Abstract
The increasing number of proteomic and DNA-microarray studies is continually providing a steady acquisition of data on the molecular abnormalities associated with human tumors. Rapid translation of this accumulating biological information into better diagnostics and more effective cancer therapeutics in the clinic depends on the use of robust function-testing strategies. Such strategies should allow identification of molecular lesions that are essential for the maintenance of the transformed phenotype and enable validation of potential drug-targets. The tetracycline regulated gene expression/ suppression systems (Tet-systems) developed and optimized by bioengineers over recent years seem to be very well suited for the function-testing purposes in cancer research. We review the history and latest improvements in Tet-technology in the context of functional oncogenomics.
Keywords: Tet on, Tet off, tetracyclines, TetR, tTA, rtTA, tTS, rtTA2S-M2
Introduction
Cancer is a systems biology disease resulting from deregulation of cellular functions (Hornberg et al. 2006). Abnormalities leading to the transformation and malignant phenotype of cancer cells accumulate over time causing irregularities in signaling networks that control normal cell behavior. The features that distinguish malignant cancer cells from their non-transformed counterparts in vivo can be defined as: self-sufficiency in growth signals, insensitivity to growth-inhibitory signals, evasion of programmed cell death, limitless replicative potential, sustained angiogenesis, and capability for tissue invasion and metastasis (Hanahan and Weinberg, 2000). The ability to recognize the molecular events that are involved in the acquisition and maintenance of these features of cancer cells should ultimately lead to better diagnostics and more effective therapies. Unfortunately our present knowledge of the biochemical events involved in malignant transformation, and of the biomarkers that could help to identify and characterize this process, is still too rudimentary. This is a consequence of three major factors: (i) an enormous molecular diversity of human cancers, (ii) limitations of the cancer model systems we use and (iii) the technical approaches we employ to study these model systems.
The molecular diversity of human cancers reflects the heterogeneity and complexity of human tissues and organs. Even cancers of the same origin (for example colorectal cancers) can display significant variability with respect to molecular lesions associated with the transformed phenotype (Boland and Goel, 2005). This unfortunately is the biological reality that we must accept. It seems reasonable to assume that we need to better characterize and understand the biological diversity of human tumors at the molecular level in order to be able to design and develop more effective drugs and therapeutic strategies.
The currently available cancer model systems can be classified into four major categories: (a) biopsies and surgically removed human tumors; (b) in vitro models based on primary cells or established cell lines; (c) human cancer cells grown in vivo as xenografts; (d) true animal models (i.e. animals, mainly rodents, in which tumor formation is triggered by genetic manipulations or treatment with carcinogens). Each of these model categories has advantages and disadvantages. Surgically removed tumor samples represent real human tumors, however they are more suitable for descriptive analysis rather than for mechanistic functional studies. In vitro models are relatively easy to investigate and well suited for high-throughput mechanistic approaches, but they are over-simplistic and prone to artifacts (Suggitt and Bibby, 2005). Xenografts of human cancer cells provide a more complex in vivo micro-environment and display many features of real tumors but they are usually in non-physiological locations in immuno-compromised animals (Becher and Holland, 2006; Sausville and Burger, 2006). True animal models provide full complexity and organ specificity of tumor development, however rodent cells are known to differ from human cells with respect to the events required for transformation (Hahn and Weinberg, 2002; Rangarajan and Weinberg, 2003). There are attempts to combine the advantages of different model systems (for example orthotopic implantation of human tumor fragments into the organs of nude mice) but they are technically challenging and their use is rather restricted (Bibby, 2004). Considering the imperfections of the different categories of cancer model systems available, it is likely that comparative approaches combining the results obtained from different types of models may be most efficient in identifying the driving genetic lesions underlying cancer development and progression. Indeed, evidence supporting this prediction is starting to accumulate (Rhodes and Chinnaiyan, 2005; Tomlins and Chinnaiyan, 2006).
Following the realization of the complexity of human carcinogenesis and the complications resulting from the limitations of the available models, it became apparent that only robust experimental approaches and improvements in cancer research technology may provide enough information to fully understand the biology of human cancers. Consequently in recent years this has resulted in the development and popularization of the “global” strategies including DNA microarrays and proteomics (Patterson and Aebersold, 2003; Tinker et al. 2006). Significant amounts of data from different cancer models have been obtained and the web-based bioinformatic resources aimed to facilitate discoveries from genome-wide and proteome-wide analyses are being developed (Rhodes and Chinnaiyan, 2004; Rhodes et al. 2004; Wiemann et al. 2004; Kihara et al. 2006; Malik et al. 2006). The advantages of these approaches over the methods used in the past are obvious and one can expect the “global analysis” trend to continue. In particular, these approaches will facilitate a deeper understanding of carcinogenesis as greater consideration will be given to quantitative and spatio-temporal data in the discovery of the control of complex signaling networks (Lazebnik, 2002).
In the light of the accumulating genomic/proteomic information there is a pressing need for the development of robust function testing technologies to distinguish causal from bystander genetic abnormalities. These technologies should be used to generate more tightly controlled model systems that would allow mechanistic functional studies of particular genetic lesions in order to precisely define their biological consequences and importance in maintaining the transformed phenotype of cancer cells. Optimally such function-testing-oriented models should be reasonably easy to generate and allow real time, quantitative introduction of genetic lesions under investigation in a precisely controlled and reversible way. Tightly controlled mechanistic models are necessary to minimize the risks of artifacts resulting from the enormous adaptatory potential of cancer cells to enforced changes in their intracellular biochemical equilibrium (Kitano, 2003).
It seems that inducible gene expression/suppression technologies developed by bioengineers over the last twenty years (for review see (Fussenegger, 2001; Toniatti et al. 2004; Weber and Fussenegger, 2006)) can now fulfill the criteria of an optimal function-testing system (Saez et al. 1997). The gene expression/suppression systems controlled by tetracycline (tc) and its analogues (Tet-systems) represent the most mature technology of this kind (Berens and Hillen, 2003). They can be precisely regulated in vitro and in vivo by a well characterized agent with favorable pharmacokinetic characteristics and facilitate elimination of non-specific compensatory mechanisms that often occur in models engineered for constitutive expression/suppression of the gene(s) of interest. We believe that they are now setting an improved technological standard in functional cancer research. The aim of this article is to provide an overview of the recent progress achieved in adopting Tet-systems to the purposes of functional oncogenomic research.
Tet-Systems: The Historical Perspective
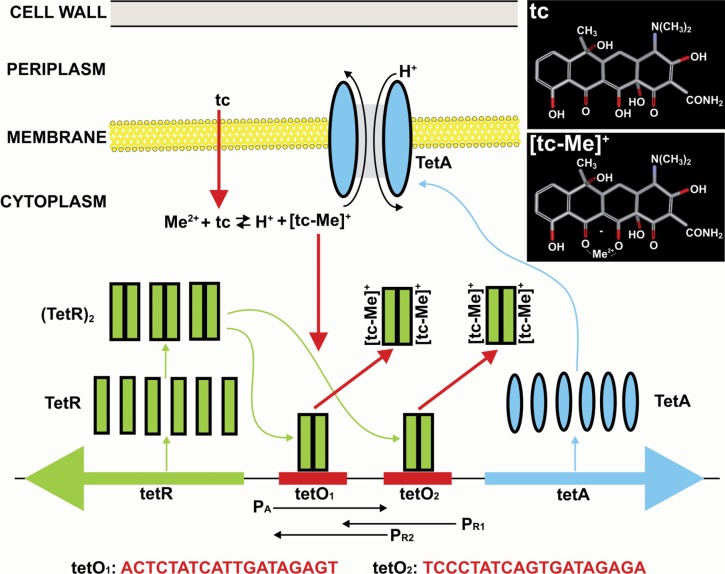
Tetracycline controlled gene expression/suppression systems in mammalian cells originate from Tet-operons in Gram-negative bacteria (Hillen and Berens, 1994; Saenger et al. 2000; Berens and Hillen, 2003). In these bacteria the resistance to the antibiotics from the tetracycline family can be acquired by expression of energy-dependent membrane associated efflux proteins known as TetA. These proteins of approximately 43kDa contain 12 hydrophobic membrane-spanning regions and function as tetracycline/metal ion – H+ anti-porters removing tetracycline/metal ion complexes ([tc-Me]+) from the cytoplasm to the periplasmatic space (Eckert and Beck, 1989) (Fig. 1). Several structurally related tetracycline/metal ion – H+ antiporter proteins have been described to date (classes: A, B, C, D, E, G, H, I, J, Y, Z, 30, 31, 33, 38, 39, reviewed in (Roberts, 1996; Chopra and Roberts, 2001; Roberts, 2005)). They are often associated with conjugative or mobilizable genetic elements (e.g. transposons) that facilitate their propagation among bacterial species. Although TetA proteins are protective against tetracyclines, they are poorly tolerated and have adverse effects on bacterial growth and viability when produced in excess (Nguyen et al. 1989). The delicate balance between the protective function of TetA against tetracyclines and its negative effects on cell growth is maintained by the precise control of TetA expression (Fig. 1). Genetic organization and the mechanisms of regulation of the tetracycline resistance determinants have been best characterized for transposon Tn10 in Escherichia coli that contains genetic elements responsible for expression of TetA(B) protein (where (B) indicates the class of the determinant). It is believed that other classes of tet-resistance determinants have a similar mode of action (Hillen and Berens, 1994).
Figure 1.
Principles of the Tn10 encoded tetracycline resistance in Escherichia coli. The top part of the figure illustrates the function of TetA protein at the cytoplasmic membrane. The lower part illustrates organization and function of the Tet-regulon. The structures of tetracycline (tc) and tetracycline-metal ion complex ([tc-Me]+) are presented in the top right corner. See text for details.
The Tet-regulon on Tn10 (illustrated in Fig. 1) consists of two genes localized next to each other and transcribed in opposite directions. One of the genes (tetA) encodes TetA antiporter whereas the other (tetR) encodes a regulatory protein known as Tet repressor (TetR). These genes are separated by a sequence of 81bp containing one promoter for tetA (tetPA) and two overlapping promoters for tetR (tetPR1 and tetPR2 respectively). The tetPA and tetPR promoters display opposite polarity and drive the expression of TetA and TetR respectively. The region containing tetPA and tetPR promoters also contains two very similar tet-operator (tetO) sequences called tetO1 and tetO2. Each of the operator sequences has a palindromic organization with a central base pair inserted on the symmetry axis and is recognized by TetR homodimers ((TetR)2) that are formed following TetR synthesis. The interaction of TetR homodimers with tetO1 and tetO2 sequences is mediated by the helix-turn-helix (HTH) motif on the TetR protein. Binding of (TetR)2 to the operator sequences interferes with the function of the promoters leading to the suppression of transcription. Thus, in the absence of tetracycline, synthesis of TetA and TetR is inhibited. When tetracyclines enter the cell they form an active tetracycline/metal ion complex [tc-Me]+ in the cytoplasm. The (TetR)2 homodimer can bind two [tc-Me]+ molecules. The affinity of TetR towards [tc-Me]+ complexes is very high with an association constant of ∼109 M−1 (Hillen et al. 1983). Following association with [tc-Me]+, the (TetR)2 undergoes conformational changes and its affinity to tetO is reduced by 6–10 orders of magnitude (Lederer et al. 1995). This results in dissociation of the TetR homodimer from the tetO sequence and in transcription of the TetA and TetR genes (Hillen and Berens, 1994; Saenger et al. 2000).
The first evidence that the Tet repressor-operator system can be functional in eukaryotic cells was provided by Gatz and Quail (Gatz and Quail, 1988). They demonstrated that in the protoplasts of Nicotiana tabacum the Tn10-encoded TetR protein (=TetR(B)) can regulate transcription from the cauliflower mosaic virus 35S promoter engineered to flank the “TATA” box with two tetO sequences (Gatz and Quail, 1988). This observation provided foundations for the development of TetR regulated RNA polymerase II (Yao et al. 1998) and RNA polymerase III promoters (Ohkawa and Taira, 2000) suitable for tetracycline regulated gene expression in mammalian cells. TetR regulated RNA polymerase II and III promoters can be used for tetracycline inducible expression of a protein of interest (Yao et al. 1998; Reeves et al. 2002), antisense RNA (Ohkawa and Taira, 2000), ribozymes (Bowden and Riegel, 2004), shRNAs (Czauderna et al. 2003; Matsukura et al. 2003; van de Wetering et al. 2003) and micro RNAs (Stegmeier et al. 2005). It is believed that in mammalian cells in the absence of tetracyclines, TetR reduces transcription from promoters engineered to include tetO sequences by steric interference with binding of RNA polymerase or other essential factors of the eukaryotic transcription machinery (Yao et al. 1998).
Recognition of the functionality of the Tet repressor-operator system in eukaryotic cells had also prompted construction of Tet repressor variants fused to the eukaryotic regulatory domains. This direction had been pioneered by Gossen and Bujard (Gossen and Bujard, 1992), and originally resulted in the generation of the tetracycline controlled transactivator tTA that is a fusion between TetR(B) protein and the C-terminal activating domain of virion protein 16 (VP16) of herpes simplex virus. In the absence of tetracycline, this transactivator enabled efficient expression of luciferase from the minimal promoter sequence of the human cytomegalovirus promoter IE preceded by seven tetO2 operators (tetO7-PhCMVmin). No luciferase activity was detected in the presence of tetracycline (Gossen and Bujard, 1992). The inducible systems based on the use of tTA (so called Tet-off systems) proved very useful in many applications (e.g. Felsher and Bishop, 1999; Oberst et al. 1999; Giavazzi et al. 2001; Honda et al. 2005), however they are compromised by the need for the sustained presence of tetracycline to maintain the un-induced state. This is very problematic, particularly in animal-based in vivo experiments. In addition, prolonged exposure to tetracyclines may have uncontrolled effects on the physiology of mammalian cells. Furthermore the kinetics of tetracycline removal required to trigger inducible gene expression are not always well controllable (Gossen et al. 1995).
To overcome the disadvantages of the Tet-off systems, a random mutagenesis of the tetR(B) and screen for Tet repressors with reversed regulatory properties was performed in Escherichia coli (Gossen et al. 1995). The screen resulted in the discovery of the first reverse Tet repressor (rTetR). The rTetR differs from the classical TetR(B) by four amino acid exchanges (Lys71 for Glu71, Asn95 for Asp95, Ser101 for Leu101, Asp102 for Gly102) and tetracyclines promote rather than abolish its binding to tet operators. Subsequent modification of the tTA construct by substitution of the TetR with rTetR resulted in the generation of the first reverse transcriptional transactivator (rtTA). The rtTA construct displays better sensitivity towards doxycycline (Dox) than towards tetracycline. Unlike tTA, rtTA induces gene expression in the presence rather than in the absence of tetracyclines (Gossen et al. 1995). The inducible systems based on the use of the reverse transcriptional transactivators are known as Tet-on systems. Similarly to Tet-off systems, the first generation Tet-on systems based on the rtTA construct proved useful in multiple applications (e.g. (Chin et al. 1999; Saam and Gordon, 1999; Wang et al. 2000; Gunther et al. 2002)). Unfortunately, their utility was restricted due to some negative properties of the rtTA construct that limit its application. These include (i) residual affinity of rtTA to tetO in the absence of Dox that results in background leakiness, (ii) relatively low stability of rtTA in mammalian cells that significantly complicates (or prevents) establishing of a functional Tet-on system in certain cell types, and (iii) the need for a relatively high Dox concentration required for full induction (1–2 μg/ml, this concentration cannot be readily achieved in some organs in vivo) (Urlinger et al. 2000).
The weaknesses of the first generation rtTA transactivator were overcome by the development of the second generation rtTAs. In Saccharomyces cervisiae, the original tTA construct was subjected to mutagenesis and functional screening for novel rtTA mutants with reduced basal activity and increased Dox sensitivity. Several new rtTAs were identified. The second generation reverse transcriptional transactivator with the most favorable properties, rtTA2S-M2, harbors five novel mutations in the TetR region (Gly12 for Ser12, Gly19 for Glu19, Pro56 for Ala56, Glu148 for Asp148, Arg179 for His179). It functions at 10-fold lower Dox concentration than rtTA, is more stable in eukaryotic cells and causes no background expression in the absence of Dox (Urlinger et al. 2000). Its coding sequence was optimized for use in human cells and the VP16 activation domain was substituted with three minimal activation domains (FFF motif) to reduce immunogeneity in vivo and to eliminate potential interactions with cellular transcription factors (Baron et al. 1997; Urlinger et al. 2000). The Tet-on systems utilizing second generation rtTA2S-M2 transactivator displayed superior properties in multiple applications in vitro and in vivo (see next section) (Knott et al. 2002; Lamartina et al. 2002; Koponen et al. 2003; Pluta et al. 2005).
In parallel to tetracycline regulated transcriptional transactivators utilizing eukaryotic transcriptional activator domains and forming the foundations of the Tet-off and Tet-on systems, tetracycline regulated transcriptional silencers (tTS) generated by fusing TetR to eukaryotic transcriptional silencing domains were also developed (Deuschle et al. 1995; Belli et al. 1998; Freundlieb et al. 1999; Ryu et al. 2001). In the absence of tetracycline these constructs exert silencing activity after binding to tetO sites placed in the proximity of the transcriptional initiation site of an eukaryotic promoter. Promoter activity is restored upon administration of tetracycline which prevents binding of tTS to the tetO sequences. Although some tTS’ enabled tetracycline controlled gene expression from appropriately engineered promoters in mammalian cells (Deuschle et al. 1995), they did not became popular as independent regulatory systems. Instead they proved very useful in combination with the Tet-on systems, reducing residual leakiness in the absence of induction (Rossi et al. 1998; Forster et al. 1999; Zhu et al. 2001; Lamartina et al. 2003; Mizuguchi et al. 2003; Rubinchik et al. 2005). The most commonly used construct of this type is the fusion of a mutant TetR and the KRAB-AB domain of the Kid-1 protein (Freundlieb et al. 1999).
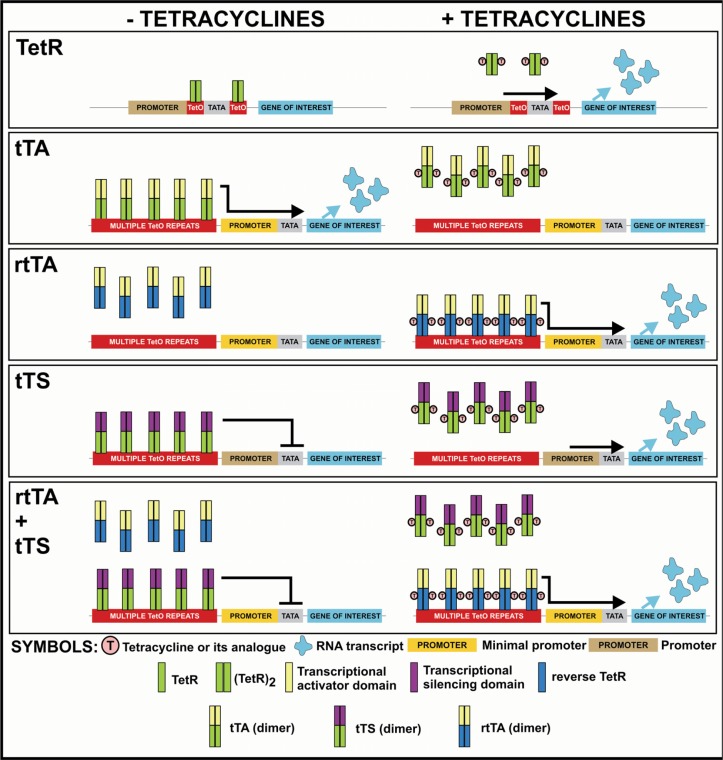
The comparison of TetR, tTA, rtTA and tTS—based systems is provided in Fig. 2. It is worth mentioning that in addition to the well established developments listed above several new promising directions in the evolution of Tet-systems have been initiated. These include (but are not restricted to): (i) changes in DNA binding specificity of Tet-transregulators (Helbl and Hillen, 1998; Helbl et al. 1998; Baron et al. 1999); (ii) Tet-transregulator setups allowing establishment of different levels of promoter activity (Knott et al. 2002; Krueger et al. 2006), (iii) transactivator mutants with altered effector specificity to allow selective regulation of more than one gene (Krueger et al. 2004a; Krueger et al. 2004b); (iv) single-chain transregulators (Krueger et al. 2003; Kamionka et al. 2006), and (v) tetracycline regulated aptamers allowing control at the translational level (Suess et al. 2003). The advances in the development of Tet-transregulators were complemented by significant progress in the generation of optimized responsive promoters and delivery systems (see next section) (Baron et al. 1995; Agha-Mohammadi et al. 2004; Qu et al. 2004).
Figure 2.
Organization and function of Tet-systems utilizing different Tet-regulatory proteins. The schematics presented illustrate the functional principles only, the details of the structure of the genetic elements encoding Tet-regulatory proteins and of Tet-responsive cassettes vary between diverse published studies. See text for a detailed description.
With over 600 papers based on the use of Tet-systems published last year (see www.tetsystems.com for a list of recent publications) the tetracycline regulated gene expression/suppression technology represents the most commonly used and the best characterized inducible platform for functional genomic research currently available. It has been successfully used in multiple model organisms (Berens and Hillen, 2003) and is presently validated for the purposes of human gene therapy (Toniatti et al. 2004; Weber and Fussenegger, 2006).
Tet-Systems in Functional Cancer Research
The use of Tet-systems in functional cancer research has been increasing steadily over recent years, reflecting perhaps the improvements in Tet-technology and growing awareness of the necessity for stringent quantitative and spatio-temporal control of gene expression in mechanistic cancer model systems. In addition to their use in protein overexpression studies (we refer to them as “classical” Tet-systems) (e.g. Hossini et al. 2003; Honda et al. 2005; Soulie et al. 2005), Tet-systems have been successfully validated in loss-of-function experiments based on RNA interference mediated by regulated expression of short hairpin RNA (shRNA) (Czauderna et al. 2003; Matsukura et al. 2003; van de Wetering et al. 2003; Wiznerowicz and Trono, 2003; Lin et al. 2004; Malphettes and Fussenegger, 2004; Amar et al. 2006; Xia et al. 2006) or shRNA imbedded in the microRNA context (Dickins et al. 2005; Stegmeier et al. 2005). Tet-vectors permitting both conditional gene expression and knockdown have been reported (Szulc et al. 2006). Development of the multicistronic Tet-responsive cassettes that in addition to the gene(s) of interest enable expression of reporter genes like luciferase, green fluorescent protein (GFP), red fluorescent protein (RFP), PET-reporters or MRI-reporters brings the promise for efficient quantitative monitoring of inducible gene expression/suppression in tumor models in vivo (Kistner et al. 1996; Sun et al. 2001; Cohen et al. 2005; Dickins et al. 2005; Tang et al. 2005; Welman et al. 2005). Tet-systems are advantageous over many other inducible technologies used in cancer research (Lewandoski, 2001; Albanese et al. 2002) also due to favorable properties of tetracyclines as inducing agents. Tetracyclines easily penetrate cellular membranes by diffusion (Argast and Beck, 1984), at low concentrations they have little or no pleiotropic effects in eukaryotic cells (Wishart et al. 2005; Goldring et al. 2006), and their biological effects in vivo are very well characterized after many years of use in the clinic (Shlaes, 2006).
The molecular lesions most frequently associated with cancer involve gene mutations and upregulation or downregulation of gene expression. As discussed above, the “classical” Tet-systems enable inducible gene expression (“upregulation”) including expression of mutated genes, whereas Tet–dependent RNA interference enables regulated gene suppression (“downregulation”). Considering the high sequence specificity of the RNA interference process (Brummelkamp et al. 2002) and a possibility of introducing silent mutations into the coding sequences of exogenously engineered constructs, simultaneous knockdown of the endogenous gene and expression of its modified analogue in a Tet-controlled manner should also be possible. Thus Tet-technology seems to provide a range of tools sufficient to mimic (or reverse) the abnormalities commonly associated with transformed phenotype. In a drug discovery setting, Tet-based systems can be applied to up or down regulate wild type or mutated drug targets in vitro or in vivo. Such experiments provide opportunities to identify on or off target drug toxicities and potentially, could facilitate the search for biomarkers of drug responses (Gonzalez-Nicolini and Fussenegger, 2005).
To date the functional cancer studies based on the use of the Tet-technology have been successfully performed using diverse genes of interest in cells grown in vitro or in vivo (as xenografts) and in animal models. Considering the amount of publications and a broad range of applications described we are unable to provide the analysis of all the functional cancer research studies that employed Tet-systems, but a representative selection is presented in Table 1.
Table 1.
A selection of representative studies illustrating the diverse applications of Tet-technology in functional cancer research.
| Model System | Regulatory Element(s) | Description of the Study | Reference |
|---|---|---|---|
| Cells in vitro and subcutaneous xenografts | tTA | Inducible over-expression of membrane-type-1 matrix metalloproteinase (MT1-MMP) in non-malignant MDCK epithelial cells is by itself sufficient to drive formation of invasive tumors. | (Soulie et al. 2005) |
| Cells in vitro and subcutaneous xenografts | tTA | Induction of fibroblast growth factor–2 (FGF-2) expression in human endometrial adenocarcinoma cells deeply affects the initial tumor growth and neovascularization but does not affect the progression of large tumors. | (Giavazzi et al. 2001) |
| Cells in vitro and orthotopic xenografts | tTA | Demonstration that actinin-4 actively increases cell motility and promotes lymph node metastasis of colorectal cancer. The Tet-off system was used to express actinin-4 in DLD-1 colorectal cancer cells. | (Honda et al. 2005) |
| Cells in vitro and subcutaneous xenografts | rtTA | This study shows that inducible expression of Bcl-Xs in human melanoma cells triggers apoptosis in vitro and delays growth of tumor xenografts in vivo. | (Hossini et al. 2003) |
| Cells in vitro and orthotopic xenografts | rtTA | Using the Tet-on system in malignant astrocytoma cells, it was shown that p125FAK can promote tumor cell proliferation in vivo and that it was not due to less apoptosis. | (Wang et al. 2000) |
| Cells in vitro and subcutaneous xenografts | tTA rtTA |
Antisense mRNA against mtCLIC/CLIC4 chloride channel protein expressed using Tet-off and Tet-on systems inhibited tumor growth and induced tumor apoptosis in human osteosarcoma cells. | (Suh et al. 2005) |
| Cells in vitro | rtTA2S-M2 tTS |
Inducible expression of GD3 ganglioside in glioblastoma cells was associated with apoptosis occurring via caspase-8 activation. | (Saqr et al. 2006) |
| Transgenic mice* | tTA | Sustained expression of the MYC transgene in hematopoietic cells resulted in the formation of malignant T cell lymphomas and acute myeloid leukemias. The subsequent inactivation of the transgene caused regression of established tumors. | (Felsher and Bishop, 1999) |
| Transgenic mice* | tTA | Inducible expression of P210 BCR-ABL in stem and progenitor cells of murine bone marrow resulted in splenomegaly, myeloid bone marrow hyperplasia and extrameddulary myeloid cell infiltration of multiple organs. This recapitulates many characteristics of human chronic myeloid leukemia (CML). | (Koschmieder et al. 2005) |
| Transgenic mice* | rtTA | Demonstration that melanoma genesis and maintenance are strictly dependent upon expression of V12 H-Ras in a Dox-inducible V12 H-Ras mouse melanoma model null for the tumor suppressor INK4a. | (Chin et al. 1999) |
| Transgenic mice* | rtTA | Inducible expression of activated receptor tyrosine kinase HER2/Neu in the mammary epithelium of transgenic mice resulted in development of multiple invasive mammary carcinomas that regressed following transgene deinduction demonstrating that Neu-initiated tumorigenesis is reversible. However the animals bearing regressed tumors ultimately developed Neu-independent recurrent tumors. | (Moody et al. 2002) |
| Cells in vitro | TetR tTA | Using breast cancer cell lines engineered to inducibly express or inducibly suppress expression of the Fra-1 gene the authors demonstrate a positive association between Fra-1 levels and cell proliferation, motility and invasiveness. | (Belguise et al. 2005) |
| Cells in vitro and orthotopic xenografts | TetR | Pol III promoter driven conditional expression of shRNA against PI-3 kinase subunits p110α and p110β showed a significant reduction in the formation of metastases following p110β but not p110α downregulation in prostate cancer cells. | (Czauderna et al. 2003) |
| Subcutaneous xenografts | TetR | Inducible downregulation of HIF-1α resulted in transcient tumor stasis and tumor regression. Inhibiting HIF-1α in early stage tumors was more efficacious than inhibiting HIF-1α in more established tumors. | (Li et al. 2005) |
| Subcutaneous xenografts | TetR | Inducible silencing of the candidate tumor suppressor KILLER/DR5 in colon cancer cell lines resulted in accelerated growth of tumor xenografts and conferred resistance to the chemotherapeutic agent 5-fluorouracil | (Wang and El-Deiry, 2004) |
| 3D cell culture in vitro and orthotopic xenografts | TetR | This study shows that a protein kinase C related molecule PKN3 is required for invasive prostate cell growth. | (Leenders et al. 2004) |
| Cells in vitro and subcutaneous xenografts | tTA rtTA |
Inducible RNA interference using microRNA-based shRNA expressed from the Pol II promoter. Tumors induced by Trp53 suppression and cooperating oncogenes regressed upon re-expression of Trp53. | (Dickins et al. 2005) |
Several additional examples of the use of Tet-transgenic mice in cancer research can be found in the recent paper by (Felsher, 2004).
Development of a fully operational Tet-regulated cancer model system requires design and construction of fit-for-purpose Tet-genetic elements and their integration into the experimental system of choice. This must be followed by an in depth validation. Decision on the experimental system to be employed to address a defined biological question might sometimes be very difficult considering the advantages and disadvantages of different cancer model categories available (see introduction). Generally, cell based in vitro functional screens are better suited for initial high-throughput experiments whereas complex animal models might be more appropriate for advanced projects. However, the final choice of a model system to be employed depends on the nature of a problem to be investigated. Detailed discussion of the applicability of different approaches to human cancer modeling have been provided elsewhere (Van Dyke and Jacks, 2002; Suggitt and Bibby, 2005; Becher and Holland, 2006; Sausville and Burger, 2006) and is beyond the scope of this article. We shall concentrate on the design, construction and genetic integration of fit-for-purpose Tet-vectors and on the validation of the engineered tetracycline regulated models.
Inducible gene expression
Inducible expression of a heterologous protein in human cervical carcinoma HeLa cells using the Tet-off system represented the first “intersection” between cancer research and Tet-technology (Gossen and Bujard, 1992). Although many scientists recognized the advantages of this inducible approach over the standard over-expression techniques its implementation as a broadly used functional tool in cancer research was rather slow. This was a consequence of difficulties in setting up functional Tet models. Generation of such models used to be achieved in two consecutive steps. In the first step a transgenic line (cell or animal) expressing a Tet regulatory protein (tTA, rtTA or TetR) was established and the line with the most favorable properties was selected. In the second step, a responsive element encoding a gene of interest under control of a promoter engineered to include tetO sequences was introduced (Gossen and Bujard, 1992; Gossen et al. 1995). Sometimes vectors encoding elements required for both steps were introduced simultaneously, although this usually resulted in models with less desirable properties (higher leakiness) (Shockett et al. 1995). Unfortunately expression of tet-regulatory proteins in many cell lines (especially many types of human cancer cells) proved notoriously difficult and unstable (Gopalkrishnan et al. 1999; Munoz et al. 2004). This very probably reflected characteristics of initially available Tet-regulatory proteins (low stability and potential cytotoxicity in mammalian cells (Shockett et al. 1995; Gallia and Khalili, 1998)) and high levels of epigenetic silencing of the expression vectors (Whitelaw et al. 2001). Suboptimal vector delivery methods as well as inefficient selection and screening protocols may have also contributed to these initial difficulties. In addition to problems associated with stable expression of Tet-regulators, variability in the efficacy of tetracycline controlled gene expression between different cell types was reported (Ackland-Berglund and Leib, 1995; Howe et al. 1995). Furthermore mice based studies revealed that the functionality of Tet-systems in transgenic animals may be affected by the mouse strain used and the position of transgene integration (Shockett et al. 1995; Robertson et al. 2002). Despite all these “early” complications, several functional cell lines and transgenic mice were successfully established (reviewed in Shockett and Schatz, 1996; Saez et al. 1997; Corbel and Rossi, 2002; Zhu et al. 2002). For example Ewald et al. using the Tet-off system demonstrated temporal requirement for viral oncogene expression in cellular transformation by SV40 large T antigen in submandibular gland (Ewald et al. 1996) and Chin et al. using first generation Tet-on system showed essential role for oncogenic Ras in tumor maintenance in a mouse melanoma model (Chin et al. 1999).
Several discoveries that followed the initial “difficult” phase of implementating Tet-systems into functional cancer research significantly increased the success rate of generating cancer model systems employing Tet-regulated inducible gene expression. The most important of these discoveries include: (i) development and characterization of the second generation transcriptional transactivators, (ii) development of the “single vector” strategies for Tet-system delivery, (iii) development and characterization of virus based Tet-system delivery methods, (iv) realization that non-viral promoters may be more effective than viral promoters in driving prolonged expression of Tet- regulators in mammalian cells, and (v) use of improved selection protocols based on the IRES principle.
Discovery of the second generation Tet-regulated transactivator rtTA2S-M2 by Urlinger et al. represented a mile-stone for Tet-technology (Urlinger et al. 2000). The superior properties of this construct have become obvious over the past five years and have enabled tightly controlled inducible gene expression in systems that resisted previous attempts based on older versions of Tet-regulators (Koponen et al. 2003; Barde et al. 2006; Goldring et al. 2006). Currently this transactivator represents the first choice of Tet-transactivator for tetracycline inducible gene expression approaches.
The original two-step strategy of the generation of Tet systems may provide more flexibility in certain applications but is time consuming and labor intensive. The feasibility of delivering both a cassette expressing tTA transactivator and a cassette containing a reporter gene under control of a Tet regulated promoter in a single vector was demonstrated for the first time in tobacco plants (Weinmann et al. 1994). Placing both cassettes in opposing orientations was sufficient to avoid high levels of leaky expression (Weinmann et al. 1994). Following this study, single step Tet-vectors applicable for mammalian cells and animal models were developed (Schultze et al. 1996). Those vectors were subsequently modified to include improved versions of Tet regulators and incorporated into highly efficient viral delivery systems (see below). It seems that the single-vector strategies might be more effective than two-step strategies in “omics” —type functional cancer research.
Many cell types cannot be efficiently transfected using standard methods like lipofection or electroporation. In addition certain cells, especially primary cells are very sensitive and cannot be subjected to a prolonged selection process. To facilitate the fast and reliable introduction of Tet-systems into such cells several groups described use of viral particles as Tet-system delivery vehicles. Viruses can be also used to efficiently transfect organs and tissues in vivo. So far efficient delivery of the Tet-systems components has been obtained using adenoviruses (Mizuguchi et al. 2003; Lee et al. 2005; Rubinchik et al. 2005), adeno-associated viruses (Chtarto et al. 2003; Chenuaud et al. 2004; Stieger et al. 2006), retroviruses (Yu et al. 1996; Lindemann et al. 1997; Pao et al. 2003), parvo viruses (Maxwell et al. 1996; Pacheco et al. 1999), lentiviruses (Koponen et al. 2003; Wiznerowicz and Trono, 2003; Markusic et al. 2005; Barde et al. 2006) and herpes viruses (Schmeisser et al. 2002; Yao et al. 2006). Each type of the virus has slightly different properties with respect to integration status of delivered DNA, the spectrum of cells it can transfect, cellular toxicity and immunogeneity in vivo. Being able to integrate into the genomes of both dividing and non-dividing cells and having no cytotoxic effects, lentiviruses seem to provide the most sophisticated viral vectors for Tet-systems delivery in functional studies (Blesch, 2004). In many studies, viral vectors were able to deliver fully operational Tet-systems to cells grown in vitro (Yu et al. 1996; Tietge et al. 2003), to diverse organs of laboratory animals (Harding et al. 1998; Favre et al. 2002; Tietge et al. 2003) and to tumor xenografts (Pao et al. 2003; Li et al. 2006).
Functionality of Tet-systems depends on continuous expression of the respective Tet-regulatory protein. Many early studies relied on the use of strong viral promoters to drive the expression of Tet regulatory proteins. More recent studies demonstrated that viral promoters are frequently silenced in human cancer cells and that use of strong non-viral promoters, for example elongation factor 1α (EF1α) or β-actin promoters, may significantly improve the efficiency of successfully generating functional Tet models (Gopalkrishnan et al. 1999; Bornkamm et al. 2005; Welman et al. 2005). A similar observation was made in mouse mammary epithelial cell line HC11 (Kenny et al. 2002). Considerable success was also achieved by using Tet-autoregulatory cassettes in which both the gene of interest and Tet-transactivator were placed under the control of a Tet-regulated promoter. Autoregulatory expression of rtTA allows extremely low levels of the transactivator and the transgene product in the absence of tetracyclines with enough rtTA molecules to induce expression upon addition of tetracy-clines (Markusic et al. 2005).
Stable cell lines selected to constitutively express Tet-regulatory protein may display its expression in only a fraction of the cell population. This heterogeneity of expression can be significantly reduced by direct selection for the presence of Tet-regulator. A few studies convincingly demonstrated that coupling the translation of a Tet-regulatory protein to a selectable reporter protein (e.g. an antibiotic resistance determinant or fluorescent marker) via an internal ribosome entry site (IRES) increases selection efficiency and enables generation of cells homogenously expressing Tet-regulator (Rossi et al. 1998; Izumi and Gilbert, 1999; Qu et al. 2004; Welman et al. 2005; Saqr et al. 2006). A protocol for high throughput generation of human cancer Tet-on cell lines based on the use of IRES principle and expression of green fluorescent protein was recently published (Welman et al. 2006a).
In addition to the developments listed above, the simultaneous use of rtTA2S-M2 transactivator and transcriptional silencer (tTS) or inclusion of insulator sequences in the Tet-responsive cassette might improve the characteristics and applicability of Tet-systems in functional studies even more by further reducing the risk of potential leaky expression (Lai et al. 2004; Qu et al. 2004; Pluta et al. 2005; Rubinchik et al. 2005). The technology that enables introduction of Tet-regulated target genes into a predetermined chromosomal loci in human cancer cells by recombinase mediated cassette exchange (RMCE) was also described recently and may provide another level of robustness in functional oncogenomics in the future (Wong et al. 2005). The RMCE methodology can be adapted for accelerated phenotype analysis in transgenic mice and in somatic cells (Toledo et al. 2006). Precise spatio-temporal and cell-lineage-specific control of inducible gene expression in mice could be obtained by integration of Cre-mediated and tetracycline-dependent expression systems (Yu et al. 2005).
Inducible gene suppression
Tetracycline regulated suppression of gene function can be achieved by: (i) inducible over-expression of dominant negative mutants, (ii) conditional knock-out approaches, or (iii) inducible knockdown of gene expression at the RNA level.
When over-expressed, dominant negative mutants are believed to interfere with physiological functions of endogenous proteins by competitive interaction with binding partners and downstream effectors. Tet-regulated expression of dominant negative mutants undergoes the principles described in the “inducible gene expression” section. This approach proved very informative in several cancer related studies (e.g. Miraux et al. 2004; Gonzalez et al. 2006) although interpretation of the results of the experiments employing dominant negative mutants may sometimes be complicated by the complex characteristics of their action (Sheppard, 1994).
Tet-controlled conditional gene knockouts represent a powerful and informative approach to study gene function, however at present they are time, cost and labor intensive and not particularly well suited for high throughput functional analyses. They have been reviewed recently (Gossen and Bujard, 2002) and we will not discuss them here.
Inhibiting endogenous proteins by knocking-down their mRNA levels represents the most robust way of functional gene suppression available at the moment. It has been successfully achieved by tetracycline inducible expression of antisense mRNAs (Lottmann et al. 2001; Suh et al. 2005) and ribozymes (Bowden and Riegel, 2004). However, the most effective way of Tet-regulated gene suppression relies on the RNA interference process with the use of short hairpin RNAs (shRNAs) (Amarzguioui et al. 2005; Dykxhoorn and Lieberman, 2005). The first generation of vectors suitable for tetracycline-regulated targeting of diverse genes of interest by expression of shRNAs utilized unmodified TetR protein and either H1 (van de Wetering et al. 2003), U6 (Czauderna et al. 2003; Matsukura et al. 2003) or 7SK (Czauderna et al. 2003) RNA polymerase III promoters engineered to include a single tetO site. These vectors proved readily applicable for both in vitro (Czauderna et al. 2003; Matsukura et al. 2003; van de Wetering et al. 2003) and in vivo (Czauderna et al. 2003; Leenders et al. 2004; Wang and El-Deiry, 2004) studies. They have been also successfully incorporated into more efficient viral delivery vehicles (Wiznerowicz and Trono, 2003; Hosono et al. 2004; Kuninger et al. 2004; Amar et al. 2006). Even more tightly controlled inducible expression of shRNA could be achieved using U6 promoter modified to include two tetO sites (Lin et al. 2004; Li et al. 2005). In some systems inducible expression of shRNA from Pol III promoters was achieved using tTS regulator (Chen et al. 2003; Szulc et al. 2006). Tet-inducible RNA interference based on the use of RNA polymerase II promoter was subsequently described (Malphettes and Fussenegger, 2004; Xia et al. 2006). The most promising recent development in the Tet-regulated RNA interference strategies is the discovery of a very high silencing potential of shRNAs embedded in the micro RNAs expressed from Pol II promoters (Dickins et al. 2005; Stegmeier et al. 2005). Regulation of Pol II promoters by components of Tet-systems can be much more sophisticated than regulation of Pol III promoters. Multicistronic co-transcription of reporter genes is also possible and might provide functional read-out and enable in vivo imaging (Dickins et al. 2005; Stegmeier et al. 2005). Tet-controlled expression of micro RNAs from Pol II promoters should enable tissue-specific, temporally regulated and reversible gene silencing in vivo (Cullen, 2005). Despite being a relatively new development, Tet-regulated RNA interference has already proven its enormous applicability in cancer research (Chen et al. 2003; Matthess et al. 2005). It seems to be particularly well suited for validating potential drug targets for cancer chemotherapy (Li et al. 2005; Ke et al. 2006).
Validation of Tet models
Due to the developments described above, generation of tightly regulated Tet cancer models is currently much less challenging than it was a few years ago. The constructed models, however, should still be carefully validated before they are used in functional studies. The validation process should comprise two steps: (i) comparison of the established Tet-model in the un-induced stage to its “parental” system from which it was generated; (ii) characterization of its functional properties.
Considering the complex nature of the manipulations required during Tet-model construction, it is essential to ensure that the characteristics of the “parental” system have not been affected beyond an acceptable level. There are reports indicating that high levels of Tet-regulators might be cytotoxic in vitro (Gallia and Khalili, 1998) and trigger pathologies (Sisson et al. 2006) and immune-responses (Latta-Mahieu et al. 2002; Lena et al. 2005) in vivo. In addition, integration of Tet-vectors into the genome of the host system may affect functions of endogenous genes, and the tight control of tetracycline-responsive expression may be compromised in some cells resulting in unacceptably high leakiness (Rang and Will, 2000; Gould and Chernajovsky, 2004). This may also lead to physiological abnormalities. The use of tetracycline regulated models that do not preserve the characteristics of the parental system in the absence of induction may lead to artificial results and should be discouraged. Even for Tet-models that closely resemble their parental system when they are in the un-induced state, it is advisable to use at least two independently generated clones/lines to ensure the reliability of the results.
Prior to more advanced mechanistic studies, the established Tet-systems should be characterized with respect to maximal induction levels and minimal concentration of tetracyclines required to achieve those levels, variation of inducibility within the system, and consistency of gene induction over time. This should confirm the model’s reliability and help in early identification of potential complications (Baron and Bujard, 2000). Published examples indicate that the levels and reversibility of transgene expression in Tet-models can be precisely controlled depending on the time of induction and concentration of tetracyclines used (Michalon et al. 2005; Krueger et al. 2006; Welman et al. 2006a). In the case of in vivo experiments the kinetics of induction may also be associated with the route of drug administration (Michalon et al. 2005). Thus a more extensive initial characterization of functional properties of the Tet-model, although time consuming, may significantly increase its utility and facilitate the design of subsequent experiments.
Concluding Remarks and Future Prospects
DNA-microarray, proteomic and genome sequencing studies can define tumor specific mutations and genes the expression levels of which are upregulated or downregulated in cancer cells as compared to their non-transformed counterparts. The functional consequences and significance of these cancer-associated genetic lesions need to be understood. It seems that Tet-systems have evolved to the point where they can now satisfy the growing demands of functional oncogenomic research for quantitative, time-controlled and reversible function-testing strategies. This is particularly important regarding the increasing awareness that cancer is a very heterogeneous and dynamic disease, and in the context of the establishment of cancer systems biology as an independent branch of cancer science. There is accumulating evidence that tumor cells represent highly variable biochemical systems that undergo constant evolution and remain in a dynamic equilibrium with their continuously changing micro-environment. The consequences of a given genetic lesion depend not only on the cell type it affects but also on the metabolic status and precise localization of that cell in the tumor mass, as well as many other factors (Anderson et al. 2006; Axelrod et al. 2006). Mutations that are essential for the maintenance of the transformed phenotype in early stages of tumorigenesis may play a less important role in later stages of tumor development (Giuriato and Felsher, 2003), and immediate consequences of gene activation/inactivation may differ from longer-term consequences (Lazzerini Denchi et al. 2005). Similarly the effects of high level activation of a given gene might be strikingly different from low-level activation (Welman et al. 2006b). All these complications force the oncology community to look for more sophisticated experimental tools and place the inducible gene regulation technologies at the forefront of modern translational cancer research. Although standardized protocols for high-throughput functional-screens based on the use of tetracycline-regulated inducible gene expression/suppression still need to be developed, the progress achieved over the last few years in adopting this technology for the purposes of translational cancer research and human gene therapy has been very impressive. Without doubt the success achieved so far in applying Tet-systems to gain important insights into basic tumor biology and to validate potential drug targets in preclinical studies grants further development. Considering the fast expansion of systems biology approaches, one can expect that the future evolution of Tet-systems will place particular emphasis on real time, non-invasive visualization of the consequences of inducible gene expression/suppression in defined tissues and organs at a single cell level in vivo. Of course this should be accompanied by further improvements in quantitative control over the levels of gene expression/suppression. Furthermore it can be expected that alternative systems will be developed and optimized to additionally enhance the achievable degree of flexibility and control. Indeed promising results have been already reported using for example systems regulated by lactose, macrolide antibiotics and Streptomyces—derived quorum-sensing components. These systems, similarly to Tet-systems, combine a high degree of specificity with little pleiotropic side-effects (Cronin et al. 2001; Mills, 2001; Weber et al. 2002; Weber et al. 2003; Higuchi et al. 2004; Caron et al. 2005; Weber et al. 2005). Combining tetracycline regulated systems with other inducible technologies may result in an even higher degree of control and provide invaluable tools necessary to unravel the intricate signalling networks involved in control of normal cell physiology and cell pathologies like cancer.
References
- Ackland-Berglund CE, Leib DA. Efficacy of tetracycline-controlled gene expression is influenced by cell type. Biotechniques. 1995;18:196–200. [PubMed] [Google Scholar]
- Agha-Mohammadi S, O’Malley M, Etemad A, et al. Second-generation tetracycline-regulatable promoter: repositioned tet operator elements optimize transactivator synergy while shorter minimal promoter offers tight basal leakiness. J. Gene. Med. 2004;6:817–28. doi: 10.1002/jgm.566. [DOI] [PubMed] [Google Scholar]
- Albanese C, Hulit J, Sakamaki T, et al. Recent advances in inducible expression in transgenic mice. Semin. Cell. Dev. Biol. 2002;13:129–41. doi: 10.1016/s1084-9521(02)00021-6. [DOI] [PubMed] [Google Scholar]
- Amar L, Desclaux M, Faucon-Biguet N, et al. Control of small inhibitory RNA levels and RNA interference by doxycycline induced activation of a minimal RNA polymerase III promoter. Nucleic Acids Res. 2006;34:e37. doi: 10.1093/nar/gkl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarzguioui M, Rossi JJ, Kim D. Approaches for chemically synthesized siRNA and vector-mediated RNAi. FEBS Lett. 2005;579:5974–81. doi: 10.1016/j.febslet.2005.08.070. [DOI] [PubMed] [Google Scholar]
- Anderson AR, Weaver AM, Cummings PT, et al. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell. 2006;127:905–15. doi: 10.1016/j.cell.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Argast M, Beck CF. Tetracycline diffusion through phospholipid bilayers and binding to phospholipids. Antimicrob Agents Chemother. 1984;26:263–5. doi: 10.1128/aac.26.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod R, Axelrod DE, Pienta KJ. Evolution of cooperation among tumor cells. Proc. Natl. Acad. Sci., U.S.A. 2006;103:13474–9. doi: 10.1073/pnas.0606053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde I, Zanta-Boussif MA, Paisant S, et al. Efficient control of gene expression in the hematopoietic system using a single Tet-on inducible lentiviral vector. Mol. Ther. 2006;13:382–90. doi: 10.1016/j.ymthe.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Baron U, Bujard H. Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol. 2000;327:401–21. doi: 10.1016/s0076-6879(00)27292-3. [DOI] [PubMed] [Google Scholar]
- Baron U, Freundlieb S, Gossen M, et al. Co-regulation of two gene activities by tetracycline via a bidirectional promoter. Nucleic Acids Res. 1995;23:3605–6. doi: 10.1093/nar/23.17.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron U, Gossen M, Bujard H. Tetracycline-controlled transcription in eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res. 1997;25:2723–9. doi: 10.1093/nar/25.14.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron U, Schnappinger D, Helbl V, et al. Generation of conditional mutants in higher eukaryotes by switching between the expression of two genes. Proc. Natl. Acad. Sci., U.S.A. 1999;96:1013–8. doi: 10.1073/pnas.96.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher OJ, Holland EC. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer Res. 2006;66:3355–8. doi: 10.1158/0008-5472.CAN-05-3827. discussion 3358–9. [DOI] [PubMed] [Google Scholar]
- Belguise K, Kersual N, Galtier F, et al. FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene. 2005;24:1434–44. doi: 10.1038/sj.onc.1208312. [DOI] [PubMed] [Google Scholar]
- Belli G, Gari E, Piedrafita L, et al. An activator/repressor dual system allows tight tetracycline-regulated gene expression in budding yeast. Nucleic Acids Res. 1998;26:942–7. doi: 10.1093/nar/26.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens C, Hillen W. Gene regulation by tetracyclines. Constraints of resistance regulation in bacteria shape TetR for application in eukaryotes. Eur. J. Biochem. 2003;270:3109–21. doi: 10.1046/j.1432-1033.2003.03694.x. [DOI] [PubMed] [Google Scholar]
- Bibby MC. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur. J. Cancer. 2004;40:852–7. doi: 10.1016/j.ejca.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Blesch A. Lentiviral and MLV based retroviral vectors for ex vivo and in vivo gene transfer. Methods. 2004;33:164–72. doi: 10.1016/j.ymeth.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Boland CR, Goel A. Somatic evolution of cancer cells. Semin. Cancer Biol. 2005;15:436–50. doi: 10.1016/j.semcancer.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Bornkamm GW, Berens C, Kuklik-Roos C, et al. Stringent doxycycline-dependent control of gene activities using an episomal one-vector system. Nucleic Acids Res. 2005;33:e137. doi: 10.1093/nar/gni137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden ET, Riegel AT. Tetracycline-regulated expression of hammerhead ribozymes in vivo. Methods Mol. Biol. 2004;252:179–94. doi: 10.1385/1-59259-746-7:179. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–7. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Caron L, Prot M, Rouleau M, et al. The Lac repressor provides a reversible gene expression system in undifferentiated and differentiated embryonic stem cell. Cell Mol. Life Sci. 2005;62:1605–12. doi: 10.1007/s00018-005-5123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stamatoyannopoulos G, Song CZ. Down-regulation of CXCR4 by inducible small interfering RNA inhibits breast cancer cell invasion in vitro. Cancer Res. 2003;63:4801–4. [PubMed] [Google Scholar]
- Chenuaud P, Larcher T, Rabinowitz JE, et al. Optimal design of a single recombinant adeno-associated virus derived from serotypes 1 and 2 to achieve more tightly regulated transgene expression from nonhuman primate muscle. Mol. Ther. 2004;9:410–8. doi: 10.1016/j.ymthe.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Chin L, Tam A, Pomerantz J, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–72. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65:232–60. doi: 10.1128/MMBR.65.2.232-260.2001. second page, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtarto A, Bender HU, Hanemann CO, et al. Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene. Ther. 2003;10:84–94. doi: 10.1038/sj.gt.3301838. [DOI] [PubMed] [Google Scholar]
- Cohen B, Dafni H, Meir G, et al. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia. 2005;7:109–17. doi: 10.1593/neo.04436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel SY, Rossi FM. Latest developments and in vivo use of the Tet system: ex vivo and in vivo delivery of tetracycline-regulated genes. Curr. Opin. Biotechnol. 2002;13:448–52. doi: 10.1016/s0958-1669(02)00361-0. [DOI] [PubMed] [Google Scholar]
- Cronin CA, Gluba W, Scrable H. The lac operator-repressor system is functional in the mouse. Genes Dev. 2001;15:1506–17. doi: 10.1101/gad.892001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR. RNAi the natural way. Nat. Genet. 2005;37:1163–5. doi: 10.1038/ng1105-1163. [DOI] [PubMed] [Google Scholar]
- Czauderna F, Santel A, Hinz M, et al. Inducible shRNA expression for application in a prostate cancer mouse model. Nucleic Acids Res. 2003;31:e127. doi: 10.1093/nar/gng127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle U, Meyer WK, Thiesen HJ. Tetracycline-reversible silencing of eukaryotic promoters. Mol. Cell. Biol. 1995;15:1907–14. doi: 10.1128/mcb.15.4.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickins RA, Hemann MT, Zilfou JT, et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 2005;37:1289–95. doi: 10.1038/ng1651. [DOI] [PubMed] [Google Scholar]
- Dykxhoorn DM, Lieberman J. The silent revolution: RNA interference as basic biology, research tool, and therapeutic. Annu. Rev. Med. 2005;56:401–23. doi: 10.1146/annurev.med.56.082103.104606. [DOI] [PubMed] [Google Scholar]
- Eckert B, Beck CF. Topology of the transposon Tn10-encoded tetracycline resistance protein within the inner membrane of Escherichia coli. J. Biol. Chem. 1989;264:11663–70. [PubMed] [Google Scholar]
- Ewald D, Li M, Efrat S, et al. Time-sensitive reversal of hyperplasia in transgenic mice expressing SV40 T antigen. Science. 1996;273:1384–6. doi: 10.1126/science.273.5280.1384. [DOI] [PubMed] [Google Scholar]
- Favre D, Blouin V, Provost N, et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J. Virol. 2002;76:11605–11. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsher DW. Reversibility of oncogene-induced cancer. Curr. Opin. Genet. Dev. 2004;14:37–42. doi: 10.1016/j.gde.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- Forster K, Helbl V, Lederer T, et al. Tetracycline-inducible expression systems with reduced basal activity in mammalian cells. Nucleic Acids Res. 1999;27:708–10. doi: 10.1093/nar/27.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlieb S, Schirra-Muller C, Bujard H. A tetracycline controlled activation/repression system with increased potential for gene transfer into mammalian cells. J. Gene. Med. 1999;1:4–12. doi: 10.1002/(SICI)1521-2254(199901/02)1:1<4::AID-JGM4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Fussenegger M. The impact of mammalian gene regulation concepts on functional genomic research, metabolic engineering, and advanced gene therapies. Biotechnol. Prog. 2001;17:1–51. doi: 10.1021/bp000129c. [DOI] [PubMed] [Google Scholar]
- Gallia GL, Khalili K. Evaluation of an autoregulatory tetracycline regulated system. Oncogene. 1998;16:1879–84. doi: 10.1038/sj.onc.1201706. [DOI] [PubMed] [Google Scholar]
- Gatz C, Quail PH. Tn10-encoded tet repressor can regulate an operator-containing plant promoter. Proc. Natl. Acad. Sci. U.S.A. 1988;85:1394–7. doi: 10.1073/pnas.85.5.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavazzi R, Giuliani R, Coltrini D, et al. Modulation of tumor angiogenesis by conditional expression of fibroblast growth factor-2 affects early but not established tumors. Cancer Res. 2001;61:309–17. [PubMed] [Google Scholar]
- Giuriato S, Felsher DW. How cancers escape their oncogene habit. Cell Cycle. 2003;2:329–32. [PubMed] [Google Scholar]
- Goldring CE, Kitteringham NR, Jenkins R, et al. Development of a transactivator in hepatoma cells that allows expression of phase I, phase II, and chemical defense genes. Am. J. Physiol. Cell. Physiol. 2006;290:C104–15. doi: 10.1152/ajpcell.00133.2005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nicolini V, Fussenegger M. In vitro assays for anti-cancer drug discovery—a novel approach based on engineered mammalian cell lines. Anticancer Drugs. 2005;16:223–8. doi: 10.1097/00001813-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Agullo-Ortuno MT, Garcia-Martinez JM, et al. Role of c-Src in human MCF7 breast cancer cell tumorigenesis. J. Biol. Chem. 2006;281:20851–64. doi: 10.1074/jbc.M601570200. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan RV, Christiansen KA, Goldstein NI, et al. Use of the human EF-1alpha promoter for expression can significantly increase success in establishing stable cell lines with consistent expression: a study using the tetracycline-inducible system in human cancer cells. Nucleic Acids Res. 1999;27:4775–82. doi: 10.1093/nar/27.24.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5547–51. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Studying gene function in eukaryotes by conditional gene inactivation. Annu. Rev. Genet. 2002;36:153–73. doi: 10.1146/annurev.genet.36.041002.120114. [DOI] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, et al. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–9. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Gould DJ, Chernajovsky Y. Endogenous GATA factors bind the core sequence of the tetO and influence gene regulation with the tetracycline system. Mol. Ther. 2004;10:127–38. doi: 10.1016/j.ymthe.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Gunther EJ, Belka GK, Wertheim GB, et al. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. Faseb. J. 2002;16:283–92. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat. Rev. Cancer. 2002;2:331–41. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Harding TC, Geddes BJ, Murphy D, et al. Switching transgene expression in the brain using an adenoviral tetracycline-regulatable system. Nat. Biotechnol. 1998;16:553–5. doi: 10.1038/nbt0698-553. [DOI] [PubMed] [Google Scholar]
- Helbl V, Hillen W. Stepwise selection of TetR variants recognizing tet operator 4C with high affinity and specificity. J. Mol. Biol. 1998;276:313–8. doi: 10.1006/jmbi.1997.1540. [DOI] [PubMed] [Google Scholar]
- Helbl V, Tiebel B, Hillen W. Stepwise selection of TetR variants recognizing tet operator 6C with high affinity and specificity. J. Mol. Biol. 1998;276:319–24. doi: 10.1006/jmbi.1997.1539. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Tsutsumi R, Higashi H, et al. Conditional gene silencing utilizing the lac repressor reveals a role of SHP-2 in cagA-positive Helicobacter pylori pathogenicity. Cancer Sci. 2004;95:442–7. doi: 10.1111/j.1349-7006.2004.tb03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W, Berens C. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 1994;48:345–69. doi: 10.1146/annurev.mi.48.100194.002021. [DOI] [PubMed] [Google Scholar]
- Hillen W, Gatz C, Altschmied L, et al. Control of expression of the Tn10-encoded tetracycline resistance genes. Equilibrium and kinetic investigation of the regulatory reactions. J. Mol. Biol. 1983;169:707–21. doi: 10.1016/s0022-2836(83)80166-1. [DOI] [PubMed] [Google Scholar]
- Honda K, Yamada T, Hayashida Y, et al. Actinin-4 increases cell motility and promotes lymph node metastasis of colorectal cancer. Gastroenterology. 2005;128:51–62. doi: 10.1053/j.gastro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Hornberg JJ, Bruggeman FJ, Westerhoff HV, et al. Cancer: a Systems Biology disease. Biosystems. 2006;83:81–90. doi: 10.1016/j.biosystems.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hosono T, Mizuguchi H, Katayama K, et al. Adenovirus vector-mediated doxycycline-inducible RNA interference. Hum. Gene. Ther. 2004;15:813–9. doi: 10.1089/1043034041648462. [DOI] [PubMed] [Google Scholar]
- Hossini AM, Eberle J, Fecker LF, et al. Conditional expression of exogenous Bcl-X(S) triggers apoptosis in human melanoma cells in vitro and delays growth of melanoma xenografts. FEBS Lett. 2003;553:250–6. doi: 10.1016/s0014-5793(03)01017-2. [DOI] [PubMed] [Google Scholar]
- Howe JR, Skryabin BV, Belcher SM, et al. The responsiveness of a tetracycline-sensitive expression system differs in different cell lines. J. Biol. Chem. 1995;270:14168–74. doi: 10.1074/jbc.270.23.14168. [DOI] [PubMed] [Google Scholar]
- Izumi M, Gilbert DM. Homogeneous tetracycline-regulatable gene expression in mammalian fibroblasts. J. Cell. Biochem. 1999;76:280–9. doi: 10.1002/(sici)1097-4644(20000201)76:2<280::aid-jcb11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kamionka A, Majewski M, Roth K, et al. Induction of single chain tetracycline repressor requires the binding of two inducers. Nucleic Acids Res. 2006;34:3834–41. doi: 10.1093/nar/gkl316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke N, Zhou D, Chatterton JE, et al. A new inducible RNAi xenograft model for assessing the staged tumor response to mTOR silencing. Exp. Cell. Res. 2006;312:2726–34. doi: 10.1016/j.yexcr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Enver T, Ashworth A. Retroviral vectors for establishing tetracycline-regulated gene expression in an otherwise recalcitrant cell line. BMC Mol. Biol. 2002;3:13. doi: 10.1186/1471-2199-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara D, Yang YD, Hawkins T. Bioinformatics resources for cancer research with an emphasis on gene function and structure prediction tools. Cancer Informatics. 2006;2:25–35. [PMC free article] [PubMed] [Google Scholar]
- Kistner A, Gossen M, Zimmermann F, et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10933–8. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H. Cancer robustness: tumour tactics. Nature. 2003;426:125. doi: 10.1038/426125a. [DOI] [PubMed] [Google Scholar]
- Knott A, Garke K, Urlinger S, et al. Tetracycline-dependent gene regulation: combinations of transregulators yield a variety of expression windows. Biotechniques. 2002;32:796, 798, 800. doi: 10.2144/02324st06. passim. [DOI] [PubMed] [Google Scholar]
- Koponen JK, Kankkonen H, Kannasto J, et al. Doxycycline-regulated lentiviral vector system with a novel reverse transactivator rtTA2S-M2 shows a tight control of gene expression in vitro and in vivo. Gene. Ther. 2003;10:459–66. doi: 10.1038/sj.gt.3301889. [DOI] [PubMed] [Google Scholar]
- Koschmieder S, Gottgens B, Zhang P, et al. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR-ABL leukemogenesis. Blood. 2005;105:324–34. doi: 10.1182/blood-2003-12-4369. [DOI] [PubMed] [Google Scholar]
- Krueger C, Berens C, Schmidt A, et al. Single-chain Tet transregulators. Nucleic Acids Res. 2003;31:3050–6. doi: 10.1093/nar/gkg421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger C, Danke C, Pfleiderer K, et al. A gene regulation system with four distinct expression levels. J Gene Med. 2006 doi: 10.1002/jgm.932. [DOI] [PubMed] [Google Scholar]
- Krueger C, Pfleiderer K, Hillen W, et al. Tetracycline derivatives: alternative effectors for Tet transregulators. Biotechniques. 2004a;37:546, 548, 550. doi: 10.2144/04374BM04. [DOI] [PubMed] [Google Scholar]
- Krueger C, Schmidt A, Danke C, et al. Transactivator mutants with altered effector specificity allow selective regulation of two genes by tetracycline variants. Gene. 2004b;331:125–31. doi: 10.1016/j.gene.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Kuninger D, Stauffer D, Eftekhari S, et al. Gene disruption by regulated short interfering RNA expression, using a two-adenovirus system. Hum. Gene. Ther. 2004;15:1287–92. doi: 10.1089/hum.2004.15.1287. [DOI] [PubMed] [Google Scholar]
- Lai JF, Cheng HY, Cheng TL, et al. Doxycycline- and tetracycline-regulated transcriptional silencer enhance the expression level and transactivating performance of rtTA. J. Gene. Med. 2004;6:1403–13. doi: 10.1002/jgm.614. [DOI] [PubMed] [Google Scholar]
- Lamartina S, Roscilli G, Rinaudo CD, et al. Stringent control of gene expression in vivo by using novel doxycycline-dependent trans-activators. Hum. Gene. Ther. 2002;13:199–210. doi: 10.1089/10430340252769734. [DOI] [PubMed] [Google Scholar]
- Lamartina S, Silvi L, Roscilli G, et al. Construction of an rtTA2(s)-m2/tts(kid)-based transcription regulatory switch that displays no basal activity, good inducibility, and high responsiveness to doxycycline in mice and non-human primates. Mol. Ther. 2003;7:271–80. doi: 10.1016/s1525-0016(02)00051-5. [DOI] [PubMed] [Google Scholar]
- Latta-Mahieu M, Rolland M, Caillet C, et al. Gene transfer of a chimeric trans-activator is immunogenic and results in short-lived transgene expression. Hum. Gene. Ther. 2002;13:1611–20. doi: 10.1089/10430340260201707. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y. Can a biologist fix a radio?—Or, what I learned while studying apoptosis. Cancer Cell. 2002;2:179–82. doi: 10.1016/s1535-6108(02)00133-2. [DOI] [PubMed] [Google Scholar]
- Lazzerini Denchi E, Attwooll C, Pasini D, et al. Deregulated E2F activity induces hyperplasia and senescence-like features in the mouse pituitary gland. Mol. Cell. Biol. 2005;25:2660–72. doi: 10.1128/MCB.25.7.2660-2672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer T, Takahashi M, Hillen W. Thermodynamic analysis of tetracycline-mediated induction of Tet repressor by a quantitative methylation protection assay. Anal. Biochem. 1995;232:190–6. doi: 10.1006/abio.1995.0006. [DOI] [PubMed] [Google Scholar]
- Lee YB, Glover CP, Cosgrave AS, et al. Optimizing regulatable gene expression using adenoviral vectors. Exp. Physiol. 2005;90:33–7. doi: 10.1113/expphysiol.2004.028209. [DOI] [PubMed] [Google Scholar]
- Leenders F, Mopert K, Schmiedeknecht A, et al. PKN3 is required for malignant prostate cell growth downstream of activated PI 3-kinase. Embo. J. 2004;23:3303–13. doi: 10.1038/sj.emboj.7600345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena AM, Giannetti P, Sporeno E, et al. Immune responses against tetracycline-dependent transactivators affect long-term expression of mouse erythropoietin delivered by a helper-dependent adenoviral vector. J. Gene. Med. 2005;7:1086–96. doi: 10.1002/jgm.758. [DOI] [PubMed] [Google Scholar]
- Lewandoski M. Conditional control of gene expression in the mouse. Nat. Rev. Genet. 2001;2:743–55. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- Li L, Lin X, Staver M, et al. Evaluating hypoxia-inducible factor-1alpha as a cancer therapeutic target via inducible RNA interference in vivo. Cancer Res. 2005;65:7249–58. doi: 10.1158/0008-5472.CAN-04-4426. [DOI] [PubMed] [Google Scholar]
- Li ZB, Zeng ZJ, Chen Q, et al. Recombinant AAV-mediated HSVtk gene transfer with direct intratumoral injections and Tet-On regulation for implanted human breast cancer. BMC Cancer. 2006;6:66. doi: 10.1186/1471-2407-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Yang J, Chen J, et al. Development of a tightly regulated U6 promoter for shRNA expression. FEBS Lett. 2004;577:376–80. doi: 10.1016/j.febslet.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Lindemann D, Patriquin E, Feng S, et al. Versatile retrovirus vector systems for regulated gene expression in vitro and in vivo. Mol. Med. 1997;3:466–76. [PMC free article] [PubMed] [Google Scholar]
- Lottmann H, Vanselow J, Hessabi B, et al. The Tet-On system in transgenic mice: inhibition of the mouse pdx-1 gene activity by antisense RNA expression in pancreatic beta-cells. J. Mol. Med. 2001;79:321–8. doi: 10.1007/s001090100229. [DOI] [PubMed] [Google Scholar]
- Malik A, Singh H, Andrabi M, et al. Databases and QSAR for Cancer Research. Cancer Informatics. 2006;2:99–111. [PMC free article] [PubMed] [Google Scholar]
- Malphettes L, Fussenegger M. Macrolide- and tetracycline-adjustable siRNA-mediated gene silencing in mammalian cells using polymerase II-dependent promoter derivatives. Biotechnol. Bioeng. 2004;88:417–25. doi: 10.1002/bit.20230. [DOI] [PubMed] [Google Scholar]
- Markusic D, Oude-Elferink R, Das AT, et al. Comparison of single regulated lentiviral vectors with rtTA expression driven by an autoregulatory loop or a constitutive promoter. Nucleic Acids Res. 2005;33:e63. doi: 10.1093/nar/gni062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura S, Jones PA, Takai D. Establishment of conditional vectors for hairpin siRNA knockdowns. Nucleic Acids Res. 2003;31:e77. doi: 10.1093/nar/gng077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthess Y, Kappel S, Spankuch B, et al. Conditional inhibition of cancer cell proliferation by tetracycline-responsive, H1 promoter-driven silencing of PLK1. Oncogene. 2005;24:2973–80. doi: 10.1038/sj.onc.1208472. [DOI] [PubMed] [Google Scholar]
- Maxwell IH, Spitzer AL, Long CJ, et al. Autonomous parvovirus transduction of a gene under control of tissue-specific or inducible promoters. Gene. Ther. 1996;3:28–36. [PubMed] [Google Scholar]
- Michalon A, Koshibu K, Baumgartel K, et al. Inducible and neuron-specific gene expression in the adult mouse brain with the rtTA2S-M2 system. Genesis. 2005;43:205–12. doi: 10.1002/gene.20175. [DOI] [PubMed] [Google Scholar]
- Mills AA. Changing colors in mice: an inducible system that delivers. Genes. Dev. 2001;15:1461–7. doi: 10.1101/gad.909301. [DOI] [PubMed] [Google Scholar]
- Miraux S, Lemiere S, Pineau R, et al. Inhibition of FGF receptor activity in glioma implanted into the mouse brain using the tetracyclin-regulated expression system. Angiogenesis. 2004;7:105–13. doi: 10.1007/s10456-004-1037-0. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H, Xu ZL, Sakurai F, et al. Tight positive regulation of transgene expression by a single adenovirus vector containing the rtTA and tTS expression cassettes in separate genome regions. Hum. Gene. Ther. 2003;14:1265–77. doi: 10.1089/104303403767740803. [DOI] [PubMed] [Google Scholar]
- Moody SE, Sarkisian CJ, Hahn KT, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–61. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- Munoz I, Gomez A, Zanuy S, et al. A one-step approach to obtain cell clones expressing tetracycline-responsive transactivators. Anal. Biochem. 2004;331:153–60. doi: 10.1016/j.ab.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Nguyen TN, Phan QG, Duong LP, et al. Effects of carriage and expression of the Tn10 tetracycline-resistance operon on the fitness of Escherichia coli K12. Mol. Biol. Evol. 1989;6:213–25. doi: 10.1093/oxfordjournals.molbev.a040545. [DOI] [PubMed] [Google Scholar]
- Oberst C, Hartl M, Weiskirchen R, et al. Conditional cell transformation by doxycycline-controlled expression of the MC29 v-myc allele. Virology. 1999;253:193–207. doi: 10.1006/viro.1998.9499. [DOI] [PubMed] [Google Scholar]
- Ohkawa J, Taira K. Control of the functional activity of an antisense RNA by a tetracycline-responsive derivative of the human U6 snRNA promoter. Hum. Gene. Ther. 2000;11:577–85. doi: 10.1089/10430340050015761. [DOI] [PubMed] [Google Scholar]
- Pacheco TR, Maxwell F, Wu MF, et al. Use of a recombinant parvovirus to facilitate screening for human melanoma cell clones expressing tetracycline-responsive transactivators. Gene. 1999;229:125–9. doi: 10.1016/s0378-1119(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Pao W, Klimstra DS, Fisher GH, et al. Use of avian retroviral vectors to introduce transcriptional regulators into mammalian cells for analyses of tumor maintenance. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8764–9. doi: 10.1073/pnas.1133333100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SD, Aebersold RH. Proteomics: the first decade and beyond. Nat. Genet. 2003;33(Suppl):311–23. doi: 10.1038/ng1106. [DOI] [PubMed] [Google Scholar]
- Pluta K, Luce MJ, Bao L, et al. Tight control of transgene expression by lentivirus vectors containing second-generation tetracycline-responsive promoters. J. Gene. Med. 2005;7:803–17. doi: 10.1002/jgm.712. [DOI] [PubMed] [Google Scholar]
- Qu Z, Thottassery JV, Van Ginkel S, et al. Homogeneity and long-term stability of tetracycline-regulated gene expression with low basal activity by using the rtTA2S-M2 transactivator and insulator-flanked reporter vectors. Gene. 2004;327:61–73. doi: 10.1016/j.gene.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Rang A, Will H. The tetracycline-responsive promoter contains functional interferon-inducible response elements. Nucleic Acids Res. 2000;28:1120–5. doi: 10.1093/nar/28.5.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Weinberg RA. Opinion: Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat. Rev. Cancer. 2003;3:952–9. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- Reeves PJ, Kim JM, Khorana HG. Structure and function in rhodopsin: a tetracycline-inducible system in stable mammalian cell lines for high-level expression of opsin mutants. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13413–8. doi: 10.1073/pnas.212519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DR, Chinnaiyan AM. Bioinformatics strategies for translating genome-wide expression analyses into clinically useful cancer markers. Ann. N.Y. Acad. Sci. 2004;1020:32–40. doi: 10.1196/annals.1310.005. [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Chinnaiyan AM. Integrative analysis of the cancer transcriptome. Nat. Genet. 2005;37(Suppl):S31–7. doi: 10.1038/ng1570. [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MC. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol. Rev. 1996;19:1–24. doi: 10.1111/j.1574-6976.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- Roberts MC. Update on acquired tetracycline resistance genes. FEMS Microbiol. Lett. 2005;245:195–203. doi: 10.1016/j.femsle.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Robertson A, Perea J, Tolmachova T, et al. Effects of mouse strain, position of integration and tetracycline analogue on the tetracycline conditional system in transgenic mice. Gene. 2002;282:65–74. doi: 10.1016/s0378-1119(01)00793-4. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Guicherit OM, Spicher A, et al. Tetracycline-regulatable factors with distinct dimerization domains allow reversible growth inhibition by p16. Nat. Genet. 1998;20:389–93. doi: 10.1038/3871. [DOI] [PubMed] [Google Scholar]
- Rubinchik S, Woraratanadharm J, Yu H, et al. New complex Ad vectors incorporating both rtTA and tTS deliver tightly regulated transgene expression both in vitro and in vivo. Gene. Ther. 2005;12:504–11. doi: 10.1038/sj.gt.3302437. [DOI] [PubMed] [Google Scholar]
- Ryu JR, Olson LK, Arnosti DN. Cell-type specificity of short-range transcriptional repressors. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12960–5. doi: 10.1073/pnas.231394998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. J. Biol. Chem. 1999;274:38071–82. doi: 10.1074/jbc.274.53.38071. [DOI] [PubMed] [Google Scholar]
- Saenger W, Orth P, Kisker C, et al. The Tetracycline Repressor-A Paradigm for a Biological Switch. Angew. Chem. Int. Ed. Engl. 2000;39:2042–2052. doi: 10.1002/1521-3773(20000616)39:12<2042::aid-anie2042>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Saez E, No D, West A, et al. Inducible gene expression in mammalian cells and transgenic mice. Curr. Opin. Biotechnol. 1997;8:608–16. doi: 10.1016/s0958-1669(97)80037-7. [DOI] [PubMed] [Google Scholar]
- Saqr HE, Omran O, Dasgupta S, et al. Endogenous GD3 ganglioside induces apoptosis in U-1242 MG glioma cells. J. Neurochem. 2006;96:1301–14. doi: 10.1111/j.1471-4159.2005.03640.x. [DOI] [PubMed] [Google Scholar]
- Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66:3351–4. doi: 10.1158/0008-5472.CAN-05-3627. discussion 3354. [DOI] [PubMed] [Google Scholar]
- Schmeisser F, Donohue M, Weir JP. Tetracycline-regulated gene expression in replication-incompetent herpes simplex virus vectors. Hum. Gene. Ther. 2002;13:2113–24. doi: 10.1089/104303402320987815. [DOI] [PubMed] [Google Scholar]
- Schultze N, Burki Y, Lang Y, et al. Efficient control of gene expression by single step integration of the tetracycline system in transgenic mice. Nat. Biotechnol. 1996;14:499–503. doi: 10.1038/nbt0496-499. [DOI] [PubMed] [Google Scholar]
- Sheppard D. Dominant negative mutants: tools for the study of protein function in vitro and in vivo. Am. J. Respir. Cell. Mol. Biol. 1994;11:1–6. doi: 10.1165/ajrcmb.11.1.8018332. [DOI] [PubMed] [Google Scholar]
- Shlaes DM. An update on tetracyclines. Curr. Opin. Investig. Drugs. 2006;7:167–71. [PubMed] [Google Scholar]
- Shockett P, Difilippantonio M, Hellman N, et al. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6522–6. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockett PE, Schatz DG. Diverse strategies for tetracycline-regulated inducible gene expression. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5173–6. doi: 10.1073/pnas.93.11.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson TH, Hansen JM, Shah M, et al. Expression of the reverse tetracycline-transactivator gene causes emphysema-like changes in mice. Am. J. Respir. Cell. Mol. Biol. 2006;34:552–60. doi: 10.1165/rcmb.2005-0378OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulie P, Carrozzino F, Pepper MS, et al. Membrane-type-1 matrix metalloproteinase confers tumorigenicity on nonmalignant epithelial cells. Oncogene. 2005;24:1689–97. doi: 10.1038/sj.onc.1208360. [DOI] [PubMed] [Google Scholar]
- Stegmeier F, Hu G, Rickles RJ, et al. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13212–7. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger K, Le Meur G, Lasne F, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol. Ther. 2006;13:967–75. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Suess B, Hanson S, Berens C, et al. Conditional gene expression by controlling translation with tetracycline-binding aptamers. Nucleic Acids Res. 2003;31:1853–8. doi: 10.1093/nar/gkg285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggitt M, Bibby MC. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clin. Cancer. Res. 2005;11:971–81. [PubMed] [Google Scholar]
- Suh KS, Mutoh M, Gerdes M, et al. Antisense suppression of the chloride intracellular channel family induces apoptosis, enhances tumor necrosis factor {alpha}-induced apoptosis, and inhibits tumor growth. Cancer Res. 2005;65:562–71. [PubMed] [Google Scholar]
- Sun X, Annala AJ, Yaghoubi SS, et al. Quantitative imaging of gene induction in living animals. Gene. Ther. 2001;8:1572–9. doi: 10.1038/sj.gt.3301554. [DOI] [PubMed] [Google Scholar]
- Szulc J, Wiznerowicz M, Sauvain MO, et al. A versatile tool for conditional gene expression and knockdown. Nat. Methods. 2006;3:109–16. doi: 10.1038/nmeth846. [DOI] [PubMed] [Google Scholar]
- Tang Y, Kim M, Carrasco D, et al. In vivo assessment of RAS-dependent maintenance of tumor angiogenesis by real-time magnetic resonance imaging. Cancer Res. 2005;65:8324–30. doi: 10.1158/0008-5472.CAN-05-0027. [DOI] [PubMed] [Google Scholar]
- Tietge UJ, Kozarsky KF, Donahee MH, et al. A tetracycline-regulated adenoviral expression system for in vivo delivery of transgenes to lung and liver. J. Gene. Med. 2003;5:567–75. doi: 10.1002/jgm.384. [DOI] [PubMed] [Google Scholar]
- Tinker AV, Boussioutas A, Bowtell DD. The challenges of gene expression microarrays for the study of human cancer. Cancer Cell. 2006;9:333–9. doi: 10.1016/j.ccr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Toledo F, Liu CW, Lee CJ, et al. RMCE-ASAP: a gene targeting method for ES and somatic cells to accelerate phenotype analyses. Nucleic Acids Res. 2006;34:e92. doi: 10.1093/nar/gkl518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins SA, Chinnaiyan AM. Of mice and men: cancer gene discovery using comparative oncogenomics. Cancer Cell. 2006;10:2–4. doi: 10.1016/j.ccr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Toniatti C, Bujard H, Cortese R, et al. Gene therapy progress and prospects: transcription regulatory systems. Gene. Ther. 2004;11:649–57. doi: 10.1038/sj.gt.3302251. [DOI] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, et al. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7963–8. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Oving I, Muncan V, et al. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 2003;4:609–15. doi: 10.1038/sj.embor.embor865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108:135–44. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- Wang D, Grammer JR, Cobbs CS, et al. p125 focal adhesion kinase promotes malignant astrocytoma cell proliferation in vivo. J. Cell. Sci. 2000;113(Pt 23):4221–30. doi: 10.1242/jcs.113.23.4221. [DOI] [PubMed] [Google Scholar]
- Wang S, El-Deiry WS. Inducible silencing of KILLER/DR5 in vivo promotes bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil. Cancer Res. 2004;64:6666–72. doi: 10.1158/0008-5472.CAN-04-1734. [DOI] [PubMed] [Google Scholar]
- Weber W, Fussenegger M. Pharmacologic transgene control systems for gene therapy. J. Gene. Med. 2006;8:535–56. doi: 10.1002/jgm.903. [DOI] [PubMed] [Google Scholar]
- Weber W, Fux C, Daoud-el Baba M, et al. Macrolide-based transgene control in mammalian cells and mice. Nat. Biotechnol. 2002;20:901–7. doi: 10.1038/nbt731. [DOI] [PubMed] [Google Scholar]
- Weber W, Malphettes L, de Jesus M, et al. Engineered Streptomyces quorum-sensing components enable inducible siRNA-mediated translation control in mammalian cells and adjustable transcription control in mice. J. Gene. Med. 2005;7:518–25. doi: 10.1002/jgm.682. [DOI] [PubMed] [Google Scholar]
- Weber W, Schoenmakers R, Spielmann M, et al. Streptomyces-derived quorum-sensing systems engineered for adjustable transgene expression in mammalian cells and mice. Nucleic Acids Res. 2003;31:e71. doi: 10.1093/nar/gng071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann P, Gossen M, Hillen W, et al. A chimeric transactivator allows tetracycline-responsive gene expression in whole plants. Plant. J. 1994;5:559–69. doi: 10.1046/j.1365-313x.1994.5040559.x. [DOI] [PubMed] [Google Scholar]
- Welman A, Barraclough J, Dive C. Generation of cells expressing improved doxycycline-regulated reverse transcriptional transactivator rtTA2S-M2. Nat. Protocols. 2006a;1:803–11. doi: 10.1038/nprot.2006.117. [DOI] [PubMed] [Google Scholar]
- Welman A, Cawthorne C, Barraclough J, et al. Construction and characterization of multiple human colon cancer cell lines for inducibly regulated gene expression. J. Cell. Biochem. 2005;94:1148–62. doi: 10.1002/jcb.20342. [DOI] [PubMed] [Google Scholar]
- Welman A, Cawthorne C, Ponce-Perez L, et al. Increases in c-Src expression level and activity do not promote the growth of human colorectal carcinoma cells in vitro and in vivo. Neoplasia. 2006b;8:905–16. doi: 10.1593/neo.06475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E, Sutherland H, Kearns M, et al. Epigenetic effects on transgene expression. Methods Mol. Biol. 2001;158:351–68. doi: 10.1385/1-59259-220-1:351. [DOI] [PubMed] [Google Scholar]
- Wiemann S, Arlt D, Huber W, et al. From ORFeome to biology: a functional genomics pipeline. Genome. Res. 2004;14:2136–44. doi: 10.1101/gr.2576704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart JA, Hayes A, Wardleworth L, et al. Doxycycline, the drug used to control the tet-regulatable promoter system, has no effect on global gene expression in Saccharomyces cerevisiae. Yeast. 2005;22:565–9. doi: 10.1002/yea.1225. [DOI] [PubMed] [Google Scholar]
- Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 2003;77:8957–61. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ET, Kolman JL, Li YC, et al. Reproducible doxycycline-inducible transgene expression at specific loci generated by Cre-recombinase mediated cassette exchange. Nucleic Acids Res. 2005;33:e147. doi: 10.1093/nar/gni145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XG, Zhou H, Xu Z. Multiple shRNAs expressed by an inducible pol II promoter can knock down the expression of multiple target genes. Biotechniques. 2006;41:64–8. doi: 10.2144/000112198. [DOI] [PubMed] [Google Scholar]
- Yao F, Svensjo T, Winkler T, et al. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum. Gene. Ther. 1998;9:1939–50. doi: 10.1089/hum.1998.9.13-1939. [DOI] [PubMed] [Google Scholar]
- Yao F, Theopold C, Hoeller D, et al. Highly efficient regulation of gene expression by tetracycline in a replication-defective herpes simplex viral vector. Mol. Ther. 2006;13:1133–41. doi: 10.1016/j.ymthe.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Yu HM, Liu B, Chiu SY, et al. Development of a unique system for spatiotemporal and lineage-specific gene expression in mice. Proc Natl Acad Sci USA. 2005;102:8615–20. doi: 10.1073/pnas.0500124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JS, Sena-Esteves M, Paulus W, et al. Retroviral delivery and tetracycline-dependent expression of IL-1beta-converting enzyme (ICE) in a rat glioma model provides controlled induction of apoptotic death in tumor cells. Cancer Res. 1996;56:5423–7. [PubMed] [Google Scholar]
- Zhu Z, Ma B, Homer RJ, et al. Use of the tetracycline-controlled transcriptional silencer (tTS) to eliminate transgene leak in inducible overexpression transgenic mice. J. Biol. Chem. 2001;276:25222–9. doi: 10.1074/jbc.M101512200. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Zheng T, Lee CG, et al. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin. Cell Dev. Biol. 2002;13:121–8. doi: 10.1016/s1084-9521(02)00018-6. [DOI] [PubMed] [Google Scholar]