Abstract
It has become widely accepted that the direction of another individual’s eye gaze induces rapid, automatic, attentional orienting, due to it being such a vital cue as to where in our environment we should attend. This automatic orienting has also been associated with the directional-arrow cues used in studies of spatial attention. Here, we present evidence that the response-time cueing effects reported for spatially non-predictive gaze and arrow cues are not the result of rapid, automatic shifts of attention. For both cue types, response-time effects were observed only for long-duration cue and target stimuli that overlapped temporally, were largest when the cues were presented simultaneously with the response-relevant target, and were driven by a slowing of responses for invalidly cued targets rather than speeding for validly cued ones. These results argue against automatic attention-orienting accounts and support a novel spatial-incongruency explanation for a whole class of rapid behavioral cueing effects.
Keywords: Attention, conflict, gaze cues, arrow cues, automatic orienting
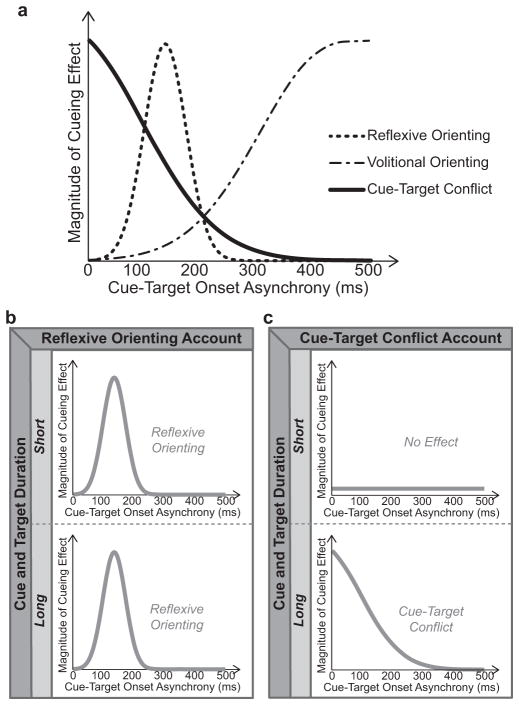
Attending to a location in space speeds responses to items that occur at that location, relative to items occurring at unattended locations (Posner, Snyder, & Davidson, 1980; Posner & Cohen, 1984). This attentional benefit can be triggered reflexively by a peripheral event that draws attention to its location or by voluntarily directing attention to a location known in advance to be the likely place for an upcoming target event. When attention is reflexively oriented, response time (RT) benefits occur rapidly (within ~100 ms) following the attention-capturing event and dissipate quickly (Fig. 1a). In contrast, when attention is shifted voluntarily, the benefit for responses takes more time to develop (>300 ms) but can be sustained for extended periods of time (Fig. 1a; Egeth & Yantis, 1997; Müller & Rabbitt, 1989; Wright & Ward, 2008).
Figure 1.
The expected pattern of cueing effects over cue-target onset asynchronies for reflexive orienting, volitional orienting, and cue-target conflict (a). The pattern of cueing effects predicted by a reflexive orienting account (b) versus a cue-target conflict account (c). For both possible outcomes, the top graph shows the expected pattern for short-duration stimuli and the bottom graph for long-duration stimuli.
The direction of another individual’s eye gaze can also be a useful guide for directing our attention to where relevant events may be occurring (Baron-Cohen, 1995; Driver et al., 1999; Langton, Watt, & Bruce, 2000). Reports of very rapid effects on the speed of responding to a target in a gazed-at location, even when the eye-gaze cues are not predictive of the target’s location, have led to the suggestion that directional eye gaze triggers a rapid, reflexive shift of spatial attention in the direction of the gaze (Friesen & Kingstone, 1998). Later, this idea of rapid automatic attention shifts was extended to simple, highly learned, arrow stimuli (Tipples, 2002), which are widely used in laboratory attentional cueing studies.
Some key evidence that eye gaze and arrows trigger reflexive shifts of attention comes from findings of faster behavioural responses for targets that occur at cued versus uncued locations as soon as 100 ms following the cue (e.g., Friesen & Kingstone, 1998). Not all evidence has supported this hypothesis, however. For example, peripheral cues that reflexively capture attention do not produce the same pattern of behavioral effects as gaze cues in normal individuals (Friesen & Kingstone, 2003; Ivanoff & Saoud, 2009) and are differentially affected by frontal-lobe damage (Vecera & Rizzo, 2006), leading to suggestions that gaze cues may evoke higher-level volitional orienting or shifts in decision criteria.
Moreover, in a recent study using spatially predictive arrow cues, we observed large RT cueing effects at very short cue-target intervals that did not appear to result from rapid attentional orienting (Green & Woldorff, 2012). In particular, rapid arrow-cueing effects were observed only when the cue and target remained on the screen together for an extended period of time, were largest when the cue and target were presented simultaneously (i.e., before attention could have shifted to the target location), and were driven by slowing of responses for invalid targets (i.e., when the cue and target contained incongruent spatial information) (Green & Woldorff, 2012). This pattern is consistent with a conflict-based process wherein the cue meaning and target location activate interfering representations that produce RT slowing when the cue and target stimuli have long, temporally overlapping durations. In contrast, at longer intervals a clear attentionally-driven pattern was observed, with RT facilitation for targets occurring in validly cued locations.
The observation of rapid conflict-like effects only for extended stimulus presentations is particularly relevant, as most studies that have reported rapid cueing effects to non-predictive eye-gaze and arrow cues have used long-duration cues and targets that remain on the screen together until the behavioral response. We propose that extended cue and target durations may result in a prolonged interaction between them, such that when they provide incongruent spatial information the responses to the target are slowed. If such a conflict account were true, then this effect should be maximal when the cue and target occur at the same time and dissipate as the time between their presentation increases (Fig. 1a; Glaser & Glaser, 1982). This temporal profile of conflict – largest with simultaneous presentation and decreasing with temporal separation of stimuli – has been demonstrated for colour/word meaning interference in the Stroop task (e.g., Glaser & Glaser, 1982) and it is likely that that cue-meaning/target-location conflict involves similar processes. Thus, the pattern of cueing effects observed across cue-target onset asynchronies for nonpredictive cues should be able to differentiate between reflexive orienting and conflict accounts.
Here, we sought to determine if the pattern of cueing effects triggered by non-predictive gaze and arrow cues are more consistent with a reflexive attentional orienting explanation or with a cue-target conflict account. Moreover, we sought to investigate whether gaze and arrows induced similar cueing-effect patterns. Due to their biological relevance as a social cue, it is possible that eye gaze could produce reflexive shifts of attention even if arrow cues do not. To this end, we had participants perform simple cued target-detection tasks using non-predictive gaze or arrow cues. For both cue types, we varied the stimulus durations and the cue-target interval, including a simultaneous cue-target condition.
Clear predictions can be made based on the expected patterns of cueing effects for different explanatory mechanisms (Fig. 1a). If rapid cueing effects are the result of reflexive orienting, then they should be maximal with a cue-target separation of ~100 ms, with no cueing effect with either simultaneous presentation or longer intervals (>300 ms; Fig. 1b). Critically, effects due to reflexive orienting should not be influenced by stimulus duration providing the cue stimulus is presented long enough for its spatial information to be extracted (e.g., ≥50 ms) (Green & Woldorff, 2012; Hommel, Pratt, Colzato, & Godijn, 2001; Müller & Rabbitt, 1989). On the other hand, cue-target conflict effects should be largest with simultaneous presentation and dissipate with increased cue-target separation (Fig. 1c). Moreover, conflict-derived effects should be reflected by a slowing for invalid/incongruent cue-target pairings rather than the speeded processing of valid/congruent targets that an attentional account would predict.
Materials & methods
Participants
Fourteen volunteers (7 female, age range 18–35 years, mean age 22.5 years, all right-handed) participated after providing informed written consent and were compensated for their participation. All procedures were approved by the Duke University Institutional Review Board.
Stimuli and procedure
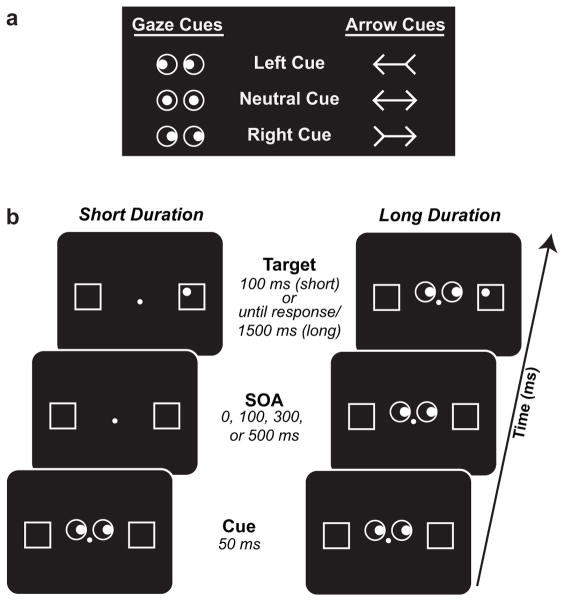
Participants were seated 57 cm from a 19″ CRT monitor in a dimly-lit, sound-attenuated chamber. Throughout each experimental block a small grey fixation cross was present in the center of the screen, along with two landmark-box outlines (each 3.5° in diameter, located 6.75° lateral to fixation). Each trial began with the presentation of an attention-directing cue 0.5° above fixation. For half of the experimental blocks, the cue consisted of an arrow (2.5° in length) pointing to the left or right of fixation, or a double-ended arrow that pointed to both locations (neutral arrow). For the other half, cues were pairs of open circles (each 1.25° in diameter, located 1.125° lateral to fixation) containing small grey dots (diameter = 0.75°), which served as schematic eyes. The dots (pupils) were presented on the right or left side of the open circles to indicate rightward or leftward gaze, respectively, or in the center of the circles (neutral gaze; see Fig. 2a). Both arrow and gaze cues were non-predictive of the location of the upcoming target (i.e., the target was equally likely to occur at the cued and uncued locations).
Figure 2.
Cue stimuli used in the current experiment. (b) Example trial sequence for the short-duration (left) and long-duration (right) conditions.
The target followed the cue at a stimulus-onset asynchrony (SOA) of 0 ms (simultaneous cue and target), or 100, 300 or 500 ms, and consisted of a small grey dot (0.5° diameter) presented in one corner of the landmark box (see Fig. 2b). Participants were instructed to press a button (right index finger) as quickly as possible when they detected the target dot. On 10% of trials no target was presented (catch trials) to ensure that participants were responding only when they actually detected the target stimulus.
The combination of cue type (arrow vs. gaze) and stimulus duration (short vs. long) resulted in four cueing conditions: Arrow/Short, Arrow/Long, Gaze/Short, and Gaze/Long. In the short-duration conditions the cue was 50 ms in duration and the target 100 ms, whereas in the long-duration conditions the cue and target remained on the screen for 1500 ms or until the participant responded to the target, whichever came first. All other task procedures remained the same across conditions. Each participant completed all four conditions in separate blocks (324 trials/block, rest break every 81 trials), with order of conditions counterbalanced across participants.
Analysis
Median response times (RTs) for each participant were first entered into a repeated-measures analysis-of-variance (ANOVA) with factors for cue type (two levels: Arrow and Gaze), stimulus duration (two levels: short and long), cue validity (three levels: valid, neutral, and invalid), and SOA (four levels: 0, 100, 300, and 500 ms). Greenhouse-Geisser-adjusted p-values are reported where appropriate. We also performed a series of planned pairwise comparisons to separate the overall cuing effect (Invalid-minus-Valid RTs) into RT costs (Invalid-minus-Neutral) and RT benefits (Neutral-minus-Valid), separately for each SOA in each of the four cueing conditions.
Results
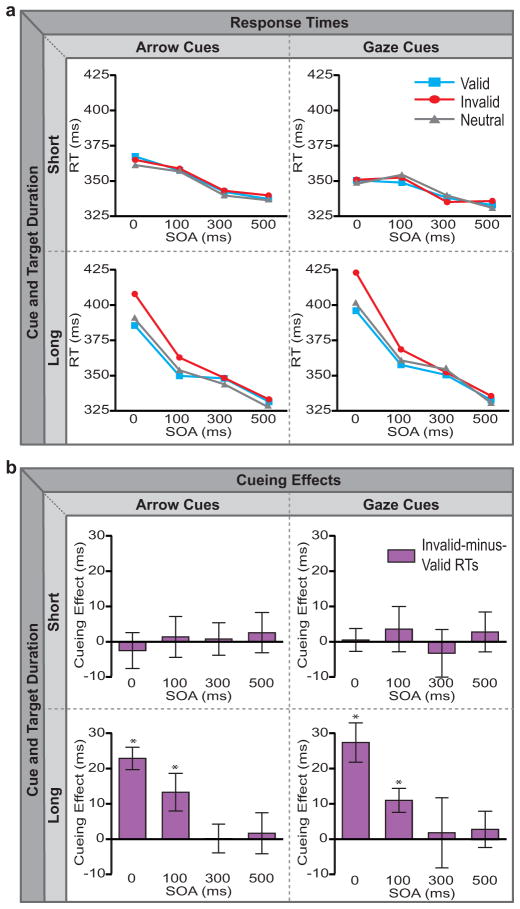
For each cue type (gaze or arrow) and duration (short or long) pairing, we examined RTs to the targets when the preceding cue pointed toward the target location (valid trials), the opposite location (invalid trials), or to both locations (neutral trials). Accuracy was near ceiling (> 97%) in all conditions and no significant differences were observed between conditions. The ANOVA showed a main effect of duration, with slower RTs in the long-duration than short-duration condition [F(1,13)=4.742, p=.048, ], but this effect was mainly driven by the slower RTs for short SOAs in the long-duration condition, particularly for invalid targets (Figure 3a). This was corroborated by significant interactions between stimulus duration and both SOA [F(3,39)=25.751, p<.0001, ] and Validity [F(2,26)=7.397, p=.008, ]. The main effect of Cue type (gaze versus arrow) was not significant [F(1,13)=.03, p=.864, ], nor were any of the interactions between Cue type and the other factors (all F’s < 2.18, all p’s >.14), indicating that gaze and arrow cues produced similar effects.
Figure 3.
Mean response times (a) and cueing effects (b) plotted as a function of SOA for arrow cues (left column) and gaze cues (right column), under both short-duration (top row) and long-duration (bottom row) conditions. Line graphs in panel (a) display mean RTs for validly, invalidly, and neutrally cued targets. Bar graphs in panel (b) depict the cueing effect (invalid-minus-valid RTs). The pattern of results corresponds to that predicted by a cue-target conflict explanation rather than by a rapid attentional orienting one (compare Fig. 3b to Fig. 1b/1c).
Planned pairwise comparisons were then performed to examine overall cueing effects (Invalid-minus-Valid RTs; see Fig. 3b), RT costs (Invalid-minus-Neutral RTs), and RT benefits (Neutral-minus-Valid RTs) in each condition. For both short-duration conditions, regardless of cue type or cue-target SOA, no significant cueing effects were observed (all t’s<−.56, all p’s>.59). For the long-duration cues, however, a different pattern emerged, which was the same for both arrow and gaze cues. No cueing effects were observed at the two longest SOAs for either the gaze cues [300 ms: t=.19, p=.85; 500 ms: t=.58, p=.58] or the arrow cues [300 ms: t=.09, p=.92; 500 ms: t=.31, p=.76]. Conversely, at the two shortest SOAs (i.e., when the cue-target SOA was 100 ms and when the cue and target were simultaneous), cueing effects were observed for both gaze cues [0 ms: t=4.91, p < .001; 100 ms: t=3.26, p=.006] and arrow cues [0 ms: t=7.17, p< .001; 100 ms: t=2.48, p=.03]. For the gaze cues considered alone, the overall cueing effects were driven by RT costs for the invalid/incongruent condition relative to neutral [0 ms: t=5.36, p< .001; 100 ms: t=3.93, p=.002] but showed no significant RT benefits for the valid/congruent case relative to neutral [0 ms: t=1.27, p=.23; 100 ms: t=.47, p=.65]. The same pattern was observed for arrow cues, with significant costs [0 ms: t=3.54, p=.004; 100 ms: t=2.39, p=.03] but no benefits [0 ms: t=1.28, p=.23; 100 ms: t=.77, p=.46].
Discussion
It has become widely accepted in the literature that eye gaze triggers a rapid reflexive shift of attention. More generally, however, the degree of automaticity in attention orienting has been widely debated. For example, although a salient-but-irrelevant item can rapidly draw attention to its location, this attentional capture can be contingent on feature similarity to the target (Folk, Remington, & Johnston, 1992) and can be avoided when the item is expected (e.g., Munneke, Van der Stigchel, & Theeuwes, 2008), suggesting that top-down processes play a role in the purported reflexive capture of attention.
Although it has been suggested that arrow-cueing effects may be modulated by top-down processes but that eye-gaze cues are immune to such influences (Ristic, Wright, & Kingstone, 2007), several recent studies have provided evidence for top-down modulation of gaze-cue effects. For example, gaze-cue effects have been shown to be modulated by social status in both monkeys and humans (Liuzza et al., 2011; Pavan et al., 2011; Shepherd et al., 2006), with larger effects when observing the gaze of high-status individuals. One recent study has even reported that gaze-cue effects vary by political leaning, (Dodd, Hibbing, & Smith, 2011). Results such as these suggest that the rapid response to gaze cues may not be evidence of a truly reflexive process.
One hypothesis is that gaze cues, and possibly arrow cues, can produce a very rapid reflexive orienting response that is followed by a later voluntary orienting response (Hill et al., 2010). Evidence for this comes largely from studies that have employed counterpredictive cueing, where the response-relevant target is actually much more likely to appear at the uncued location, requiring voluntary attentional orienting to the invalidly cued location. Rapid cueing effects have been reported under such conditions, which has been taken as evidence that attention was first reflexively oriented to the cued location before being volitionally oriented to the uncued one (Friesen, Ristic, & Kingstone, 2004; Tipples, 2008).
The conclusion that these effects reflect reflexive orienting, however, rests on the assumption that a difference in behavioural responses to cued and uncued targets at short cue-target intervals is necessarily the result of the rapid orienting of attention toward the cued location. However, other cognitive processes, such as changes in decision or response criteria (Ivanoff & Saoud, 2009) or spatial compatibility (Downing, Dodds, & Bray, 2004), can also produce faster responses for cued-location targets. In our previous study, we suggested that one such process is the conflict between the spatial information provided by the cue and target (Green & Woldorff, 2012). This spatial conflict effect was observed when the cue and target remained on the screen together, and so may be related to cue-target perceptual-integration explanations forwarded by other researchers (Crump, Milliken, & Ansari, 2007). In various stimulus-conflict paradigms, conflict tends to be maximal when the items are presented simultaneously (or are perceived as simultaneous; e.g., Glaser & Glaser, 1982). Thus, differentiating between reflexive-orienting and spatial-conflict accounts requires a closer examination of the timing and nature of the cueing effects at short intervals and of the influence of stimulus duration.
The expected time-courses of cueing effects (see Fig. 1a) are such that reflexive orienting and cue-target conflict for nonpredictive cues should produce distinct and opposing patterns (Fig. 1b versus Fig. 1c). Our observed pattern of results (Fig. 3b) are clearly consistent with a cue-target conflict explanation rather than a reflexive-orienting account. Cueing effects were observed only for long-duration cue and target stimuli with substantial temporal overlap, were largest when the cue and target were presented simultaneously, and were attributable to slowing for the invalid/incongruent condition rather than facilitation for the valid/congruent case. Moreover, the patterns observed for arrow and gaze cues were nearly identical. These results strongly suggest that RT cueing effects rapidly elicited by both gaze and arrow cues result from conflict between the spatial information contained in the cue and target stimuli, rather than from very rapid reflexive attentional orienting. It appears that when the cue and target stimuli overlap extensively in time, the conflicting spatial information slows responses and produces a valid-versus-invalid reaction-time difference that can be difficult to distinguish from reflexive orienting without the additional conditions and analyses presented here.
It should be noted that the gaze-cue stimuli used here were highly schematized, so it is unknown whether the same conflict-like pattern would be observed with directional-gaze cues from real face stimuli, a topic that would be valuable to pursue in future studies. Regardless, the present results strongly question the widely accepted view that nonpredictive eye-gaze and arrow cues trigger very rapid and reflexive attentional orienting. Rather, the rapid RT cueing effects observed with either gaze or arrow cues are likely due to conflict caused by incongruent spatial information between the cue and the target.
Acknowledgments
This work was supported by NIH Grants R01-NS051048 and R01-MH060415 to M.G.W. and an NSERC postdoctoral fellowship to J.J.G.
References
- Baron-Cohen S. The eye direction detector (EDD) and the shared attention mechanism (SAM): Two cases for evolutionary psychology. In: Moore C, Dunham PJ, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. [Google Scholar]
- Crump MJC, Milliken B, Ansari I. Shifting views on the symbolic cueing effect: Cueing attention through recent prior experience. Psicológica. 2007;29(1):97–114. [Google Scholar]
- Dodd MD, Hibbing JR, Smith KB. The politics of attention: gaze-cuing effects are moderated by political temperament. Attention, perception & psychophysics. 2011;73(1):24–29. doi: 10.3758/s13414-010-0001-x. [DOI] [PubMed] [Google Scholar]
- Downing P, Dodds C, Bray D. Why does the gaze of others direct visual attention? Visual Cognition. 2004;11(1):71–79. doi: 10.1080/13506280344000220. [DOI] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Visual Cognition. 1999;6(5):509–540. doi: 10.1080/135062899394920. [DOI] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annual review of psychology. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal Of Experimental Psychology-Human Perception And Performance. 1992;18(4):1030–1044. doi: 10.1037/0096-1523.18.4.1030. [DOI] [PubMed] [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review. 1998;5(3):490–495. doi: 10.3758/BF03208827. [DOI] [Google Scholar]
- Friesen CK, Kingstone A. Abrupt onsets and gaze direction cues trigger independent reflexive attentional effects. Cognition. 2003;87(1):B1–10. doi: 10.1016/s0010-0277(02)00181-6. [DOI] [PubMed] [Google Scholar]
- Friesen CK, Ristic J, Kingstone A. Attentional effects of counterpredictive gaze and arrow cues. Journal of Experimental Psychology-Human Perception and Performance. 2004;30(2):319–329. doi: 10.1037/0096-1523.30.2.319. [DOI] [PubMed] [Google Scholar]
- Glaser MO, Glaser WR. Time course analysis of the Stroop phenomenon. Journal of Experimental Psychology-Human Perception and Performance. 1982;8(6):875–894. doi: 10.1037//0096-1523.8.6.875. [DOI] [PubMed] [Google Scholar]
- Green JJ, Woldorff MG. Arrow-elicited cueing effects at short intervals: Rapid attentional orienting or cue-target stimulus conflict? Cognition. 2012;122(1):96–101. doi: 10.1016/j.cognition.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JL, Patel S, Gu X, Seyedali NS, Bachevalier J, Sereno AB. Social orienting: reflexive versus voluntary control. Vision Research. 2010;50(20):2080–2092. doi: 10.1016/j.visres.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Pratt J, Colzato L, Godijn R. Symbolic Control of Visual Attention. Psychological Science. 2001;12(5):360–365. doi: 10.1111/1467-9280.00367. [DOI] [PubMed] [Google Scholar]
- Ivanoff J, Saoud W. Nonattentional effects of nonpredictive central cues. Attention, Perception & Psychophysics. 2009;71(4):872–880. doi: 10.3758/APP.71.4.872. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Watt RJ, Bruce V. Do the eyes have it? Cues to the direction of social attention. Trends in Cognitive Sciences. 2000;4(2):50–59. doi: 10.1016/s1364-6613(99)01436-9. [DOI] [PubMed] [Google Scholar]
- Liuzza MT, Cazzato V, Vecchione M, Crostella F, Caprara GV, Aglioti SM. Follow My Eyes: The Gaze of Politicians Reflexively Captures the Gaze of Ingroup Voters. PLoS ONE. 2011;6(9):e25117. doi: 10.1371/journal.pone.0025117.t002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munneke J, Van der Stigchel S, Theeuwes J. Cueing the location of a distractor: an inhibitory mechanism of spatial attention? Acta Psychologica. 2008;129(1):101–107. doi: 10.1016/j.actpsy.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Müller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. Journal of Experimental Psychology-Human Perception and Performance. 1989;15(2):315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Pavan G, Dalmaso M, Galfano G, Castelli L. Racial Group Membership Is Associated to Gaze-Mediated Orienting in Italy. PLoS ONE. 2011;6(10):e25608. doi: 10.1371/journal.pone.0025608.g003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. Journal of Experimental Psychology. 1980;109(2):160–174. [PubMed] [Google Scholar]
- Posner M, Cohen Y. Components of Visual Orienting. In: Bouma H, Bowhuis D, editors. Attention and Performance X. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Ristic J, Wright A, Kingstone A. Attentional control and reflexive orienting to gaze and arrow cues. Psychonomic Bulletin & Review. 2007;14(5):964–969. doi: 10.3758/bf03194129. [DOI] [PubMed] [Google Scholar]
- Shepherd SV, Deaner RO, Platt ML. Social status gates social attention in monkeys. Current Biology : CB. 2006;16(4):R119–20. doi: 10.1016/j.cub.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Tipples J. Eye gaze is not unique: automatic orienting in response to uninformative arrows. Psychonomic Bulletin & Review. 2002;9(2):314–318. doi: 10.3758/bf03196287. [DOI] [PubMed] [Google Scholar]
- Tipples J. Orienting to counterpredictive gaze and arrow cues. Perception and Psychophysics. 2008;70(1):77–87. doi: 10.3758/pp.70.1.77. [DOI] [PubMed] [Google Scholar]
- Vecera SP, Rizzo M. Eye gaze does not produce reflexive shifts of attention: evidence from frontal-lobe damage. Neuropsychologia. 2006;44(1):150–159. doi: 10.1016/j.neuropsychologia.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Wright RD, Ward LM. Orienting of attention. Oxford University Press; USA: 2008. [Google Scholar]