Acknowledgments
This work was supported by IPA NIH Grant No. GM082974.
References
- 1.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008 Feb 15;5(2):136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dopp JM, Reichmuth KJ, Morgan BJ. Obstructive sleep apnea and hypertension: mechanisms, evaluation, and management. Curr Hypertens Rep. 2007 Dec;9(6):529–34. doi: 10.1007/s11906-007-0095-2. [DOI] [PubMed] [Google Scholar]
- 3.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009 Jun 15;5(3):263–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993 Aug;148(2):462–6. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 5.Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clin Chest Med. 2010 Jun;31(2):203–20. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993 Apr 29;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 7.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010 Jul 15;182(2):269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher EC. The relationship between systemic hypertension and obstructive sleep apnea: Facts and theory. Am J Med. 1995;98(2):118–28. doi: 10.1016/S0002-9343(99)80395-7. [DOI] [PubMed] [Google Scholar]
- 9.Pankow W, Nabe B, Lies A, Becker H, Kohler U, Kohl FV, Lohmann FW. Influence of sleep apnea on 24-hour blood pressure. Chest 1997. 1997 Nov 1;112(5):1253–8. doi: 10.1378/chest.112.5.1253. [DOI] [PubMed] [Google Scholar]
- 10.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS, Bradley TD. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001 Dec;19(12):2271–7. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Neubauer JA. Invited review: physiological and pathophysiological responses to intermittent hypoxia. J Appl Physiol. 2001 Apr;90(4):1593–9. doi: 10.1152/jappl.2001.90.4.1593. [DOI] [PubMed] [Google Scholar]
- 12.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997 Jan 1;99(1):106–9. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992 Jun;19(6 Pt 1):555–61. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 14.Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol 1997. 1997 Jul 1;83(1):95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension. 1992 Nov;20(5):612–9. doi: 10.1161/01.hyp.20.5.612. [DOI] [PubMed] [Google Scholar]
- 16.Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl. 1988 Dec;6(4):S529–31. doi: 10.1097/00004872-198812040-00166. [DOI] [PubMed] [Google Scholar]
- 17.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995 Oct;96(4):1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waradekar NV, Sinoway LI, Zwillich CW, Leuenberger UA. Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am J Respir Crit Care Med. 1996 Apr;153(4 Pt 1):1333–8. doi: 10.1164/ajrccm.153.4.8616563. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 1994. 1994 Oct 1;74(4):829–98. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 20.Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol. 2005 May-Jun;32(5–6):447–9. doi: 10.1111/j.1440-1681.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol. 1999 Jan;86(1):298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- 22.Rey S, Del Rio R, Iturriaga R. Contribution of endothelin-1 to the enhanced carotid body chemosensory responses induced by chronic intermittent hypoxia. Brain Res. 2006 May 1;1086(1):152–9. doi: 10.1016/j.brainres.2006.02.082. [DOI] [PubMed] [Google Scholar]
- 23.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006 Dec 1;577(Pt 2):705–16. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol. 2003 Jun;94(6):2342–9. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- 25.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004 Feb;27(1):123–8. [PubMed] [Google Scholar]
- 26.Jordan W, Cohrs S, Degner D, Meier A, Rodenbeck A, Mayer G, Pilz J, Ruther E, Kornhuber J, Bleich S. Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J Neural Transmission. 2006;113(2):239–54. doi: 10.1007/s00702-005-0316-2. [DOI] [PubMed] [Google Scholar]
- 27.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007. 2007 Jan 1;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 28.Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 2009. 2009 Apr 15;29(15):4903–10. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia augments acute hypoxic sensing via HIF-mediated ROS. Respir physiol Neurobiol. 2010;174(3):230–4. doi: 10.1016/j.resp.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng Y-J, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: Implications for recurrent apneas. Proc Natl Acad Sci U S A 2003. 2003 Aug 19;100(17):10073–8. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol. 2009 Mar;296(3):R735–42. doi: 10.1152/ajpregu.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenza GL. Oxygen sensing, homeostasis, and disease. New Engl J Med. 2011;365(6):537–47. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia augments acute hypoxic sensing via HIF-mediated ROS. Respir Physiol Neurobiol. 2010;174(3):230–4. doi: 10.1016/j.resp.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1α expression by intermittent hypoxia: Involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217(3):674–85. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia-mediated plasticity of acute O2 sensing requires altered red-Ox regulation by HIF-1 and HIF-2. Ann NY Acad Sci. 2009;1177(1):162–8. doi: 10.1111/j.1749-6632.2009.05034.x. [DOI] [PubMed] [Google Scholar]
- 36.Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2α via calpains resulting in oxidative stress: Implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A 2009. 2009 Jan 27;106(4):1199–204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003 Dec;35(4):331–40. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 38.Rey S, Corthorn J, Chacon C, Iturriaga R. Expression and immunolocalization of endothelin peptides and its receptors, ETA and ETB, in the carotid body exposed to chronic intermittent hypoxia. J Histochem Cytochem. 2007 Feb;55(2):167–74. doi: 10.1369/jhc.6A7079.2006. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002 Jun;282(6):L1314–23. doi: 10.1152/ajplung.00454.2001. [DOI] [PubMed] [Google Scholar]
- 40.Lam SY, Leung PS. A locally generated angiotensin system in rat carotid body. Regul Pept. 2002 Jul 15;107(1–3):97–103. doi: 10.1016/s0167-0115(02)00068-x. [DOI] [PubMed] [Google Scholar]
- 41.Lin L, Finn L, Zhang J, Young T, Mignot E. Angiotensin-converting enzyme, sleep-disordered breathing, and hypertension. Am J Respir Crit Care Med. 2004 Dec 15;170(12):1349–53. doi: 10.1164/rccm.200405-616OC. [DOI] [PubMed] [Google Scholar]
- 42.Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol. 2010 Apr 15;171(1):36–45. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iturriaga R, Villanueva S, Mosqueira M. Dual effects of nitric oxide on cat carotid body chemoreception. J Appl Physiol. 2000 Sep;89(3):1005–12. doi: 10.1152/jappl.2000.89.3.1005. [DOI] [PubMed] [Google Scholar]
- 44.Neubauer JA, Sunderram J. Oxygen-sensing neurons in the central nervous system. J Appl Physiol. 2004 Jan;96(1):367–74. doi: 10.1152/japplphysiol.00831.2003. [DOI] [PubMed] [Google Scholar]
- 45.Kelz MB, Nestler EJ. deltaFosB: a molecular switch underlying long-term neural plasticity. Curr Opin Neurol. 2000 Dec;13(6):715–20. doi: 10.1097/00019052-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 46.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/ΔFosB in central autonomic regions. Am J Physiol 2011. 2011 Jul 1;301(1):R131–9. doi: 10.1152/ajpregu.00830.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zucker IH. Brain angiotensin II: new insights into its role in sympathetic regulation. Circ Res. 2002 Mar 22;90(5):503–5. doi: 10.1161/01.res.0000014287.96335.21. [DOI] [PubMed] [Google Scholar]
- 48.Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992 Jun;262(6 Pt 1):E763–78. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- 49.Weiss JW, Liu MD, Huang J. Physiological basis for a causal relationship of obstructive sleep apnoea to hypertension. Exp Physiol. 2007 Jan;92(1):21–6. doi: 10.1113/expphysiol.2006.035733. [DOI] [PubMed] [Google Scholar]
- 50.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002 Nov 29;91(11):1038–45. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 51.Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005 Oct 14;97(8):772–80. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- 52.Huang J, Xie T, Wu Y, Li X, Lusina S, Ji ES, Xiang S, Liu Y, Gautam S, Weiss JW. Cyclic intermittent hypoxia enhances renal sympathetic response to ICV ET-1 in conscious rats. Respir Physiol Neurobiol. 2010 Apr 30;171(2):83–9. doi: 10.1016/j.resp.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. J Physiol. 1997 Mar 15;499( Pt 3):733–46. doi: 10.1113/jphysiol.1997.sp021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol. 1998 Sep;275(3 Pt 2):R728–34. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 55.Coleman CG, Wang G, Park L, Anrather J, Delagrammatikas GJ, Chan J, Zhou J, Iadecola C, Pickel VM. Chronic Intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci 2010. 2010 Sep 8;30(36):12103–12. doi: 10.1523/JNEUROSCI.3367-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun MK, Jeske IT, Reis DJ. Cyanide excites medullary sympathoexcitatory neurons in rats. Am J Physiol. 1992 Feb;262(2 Pt 2):R182–9. doi: 10.1152/ajpregu.1992.262.2.R182. [DOI] [PubMed] [Google Scholar]
- 57.D’Agostino D, Mazza E, Jr, Neubauer JA. Heme oxygenase is necessary for the excitatory response of cultured neonatal rat rostral ventrolateral medulla neurons to hypoxia. Am J Physiol Regul Integr Comp Physiol. 2009 Jan;296(1):R102–18. doi: 10.1152/ajpregu.90325.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sunderram J, Semmlow J, Thakker-Varia S, Bhaumik M, Hoang-Le O, Neubauer JA. Heme oxygenase-1-dependent central cardiorespiratory adaptations to chronic hypoxia in mice. Am J Physiol. 2009 Aug;297(2):R300–12. doi: 10.1152/ajpregu.90737.2008. [DOI] [PubMed] [Google Scholar]
- 59.Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2004 Jan;286(1):H388–93. doi: 10.1152/ajpheart.00683.2003. [DOI] [PubMed] [Google Scholar]
- 60.Lattimore JL, Wilcox I, Skilton M, Langenfeld M, Celermajer DS. Treatment of obstructive sleep apnoea leads to improved microvascular endothelial function in the systemic circulation. Thorax. 2006 Jun;61(6):491–5. doi: 10.1136/thx.2004.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Solh AA, Saliba R, Bosinski T, Grant BJ, Berbary E, Miller N. Allopurinol improves endothelial function in sleep apnoea: a randomised controlled study. Eur Respir J. 2006 May;27(5):997–1002. doi: 10.1183/09031936.06.00101005. [DOI] [PubMed] [Google Scholar]
- 62.Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, Schulz R. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006 Apr 15;173(8):897–901. doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- 63.Ip MS, Lam B, Chan LY, Zheng L, Tsang KW, Fung PC, Lam WK. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000 Dec;162(6):2166–71. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 64.Moller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens. 2003 Apr;16(4):274–80. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 65.Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001 Feb;37(2 Part 2):511–5. doi: 10.1161/01.hyp.37.2.511. [DOI] [PubMed] [Google Scholar]
- 66.Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999 Jan;17(1):61–6. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 67.Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol. 2007 Nov;293(5):H2971–6. doi: 10.1152/ajpheart.00219.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/ c4 pattern the developing vasculature. Cell. 2001 Jun 29;105(7):863–75. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 69.de Frutos S, Spangler R, Alo D, Bosc LV. NFATc3 mediates chronic hypoxia-induced pulmonary arterial remodeling with alpha-actin up-regulation. J Biol Chem. 2007 May 18;282(20):15081–9. doi: 10.1074/jbc.M702679200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. I. models of respiratory neurons. J Neurophysiol. 1997 Apr;77(4):1994–2006. doi: 10.1152/jn.1997.77.4.1994. [DOI] [PubMed] [Google Scholar]
- 71.Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. II. network models of the central respiratory pattern generator. J Neurophysiol. 1997 Apr;77(4):2007–26. doi: 10.1152/jn.1997.77.4.2007. [DOI] [PubMed] [Google Scholar]
- 72.Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. III. Comparison of model performances during afferent nerve stimulation. J Neurophysiol. 1997 Apr;77(4):2027–39. doi: 10.1152/jn.1997.77.4.2027. [DOI] [PubMed] [Google Scholar]
- 73.Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. I. Bursting pacemaker neurons. J Neurophysiol. 1999 Jul;82(1):382–97. doi: 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- 74.Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. II. populations of coupled pacemaker neurons. J Neurophysiol. 1999 Jul;82(1):398–415. doi: 10.1152/jn.1999.82.1.398. [DOI] [PubMed] [Google Scholar]
- 75.Del Negro CA, Johnson SM, Butera RJ, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. III. experimental tests of model predictions. J Neurophysiol. 2001 Jul;86(1):59–74. doi: 10.1152/jn.2001.86.1.59. [Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 76.Huang Y, White DP, Malhotra A. Use of computational modeling to predict responses to upper airway surgery in obstructive sleep apnea. Laryngoscope. 2007 Apr;117(4):648–53. doi: 10.1097/MLG.0b013e318030ca55. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fink M, Batzel JJ, Tran H. A respiratory system model: parameter estimation and sensitivity analysis. Cardiovasc Eng. 2008 Jun;8(2):120–34. doi: 10.1007/s10558-007-9051-7. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- 78.Batzel JJ, Tran HT. Stability of the human respiratory control system. II. analysis of a three-dimensional delay state-space model. J Math Biol. 2000 Jul;41(1):80–102. doi: 10.1007/s002850000045. [DOI] [PubMed] [Google Scholar]
- 79.Batzel JJ, Tran HT. Stability of the human respiratory control system. I. analysis of a two-dimensional delay state-space model. J Math Biol. 2000 Jul;41(1):45–79. doi: 10.1007/s002850000044. [DOI] [PubMed] [Google Scholar]
- 80.Seta KA, Millhorn DE. Functional genomics approach to hypoxia signaling. J Appl Physiol. 2004 Feb;96(2):765–73. doi: 10.1152/japplphysiol.00836.2003. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- 81.van der Meer DL, van den Thillart GE, Witte F, de Bakker MA, Besser J, Richardson MK, Spaink HP, Leito JT, Bagowski CP. Gene expression profiling of the long-term adaptive response to hypoxia in the gills of adult zebrafish. Am J Physiol Regul Integr Comp Physiol. 2005 Nov;289(5):R1512–9. doi: 10.1152/ajpregu.00089.2005. [DOI] [PubMed] [Google Scholar]
- 82.Ganfornina MD, Perez-Garcia MT, Gutierrez G, Miguel-Velado E, Lopez-Lopez JR, Marin A, Sanchez D, Gonzalez C. Comparative gene expression profile of mouse carotid body and adrenal medulla under physiological hypoxia. J Physiol. 2005 Jul 15;566(Pt 2):491–503. doi: 10.1113/jphysiol.2005.088815. [Comparative Study Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qutub AA, Popel AS. A computational model of intracellular oxygen sensing by hypoxia-inducible factor HIF1 alpha. J Cell Sci. 2006 Aug 15;119(Pt 16):3467–80. doi: 10.1242/jcs.03087. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004 Aug;19:176–82. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q, Pi J, Woods CG, Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. 2010 Apr 1;244(1):84–97. doi: 10.1016/j.taap.2009.08.018. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O-2 tension. Am J Physiol-Cell Ph. 1996 Oct;271(4):C1172–80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 87.Kohn KW, Riss J, Aprelikova O, Weinstein JN, Pommier Y, Barrett JC. Properties of switch-like bioregulatory networks studied by simulation of the hypoxia response control system. Mol Biol Cell. 2004 Jul;15(7):3042–52. doi: 10.1091/mbc.E03-12-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu Y, Wang G, Simha R, Peng W, Turano F, Zeng C. Pathway switching explains the sharp response characteristic of hypoxia response network. PLoS Comput Biol. 2007 Aug;3(8):e171. doi: 10.1371/journal.pcbi.0030171. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Namas R, Zamora R, Namas R, An G, Doyle J, Dick TE, Jacono FJ, Androulakis IP, Nieman GF, Chang S, Billiar TR, Kellum JA, Angus DC, Vodovotz Y. Sepsis: Something old, something new, and a systems view. J Crit Care. 2012 Jun;27(3):314.e1–11. doi: 10.1016/j.jcrc.2011.05.025. Epub 2011 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]