Abstract
Purpose.
To determine the intracellular signaling pathways that vasoactive intestinal peptide (VIP) uses to stimulate high molecular weight glycoconjugate secretion from cultured rat conjunctival goblet cells.
Methods.
Goblet cells from rat bulbar and forniceal conjunctiva were grown in organ culture. Presence and localization of VIP receptors (VPAC1 and 2) were determined by RT-PCR, immunofluorescence microscopy and Western blot analysis. Intracellular [Ca2+] ([Ca2+]i) was measured using fura-2. Extracellular signal-regulated kinase (ERK)-1/2 activity was determined by Western blot analysis. High molecular weight glycoconjugate secretion was measured with an enzyme-linked lectin assay on cultured goblet cells that were serum-starved for 2 hours before stimulation with VIP, VPAC1-, or VPAC2-specific agonists. Inhibitors were added 30 minutes prior to VIP. Activation of epidermal growth factor receptor (EGFR) was measured by immunoprecipitation using an antibody against pTyr followed by Western blot analysis with an antibody against EGFR.
Results.
Both VIP receptors were present in rat conjunctiva and cultured goblet cells. VIP- and VPAC-specific agonists increased [Ca2+]i and secretion in a concentration-dependent manner. VIP also increased ERK1/2 activity, VIP-stimulated increase in [Ca2+]i. Secretion, but not ERK1/2 activity, was inhibited by the protein kinase A inhibitor, H89. VIP-stimulated secretion was inhibited by siRNA for ERK2 but not by siRNA for EGFR. VIP did not increase the phosphorylation of the EGFR.
Conclusions.
In conclusion, in cultured rat conjunctival goblet cells, VPAC1 and 2 receptors are functional. VIP stimulates a cAMP-dependent increase in [Ca2+]i and glycoconjugate secretion, but not ERK1/2 activation. VIP does not activate with EGFR.
Keywords: goblet cells, conjunctiva, ERK, intracellular Ca2+
This study identifies the signaling pathways used VIP to induce glycoconjugate secretion in rat cultured conjunctival goblet cells.
Introduction
Vasoactive intestinal peptide (VIP) functions as a parasympathetic neurotransmitter that can be released along with acetylcholine to stimulate cells innervated by parasympathetic nerves, as are conjunctival goblet cells. Activation of afferent sensory nerves in the cornea or conjunctiva in response to thermal, chemical, mechanical, or pathogenic stimuli stimulates efferent parasympathetic nerves that surround the conjunctival goblet cells. Neural stimuli induce these cells to secrete mucins and other glycoconjugates into the tear film that overspreads the cornea and conjunctiva to neutralize environmental threats. We previously found that acetylcholine and other cholinergic agonists use muscarinic receptors that are present on conjunctival goblet cells.1–3 Activation of these receptors stimulates phospholipase β to increase Ca2+ and transactivate the epidermal growth factor receptor (EGFR).4 The active, phosphorylated EGFR stimulates the protein kinase cascade of Ras, Raf, and mitogen activated protein kinase kinase (MEK1/2) that ultimately induces extracellular regulated kinase (ERK)-1/2 (also known as p42/p44 mitogen activated protein kinase, MAPK), and causes goblet cell secretion.4
VIP is a well-known secretagogue in a variety of tissues. VIP activates protein, as well as fluid secretion from the lacrimal gland, as well as electrolyte and water secretion from the salivary gland and exocrine pancreas.5–8 In other tissues from the gastrointestinal tract, VIP stimulates goblet cell secretion from a colonic cell line,9 rat nasal mucosa,10 pancreatic duct,11 and some species of tracheal epithelium.12
Parasympathetic nerve release of VIP also stimulates conjunctival goblet cell secretion.13 We detected the VIP receptor subtypes VPAC2, but not VPAC1, on goblet cells in the rat conjunctiva and found that VIP stimulates high molecular weight glycoconjugate secretion that includes mucins from pieces of rat conjunctiva.1 Finally, VIP applied topically to rat conjunctiva in vivo stimulated goblet cell secretion.1,13 In all previous studies on the role of VIP in conjunctival secretion, the whole conjunctival epithelium and underlying stroma were used. As this tissue contains stratified squamous cells, stromal fibroblasts, and nerve endings in addition to the goblet cells, it is not possible to use conjunctival tissue pieces to study the signaling mechanisms specific to goblet cells. Thus we developed a method to culture an essentially pure population of conjunctival goblet cells.14 Herein we investigate the cellular signaling mechanisms activated by VIP in conjunctival goblet cells in culture.
VIP works by binding to its receptors VPAC1 or 2 to activate adenylyl cyclase (AC). An increase in adenylyl cyclase activity produces cAMP from ATP, which then activates protein kinase A (PKA) to phosphorylate proteins that stimulate secretion or other cellular functions.15 A second substrate of cAMP, in addition to PKA, is the guanine nucleotide exchange factors Epac1 and 2.16 More recently VIP has been shown to elevate intracellular Ca2+ ([Ca2+]i) by multiple, different mechanisms.17,18 Thus VIP can function by increasing [Ca2+]i in a cAMP-dependent manner.
In the present study, we used cultured conjunctival goblet cells, in contrast to conjunctival tissue, to demonstrate both VPAC1 and 2 receptors are functional and that VIP stimulates high molecular weight glyconjugate secretion by elevating the cellular concentration of cAMP that increases [Ca2+]i. VIP activation of ERK1/2 is cAMP-independent. We found that compared with cholinergic agonists, VIP does not transactivate the EGFR. VIP thus activates a complex signaling pathway distinct from cholinergic agonists for stimulating goblet cell secretion.
Materials
VIP and antibodies to VPAC1 and VPAC2 were purchased from EMD Millipore Corporation (Billerica, MA), while phosphorylated ERK1/2 and ERK2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies to EGFR and phosphorylated Tyr (pTyr) were from Cell Signaling Technologies (Beverly, MA). Primers for RT-PCR were purchased from Integrated DNA Technologies (Coralville, IA). [Ala2,8,9,11,19,22,24,25,27,28]-VIP and Bay 55-9837 were purchased from R&D Systems (Minneapolis, MN). Fura-2AM was purchased from Invitrogen (Carlsbad, CA). RPMI 1640 media is from Lonza (Walkersville, MD). siRNA and transfection reagents were purchased from Dharmacon (Lafayette, CO) while FITC-conjugated Ulex europaeus agglutinin (UEA)-1 lectin and H89 were from Sigma-Aldrich (St. Louis, MO). AG1478 was from LC Services (Waltham, MA). U0126 was from Tocris (Minneapolis, MN). Rat MUC5AC ELISA kit was purchased from BIOTANG Inc. (Waltham, MA).
Methods
Animals
Male Sprague-Dawley rats (Taconic Farms, Hudson, NY) weighing between 125 and 150 g were anesthetized with CO2 for 1 minute, decapitated, and the bulbar conjunctiva and fornix removed from both eyes. All experiments adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research and were approved by the Schepens Eye Research Institute Animal Care and Use Committee.
Cell Culture
Goblet cells from rat bulbar and forniceal conjunctiva were grown in organ culture as described previously.14,19 The tissue plug was removed after nodules of cells were observed. First-passage goblet cells were used in all experiments. Cultured cells were periodically checked by evaluating staining with antibody to cytokeratin 7 (detects goblet cell bodies) and the lectin UEA-1 (detects goblet cell secretory product) to ensure that goblet cells predominated.
RT-PCR
Rat conjunctiva and cultured goblet cells were homogenized in TRIzol and total RNA was isolated according to manufacturer's instructions. One microgram of purified total RNA was used for complementary DNA (cDNA) synthesis using a cDNA synthesis kit (Superscript First-Strand Synthesis system for RT-PCR; Invitrogen). The cDNA was amplified by the PCR using primers specific to VPAC1 and VPAC2 receptors using a commercial reaction mix (Jumpstart REDTaq ReadyMix Reaction Mix; Sigma-Aldrich) in a thermal cycler (Master Cycler; Eppendorf, Hauppauge, NY). The primers used for VPAC receptors were derived from previously published sequences.20 The primers for VPAC1 were: GTGAAGACCGGCTACACCAT and TGAAGAGGGCCATATCCTTG with a product of 178 bp. The primers for VPAC2 were: AGAGCCATCTCTGTGCTGGT and AGGTAGGCCAGGAAACACCT, with a product of 221 bp. The conditions were as follows: 5 minutes at 95°C followed by 35 cycles of 1 minute at 94°C, 30 seconds at an annealing temperature of 62°C, and 1 minute at 72°C with a final hold at 72°C for 10 minutes. Samples with no cDNA served as the negative control while the presence of β-actin was the positive control. Amplification products were separated by electrophoresis on a 1.5% agarose gel and visualized by ethidium bromide staining.
Western Blot Analysis for VIP Receptors
Rat conjunctiva and cultured goblet cells were homogenized in RIPA buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, and 1 mM EDTA) in the presence of a protease inhibitor cocktail (Sigma-Aldrich). The homogenate was centrifuged at 2000g for 30 minutes at 4°C. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and processed for Western blotting as described previously.24 The membranes were blocked in 5% dried milk in TBST (10 mM Tris-HCl pH 8, 500 mM NaCl, 0.05% Tween-20) and incubated with antibodies to either VIPAC1 or VIPAC2 overnight at 4°C. The membranes were washed three times in TBST before incubation for 1 hour with the secondary antibody conjugated to horseradish peroxidase. The immunoreactive bands were visualized by the chemiluminescence method.
Immunofluorescence Experiments
Goblet cells were grown on glass cover slips as described previously.21–23 Cells were fixed in 4% formaldehyde in PBS before use. VPAC1 and VPAC2 antibodies were used at 1:100 or 1:400 dilution, respectively, overnight at 4°C. UEA-1 conjugated to FITC was used at a dilution of 1:500 and identified goblet cell secretory product. Secondary antibody was conjugated to Cy 3 (Jackson ImmunoResearch Laboratories, West Grove, PA) and was used at a dilution of 1:150 for 1.5 hours at room temperature. To assess specificity of the primary antibody, experiments were performed using the isotype control of the primary antibodies. Cellular location of immunofluorescence was determined by viewing labeled cells using fluorescence microscopy (Eclipse E80i; Nikon, Tokyo, Japan) and immunofluorescent micrographs were taken with a digital camera (Spot; Diagnostic Instruments, Inc., Sterling Heights, MI).
Western Blot Analysis for ERK1/2 Activity
Cultured goblet cells were serum-starved for 2 hours before preincubation with and without inhibitors dissolved in KRB-HEPES and stimulated with VIP for indicated time. Cells were lysed in ice-cold RIPA buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, and 1 mM EDTA) in the presence of a protease inhibitor cocktail (Sigma-Aldrich). The homogenate was centrifuged at 2000g for 30 minutes at 4°C. Proteins were separated by SDS-PAGE and processed for Western blotting. The primary antibodies used were against phosphorylated ERK1/2 and EGFR. Antibodies were diluted 1:1000. The membranes were blocked in 5% dried milk in TBST (10 mM Tris-HCl pH 8, 500 mM NaCl, 0.05% Tween-20) and incubated with antibodies overnight at 4°C. The membranes were washed 3 times in TBST before incubation for 1 hour with the secondary antibody conjugated to horseradish peroxidase. The immunoreactive bands were visualized by the chemiluminescence method. The films were analyzed with a Java-based software program (ImageJ; provided in the public domain by National Institutes of Health, Bethesda, MD; http://rsbweb.nih.gov/ij/).
Measurement of [Ca2+]i
Goblet cells were incubated for 1 hour at 37°C with Krebs-Ringer bicarbonate buffer with HEPES (KRB-HEPES; 119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 MM NaHCO3, 10 mM HEPES, and 5.5 mM glucose [pH 7.45]) plus 0.5% BSA containing 0.5 μM fura 2/AM, 8 μM pluronic acid F127, and 250 μM sulfinpyrazone followed by washing in KRB-HEPES containing sulfinpyrazone. Calcium measurements were made with a ratio imaging system (InCyt Im2; Intracellular Imaging, Inc., Cincinnati, OH) using wavelengths of 340 and 380 nm and an emission wavelength of 505 nm. At least 10 cells were used for each condition. Inhibitors, dissolved in KRB-HEPES, were added 30 minutes before agonists. After addition of agonists, data were collected in real time. Data are presented as the actual [Ca2+]i with time or as the change in peak [Ca2+]i. Change in peak [Ca2+]i was calculated by subtracting the average of the basal value (no added agonist) from the peak [Ca2+]i. Although data are not shown, the plateau [Ca2+]i was affected similarly to the peak [Ca2+]i.
Measurement of High-Molecular Weight Glycoconjugate and MUC5AC Secretion
Cultured goblet cells were serum-starved for 2 hours before use, preincubated with inhibitors for 30 minutes or siRNA as described above, and then stimulated with agonists in serum-free RPMI 1640 supplemented with 0.5% BSA for 0 to 4 hours. Goblet cell secretion was measured using an enzyme-linked lectin assay (ELLA) with the lectin UEA-I. UEA-1 detects high molecular weight glycoconjugates including mucins produced by rat goblet cells. The media were collected and analyzed for the amount of lectin-detectable glycoconjugates, which quantifies the amount of goblet cell secretion as described earlier.1,4,21 Glycoconjugate secretion was expressed as fold increase over basal that was set to 1.
To measure MUC 5AC secretion, goblet cells were also serum-starved for 2 hours before use and then stimulated with VIP or carbachol (CCh) in serum-free RPMI 1640 supplemented with 0.5% BSA for 1 hour. The supernatant was collected and assayed for MUC 5AC by ELISA according to the manufacturer's instructions. The amount of MUC 5AC was standardized to the amount of total protein in each well as determined by Bradford assay.25
siRNA Experiments
First passage goblet cells were grown in six well plates. siRNA against either scrambled sequence, ERK2 or EGFR, was added at a final concentration of 100 nM in antibiotic-free RPMI 1640 as described previously.3 Media was removed after 18 hours and replaced with fresh, complete RPMI 1640 and incubated for 48 hours before use.
Immunoprecipitation Experiments
Cultured rat goblet cells were serum-starved for 2 hours prior to stimulation with either EGF (10−7 M) for 5 minutes or VIP (10−7 M) for either 1 or 5 minutes. The experiment was terminated by scraping cells in ice-cold RIPA buffer. The homogenate was centrifuged at 2000g for 30 minutes at 4°C and supernatant was incubated overnight at 4°C with an antibody against pTyr with gentle agitation. Protein A agarose beads were added and incubated for 2 hours at 4°C with gentle agitation. The beads were washed three times with RIPA buffer, and Western blot analysis was performed as described using an antibody against EGFR.
Statistical Analysis
Results were expressed as the fold increase above basal. Results are presented as mean ± SEM. Data were analyzed by Student's t-test. P < 0.05 was considered statistically significant.
Results
Presence of VIP Receptors on Cultured Conjunctival Goblet Cells and Conjunctiva
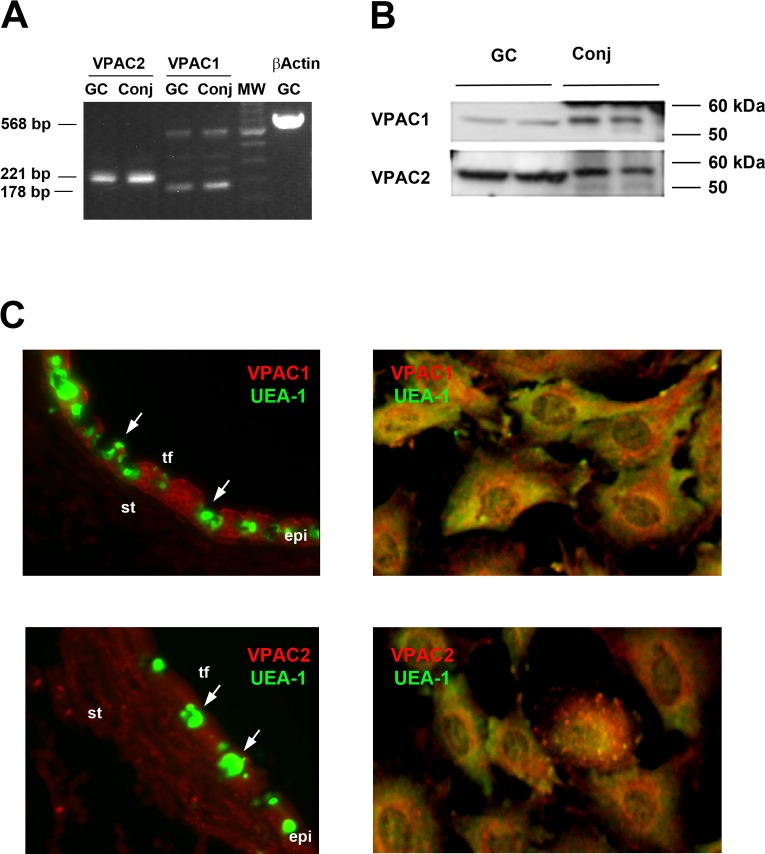
RT-PCR, Western blotting analysis, and immunofluorescence microscopy were used to determine if the VIP receptor subtypes VPAC1 and 2 were present on conjunctival goblet cells in culture and in rat conjunctiva. RT-PCR demonstrated that both VPAC1 and 2 were present in both the cultured goblet cells and in conjunctival tissue (Fig. 1A) at the expected size. Along with the expected length product of 178 bp, a second larger band was seen for VPAC1 by RT-PCR. Western blotting analysis was also performed on both goblet cell and conjunctival tissue homogenates using antibodies to VPAC1 and 2. VPAC1 and 2 were each present as a single band at approximately 52 kDa in both goblet cells and conjunctiva (Fig. 1B). In the conjunctiva, both receptors were detected at a slightly greater molecular weight than in the goblet cells. This difference could represent a differential glycosylation of the receptor in the native conjunctiva compared with the cultured goblet cells or a difference between the stratified squamous cells in the conjunctiva compared with the goblet cells, as stratified squamous cells and stromal fibroblasts together outnumber goblet cells in intact conjunctiva.
Figure 1. .
Presence and localization of VPAC1 and VPAC2 in cultured rat conjunctival goblet cells. The presence of VPAC1 and VPAC2 in cultured rat goblet cells (GC) and rat conjunctiva (Conj) was determined by RT-PCR (A) and Western blot analysis (B). Each lane represents an individual animal. Localization of VPAC1 and VPAC2 (shown in red) in conjunctival epithelium (left) and cultured rat goblet cells (right; [C]). Goblet cells were identified by their secretory product which binds to the lectin UEA-1, shown in green. Arrows indicate clusters of goblet cells. Micrographs are representative of three individual animals. Magnification ×400. Tf, tear film; st, stroma; epi, epithelium.
Using immunofluorescence microscopy, we previously found only VPAC2, but not VIPAC1 on goblet cells in conjunctival tissue sections.1 To confirm those studies, we repeated immunofluorescence experiments in rat conjunctival tissue using currently available VPAC1 and 2 receptors. With these antibodies, both VPAC1 and 2 were found in the conjunctiva. VPAC1 was seen in the stratified epithelial cells and goblet cells while VPAC2 was seen not only in the stratified squamous and goblet cells, but also in the stromal cells (Fig. 1C). Using goblet cells grown on cover slips we also found both subtypes of VIP receptors in conjunctival goblet cells (Fig. 1C). Both VPAC receptors were present in punctate pattern throughout the goblet cells. To ensure that the cells—both in the conjunctiva and in culture—were goblet cells, they were double labeled with the lectin UEA-1. All cultured cells contained immunoreactivity to both VIP receptor and UEA-1. The punctate VPAC receptor pattern was distinct from that of the secretory granules labeled with UEA-1.
When measured by RT-PCR, Western blotting analysis and immunofluorescence microscopy, both VPAC1 and 2 are localized to conjunctival goblet cells in conjunctival tissue and in culture.
Effect of VPAC-Specific Agonists and VIP on [Ca2+]i in Cultured Conjunctival Goblet Cells
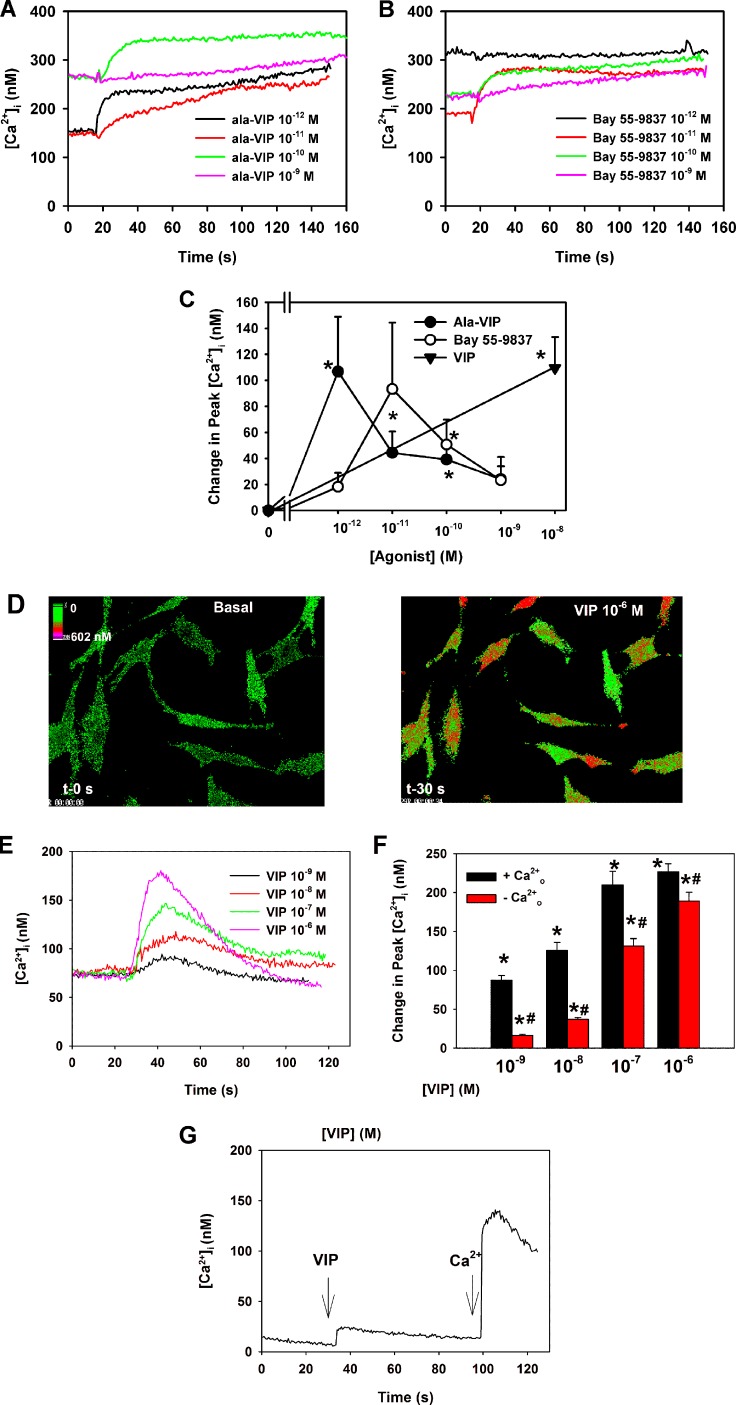
As there are no specific antagonists for the VPAC receptors, cultured goblet cells were stimulated with either the VPAC1-specific agonist [Ala2,8,9,11,19,22,24,25,27,28]-VIP (Ala-VIP) or the VPAC2 specific agonist Bay 55-9837, and [Ca2+]i was measured in conjunctival goblet cells in culture using the calcium sensitive dye fura-2. Both agonists increased [Ca2+]i (Figs. 2A, 2B). When peak Ca2+ was determined, Ala-VIP significantly increased [Ca2+]i a maximum of 106.8 ± 42.2 nM at 10−12 M (Fig. 2C). While Bay 55-9837 increased [Ca2+]i at 10−11 M, this increase was not significantly different from basal. However, the increase at 10−10 M of 50.7 ± 19.2 nM was significantly different from basal (Fig. 2C). In the same animals, VIP 10−8 M significantly increased [Ca2+]i by 109.5 ± 23.7 nM. Thus, activation of both VPAC1 and VPAC2 receptors results in an increase [Ca2+]i.
Figure 2.
Effect of VPAC1 and 2 agonists and VIP on [Ca2+]i. Cultured rat conjunctival goblet cells were loaded with fura-2 before addition of the VPAC1-specific agonist [Ala2,8,9,11,19,22,24,25,27,28]-VIP (Ala-VIP, [A, C]) or the VPAC2-specific agonist Bay 55-9837 (B, C) or VIP (D–G). Traces in (A, B, E, G) are from a single, representative animal. Change in peak [Ca2+]i was calculated in the presence of Ala-VIP 10−12 to 10−9 M stimulation and is shown in (C). Data is mean ± SEM from four individual experiments. [Ca2+]i over time after Bay 55-9837 10−12 to 10−9 M addition is shown in (B). Change in peak [Ca2+]i was calculated in the presence of Bay 55-9837 10−12 to 10−9 M stimulation and is shown in (C). Data is mean ± SEM from four individual experiments. Pseudocolor images of [Ca2+]i before (left panel) and after (right panel) addition of VIP 10−6 M are shown in (D). [Ca2+]i over time after VIP 10−9 to 10−6 M addition is shown in (E). Change in peak [Ca2+]i was calculated in the presence and absence of extracellular Ca2+o during VIP 10−9 to 10−6 M stimulation and is shown in (F). Data is mean ± SEM from three individual experiments. [Ca2+]i over time with VIP 10−8 M stimulation in the absence of Ca2+o (first arrow) followed by reintroduction of Ca2+o (second arrow) is shown in (G). *Indicates significant difference from zero. #Indicates significant difference of VIP in the presence of Ca2+o.
VIP (10−9 − 10−6 M) significantly increased [Ca2+]i in a concentration-dependent manner (Figs. 2D–F) with 10−6 M VIP causing the highest response with an increase in peak [Ca2+]i over basal of 226.7 ± 10.4 nM. To determine the cellular source of Ca2+, extracellular Ca2+ (Ca2+o) was removed and cells stimulated with VIP. This maneuver significantly decreased the VIP-induced increase in [Ca2+]i, but a response to VIP did occur especially at the higher concentrations of VIP, suggesting the release of intracellular Ca2+ stores (Fig. 2F). When Ca2+o was added back after VIP stimulation in the absence of extracellular Ca2+, a large increase in [Ca2+]i occurred that represented an influx of extracellular Ca2+ to refill the intracellular stores (Fig. 2G). These data demonstrated that VIP releases Ca2+ from intracellular stores and that emptying of these stores stimulates the influx of extracellular Ca2+.
Effect of VIP on Activation of ERK1/2
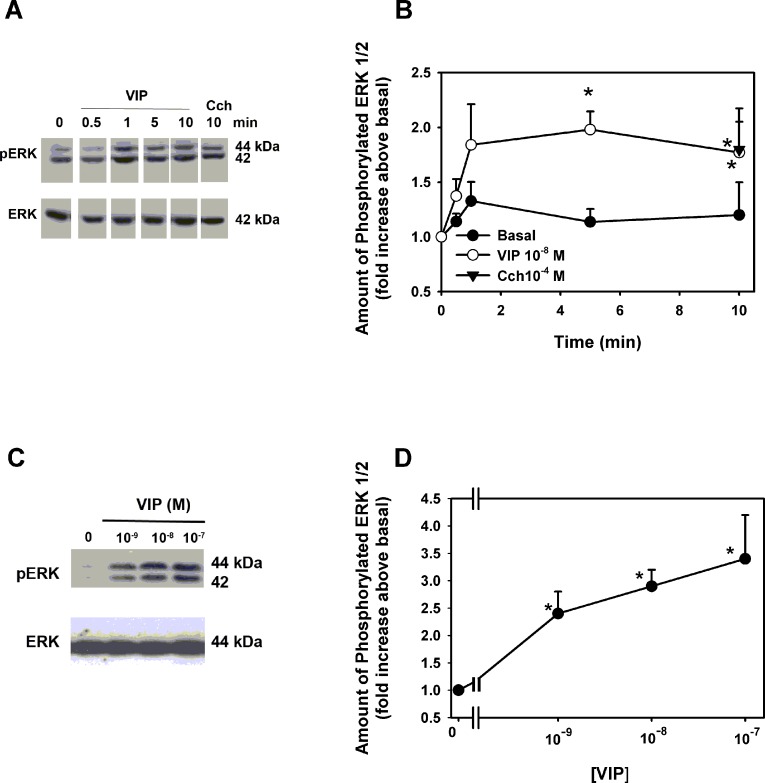
ERK1/2 activity was measured in cultured conjunctival goblet cells using an antibody specific to phosphorylated (active) ERK1/2. VIP at 10−8 M caused a time-dependent significant increase in ERK1/2 activity at 5 and 10 minutes of stimulation, with a maximum fold increase in activity of 2.0 ± 0.2-fold above basal occurring at 5 minutes (Figs. 3A, 3B). The cholinergic agonist CCh at 10−4 M caused a significant stimulation of 1.8 ± 0.4-fold that was the same as the VIP stimulation. VIP at 10−9 to 10−7 M significantly increased ERK1/2 activity with 10−7 M increasing ERK1/2 activity 3.4 ± 0.8-fold above basal (Figs. 3C, 3D).
Figure 3. .
Effect of VIP on activation of ERK1/2. Cultured rat goblet cells were incubated with VIP 10−8 M for 0 to 10 minutes or the muscarinic agonist carbachol (10−4 M) as a positive control for 10 minutes. Blot shown in (A) is representative of three individual experiments. Data shown in (B) is mean ± SEM from three individual experiments. Cultured rat goblet cells were also incubated for 5 minutes with VIP 10−9 to 10−7 M VIP. Blot in (C) is representative of eight individual experiments. Data shown in (D) are mean ± SEM from eight individual experiments. *Indicates significant difference from basal.
Effect of Removal of Extracellular Ca2+ on VIP-Stimulated ERK1/2 Activation in Cultured Conjunctival Goblet Cells
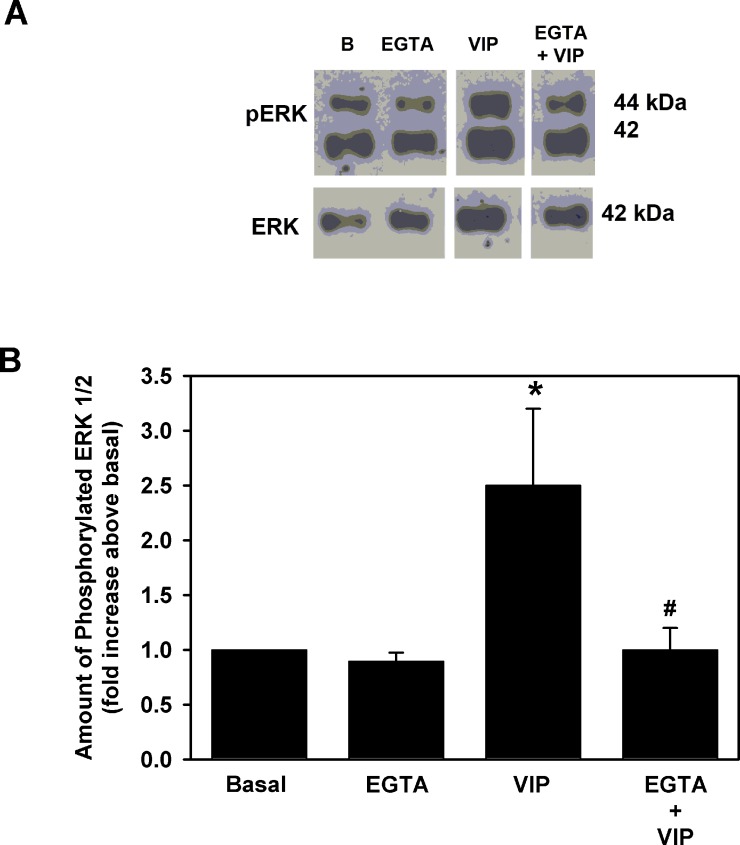
To determine the role that Ca2+ plays in ERK1/2 activation, extracellular Ca2+ was chelated with 2 mM EGTA for 5 minutes prior to addition of VIP (10−8 M) to cultured goblet cells. After 5 minutes, the cells were homogenized and Western blot analysis was performed using antibodies to phosphorylated and total ERK1/2. Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) alone had no effect on basal ERK1/2 activity (Figs. 4A, 4B). VIP increased phosphorylation of ERK1/2 to 2.5 ± 0.6-fold above basal. Chelation of Ca2+ significantly decreased this activation to 1.0 ± 0.2-fold above basal (Fig. 4B).
Figure 4. .
Effect of chelation of extracellular Ca2+ on VIP-stimulated ERK1/2 activity. Cultured rat goblet cells were preincubated in either Ca2+ free, KRB containing 2 mM EGTA, or normal Ca2+ containing KRB for 5 minutes prior to addition of VIP 10−8 M for 5 minutes. Blot shown in (A) is representative of four individual animals. Please note that lanes in blot are from the same experiment but have been rearranged for clarity. Data shown in (B) are mean ± SEM from four individual experiments. *Indicates significant difference from basal. #Indicates significance from VIP.
These results demonstrate that VIP increases [Ca2+]i and activates ERK1/2, the same pathways as activated by other stimuli of goblet cell secretion including cholinergic agonists, histamine, EGF, and cysteinyl leukotrienes.3,21,24
Effect of VPAC Specific Agonists and VIP on High Molecular Weight Glycoconjugate Secretion From Conjunctival Goblet Cells
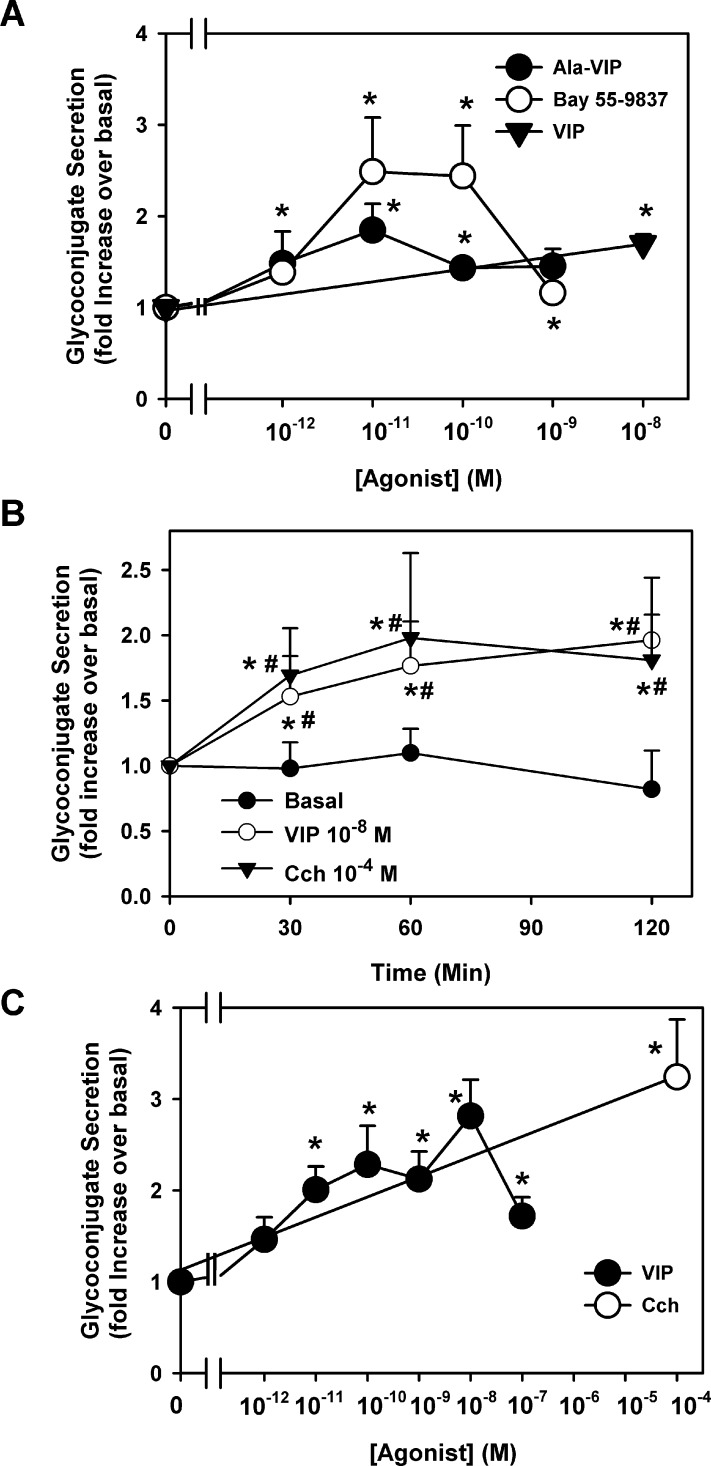
To determine if activation of VPAC1 and 2 receptors stimulate high molecular weight glycoconjugate secretion, goblet cells were stimulated with Ala-VIP (10−12 to 10−9 M); Bay 55-9738 (10−12 to 10−9 M); or VIP (10−8 M) as a positive control. Both receptor subtype agonists stimulated secretion in a concentration-dependent manner with a maximum secretion at 10−11 M (Fig. 5A). Ala-VIP significantly stimulated secretion a maximum of 1.8 ± 0.3-fold above basal while Bay 55-9837 significantly stimulated secretion a maximum of 2.5 ± 0.6-fold above basal. In the same cells, VIP significantly increased secretion 1.7 ± 0.1-fold above basal.
Figure 5. .
Effect of VPAC1 and 2 agonists and VIP on glycoconjugate secretion. Cultured rat conjunctival goblet cells were serum-starved for 2 hours before stimulation with the VPAC1 agonist Ala-VIP (10−12 to 10−9 M), the VPAC2 agonist Bay 55-9837 (10−12 to 10−9 M) or VIP 10−8 M for 60 minutes (A). Goblet cells were also stimulated with no additions (basal), VIP 10−8 M or the cholinergic agonist CCh (10−4 M) for 0 to 120 minutes (B) or VIP 10−12 to 10−7 M for 60 minutes, and/or CCh (10−4 M) for 120 minutes (C). Data shown are mean ± SEM from four individual experiments (A), nine individual experiments (B), or 3 to 6 individual experiments (C). *Indicates significant difference from t = 0. #Indicates significance from basal at indicated time.
The effect of VIP on high molecular weight glycoconjugate secretion was determined by incubating goblet cells with VIP at 10−8 M for 0 to 120 minutes (Fig. 5B). VIP significantly stimulated secretion at all time points measured. A maximum of 2.0 ± 0.5-fold was obtained at 120 minutes of stimulation. Over the 120-minute period, basal secretion (buffer alone) did not significantly change. As a positive control, CCh at 10−4 M significantly increased secretion at 30, 60, and 120 minutes. Carbachol-stimulated secretion at 120 minutes was 1.8 ± 0.3-fold over basal.
The effect of VIP concentration on goblet cell secretion was determined at 60 minutes of incubation time. VIP from 10−12 to 10−7 M stimulated secretion in a concentration-dependent manner, with 10−11 to 10−7 M VIP having significant effects compared with basal (no additions; Fig. 5C). The highest fold stimulation was obtained with 10−8 M VIP, which stimulated secretion 2.8 ± 0.4-fold compared with basal. As a positive control, CCh at 10−4 M significantly stimulated secretion 3.2 ± 0.6-fold when incubated for 120 minutes.
To ensure that MUC5AC is a component of the glycoconjugate proteins secreted by stimulation of VIP, cultured goblet cells were serum-starved for 2 hours and stimulated by VIP (10−7 M) or, as a positive control, CCh 10−4 M. The amount of MUC5AC in the supernatant was determined by ELISA. VIP stimulated MUC5AC secretion 8.3-fold above basal while CCh stimulated MUC5AC secretion 6.5-fold above basal (data not shown).
These results suggest that specific VPAC receptor subtypes agonists and VIP stimulate high molecular weight glycoconjugate secretion from cultured conjunctival goblet cells. VIP specifically stimulates MUC5AC secretion.
Effect of Inhibition of PKA on VIP-Stimulated Cultured Conjunctival Goblet Cell Function
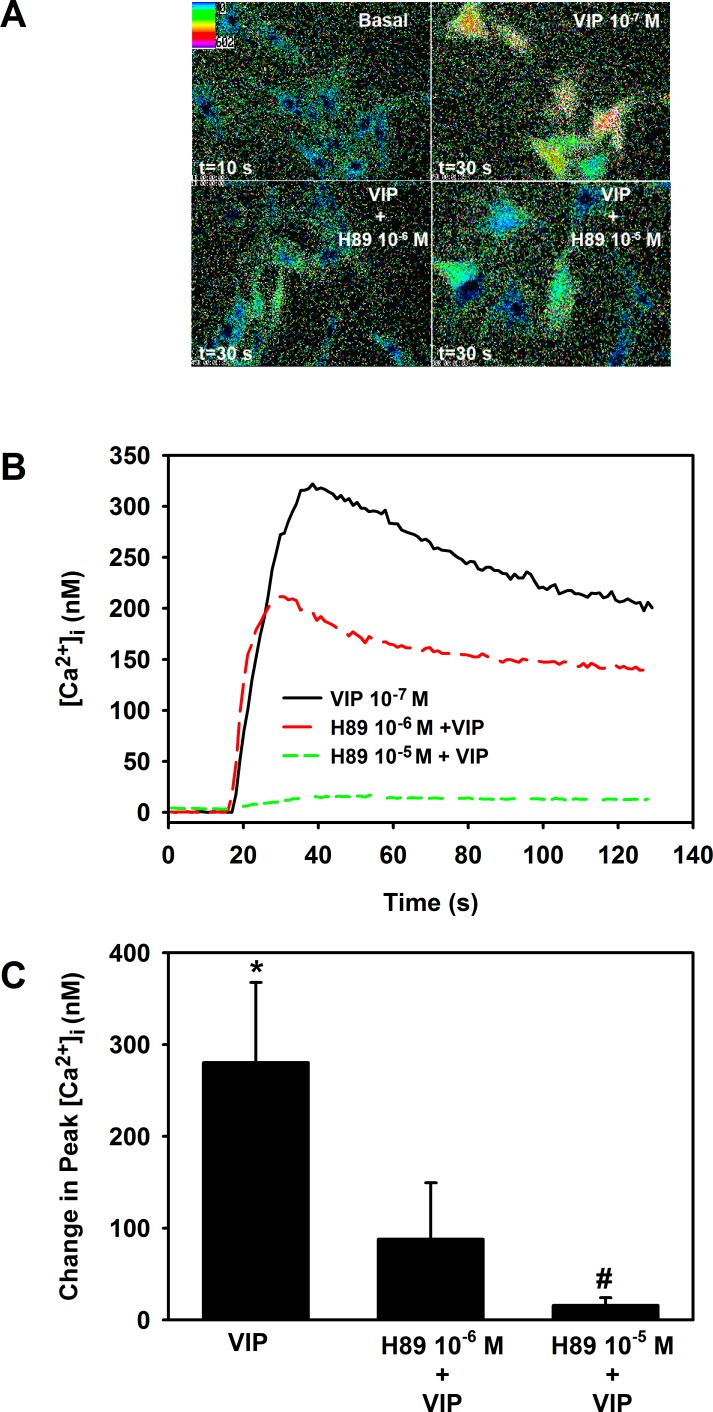
It is well established that VIP exerts its effects through activation of PKA and generation of cAMP. To determine if this occurs in goblet cells, cells were preincubated with the PKA inhibitor H89 for 30 minutes before addition of VIP. In the first set of experiments, goblet cells were also loaded with fura-2 and [Ca2+]i was measured. As shown in Figure 6, VIP 10−7 M significantly increased [Ca2+]i by 280.2 ± 87.4 nM. H89 at 10−6 M substantially decreased—though not significantly—this response to 87.9 ± 61.6 nM. H89 at 10−5 M significantly decreased the VIP response to 15.9 ± 8.3 nM.
Figure 6. .
Effect of inhibition of PKA on VIP-stimulated increase in [Ca2+]i. Cultured rat conjunctival goblet cells were loaded with fura-2 and preincubated with H89 10−6 and 10−5 M for 30 minutes prior to stimulation with VIP 10−7 M. Pseudocolor images of cells are shown in (A). [Ca2+]i over time is shown in (B). Traces are from a single animal and representative of three animals. Change in peak [Ca2+]i was calculated and is shown in (C). Data are mean ± SEM from three individual experiments. *Indicates significant difference from zero. #Indicates significant difference from VIP.
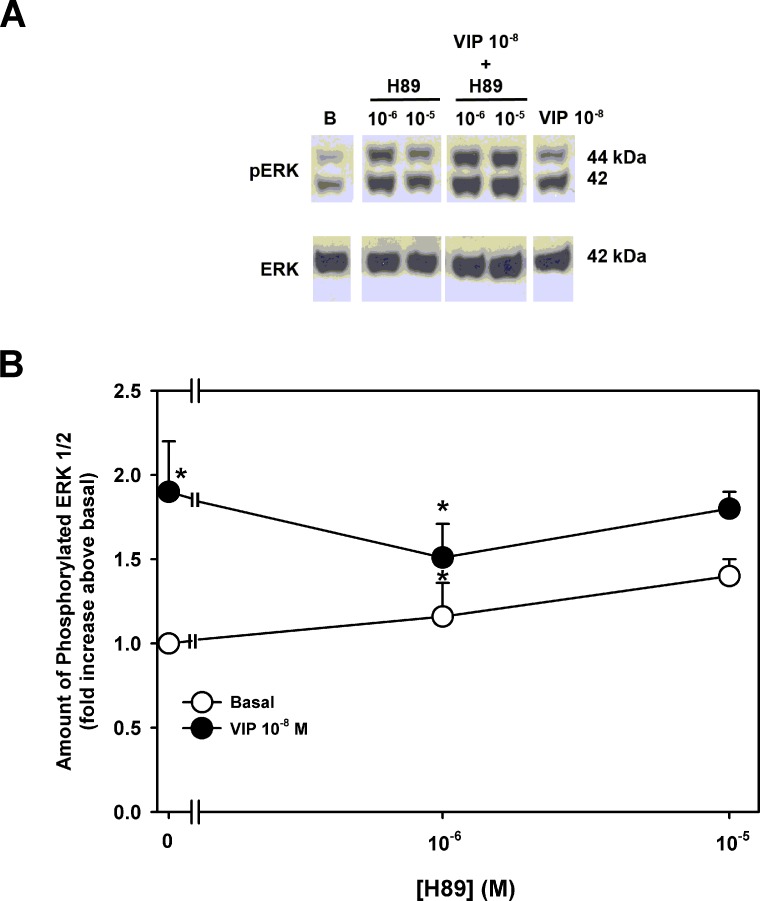
In a second set of experiments, the activity of ERK1/2 was measured after a 30-minute preincubation with H89. VIP (10−8 M) alone significantly increased ERK1/2 activity by 1.9 ± 0.3-fold above basal (Figs. 7A, 7B). Incubation with H89 alone (10−6 to 10−5 M) had no significant effect on basal ERK activity (Fig. 7B). No concentration of H89 altered VIP-stimulated ERK activity (Fig. 7B).
Figure 7. .
Effect of inhibition of PKA on VIP-stimulated increase in ERK1/2 activity. Cultured rat goblet cells were serum-starved for 2 hours and preincubated with H89 (10−6 to 10−4 M) for 30 minutes. Either buffer (open circles) or VIP 10−8 M (closed circles) were added for 5 minutes. Blot in (A) is representative of three individual experiments. Data shown in (B) are mean ± SEM from three individual experiments. *Indicates significant difference from basal.
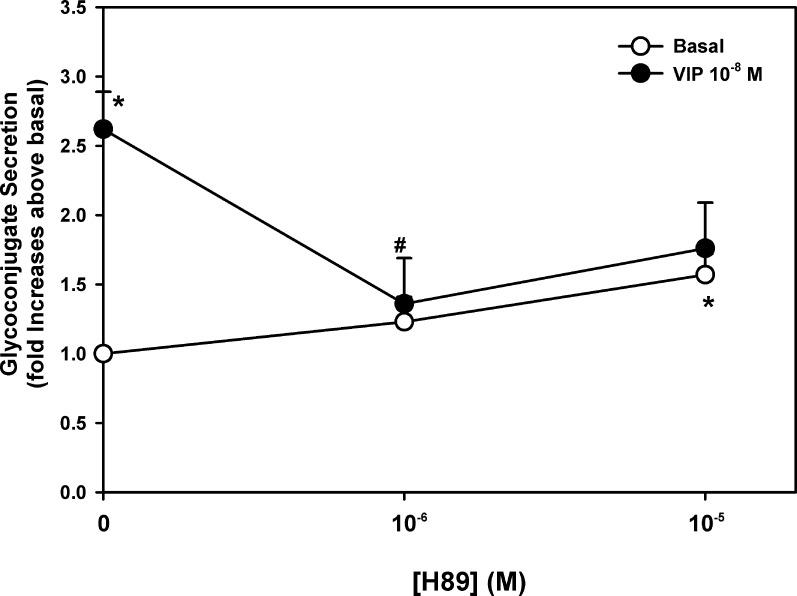
The effect of H89 on VIP-stimulated glycoconjugate secretion was also tested. H89 alone had a minimal effect on basal secretion (Fig. 8). In contrast VIP (10−8 M) significantly increased mucin secretion 2.6 ± 0.3-fold above basal. A 30-minute preincubation with H89 at 10−6 significantly decreased VIP-stimulated section to 1.4 ± 0.3-fold above basal (Fig. 8). While H89 10−5 M also decreased VIP-stimulated secretion, the results did not reach statistical significance.
Figure 8.
Effect of inhibition of PKA on VIP-stimulated glycoconjugate secretion. Cultured rat goblet cells were serum-starved for 2 hours and preincubated with H89 (10−6 to 10−5 M) for 30 minutes. Either buffer (open circles) or VIP 10−8 M (closed circles) were added for 60 minutes. Data shown are mean ± SEM from seven individual experiments. *Indicates significant difference from basal. †Indicates significant difference from VIP.
These data demonstrate that PKA plays a role VIP-stimulated increase in [Ca 2+]i and glycoconjugate secretion, but not in ERK1/2 activation.
Effect of Inhibition of ERK1/2 on VIP-Stimulated High Molecular Weight Glycoconjugate Secretion From Cultured Conjunctival Goblet Cells
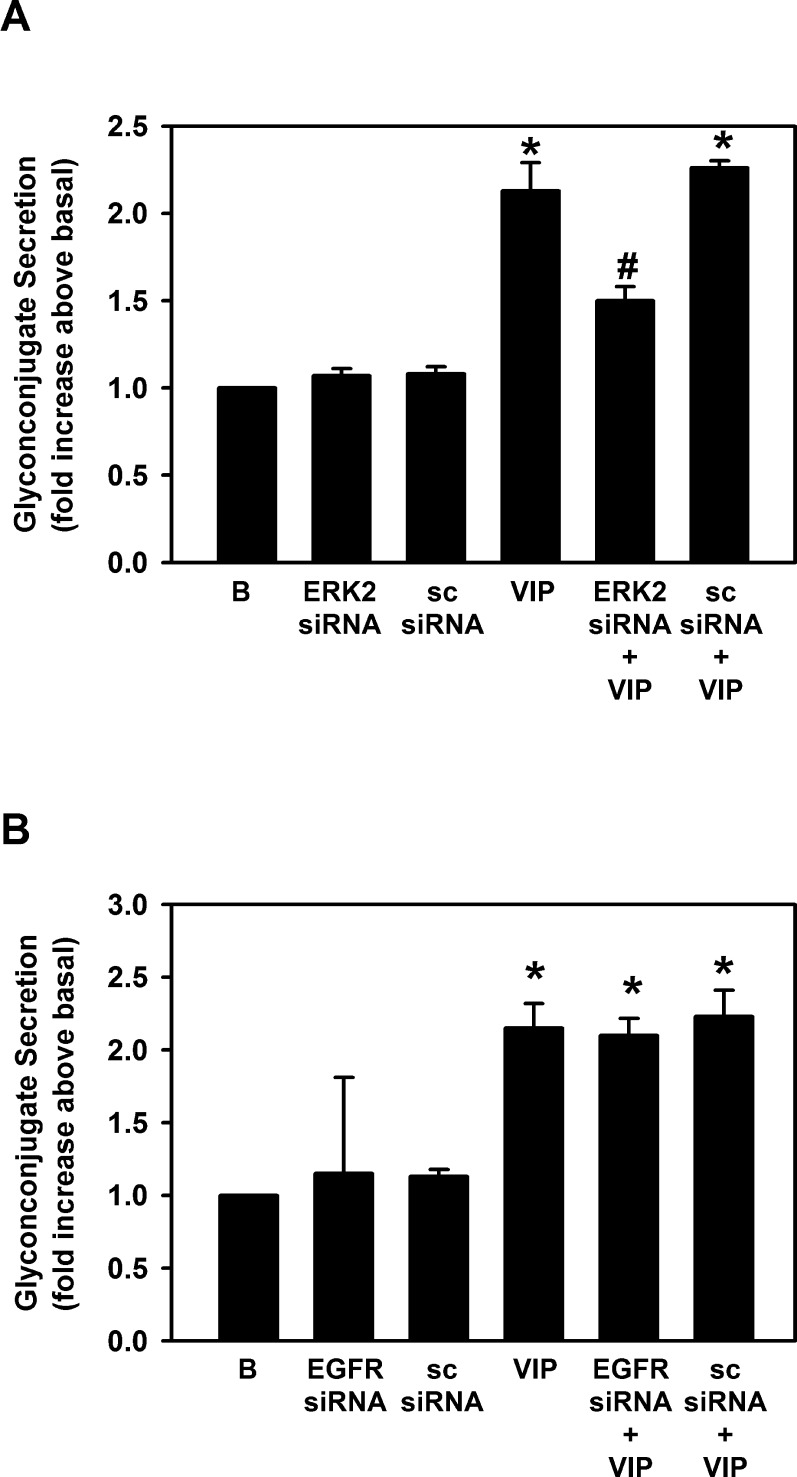
As VIP activates ERK1/2 in cultured conjunctival goblet cells, we determined if VIP-stimulated increase in ERK1/2 leads to high molecular weight glycoconjugate secretion from cultured conjunctival goblet cells. To this end, we used ERK2 siRNA as used previously.3 Neither ERK2 siRNA alone nor SC siRNA altered goblet cell secretion (Fig. 9A). VIP at 10−7 M significantly stimulated goblet cell secretion 2.1 ± 0.2-fold (Fig. 9A). When VIP was added after ERK2 siRNA, its effect was significantly blocked to 1.5 ± 0.1-fold above basal. The negative control, SC siRNA, did not alter VIP-stimulated secretion. These results suggest that VIP activates ERK1/2 to stimulate high molecular weight glycoconjugate secretion from conjunctival goblet cells.
Figure 9. .
Effect of inhibition of ERK1/2 and EGF receptor on VIP-stimulated glycoconjugate secretion. (A) Cultured rat goblet cells were treated with siRNA for a scrambled sequence (SC siRNA) or siRNA for ERK2. Cells were serum-starved for 2 hours and stimulated with VIP 10−7 M for 60 minutes. Glycoconjugate secretion was measured. (B) Cultured rat goblet cells were treated with siRNA for a scrambled sequence (SC siRNA) or siRNA for EGFR. Cells were serum-starved for 2 hours and stimulated with VIP 10−7 M for 60 minutes. Glycoconjugate secretion was measured. Data shown are mean ± SEM from three individual experiments. *Indicates significant difference from basal. #Indicates significant difference from VIP.
Effect of Inhibition of the EGF Receptor on VIP-Stimulated High Molecular Weight Glycoconjugate Secretion From Cultured Conjunctival Goblet Cells
Cholinergic agonists stimulate conjunctival goblet cell secretion by transactivating the EGFR that then engages the kinase cascade leading to activation of ERK1/2.4 To determine if VIP activates the EGFR to stimulate secretion, we used EGFR siRNA as used previously.3 Neither the SC siRNA nor the EGFR siRNA alone altered goblet cell secretion (Fig. 9B). VIP significantly stimulated goblet cell secretion by 2.2 ± 0.2-fold above basal, while neither SC siRNA nor EGFR siRNA altered VIP-stimulated secretion (Fig. 9B).
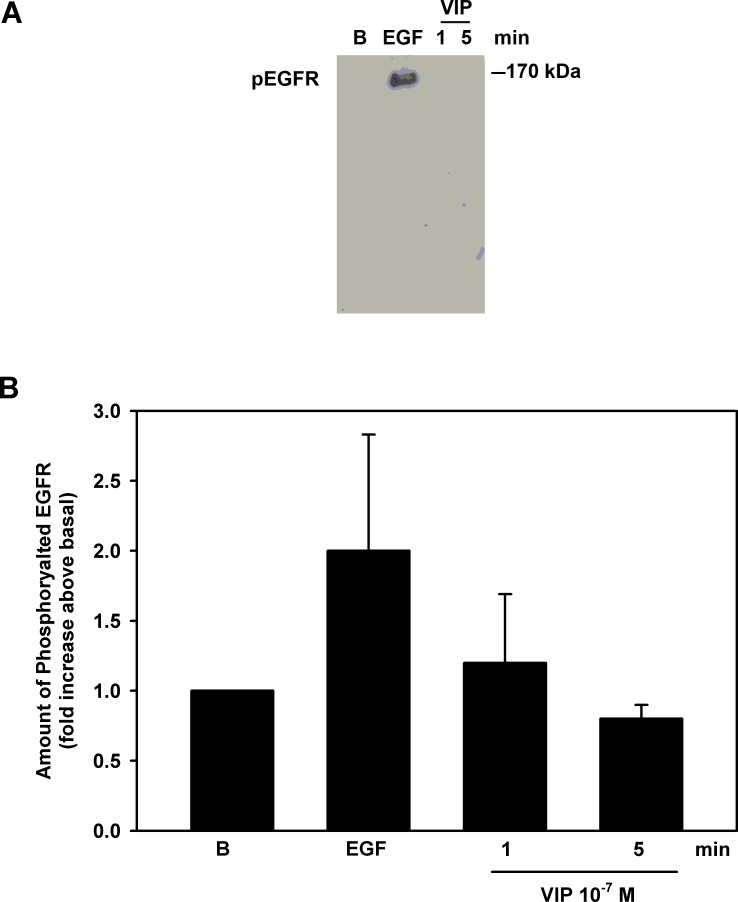
To confirm the results obtained with EGFR siRNA, we examined whether VIP activated the EGFR by immunoprecipitating proteins phosphorylated on tyrosine residues followed by Western blot analysis using an antibody to the EGFR. As a positive control, EGF (10−7 M) used for 5 minutes increased the phosphorylation of the EGFR by 2.0 ± 0.8-fold above basal (Fig. 10). VIP (10−7 M) incubated for 1 or 5 minutes did not change phosphorylation of the EGFR (Fig. 10).
Figure 10. .
Effect of VIP on phosphorylation of EGFR. Cultured rat goblet cells were serum-starved for 2 hours and stimulated with EGF for 5 minutes or VIP (10−7 M) for 1 or 5 minutes. The samples were immunoprecipitated with pTyr antibody and samples probed with an antibody against EGFR. Blot shown in (A) is representative of three individual experiments. Data in (B) are mean ± SEM from three individual experiments.
We conclude that VIP does not transactivate the EGFR and thus ERK1/2 is not activated by the EGFR stimulated pathway.
Discussion
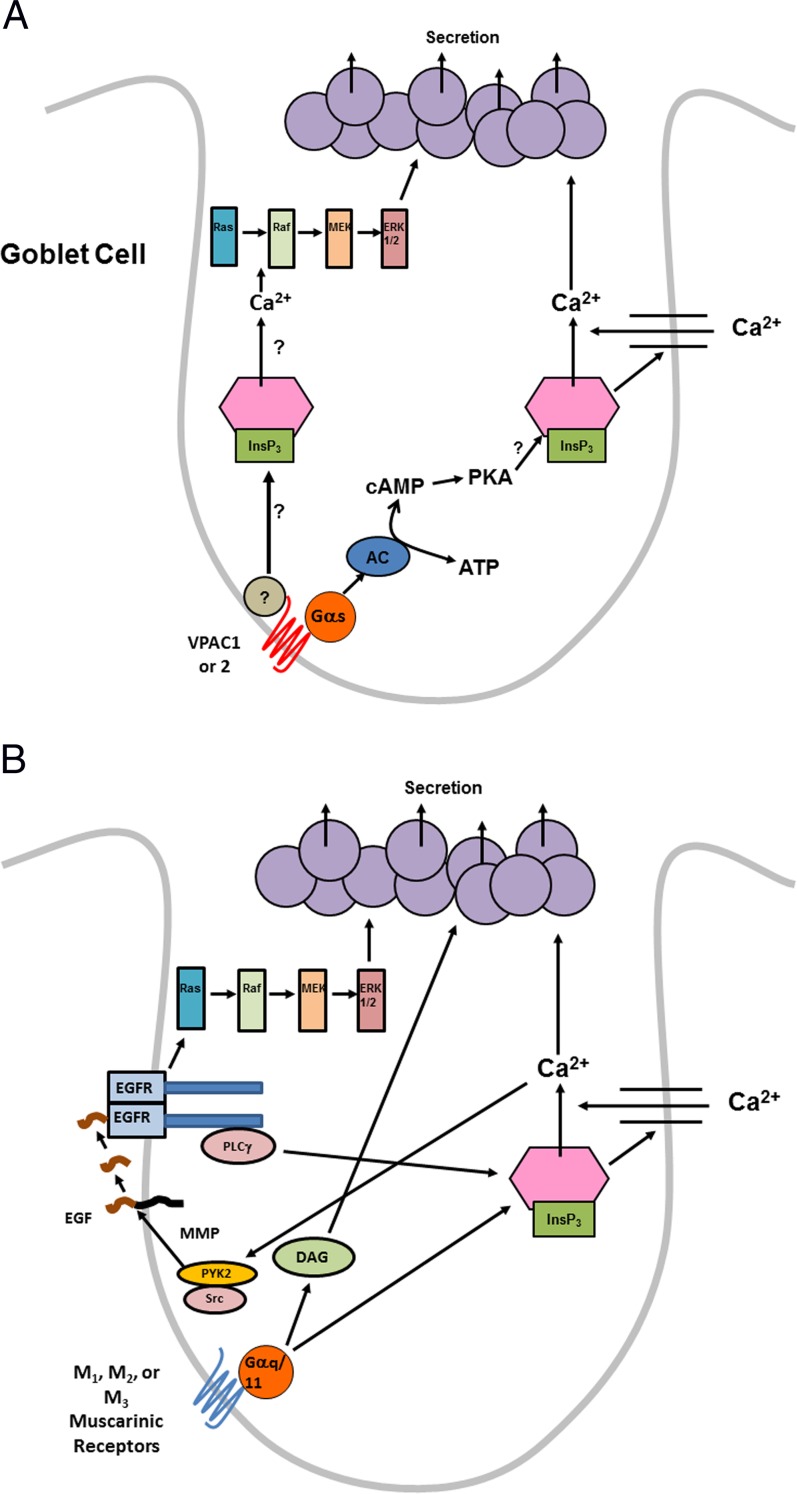
In conjunctival goblet cells in culture, both parasympathetic neurotransmitters cholinergic agonists and VIP, stimulate secretion of high molecular weight glycoconjugates including MUC5AC in agreement with our previous findings in pieces of conjunctiva in vitro and with topical application in vivo.1,3,4,13 However, using cultured conjunctival goblet cells uncontaminated by stratified squamous cells and stromal fibroblasts, we are now able to demonstrate that VIP and cholinergic agonists use differing cellular signaling pathways. VIP interacts with its receptors VPAC1 and 2 to activate adenylyl cyclase to increase cellular levels of cAMP (Fig. 11A). The increased levels of cellular cAMP activate PKA. PKA increases [Ca2+]i, perhaps by interacting directly with the inositol trisphosphate (InsP3) receptor to release intracellular Ca2+ that causes Ca2+ influx (Fig. 11A). VIP, independent of cAMP and the cAMP-dependent Ca2+ store, also activates the Raf, Ras, MEK, and ERK1/2 cascade at an unknown step. The elevated levels of cellular cAMP, the increased [Ca2+]i, and the activated ERK1/2 stimulate goblet cell secretion (Fig. 11A). VIP does not transactivate the EGFR to stimulate secretion or to activate the ERK1/2 signaling cascade.
Figure 11.
(A) Schematic diagram of pathways activated by VIP in rat conjunctival goblet cells. Gαs, alpha subunit of stimulatory G protein; ATP, adenosine triphosphate. (B) Schematic diagram of pathways activated by cholinergic agonists in rat conjunctival goblet cells. Gαq/11, alpha subunit of q/11 G protein; DAG, diacylglycerol.
In contrast, cholinergic agonists interact with M1AchR, and to a lesser extent M2AchR and M3AchR, to release intracellular Ca2+ stores and increase Ca2+ influx (Fig. 11B). This increase in [Ca2+]i activates the nonreceptor tyrosine kinases Pyk2 and Src.4 Activated PYK2 and Src stimulate matrix metalloproteinase (MMP) activity to cleave pro-EGF localized in the cell membranes. Released EGF interacts with EGFR to activate PLCγ to increase [Ca2+]i. The active EGFR also induces the Raf, Ras, MEK, and ERK1/2 cascade. Activation of these different pathways leads to secretion. Compared to VIP, cholinergic agonists do not use cAMP, but do transactivate the EGFR. The use of different signaling pathways by VIP and cholinergic agonists provide backup mechanisms to stimulate secretion and ensure that goblet cell secretion is not compromised.
In multiple tissues cAMP increases [Ca2+]i, but does so by several different mechanisms. First cAMP can activate either PKA or Epac.16 PKA can also activate the ryanodine or InsP3 receptors to increase the release of intracellular Ca2+26 as PKA, along with two phosphatases, is a component of an InsP3 receptor macromolecular complex that regulates Ca2+ release by this receptor.27 PKA can phosphorylate the InsP3 receptor to increase its sensitivity to InsP3 so that basal levels of InsP3 can cause Ca2+ release.18 The cAMP-dependent phosphorylation of the InsP3 receptor is independent of the isoform of InsP3 receptor present as all three subtypes are targets of cAMP.28 Epac is another target of cAMP. In endothelial cells, β2-adrenergic agonists activate Epac to release intracellular Ca2+ via an interaction with InsP3 receptors.29 Epac can also activate Ca2+calmodulin–dependent protein kinase II to interact with the ryanodine receptor and release intracellular Ca2+.30,31 In the present study, VIP-stimulated PKA activity increased [Ca2+]i in conjunctival goblet cells. We did not determine if Epac plays a similar role. We also did not determine the mechanism VIP uses to increase [Ca2+]i.
As presented here, we found that VIP did not transactivate the EGFR. However, there are other pathways to activate ERK1/2 independent of the EGFR. For example, in a human salivary gland cell line, Yeh et al. demonstrate that β-adrenergic agonists (which also increase intracellular cAMP levels), activate Epac which in turn phosphorylates Src, Rap-1, Raf, MEK1/2, and finally ERK1/2.32 In addition, Ras, Raf, MEK, and ERK1/2 are well-known targets of cAMP itself. We did not explore theses mechanisms of VIP stimulation of ERK1/2 in this study.
In the present study, both VIP receptors were detected in cultured conjunctival goblet cells and homogenized conjunctiva by RT-PCR, Western blotting analysis, and cultured conjunctival goblet cells by immunofluorescence microscopy. Previous studies demonstrated VPAC1, but not VPAC2, immunoreactivity in both goblet and stratified squamous cells in intact conjunctival epithelium.1 When these experiments were repeated with the currently available antibodies for VPAC1 and 2, both receptors were detected in the rat conjunctiva as well as cultured goblet cells. These findings were confirmed by RT-PCR. Thus, both VIP receptors are present in conjunctival goblet cells and in goblet cells in situ.
While it is not clear if VIP binds to both VPAC1 and 2 or preferentially uses one over the other, activation of VPAC1 and 2 by specific agonists increase both [Ca2+]i and high molecular weight glycoconjugate secretion. Interestingly VIP, Ala-VIP, and Bay 55-9738 all increased [Ca2+]i to the same magnitude, though the specific agonists did so at a much lower concentration. This was also true for secretion where maximum secretion occurred at 10−8 M VIP but at 10−11 M for the specific agonists. One explanation for this is that the receptor subtype agonists have a higher affinity for their receptors than VIP. Another explanation is that when both receptors are activated together, as could occur with VIP, the receptors have an antagonist effect toward one another requiring a higher concentration of agonist to achieve the same effect as when only one receptor is activated. This might be important to limit the amount of secretion under normal circumstances.
We conclude that in cultured conjunctival goblet cells, both VPAC1 and 2 receptors are functional in goblet cells. VIP stimulates secretion by increasing cAMP levels that release Ca2+i thus stimulating Ca2+ influx to induce secretion. The increase in [Ca2+]i but not the elevation of cAMP activates the ERK1/2 signaling cascade to induce secretion. The cellular signaling pathway induced by VIP differs from that activated by cholinergic agonists that transactivate the EGFR, but do not use cAMP.
Acknowledgments
The authors thank J. David Rios for technical assistance.
Supported by NIH EY019470 and EY09570. The authors alone are responsible for the content and writing of the paper.
Disclosure: D. Li, None; J. Jiao, None; M.A. Shatos, None; R.R. Hodges, None; D.A. Dartt, None
References
- 1. Rios JD, Zoukhri D, Rawe IM, et al. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci. 1999; 40: 1102–1111 [PubMed] [Google Scholar]
- 2. Horikawa Y, Shatos MA, Hodges RR, et al. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003; 44: 2535–2544 [DOI] [PubMed] [Google Scholar]
- 3. Hodges RR, Bair JA, Carozza RB, et al. Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp Eye Res. 2012; 103: 99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanno H, Horikawa Y, Hodges RR, et al. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol. 2003; 284: C988–C998 [DOI] [PubMed] [Google Scholar]
- 5. Dartt DA, Baker AK, Vaillant C, et al. Vasoactive intestinal polypeptide stimulation of protein secretion from rat lacrimal gland acini. Am J Physiol. 1984; 247 (5 pt 1): G502–G509 [DOI] [PubMed] [Google Scholar]
- 6. Hallden G, Abens J, Engstrom C, et al. Iodinated derivatives of the vasoactive intestinal polypeptide (VIP) are agonists at the cat pancreas and the rat submandibular salivary gland. Regul Pept. 1986; 16: 183–188 [DOI] [PubMed] [Google Scholar]
- 7. Scott J, Baum BJ. Involvement of cyclic AMP and calcium in exocrine protein secretion induced by vasoactive intestinal polypeptide in rat parotid cells. Biochim Biophys Acta. 1985; 847: 255–262 [DOI] [PubMed] [Google Scholar]
- 8. Turner JT, Camden JM. Regulation of secretion by vasoactive intestinal peptide in isolated perfused rat submandibular glands. Arch Oral Biol. 1992; 37: 281–287 [DOI] [PubMed] [Google Scholar]
- 9. McCool DJ, Marcon MA, Forstner JF, et al. The T84 human colonic adenocarcinoma cell line produces mucin in culture and releases it in response to various secretagogues. Biochem J. 1990; 267: 491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamijo A, Terakawa S, Hisamatsu K. Neurotransmitter-induced exocytosis in goblet and acinar cells of rat nasal mucosa studied by video microscopy. Am J Physiol. 1993; 265 (2 pt 1): L200–L209 [DOI] [PubMed] [Google Scholar]
- 11. Wilkins TJ, de Ondarza J, Hootman SR. Intracellular mediators of goblet cell degranulation in isolated pancreatic ducts. Biochem Biophys Res Commun. 1994; 205: 423–428 [DOI] [PubMed] [Google Scholar]
- 12. Lazarus SC, Basbaum CB, Barnes PJ, et al. cAMP immunocytochemistry provides evidence for functional VIP receptors in trachea. Am J Physiol. 1986; 251 (1 pt 1): C115–C119 [DOI] [PubMed] [Google Scholar]
- 13. Dartt DA, Kessler TL, Chung EH, et al. Vasoactive intestinal peptide-stimulated glycoconjugate secretion from conjunctival goblet cells. Exp Eye Res. 1996; 63: 27–34 [DOI] [PubMed] [Google Scholar]
- 14. Shatos MA, Rios JD, Tepavcevic V, et al. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001; 42: 1455–1464 [PubMed] [Google Scholar]
- 15. Billington CK, Hall IP. Novel cAMP signalling paradigms: therapeutic implications for airway disease. Br J Pharmacol. 2012; 166: 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holz GG, Kang G, Harbeck M, et al. Cell physiology of cAMP sensor Epac. J Physiol. 2006; 577 (pt 1): 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wojcikiewicz RJ, Luo SG. Phosphorylation of inositol 1,4,5-trisphosphate receptors by cAMP-dependent protein kinase. Type I, II, and III receptors are differentially susceptible to phosphorylation and are phosphorylated in intact cells. J Biol Chem. 1998; 273: 5670–5677 [DOI] [PubMed] [Google Scholar]
- 18. Lee RJ, Foskett JK. cAMP-activated Ca2+ signaling is required for CFTR-mediated serous cell fluid secretion in porcine and human airways. J Clin Invest. 2010; 120: 3137–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shatos MA, Rios JD, Horikawa Y, et al. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003; 44: 2477–2486 [DOI] [PubMed] [Google Scholar]
- 20. Castorina A, Tiralongo A, Giunta S, et al. PACAP and VIP prevent apoptosis in schwannoma cells. Brain Res. 2008; 1241: 29–35 [DOI] [PubMed] [Google Scholar]
- 21. Dartt DA, Hodges RR, Li D, et al. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011; 186: 4455–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shatos MA, Hodges RR, Bair JA, et al. Stimulatory role of PKCalpha in extracellular regulated kinase 1/2 pathway in conjunctival goblet cell proliferation. Invest Ophthalmol Vis Sci. 2009; 50: 1619–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shatos MA, Hodges RR, Oshi Y, et al. Role of cPKCalpha and nPKCepsilon in EGF-stimulated goblet cell proliferation. Invest Ophthalmol Vis Sci. 2009; 50: 614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayashi D, Li D, Hayashi C, et al. Role of histamine and its receptor subtypes in stimulation of conjunctival goblet cell secretion. Invest Ophthalmol Vis Sci. 2012; 53: 2993–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72: 248–254 [DOI] [PubMed] [Google Scholar]
- 26. Bugrim AE. Regulation of Ca2+ release by cAMP-dependent protein kinase. A mechanism for agonist-specific calcium signaling? Cell Calcium. 1999; 25: 219–226 [DOI] [PubMed] [Google Scholar]
- 27. DeSouza N, Reiken S, Ondrias K, et al. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem. 2002; 277: 39397–39400 [DOI] [PubMed] [Google Scholar]
- 28. Taylor CW, Tovey SC. From parathyroid hormone to cytosolic Ca2+ signals. Biochem Soc Trans. 2012; 40: 47–152 [DOI] [PubMed] [Google Scholar]
- 29. Mayati A, Levoin N, Paris H, et al. Induction of intracellular calcium concentration by environmental benzo(a)pyrene involves a beta2-adrenergic receptor/adenylyl cyclase/Epac-1/inositol 1,4,5-trisphosphate pathway in endothelial cells. J Biol Chem. 2012; 287: 4041–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah AU, Grant WM, Latif SU, et al. Cyclic AMP accelerates calcium waves in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008; 294: G1328–G1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pereira L, Metrich M, Fernandez-Velasco M, et al. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol. 2007; 583 (pt 2): 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yeh CK, Ghosh PM, Dang H, et al. beta-Adrenergic-responsive activation of extracellular signal-regulated protein kinases in salivary cells: role of epidermal growth factor receptor and cAMP. Am J Physiol Cell Physiol. 2005; 288: C1357–C1366 [DOI] [PubMed] [Google Scholar]