Abstract
Urokinase-type plasminogen activator (uPA) is associated with cancer recurrence where the most evidence comes from studies in breast cancer. According to the European Organization for Research and Treatment of Cancer, uPA is considered one of the most prominent biomarkers for cancer recurrence and therefore new agents are needed to inhibit it. Whether uPA is also expressed in pediatric cancers is yet unknown. If it is then uPA inhibitors might also help children with recurrent cancers. In this study, we addressed whether the integrin-linked kinase inhibitor (ILK), QLT0267, could suppress uPA. We previously showed that uPA expression is maximally inhibited when both the Akt and MAP kinase pathways were blocked which we anticipated can be achieved via QLT0267. In MDA-MB-231 breast cancer cells, QLT0267 blocked signaling through Akt and MAP kinase with a correlative decrease in uPA protein and mRNA, which corresponded to an inhibition of c-Jun phosphorylation. Consistent with these findings, cellular invasion was inhibited with either QLT0267 or with small interfering RNA against ILK. We then questioned whether uPA was commonly expressed in childhood sarcomas and if QLT0267 might be effective in this setting. We determined for the first time that uPA was highly expressed in rhabdomyosarcomas (RMS), but not Ewings sarcomas by screening cell lines (n = 31) and patient samples (n = 200) using Affymetrix microarrays. In alveolar RMS (ARMS) cell lines, QLT0267 blocked cell signaling, uPA production, invasion and ultimately survival. We concluded that QLT0267 blocks the production of uPA providing a new target for the management of recurrent cancers.
Keywords: urokinase-type plasminogen activator, integrin-linked kinase, breast cancer, rhabdomyosarcoma, recurrence
Introduction
Early detection and adjuvant systemic therapies have markedly improved survival for cancer patients; however, success of treatment is hindered by metastatic disease and high rates of relapse. Approximately 1.1 million women will be diagnosed with breast cancer this year worldwide and the survival rates for localized regional and distant stages of breast cancer are 98%, 81% and 26%, respectively (1). This same can be argued for cancers that affect children. For example, children with rhabdomyosarcomas frequently develop metastatic disease, which is associated with grim overall survival (2). The expected rate of recurrence for children with rhabdomyosarcomas is ∼30% (3). Thus, it is imperative to identify novel therapeutic strategies to improve survival rates.
uPA is a serine protease that promotes the growth and eventual dissemination of breast cancers (4–6), as well as other malignancies of the prostate, lung, ovaries and colon (7, 8). In a study of approximately 3000 breast cancer cases, from nine different centers around the world, uPA was consistently found to have prognostic significance for poor overall survival (6) and its expression correlated with recurrence (4, 9, 10). The supporting evidence for uPA and breast cancer places it in the Level I category as a biomarker for cancer according to the European Organization for Research and Cancer Therapeutics (11). Thus identifying uPA inhibitors could provide novel opportunities for treating aggressive types of cancer.
It is known that uPA has several functional roles, including proteolysis and signal transduction. The serine protease uPA binds to receptor uPAR and converts inactive plasminogen to plasmin that can degrade proteins comprising the extracellular matrix (ECM) (12). In addition uPA can activate precursor forms of matrix metalloproteinases (MMPs) including MMP-3, MMP-9, MMP-12 and MMP-13, proteins that are also involved in degradation of ECM (13). As such, the uPA/uPAR pathway plays a role in cancer invasion and metastasis.
The strong consensus that uPA is widely expressed in cancers has led to the question of tumor dependency and therapeutic targeting. Strategies to suppress uPA production have resulted in an inhibition of tumorigenesis indicating that this could be a valuable therapeutic target. For example, inhibiting uPA with small interfering RNA (siRNA) suppresses the growth of prostate cancer in preclinical model (14). The peptide inhibitor A6, designed to specifically bind to the amino acid sequence essential for uPA to bind to uPAR, has shown promise in phase I clinical studies in ovarian cancer (15). Similarly, a diptheria toxin fusion protein that binds uPAR and induces apoptosis in vitro while in mice it suppressed the growth of glioblastoma cells (16). Other drugs including herbimycin A (17), 17-allylamino geldanamycin (17-AAG) (18) and celecoxib (19) suppress uPA production; however, they are hindered by formulation and cytotoxicity problems; as such they have limitations in the clinic.
The overarching goal of the study described herein was to identify new ways in which to inhibit uPA. We previously reported that the regulation of uPA depends on a convergence of Pi3K/Akt and MAP kinase pathways, leading to the activation of the uPA promoter encoding activator protein -1 (AP-1) and PEA3 sites (17). Inhibition of both Pi3K/Akt and MAP kinase optimally inhibited the uPA-reporter activity (17). This observation builds on previous reports indicating that the AP-1 site is required for uPA induction leading to the stimulation of the AP-1 complex, containing transcription factors c-fos and c-Jun (20). Therefore, we chose to study integrin-linked kinase (ILK) because it activates the Pi3K/Akt pathway by phosphorylating Akt on Ser-473 (21, 22). The over-expression of ILK in transgenic mice has been shown to induce mammary cancer (23) and promote tumor cell invasion making it an attractive molecular target for cancer therapy (24). The tumors derived from these mice also had hyperactivation of both Pi3K/Akt and MAP kinase pathways (23). Conversely, inhibiting ILK with the small molecules KP-SD-1 (25) or QLT0267 (26) blocks the invasion of glioblastoma that correlated with decreased MMP-9 and MMP-2, respectively (26). Whether or not inhibiting ILK with QLT0267 blocks uPA and therefore cellular invasion has not been previously addressed. We demonstrate herein that QLT0267 or ILK siRNA suppresses uPA production, invasion and ultimately the growth tumor cells representing exquisitely aggressive models of cancer. Importantly, we analyzed a large cohort of pediatric primary tumors (n = 200) and cell lines (n = 22) by Affymetrix microarrays to find that uPA is frequently expressed at high levels in rhabdomyosarcomas (RMS). QLT0276 may therefore be a novel targeted therapy for the treatment of cancers associated with high rates of recurrence.
Materials and Methods
Drugs and small interfering RNA
QLT0267 was kindly provided by QLT Inc. (Vancouver, BC. Canada) and dissolved in dimethyl sulphoxide (DMSO). QLT0267 has been shown to inhibit the kinase activity of ILK in a cell-free assay at 26 nmol/L and preliminary experiments suggest that it has 1,000-fold selectivity over other kinases tested under similar conditions, including CK2, CSK, DNA-PK, PIM1, protein kinase B or AKT kinase, and PKC; 100-fold selectivity over extracellular signal-regulated kinase 1, GSK3ß, LCK, PKA, p70S6K, and RSK1. Of those tested, CDK1, CDK2, and CDK5 show the greatest inhibition by QLT0267, but the selectivity window is still at least 10-fold (27). To knock-down ILK protein expression, transfections with siRNA oligos were performed using Dharmacon’s protocol. The ILK specific oligo was a 21 mer-double stranded RNA with d(TT) overhang and selected for its ability to knock-down ILK expression (28). The sense sequences for ILK siRNA was 5′ -CCUGACGAAGCUCAAC-GAGAA-3′ (Dharmacon Inc. Lafayette, CO) and control siRNA was 5′-AAUUCUCCGAACGU-GUCACGU-3′ (Qiagen, Inc., Mississauga, ON).
Cell line and culture conditions
MDA-MB-231, HBL100, RD (ATCC# CCL-136), and SJCRH30 (ATCC # CRL2061 contains PAX3-FKHR fusion protein) cells were obtained from the American Tissue Culture Collection (Rockville, MD). CW9019 (containing PAX7/FKHR gene fusion) alveolar rhabdomyosarcoma cell line was a generous gift from Dr. Frederic Barr. The cell lines were grown in Dulbecco’s modified Eagle medium (DMEM, Invitrogen Canada Inc., Burlington, ON) and supplemented with 10% fetal bovine serum containing 1% penicillin and streptomycin at 37 °C in a humidified air containing 5% CO2.
Cytoplasmic lysates and immunoblot analyses
Whole cell extracts were prepared according to the protocol from Cell Signaling Technology, Inc. (Danvers, MA). Protein expression was analyzed by separating 50 μg total protein on a 12% acrylamide gel and transferred to a nitrocellulose membrane. Membrane blots were blocked for 1 hour with 5% nonfat milk in PBS with 1% Tween-20 and then incubated overnight at 4 °C with the following primary antibodies at 1:1000 dilution; p-Akt Ser473, Akt, p-S6 ribosomal protein Ser235/236, p-ERK 1/2Thr202/Tyr204, total ERK 1/2, p-mTOR2448, mTOR, p-c-Jun Ser63 (Cell Signaling Technology, Danvers, MA), ILK monoclonal antibody (BD Transduction Labs, Lexington, KY), and vinculin (Santa Cruz Biotechnology, CA). Total c-Jun (Upstate Biotechnology, NY) was generously provided Dr. Cristina Tognon. Prior to developing, immunoblots were incubated with the appropriate horseradish peroxidase conjugated secondary antibody (anti-rabbit 1:2000 or anti-mouse 1:5000) for 1 hour at room temperature and proteins were localized by using enhanced chemiluminescence (ECL)-western blotting detection system (Amersham Biosciences Corp., Piscataway, NJ).
uPA mRNA and protein detection
MDA-MB-231 cells (70,000 cells/well in 96 well plate) were treated with varying concentrations of QLT0267, ILK siRNA or control siRNA for 24 or 48 hrs. Transfections with siRNA were performed using Lipofectamine™ 2000 reagent. (Invitrogen,Inc. Burlington, ON). Conditioned media was collected and stored at −70 °C. The media was diluted 1:5 and uPA protein concentrations were quantified by ELISA according to the manufacturer’s protocol (Imunobind uPA ELISA kit; American Diagnostica Inc., Montreal, QC).
qRT-PCR analysis
MDA-MB-231 and rhabdomyosarcoma cells were treated with DMSO, 12.5 μM or 25 μM QLT0267 for 24 hrs, RNA was isolated (Qiagen, Inc., Mississauga, ON) and reverse transcribed (Superscript™ III First-Strand Synthesis System, Invitrogen). The cDNA was amplified (ABI Prism® 7000 Sequence Detection System, Applied Biosystems, Foster City, CA) using uPA Assay on Demand (Applied Biosystems, Streetsville, ON) according to our previously reported method (19). Each treatment was analyzed in triplicate on two separate occasions. The TATA box binding protein (TBP) or 18S ribosomal protein was used as controls to assess differences in sample input (29).
Cell proliferation assays
Cell viability was measured using the CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (Promega Corp., Madison, WI) containing 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) salt. In brief, 7,000 MDA-MB-231 cells and 5,000 HBL-100 cells were plated per 100 μl in Falcon® 96 well microtiter plates (Becton Dickinson Biosciences, Mississauga, ON) and allowed to adhere overnight prior to treatment with varying concentrations (6.25 – 25 μM) of QLT0267 for 72 hrs. Cell viability following treatment was normalized to untreated controls. All assays were performed in triplicate.
Invasion assays
The Matrigel invasion assays were performed as previously described (30). In brief, MDA-MB-231 and SJCRH30 cells were treated with varying concentrations of QLT0267 or siRNA (10 nM and 25 nM ILK or control) and plated at 1 × 106 cells/ well μl in DMEM media containing 0.1% bovine serum albumin (Sigma Chemical Co., St. Louis, MO). Transfections with ILK siRNA were performed using Lipofectamine™ 2000 (Invitrogen Canada, Inc. Burlington, ON) reagent for 48 hrs. In a transwell chamber (Becton Dickenson Biosciences, Mississauga, Ontario) containing a filter 6.5 mm in diameter and 8 μm pores coated with Matrigel (Collaborative Research Co., Bedford, MA) at concentration of 10 μg/100 μl/ well. The cells were incubated for 24 hrs at 37 °C in humidified air with 5% CO2. Following incubation, cells on the upper chamber were removed with cotton swabs. The cells that invaded the lower surface of the membrane were fixed with methanol and stained with Hoechst 33258 (Sigma Chemical Co., Bedford, St. Louis, MA). Cellular invasion was measured by counting the cells within the field of view of 40X magnification.
Affymetrix array for rhabdomyosarcoma cell lines and primary tumors
Fresh-frozen primary tumor tissues were obtained before therapy from the Children’s Hospital Los Angeles (CHLA) institutional tumor bank, Los Angeles, CA, U.S.A. Patient consent and relevant IRB approval were obtained. Samples were collected from patients with an unequivocal diagnosis based on a combination of confirmed primary site, review of histology and immunohistochemical staining and were stored in liquid nitrogen freezers. Histopathologic diagnoses were based on the International Classification of Rhabdomyosarcoma criteria. RNA was extracted from excised tumors. All tumor samples contained >80% tumor cells. The PAX3/FKHR and PAX7/ FKHR fusion gene status was determined by RT-PCR. Frozen tumor tissues were obtained from Children’s Hospital Los Angeles with full Institution Review Board approval for research use of anonymous specimens. The preparation and processing of labeled and fragmented cRNA targets is detailed by Affymetrix (Santa Clara, CA). Briefly, total RNAs were prepared from cells using RNA STAT-60 (Tel-Test, Inc, Friendswood, TX). A T7-(dT)24 primer containing a T7 RNA-polymerase promoter site (Genset Oligos, La Jolla, CA, U.S.A.) was used to synthesize double-stranded, T7-tailed cDNAs directly from 10 μg of each total RNA as previously described (31) (Superscript™ Choice System for cDNA synthesis, Invitrogen, Carlsbad, CA). Biotinylated cRNAs were in vitro transcribed from 0.5 μg of each cDNA (biotinylated-11-CTP and -16-UTP; BioArray™ HighYield™ RNA Transcript Labeling Kit, Enzo Diagnostics, Farmingdale, NY) and fragmented. Cocktails including labeled and fragmented cRNAs (10 μg) and hybridization controls were hybridized to Affymetrix Human Genome U133A Arrays for 16 hrs at 45 °C (GeneChip® Hybridization Oven 640). The microarrays were washed as required (GeneChip® Fluidics Station 450) and stained using 10 μg/ml streptavidin-phycoerythrin (Molecular Probes, OR). Microarray images were obtained (GeneChip® Scanner 3000) and translated into expression analysis files using GeneChip® Operating System (GCOS) 2.0. To permit the direct comparison of all samples, the average fluorescence intensity of the entire arrays were scaled.
Genetrix™ (EpiCenter Software, Los Angeles, U.S.A.) is a data management, analysis and visualization tool that incorporates clinical and biological variables in conjunction with expression microarray data. Genetrix™ employs a package called Probe Profiler (Corimbia Inc, Berkeley, CA) that models probe intensities across all samples simultaneously to provide improved signal to noise ratios, estimates of standard errors, identification of outliers, and adjustments for scanner saturation. The raw data were collected from 75 alveolar rhabdomyosarcoma (ARMS), 77 embryonal rhabdomyosarcoma (ERMS), and 48 Ewing’s sarcoma (EWS), and they were imported into Genetrix™ via Probe Profiler using a principle component analysis (PCA) model and log-transformed. All data values are reported as mean ± standard deviation (S.D.). Student’s t test was used to measure statistical significance between two groups. For multiple comparisons, a standard one-way analysis of variance (ANOVA) was used to determine statistically significant differences of the means (InStat GraphPad). For multiple comparisons, posthoc analysis using the Tukey-Kramer test was performed. P < 0.05 was considered significant for all statistical tests.
Results and Discussion
Blocking ILK by the small molecule inhibitor QLT0267 suppresses Pi3K/ AKT and MAP kinase signaling, uPA production, invasion and cell viability
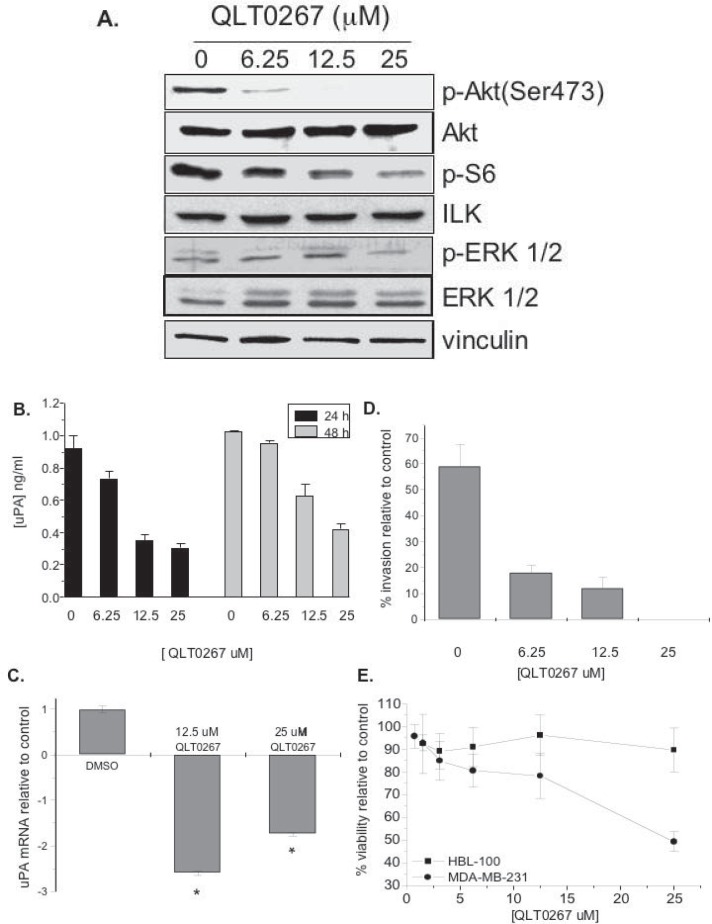
UPA protein expression requires activation of both Pi3K/Akt and MAP kinase pathways (17) and thus we examined the effect of ILK inhibition on downstream signaling of the Pi3K/Akt pathway following treatment with QLT0267, a small molecule inhibitor of ILK, in MDA-MB-231 cells. This was initially done in MDA-MB-231 breast cancer cells because they produce sufficient quantities of uPA and are highly invasive (17). We also recently showed that QLT0267 inhibits ILK kinase activity in a dose dependent manner in MDA-MB-231 cells (32) while others have reported this in models of thyroid cancer (27). Herein, QLT0267 inhibited the phosphorylation of AktSer473, S6 ribosomal protein and Erk1/2 (Fig. 1A). The inhibition was shown to be concentration-dependent with the greatest suppression observed when cells were treated with 25 μM QLT0267. The drug had no effect on vinculin, Akt, ERK 1/2, or ILK protein levels. Following an inhibition in signal transducition, QLT0267 decreased uPA in a concentration-dependent manner when cells were treated for either 24 or 48 hrs (Fig.1B). There was approximately a 3-fold reduction in uPA protein concentration (ng/ml) when cells were treated with QLT0267 (25 μM) at both time points. The decreased production of uPA in the conditioned media of cells treated with 12.5 μM and 25 μM QLT0267 was statistically significant as compared to untreated cells (p < 0.01). QLT0267 also inhibited uPA mRNA 2.60-fold and 1.75-fold when treated with 12.5 μM and 25 μM of the drug for 24 hr, respectively (Fig. 1C). Consistent with this observation, invasion of MDA-MB-231 through Matrigel was inhibited by treatment with QLT0267 for 24 hrs (Fig. 1D). Finally, QLT0267 (25 μM) decreased cell viability 50% in MDA-MB-231 cells; whereas viability was 90% in HBL-100 cells (Fig. 1E). As an aside, HBL100 cells are immortalized, non-tumorigenic breast epithelial cells. This is consistent with our recent study showing that QLT0267 does not kill immortalized breast epithelial cells, MCF-10A, 184htrt or primary breast epithelial cell isolates from patients that underwent reduction mammoplasties (32). In contrast, all of the cancer cell lines tested (MDA-MB-231, MDA-MB-435, BT-549, and MDA-MB-468) were sensitive to QLT0267 (32). Furthermore inhibiting Ilk by a dominant negative approach caused MDA-MB-231 cells to undergo apoptosis while 184htrt immortalized breast epithelial cells did not die. Taken together, QLT0267 has the potential to inhibit the growth of uPA-producing breast cancer cells suggesting that ILK may be a molecular target for cancers that are prone to recurrence.
Figure 1.
QLT0267 inhibits MDA-MB-231 signaling, uPA mRNA, invasion and ultimately cell viability. A) QLT0267 demonstrated a dose-dependent decrease in phosphorylation of p-Akt, p-S6 ribosomal protein, or p-ERK 1/2, while no changes were observed in total levels of Akt, ERK, ILK or vinculin. B) QLT0267 suppressed uPA production in conditioned media. uPA concentration was normalized for cell viability at 48 hrs, no cell death was observed following 24 hrs. C) uPA mRNA levels decreased 2.6-fold and 1.75-fold when MDA-MB-231 cells were treated with 12.5 μM and 25 μM QLT0267, respectively. Inhibition of ILK significantly decrease uPA mRNA at both concentrations tested when compared to the DMSO control (p < 0.01, ANOVA). D) Cell invasion was inhibited by QLT0267 (6.25, 12.5 or 25 μM) after being exposed to the drug for 24 hr. E) Growth of MDA-MB-231 cells were inhibited by the drug in a dose dependent manner whereas the immortalized breast epithelial HBL-100 cells continued to grow. All experiments were performed in triplicate, values represent the mean and standard deviation, **, p < 0.01.
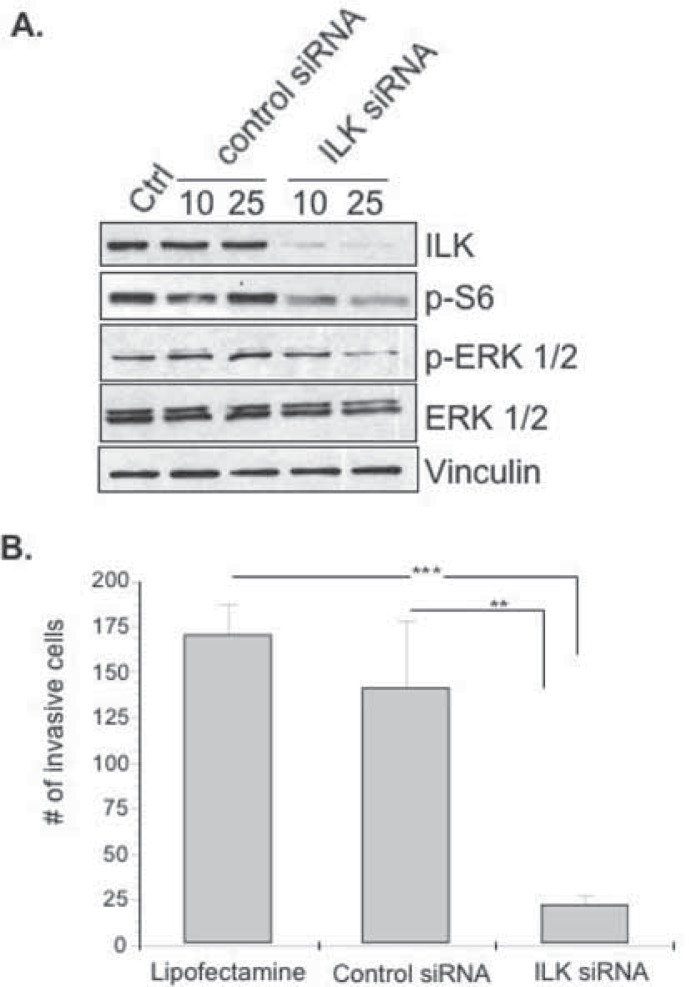
Knock-down of ILK with siRNA inhibits Pi3K/Akt and MAP kinase signaling resulting in decreased uPA production and invasion
To validate our results suggesting that ILK is a critical protein involved in uPA production and cell invasion we used siRNA as an additional means of inhibiting this target. We observed that >80% knockdown of ILK occurred following 48 hrs with 10 nM and 25 nM ILK siRNA (Figure 2A, lanes 4 and 5). Under these optimized conditions, knockdown of ILK expression was not observed in cells treated with Lipofectamine™ 2000 (Figure 2a, lane 1) or control siRNA (Figure. 2A, lanes 2 and 3). In agreement with our previous results with the small molecule ILK inhibitor, knock down of ILK resulted in a decreased expression of p-S6 ribosomal protein and p-ERK 1/2 while total ERK and vinculin protein levels remained unchanged (Fig. 2A). Similarly, uPA protein was inhibited 2.5 and 1.8 fold following ILK knock down with 10 nM and 25 nM siRNA (data not shown). As expected, silencing ILK (25 nM) inhibited invasion when compared to the control siRNA (p < 0.01) or Lipofectamine™ 2000 (p < 0.001) (Fig. 2B). We therefore conclude that inhibiting ILK with siRNA mirrors the inhibitory effect observed with QLT0267 indicating that the drug was specific.
Figure 2.
ILK siRNA suppresses Pi3K/Akt and MAPK signaling and cell invasion. A) MDA-MB-231 cells were treated with ILK siRNA for 48 hrs and signal transduction was evaluated by immunoblotting. B) For invasion assays, cells were transfected with siRNA for 48 hrs and subsequently plated on transwell inserts containing Matrigel for 24 hrs. Treatment with 25 nM ILK siRNA was significantly lower (***p < 0.005) than lipofectamine-treated or 25 nM control siRNA-treated cells (**p < 0.01). Statistical significance was determined using ANOVA.
During the writing of this manuscript, a study was published showing that inhibiting ILK with siRNA suppresses uPA expression in a model of metastatic mammary cancer (33) further validating our observations made with QLT0267. We also used a different siRNA to inhibit ILK. In that study, they found that the metastatic mammary cancer cell line 4T1 expressed high levels of uPA and MMP2 whereas the non-metastatic cell line 4T07 did not (33). The authors further pursued the mechanism whereby ILK inhibition led to the suppression of uPA mRNA by focusing on its effect on the AP-1 complex, which is known to be important for the induction of this invasion protease. For example, it has previously been characterized that the uPA promoter contains two AP-1/PEA3 sites (34) and as such we later reported that the Pi3K and MAP kinase pathways converge on these sites using a minimal uPA promoter (17). This could begin to explain why the introduction of wild-type ILK into mouse mammary epithelial cells stimulates the AP-1 reporter (35). Conversely, inhibiting ILK with either a kinase dead mutant (25) or siRNA (33) decreases AP-1 activity. To study this pathway in further detail, Mi et al. showed that blocking ILK with a dominant negative inhibitor prevented c-Jun from binding to the AP-1 sites on uPA and MMP-2 using chromatin immunoprecipitation (33). Given this, we pursued the possibility that QLT0267 similarly inhibits uPA mRNA by blocking the phosphorylation of c-Jun because studies have shown that it must be phosphorylated in order to be actively form the AP-1 complex (36, 37).
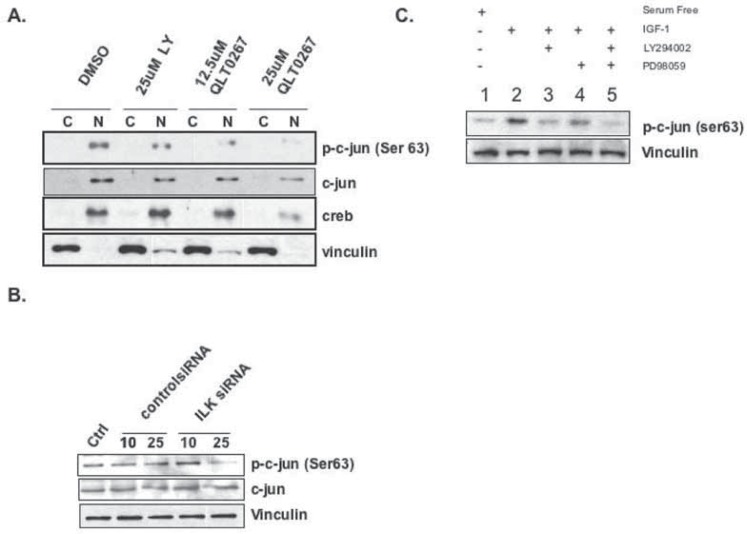
ILK modulates uPA production by phosphorylation of transcription factor c-Jun
QLT0267 inhibited the phosphorylation of c-Jun on Ser63 in a dose-dependent manner (Fig. 3A). Likewise, silencing ILK with siRNA inhibited c-Jun phosphorylation (Fig. 3B). Given this we also show that inhibiting the Pi3K and MAP Kinase pathways with LY294002 and PD098059 suppresses the phosphorylation of c-Jun (Figure 3C, lanes 3 and 4). However the most pronounced effect was observed when the two compounds were combined (Figure 3C, lane 5). These data thereby begin to explain why QLT0267 inhibits uPA mRNA. These results are in accordance with Troussard et al. who showed that ILK regulates c-Jun activity (35). We therefore suspect that the mechanism whereby QLT0267 inhibits uPA mRNA is at least in part by inhibiting c-Jun from binding to the AP-1 binding sites on the uPA promoter.
Figure 3.
ILK inhibition suppresses c-Jun activation. A) MDA-MB-231 cells were treated with 25 μM LY294002, 12.5 μM QLT0267 or 25 μM QLT0267. Cells were fractionated to separate cytoplasmic (C) and nuclear (N) compartments and analyzed by western blotting. Phospho-c-Jun (Ser63) expression decreased when treated with QLT0267. B) Treatment with 25 nM ILK siRNA, inhibited phospho-c-Jun (Ser63) expression while there was no effect on total c-Jun. C) Cells were serum starved (lane 1) or stimulated with IGF-1 to induce c-Jun phosphorylation (lane 2). The addition of LY294002 (20 μM) or PD98059 (20 μM, lanes 3&4) partially inhibited c-Jun phosphorylation following IGF-1 stimulation. Both compounds maximally attenuated the phosphorylation of c-Jun (lane 5).
It is now generally accepted that uPA is highly expressed in primary breast tumors (11, 18) and even in early ductal carcinoma in situ, (38) however studying it’s expression in cell-based models is confounded by the fact that most breast cancer cell lines do not express this protein. In keeping with this, we previously screened 11 different breast cancer cell lines and determined that MDA-MB-231 cell line was the only one that made significant amounts of uPA (17). It is thought that uPA commonly silenced in culture due to methylation (39). We therefore sought to verify the effect of QLT0267 in another type of cancer that produces uPA to confirm and extend our initial observations. We were drawn to pediatric sarcomas because we had unpublished results showing that serum uPA is elevated in a patient with recurrent rhabdomyosarcoma (RMS) leading us to suspect that because this is a secreted protein the primary tumor may also express it. Upon further investigation, we noted that uPA was previously reported in the pediatric RMS cell line RD (40, 41) however this was the limit of the association. RMS is similar to breast cancer in that approximately one third of tumors will recur (3) and patients eventually die of metastatic disease.
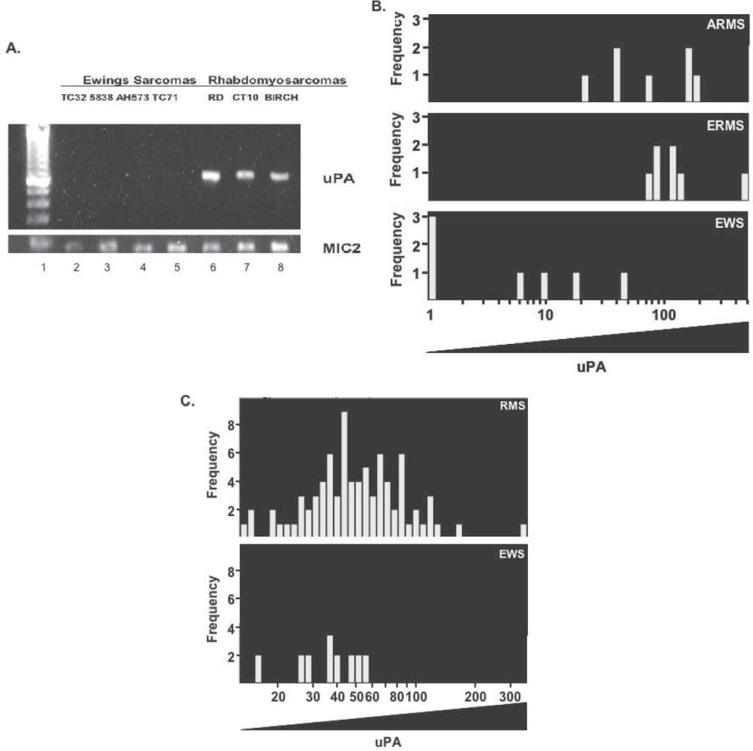
Urokinase plasminogen activator is a feature of rhabdomyosarcoma (RMS) and ILK inhibition suppresses cell growth
RMS is the most common type of pediatric sarcoma and is characterized by aggressive and recurrent disease (42). We asked the question whether uPA was produced in common pediatric cancers including RMS or Ewings sarcoma (EWS). Initially, we screened seven sarcoma cell lines by RT-PCR and detected uPA in all of the RMS samples (Figure 4A, lanes 6–8) while it was not detectable in the EWS cell lines (Figure 4A, lanes 2–5). This prompted us to use Affymetrix arrays, to further assess the intensity and frequency of uPA expression in alveolar (ARMS) and embryonal rhabdomyosarcoma (ERMS) cell lines (n = 22). Comparisons were made to EWS cell lines (n = 9). This approach revealed that uPA was frequently expressed at high levels in RMS and more so than Ewings sarcoma (p = 0.00004, Figure 4B). ARMS and ERMS both expressed high levels of uPA indicating that there are not differences within the RMS subtypes. This trend was further confirmed when we analyzed primary RMS (n = 152 cases) and EWS (n = 48 cases) from patients (p = 0.019, Figure 4C). This is a rather remarkable finding given that we were able to survey such a large number of tumors from relatively rare cancer types. For example, there were only ∼290 cases of RMS detected in the United States in 2006 (2) compared to ∼213,000 new cases of breast cancer (43).
Figure 4.
uPA is highly expressed in pediatric rhabdomyosarcomas (RMS). A) Ewings sarcoma (EWS) cell lines, TC32, 5838, AH573 and TC71 were compared to rhabdomyosarcoma (RMS) cell lines RD, CT10 and Birch for uPA mRNA which was detected by RT-PCR. uPA mRNA was expressed in pediatric RMS (lanes 6–8) but not EWS cell lines (lanes 2–5). The loading control MIC2 was used to ensure that the input cDNA was the same between samples. B) Expression profiles of pediatric sarcoma cell lines (n = 22 RMS and n = 9 EWS) were queried for uPA using Affymetrix arrays. uPA is highly expressed in RMS compared to Ewings sarcoma (EWS), p = 0.00004. Alveolar rhabdomyosarcomas (ARMS) and embryonal rhabdomyosarcomas (ERMS) both expressed high levels of uPA. C) Primary RMS (n = 152) were compared to EWS (n = 48) for uPA expression. uPA was more commonly expressed in RMS (p = 0.019).
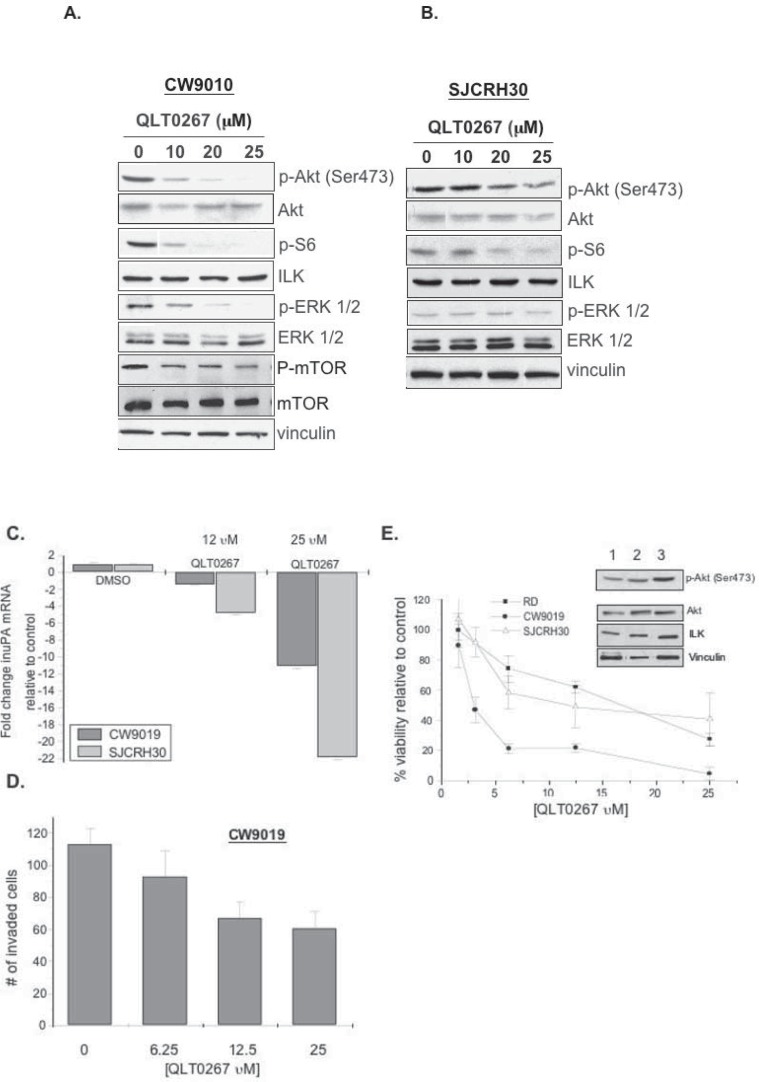
Our studies indicate that uPA is produced specifically in RMS cell lines and primary tumors; however, uPA is not a feature in EWS. Given this, we treated RMS cell lines with QLT0267 and observed a decrease in p-Akt, p-S6 ribosomal protein and p-ERK 1/2, p-mTOR, while total Akt, ERK, ILK, mTOR and vinculin protein levels remained unchanged in CW9019 cells (Fig. 5A). It is apparent that SJCRH30 were not as sensitive to changes in phosphorylated Akt, S6 ribosomal and ERK 1/2 proteins (Fig. 5A & B). QLT0267 also inhibited uPA mRNA in both cell lines (Fig. 5C). Similarly, inhibiting ILK with QLT0267 attenuated the SJCRH30 cells from invading through Matrigel (Fig. 5D). This led us to address whether the cells would ultimately die when exposed to the drug for longer time intervals. The CW9019 cells (circles, IC50 = 3 μM) were much more sensitive to QLT0267 treatment as compared to either SJCRH30 (squares, IC50 = 25 μM) or RD cells (triangles, IC50 = 25 μM) (Figure 5E). Variation in the drug sensitivities may be attributed to differences in p-Akt and ILK expression levels because RD or SJCRH30 cells have lower levels the CW9019 cells (Figure 5E insert; lanes 1–3 respectively). Higher expression of p-Akt and ILK suggest that these cells are dependent on this pathway for cellular transformation and thus disruption enhances drug sensitivity; a phenomenon termed “oncogene addiction” (44, 45). These data bring to light that uPA is highly expressed in primary RMS and that tumor derived cells lines are exquisitely sensitive to ILK inhibition because they produce less uPA, are less invasive and ultimately succumb to the effects of the drug by ceasing to grow.
Figure 5.
QLT0267 inhibits RMS signaling, uPA mRNA, invasion and ultimately cell viability. A–B) CW9019 and SJCRH30 cells were treated for 1 hr with QLT0267 and analyzed by immunoblotting. The drug decreased expression of p-Akt, p-S6 ribosomal protein p-ERK 1/2, and p-mTOR. CW9019 were more sensitive to the drug than the SJCRH30 cells. C) Cells were treated with either 12.5 or 25 μM of QLT0267 for 24 hrs and then uPA mRNA was evaluated by qRT-PCR. QLT0267 inhibited uPA mRNA in both cell lines tested. D) CW9019 cells were treated with increasing concentrations of QLT0267 and the effect on cellular invasion through Matrigel was assessed 24 hrs later. QLT0267 inhibited the cells from invading in a dose-dependent manner. E) RMS cell lines were treated with varying concentrations of QLT0267 for 72 hrs and inhibited cell viability in a dose-dependent manner. Symbols represent CW9019 (circles, IC50 = 3 μM), SJCRH30 (squares, IC50 = 25 μM) and RD (triangles, IC50 = 25 μM). Each of the cell lines was sensitive to the drug however the CW9019 cells were the most responsive. Insert: RD, SJCRH30 and CW9019 cells (lanes 1–3) were examined for potential differences in either p-Akt and/or ILK.
We demonstrated that uPA expression is a common feature of pediatric RMS in both cell lines and primary tumors, a feature not present in EWS. Thus, uPA could be used as an additional marker to distinguish between these tumor types. Most alveolar RMS are thought to arise from PAX3/FKHR or PAX7/FKHR gene fusions (42), whether either of these gene products directly influence the production of uPA is not known. It is also possible that these fusion genes stimulate AP-1 however that too has not been investigated to date. Another possibility is that uPA is being induced by a growth factor(s) such as insulin-like growth factor-1 (IGF-1) in the RMS as we previously reported in breast cancer (17). This is a distinct possibility given that PAX3/FKHR induces several components of the IGF-1 pathway including IGF-2 as well as the IGF-1 binding proteins 2 and 5 (IGFBP-5) (46). Subsequently, IGF-2 and IGF-BP5 clustered in with RMS tumors by gene expression profiling (47). In keeping with this, IGF-1 (48) and insulin (21) are known to stimulate ILK activity. Enhanced ILK activity could also explain why we find that uPA is highly expressed in breast tumors with coordinately elevated levels of IGF-1R (18). Given this, we propose that QLT0267 could be used to interrupt the IGF-1R/uPA pathway.
In closing, there is strong evidence that uPA is associated with in many types of cancer affecting adults and children. In the case of RMS, inhibiting ILK might therefore improve the grim outcome predicted for children. Children with PAX3-FKHR fusion positive tumors currently have shockingly low survival rates where only 8% of patients are expected to live beyond 4-years (49). It is likely that uPA plays a role in the pathogenesis of this disease given that the majority of children have metastases rather than localized disease (49). Thus there is a high demand for improving the treatment of children with this disease. QLT0267 provides and exciting opportunity for the treatment of many types of cancers and as such pre-clinical studies have been reported. For example, QLT0267 was recently reported to suppress the growth of thyroid cancer in mice (27). The studies that we have performed thus far provide the groundwork for addressing whether QLT0267 has anti-tumor activity against cancers that express high levels of uPA. It would also be very interesting to test this inhibitor in models of cancer recurrence (50). In conclusion, QLT0267 inhibits the production of uPA in aggressive forms of cancer resulting in a suppression of tumor cell invasion and ultimately survival. Taken together, inhibiting ILK with small molecules such as QLT0267 provide a new therapeutic strategy for suppressing uPA which would be used to treat patients that have a high risk of recurrence.
Acknowledgments
We thank QLT Inc. for providing the small molecule ILK inhibitor QLT0267. Our sincere appreciation to Dr. Tim Daynard at QLT for synthesizing QLT0267. We would also like to thank Ms. Joan Mathers from BC Children’s Hospital for providing the RMS and EWS cDNA for the initial survey of uPA mRNA performed by RT-PCR. Our gratitude also goes to the Michael Cuccione Foundation for partial support of this project.
Abbreviations:
- ARMS
alveolar rhabdomyosarcoma;
- BSA
bovine serum albumin;
- DCIS
ductal carcinoma in situ;
- DMSO
dimethyl sulfoxide;
- ECM
extracellular matrix;
- ELISA
enzyme-linked immunosorbent assay;
- ERK 1/2
extracellular regulated kinase 1 and 2;
- ERMS
embryonal rhabdomyosarcoma;
- EWS
Ewing’s sarcoma;
- FBS
fetal bovine serum;
- Hsp90
heat shock protein 90;
- ILK
integrin-linked kinase;
- MAPK
mitogen-activated protein kinase;
- mTOR
mammalian target of rapamycin;
- MTS
3-(4,5 dimethylthiazol-2-yl)-5-(3 carboxymethoxyphenyl)-2-(4 sulfophenyl)-2H-tetrazolium;
- p-AKT
phosphorylated Akt;
- PBS
phosphate buffered saline;
- Pi3K
phosphatidylinositol-3′ kinase;
- PMS
phenazine metho-sulfate;
- PTEN
phosphatase and tensin homologue;
- qRT-PCR
quantitative real time polymerase chain reaction;
- RMS
rhabdomyosarcoma;
- RNA
ribonucleic acid;
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis;
- siRNA
small interfering RNA;
- TBP
TATA box binding protein;
- TMA
tissue microarray;
- uPA
urokinase-type plasminogen activator;
- uPAR
urokinase-type plasminogen activator receptor.
Footnotes
Grant support
This study was funded by QLT, Inc., Translational Acceleration Grant II, Canadian Breast Cancer Research Alliance (CBCRA), NIH director challenge grant, the Michael Cuccione Foundation and Rethink Breast Cancer.
References
- [1].Society AC. Cancer Facts. 2005. http://www.cancer.org/downloads/STT/CAFF2005f4PWSecured.pdf.
- [2].Dagher R, Helman L. Rhabdomyosarcoma: An overview. Oncologist. 1999;4:34–44. [PubMed] [Google Scholar]
- [3].Ruymann FB, Grovas AC. Progress in the diagnosis and treatment of rhabdomyosarcoma and related soft tissue sarcomas. Cancer Invest. 2000;18:223–241. doi: 10.3109/07357900009031827. [DOI] [PubMed] [Google Scholar]
- [4].Duffy MJ, O’Grady P, Devaney D, et al. Urokinase-plasminogen activator, a marker for aggressive breast carcinomas. Preliminary report. Cancer. 1988;62(3):531–533. doi: 10.1002/1097-0142(19880801)62:3<531::aid-cncr2820620315>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- [5].Look MP, van Putten WL, Duffy MJ, et al. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J. Natl. Cancer Inst. 2002;94:116–128. doi: 10.1093/jnci/94.2.116. [DOI] [PubMed] [Google Scholar]
- [6].Foekens JA, Peters HA, Look MP, et al. The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients. Cancer Res. 2000;60(3):636–643. [PubMed] [Google Scholar]
- [7].Rabbani SA, Xing RH. Role of urokinase (uPA) and its receptor (uPAR) in invasion and metastasis of hormone-dependent malignancies (Review) Int. J. Oncology. 1998;12:911–920. doi: 10.3892/ijo.12.4.911. [DOI] [PubMed] [Google Scholar]
- [8].Blasi F, Carmeliet P., uPAR A versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 2002;3(12):932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- [9].Dazzi C, Cariello A, Maioli P, et al. A high cytosol value of urokinase-plasminogen activator (uPA) may be predictive of early relapse in primary breast cancer. Cancer Invest. 2003;21(2):208–216. doi: 10.1081/cnv-120016417. [DOI] [PubMed] [Google Scholar]
- [10].Janicke F, Schmitt M, Ulm K, Gossner W, Graeff H. Urokinase-type plasminogen activator antigen and early relapse in breast cancer. Lancet. 1989 Oct;28:1049. doi: 10.1016/s0140-6736(89)91070-2. [DOI] [PubMed] [Google Scholar]
- [11].Harbeck N, Kates RE, Guager K, et al. Urokinase-type plasminogen activator (uPA) and its inhibitor PAI-1: Novel tumor derived factors with high prognostic and predictive impact in breast cancer. Thromb. Haemost. 2004;91(3):450–456. doi: 10.1160/TH03-12-0798. [DOI] [PubMed] [Google Scholar]
- [12].Duffy MJ. Urokinase plasminogen activator and its inhibitor, PAI-1, as prognostic markers in breast cancer: From pilot to Level 1 evidence studies. Clin. Chem. 2002;48(8):1194–1197. [PubMed] [Google Scholar]
- [13].Carmeliet P, Moons L, Lijnen R, et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat. Genetics. 1997;17(4):439–444. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- [14].Pulukuri SM, Gondi CS, Lakka SS, et al. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival and tumorigenicity in vivo. J. Biol. Chem. 2005;280(43):36529–36540. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [15].Berkenblit A, Matulonis WA, Droener JF, et al. A6, a urokinase plasmonogen activator (uPA)-derived peptide in patients with advanced gynecologic cancer: A Plase I trial. Gyencol. Oncol. 2005;99(1):50–57. doi: 10.1016/j.ygyno.2005.05.023. [DOI] [PubMed] [Google Scholar]
- [16].Vallera DA, Li C, Jin N, Panoskaltsis-Mortari A, Hall WA. Targeting urokinase-type plasminogen activator receptor on human glioblastoma tumors with diptheria toxin fusion protein DTAT. J. Natl. Cancer Inst. 2002;94(8):597–606. doi: 10.1093/jnci/94.8.597. [DOI] [PubMed] [Google Scholar]
- [17].Dunn SE, Torres JV, Barrett JC. Up-regulation of urokinase type plasminogen activator by insulin-like growth factor-1 depends upon phosphotidyl inositol-3 kinase and Map kinase kinase. Cancer Res. 2001;61:1367–1374. [PubMed] [Google Scholar]
- [18].Nielsen T, Andrews H, Cheang M, et al. Expression of the insulin-like growth factor-1 receptor and urokinase plasminogen activator in breast cancer is associated with poor survival: Potential for intervention with 17 allylamino 17-demethoxy geldanamycin. Cancer Res. 2004;64:286–291. doi: 10.1158/0008-5472.can-03-1242. [DOI] [PubMed] [Google Scholar]
- [19].Andrews HN, Habibi G, Kucab JE, Dunn SE. Celecoxib inhibits urokinase-plasminogen activator (uPA) production in MDA-MB-231 breast cancer cells. Breast Cancer Res. Treat. 2005;94(1):47–52. doi: 10.1007/s10549-005-6941-5. [DOI] [PubMed] [Google Scholar]
- [20].Nerlov C, De Cesare D, Pergola F, et al. A regulatory element that mediates co-operation between a PEA3-AP-1 element and an AP-1 site is required for phorbol ester induction of urokinase enhancer activity in HepG2 hepatoma cells. EMBO J. 1992;11(12):4573–4582. doi: 10.1002/j.1460-2075.1992.tb05559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Delcommenne M, Tan C, Gray V, et al. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin linked kinase. Proc. Nat. Acad. Sci. U.S.A. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lynch DK, Ellis CA, Edwards PA, Hilles ID. Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene. 1999;18(56):8024–8032. doi: 10.1038/sj.onc.1203258. [DOI] [PubMed] [Google Scholar]
- [23].White DE, Cardiff RD, Dedhar S, Muller WJ. Mammary epithilial-specific expression of the integrin linked kinase (ILK) results in the induction of mammary gland hyperplasia and tumors in transgenic mice. Oncogene. 2001;20:7064–7072. doi: 10.1038/sj.onc.1204910. [DOI] [PubMed] [Google Scholar]
- [24].Hannigan GE, Troussard A, Dedhar S. Integrin-linked kinase: A cancer therapeutic target unique amongs its ILK. Nat. Rev. Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- [25].Troussard A, Costello P, Yoganathan N, et al. The integrin linked kinase (ILK) induces an invasion phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase 9 (MMP-9) Oncogene. 2000;19:5444–5452. doi: 10.1038/sj.onc.1203928. [DOI] [PubMed] [Google Scholar]
- [26].Koul D, Shen R, Bergh S, et al. Targeting integrin-linked kinase inhibits Akt signaling pathways and decreases tumor progression of human glioblastoma. Mol. Cancer Ther. 2005;4(11):1681–1688. doi: 10.1158/1535-7163.MCT-05-0258. [DOI] [PubMed] [Google Scholar]
- [27].Younes MN, S, K, Yigitbasi OG, et al. Integrin-linked kinase is a potential therapeutic target for anaplastic thyroid cancer. Mol. Cancer Ther. 2005;4(8):1146–1156. doi: 10.1158/1535-7163.MCT-05-0078. [DOI] [PubMed] [Google Scholar]
- [28].Troussard AA, Mawji NM, Ong C, et al. Conditional knockout of integrin-linked kinase demonstrates an essential role in protein kinaseB/Akt activation. J. Biol. Chem. 2003;278(25):22374–22378. doi: 10.1074/jbc.M303083200. [DOI] [PubMed] [Google Scholar]
- [29].Oh JS, Buchel P, Martin K, et al. Insulin-like growth factor-1 inscribes a gene expression profile for angiogenic factors and cancer progression in breast epithelial cells. Neoplasia. 2002;4(3):204–217. doi: 10.1038/sj.neo.7900229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dunn SE, Torres JV, Nihei N, Barrett JC. The insulin-like growth factor-1 elevates urokinase-type plasminogen activator-1 in human breast cancer cells: A new avenue for Breast Cancer Therapy. Mol. Carcinogenesis. 2000;27:10–17. doi: 10.1002/(sici)1098-2744(200001)27:1<10::aid-mc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [31].Mahadevappa M, Warrington JA. A high-density probe array sample preparation method using 10-100-fold fewer cells. Nat Biotechnol. 1999;18(6) doi: 10.1038/15124. [DOI] [PubMed] [Google Scholar]
- [32].Troussard A, McDonald PC, Wederell ED, et al. Preferential dependence of breast cancer cells versus normal cells on integrin-linked kinase for protein kinase B/Akt activation and cell survival. Cancer Res. 2006;66(1):393–403. doi: 10.1158/0008-5472.CAN-05-2304. [DOI] [PubMed] [Google Scholar]
- [33].Mi Z, Guo H, Wai PY, Gao C, Kuo PC. Integrin-linked kinase regulates osteopontin-dependent MMP-2 and uPA expression to convey metastatic function in murine mammary epithelial cancer cells. Carcinogenesis. 2006;27(6):1134–1145. doi: 10.1093/carcin/bgi352. [DOI] [PubMed] [Google Scholar]
- [34].Miralles F, Ibanez-Tallon I, Parra M, et al. Transcriptional regulation of the murine urokinase-type plasminogen activagor gene in skeletal myoblasts. Thromb. Haemost. 1999;81(5):767–774. [PubMed] [Google Scholar]
- [35].Troussard A, Tan C, Yoganathan N, Dedhar S. Cell-extracellular matrix interactions stimulate the AP-1 transcription factor in an integrin-linked kinase and glycogen synthase kinase 3-dependent manner. Mol. Cell. Biol. 1999;19(11):7420–7427. doi: 10.1128/mcb.19.11.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Smeal T, Binetruy B, Mercola DA, Sirrer M, Karin M. Oncogenic and transcriptional cooperation with Ha-ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 354(6353):494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- [37].Smeal T, Binetruy B, Mercola DA, et al. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol. Cell. Biol. 1992;12(8):3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kucab JE, Dunn SE. Role of IGF-1R in mediating breast cancer invasion and metastasis. Breast Disease. 2003;17:41–47. doi: 10.3233/bd-2003-17105. [DOI] [PubMed] [Google Scholar]
- [39].Xing RH, Rabbani SA. Transcriptional regulation of urokinase (uPA) gene expression in breast cancer cells: Role of DNA methylation. Int. J. Cancer. 1999;81:442–450. doi: 10.1002/(sici)1097-0215(19990505)81:3<443::aid-ijc19>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- [40].Bouche M, Canipari R, Malchionna R, et al. TGF-b autocrine loop regulates growth and myogenic differentiation in human rhabdomyosarcoma cells. FASEB J. 2000;14:1147–1158. doi: 10.1096/fasebj.14.9.1147. [DOI] [PubMed] [Google Scholar]
- [41].Pollanen J, Vaheri A, Tapiovaara H, et al. Prourokinase activation on the surface of human rhabdoyosarcoma cells: Localization and inactivation of newly formed urokinase-type plasminogen activator by recombinant class 2 plasminogen activator inhibitor. Proc. Nat. Acad. Sci., U.S.A. 1990;87:2230–2234. doi: 10.1073/pnas.87.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Merlino GT, Helman L. Rhabdomyosarcoma—working out the pathways. Oncogene. 1999;18:5340–5348. doi: 10.1038/sj.onc.1203038. [DOI] [PubMed] [Google Scholar]
- [43].http://www.cancer.org/docroot/STT/stt_0.asp Cancer Statistics 2006.
- [44].Weinstein IB. Addition to Oncogenes—The achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- [45].Sharma SV, Fischbach MA, Haber DA, Settleman J. “Oncogenic Shock”: Explaining oncogene addiction through differential signal attenuation. Clin. Cancer Res. 2006;12(14 Suppl):4392–4395. doi: 10.1158/1078-0432.CCR-06-0096. [DOI] [PubMed] [Google Scholar]
- [46].Khan J, Bittner ML, Saal LH, et al. cDNA microarrays detect activation of a myogenic transcription proram by the Pax3/FKHR fusion oncogene. Proc. Nat. Acad. Sci., U.S.A. 1999;96:13264–13269. doi: 10.1073/pnas.96.23.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Khan O, Wei JS, Ringner M, et al. Classification and diagnostic prediction of cancers using gene expression profiling and artificial neural networks. Nat. Med. 2001;7(6):673–679. doi: 10.1038/89044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Leung-Hagesteijn CAM, Naruszewicz E, Hannigan GE. Modulation of integrin signal transduction by ILKAP, a protein phosphatase 2C associating with the integrin-linked kinase, ILK1. EMBO, J. 2001;20(9):2160–2170. doi: 10.1093/emboj/20.9.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sorensen PHB, Lynch JC, Qualman SJ, et al. Pax3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcomas: A report from the children’s oncology group. J. Clin. Oncology. 2002;20(11):2672–2679. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- [50].Moody SE, Perez D, Pan T, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]