SUMMARY
Invasive fungal infections (IFIs) are a major cause of death in organ transplant patients. The murine hydrocortisone-mediated immunosuppression model of pulmonary aspergillosis is commonly used to characterise IFIs in these patients. However, this model does not take into account the effects of calcineurin inhibitors on transplant immunity to IFIs or the fungal calcineurin pathway, which is required for both virulence and antifungal drug resistance. To address these two issues, a new and clinically relevant transplant immunosuppression model of tacrolimus (FK506) and hydrocortisone-associated pulmonary aspergillosis was developed. We first characterised IFIs in 406 patients with a lung transplant. This showed that all of the patients with pulmonary aspergillosis were immunosuppressed with calcineurin inhibitors and steroids. Murine pharmacokinetic studies demonstrated that an ideal dose of 1 mg/kg/day of FK506 intraperitoneally produced blood trough levels in the human therapeutic range (5–12 ng/ml). There was increased mortality from pulmonary aspergillosis in a transplant-relevant immunosuppression model using both FK506 and hydrocortisone as compared with immunosuppression using hydrocortisone only. Lung histopathology showed neutrophil invasion and tracheobronchitis that was associated with reduced lung tumour necrosis factor-α (TNFα), JE (homologue of human MCP-1) and KC (homologue of human IL-8) at 24 hours, but increased lung TNFα, JE and KC at 48 hours when fungal burden was high. Furthermore, FK506 directly impaired fungal killing in alveolar macrophages in vitro, with FK506-mediated inhibition of the radial growth of Aspergillus fumigatus in vitro occurring at the low concentration of 5 ng/ml. Taken together, these findings show that the immunosuppressive activity of FK506 outweighs its antifungal activity in vivo. These observations demonstrate that FK506 impairs innate immune responses and leads to an incremental increase in susceptibility to IFIs when it is combined with steroids. This new and clinically relevant mouse model of invasive aspergillosis is a valuable addition to the further study of both fungal immunity and antifungal therapy in organ transplantation.
INTRODUCTION
Since the pioneering experiments of Peter Medawar in the 1950s, immunosuppressive drugs have been used to inhibit the immune rejection of organ transplants in man (Medawar, 1958). Although early approaches were based upon cortisone and azathioprine, the discovery of the calcineurin inhibitor cyclosporin A in the 1970s was a major breakthrough that enabled transplantation to become an established therapy (Green and Allison, 1978). In the subsequent four decades, organ transplantation has emerged as an effective treatment for end-stage organ failure, as well as for diseases such as type 1 diabetes mellitus (Watson and Dark, 2012). In the USA there were more than 28,000 organ transplants in 2008 (http://www.srtr.org/) with this number increasing every year. The macrolide calcineurin inhibitor tacrolimus (FK506) was licensed for use in transplantation by the FDA in 1994, and it has now replaced cyclosporin A as the first-line calcineurin inhibitor in solid organ transplantation because of improved outcomes (Moore and Lord, 1994; Watson and Dark, 2012). FK506 binds to FKBP12 in T cells and this complex mediates calcineurin inhibition, and also blocks T cell signal transduction via NFAT and interleukin-2 (IL-2) transcription (Tamura et al., 1994). Thus, the major mechanism of action of FK506 is to inhibit T-cell-dependent transplant rejection. However, recent studies have also demonstrated that calcineurin negatively regulates the Toll-like receptor signalling pathway (Kang et al., 2007) that is required for innate C-type lectin signalling (Greenblatt et al., 2010). These observations suggest a more widespread effect of calcineurin inhibitors on both innate as well as adaptive immune responses.
Organ transplant recipients are at an increased risk of a number of different bacterial, viral and opportunistic fungal infections (Fishman, 2011). There is a clear association between calcineurin inhibitors and the opportunistic infections cytomegalovirus, hepatitis C, BK virus and invasive fungal pathogens (Kotton and Fishman, 2005; Fishman, 2007; Pappas et al., 2010). Comprehensive surveillance data from the US TRANSNET registry shows that solid organ transplant patients have an overall risk of 3.1% per annum for proven or probable IFIs, and that this risk continues for at least 3 years post-transplantation (Pappas et al., 2010). The overall mortality from IFIs in these patients is ∼40%. The primary pathogens responsible are Candida spp., A. fumigatus and Cryptococcus neoformans. Furthermore, and in contrast to stem cell transplantation, neutropenia is not a significant driver for IFI in organ transplantation, suggesting that the immunological determinants of susceptibility to infection are different to those that have already been defined (Baddley et al., 2010). Animal models that accurately represent the immunosuppressive drug regimens in current clinical use in solid organ transplantation patients are therefore needed to understand the pathogenesis of these life-threatening infections.
TRANSLATIONAL IMPACT.
Clinical issue
Invasive fungal infections (IFIs) are a major cause of death among organ transplant recipients, who have to be immunosuppressed to inhibit T-cell-mediated organ rejection. Recipients of solid organ transplants have an overall risk of about 3% per annum of IFIs, and their mortality from IFIs is about 40%, despite the availability of antifungal drugs with good in vitro activity. Studies in humans suggest that organ transplant recipients who succumb to IFIs have an impaired peripheral blood interferon-γ response and that such patients benefit from adjunctive recombinant human interferon-γ therapy. Immunosuppression for organ transplantation currently consists of a combination of a steroid and a calcineurin inhibitor such as tacrolimus (FK506). Importantly, in addition to immunosuppressive activity, calcineurin inhibitors have antifungal activity and interact with other antifungals in vitro.
Results
Most studies of fungal infections in organ transplantation are undertaken in a hydrocortisone-mediated immunosuppression murine model of invasive pulmonary aspergillosis, a model that fails to take into account the effects of calcineurin inhibitors. In this study, the authors develop a FK506-hydrocortisone combination murine model of invasive aspergillosis. They determine the FK506 dose that achieves a blood trough in male BALB/c mice equivalent to human therapeutic levels in transplantation and show that the combination of FK506 and hydrocortisone increases mortality in mice with invasive pulmonary aspergillosis compared with hydrocortisone alone. In mice immunosuppressed with FK506 and hydrocortisone with invasive pulmonary aspergillosis, examination of the lung histopathology, analysis of the expression of pulmonary inflammatory cytokines and chemokines in the mice, and Aspergillus fumigatus infection assays in murine alveolar macrophages indicate that the immunosuppressive activity of FK506 outweighs its antifungal activity in vivo and in vitro.
Implications and future directions
These findings introduce a new and clinically relevant murine model for invasive pulmonary aspergillosis. Despite the in vitro antifungal activity of calcineurin inhibitors, these findings show that FK506-mediated immunosuppression in mice with invasive pulmonary aspergillosis leads to heightened inflammation and necrosis in the lung and tracheobronchitis, and increases mortality from pulmonary aspergillosis, probably because of a calcineurin-dependent defect of fungal killing in alveolar macrophages. This new murine model has the potential to provide insights into the immunity to IFIs in organ transplant recipients, and can also be used for preclinical studies of antifungal drug efficacy.
Cyclosporin A was first isolated from the soil fungus Tolypocladium inflatum, by Novartis in 1972 during an extensive screen for immunosuppressive compounds (Rüegger et al., 1976). Cyclosporin A complexes with cyclophilin and directly inhibits calcineurin. Similarly, FK506 was first isolated from the actinomycete Streptomyces tsukabaensis (Ochiai et al., 1987). In fungi, both drugs are known to inhibit calcinueurin signalling, which is required for conidial germination, hyphal growth and tissue invasion (Fox and Heitman, 2002). Previous studies in a murine model of haematogenous aspergillosis indicated that cyclosporin A reduces the length of survival but that FK506 has no net effect (High and Washburn, 1997). However, aspergillosis is not acquired by the haematogenous route, but by the pulmonary route. Although earlier studies showed that neither immunosuppressive drug had an effect on invasive candidiasis, recent studies have shown that cyclosporin A impairs innate immunity (Greenblatt et al., 2010). In these studies, the effect of steroids combined with calcineurin inhibitors in transplantation was not investigated.
Taken together, these observations suggest a complex relationship between the effects of calcineurin inhibitors on fungal virulence, host defence and antifungal therapy. Therefore, clinically relevant models of transplant invasive fungal infections are now required to better understand these crucial interactions. The key requirements for such a model are: (i) to account for the compound effects of different transplant immunosuppressive drugs on disease outcome; (ii) to enable characterisation of immunosuppressive drug pharmacokinetics so that they are relevant to human transplantation; (iii) an appropriate route of infection and organ specificity of the disease; (iv) the genetic tractability of the murine model, (v) a disease pathology in mice that accurately reflects the disease in humans; and (vi) cross-correlation with existing models of aspergillosis in mice. This study has addressed all these issues in a new murine model of transplant pulmonary aspergillosis.
RESULTS
Lung transplant patients immunosuppressed with steroids and FK506 are at high risk of pulmonary aspergillosis
We first characterised the immunosuppressive drug regimens and the incidence of invasive aspergillosis in a cohort of lung transplant recipients. Prospective surveillance of a cohort of 406 lung transplant patients was undertaken over a 6-month period to determine their incidence of proven or probable pulmonary aspergillosis using the revised EORTC criteria (De Pauw et al., 2008). Proven cases were defined as biopsy-proven invasive aspergillosis. Probable cases were defined as patients with a positive BAL (broncho-alveolar lavage) culture for Aspergillus sp. and radiological evidence of invasive aspergillosis (macronodules, halo sign or air crescent sign) or tracheobronchitis (tree-in-bud). We did not include possible cases. There were 39 patients with proven or probable IFIs, of whom 19 had pulmonary aspergillosis; this was equivalent to a 12-month incidence of 9% for pulmonary aspergillosis. Eleven patients had Aspergillus tracheobronchitis, and eight patients had invasive aspergillus disease. All of the patients with aspergillosis had been maintained on prednisolone-mediated immunosuppression and calcineurin inhibitors. Sixteen patients were receiving FK506 and three patients were receiving cyclosporine. A further 12 patients were also receiving mycophenolate mofetil, and three patients azathioprine.
FK506 inhibits A. fumigatus growth in vitro at transplant-relevant concentrations
To determine whether the immunosuppressive drug FK506 has significant antifungal activity at concentrations that are physiologically relevant to clinical transplantation, we first defined the minimum inhibitory concentration for FK506 against A. fumigatus CEA10 using the EUCAST microbroth dilution method (Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing, 2008). The minimum inhibitory concentration (MIC) for FK506 was 16 μg/ml; however, morphological changes (microcolonies and increased frequency of hyphal branching) could be seen at doses down to 0.5 μg/ml. For this reason, we then further analysed the effects of FK506 with the more sensitive radial growth assay on both Sabaraud and RPMI agar (Fig. 1). Using this approach, we found that the radial growth of A. fumigatus CEA10 was significantly inhibited by FK506 at concentrations that were equivalent to the trough serum levels in patients on maintenance drug therapy to prevent organ rejection (5–12 ng/ml; Fig. 1). There was no significant effect on growth rates for hydrocortisone at concentrations from 0.5 to 1000 μg/ml (data not shown).
Fig. 1.
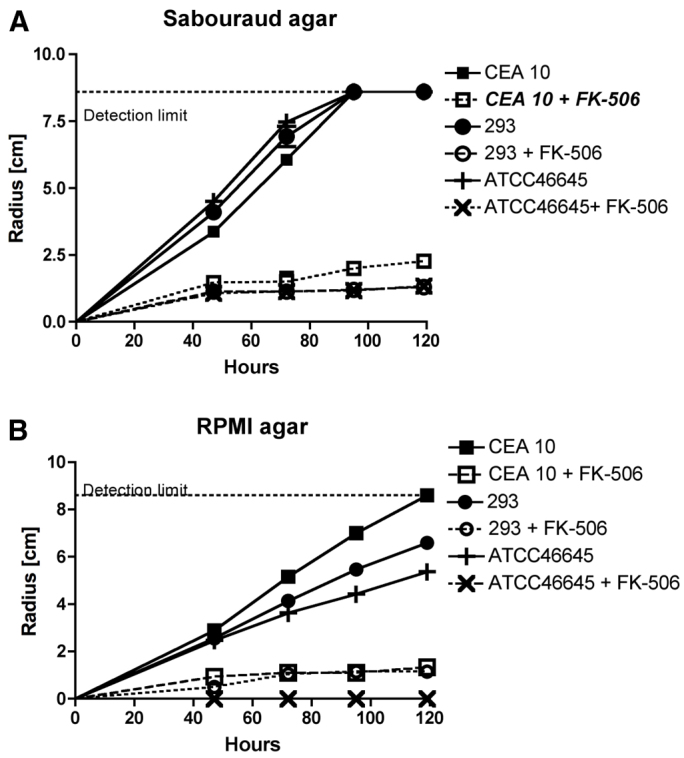
Determination of the effect of FK506 on A. fumigatus growth at a human-therapeutic trough concentration by radial growth assay. (A,B) Radial growth of A. fumigatus strains CEA10, AF293 and ATCC46645 was measured at defined time points after inoculation of 1×103 conidia onto Sabaroud (A) or RPMI (B) media with or without 5 ng/ml FK506 and incubation at 37°C. Data are shown for triplicates measurements for each of the three strains tested. The detection limit indicates the width of the Petri dish.
Human-equivalent FK506-mediated immunosuppression does not enable establishment of murine pulmonary aspergillosis with a standard inoculum of A. fumigatus
In order to produce a clinically relevant model of transplant aspergillosis, we then undertook dose-ranging studies for FK506 to define the ideal dosing regimen required to produce a human-equivalent 12-hour therapeutic trough level. FK506 was given as daily and incremental intraperitoneal (i.p.) doses. All trough concentrations were assayed on whole blood taken 12 hours after the third dose (84 hours). These initial dose-ranging studies demonstrated that, for FK506, the ideal human-relevant dosage was 1 mg/kg i.p. daily (mean trough concentration 6.9 ng/ml, s.e.m. 0.58; Fig. 2). Incremental dose-finding studies defined the LD50 (dose at which 50% of animals die) for tacrolimus as 20 mg/kg daily i.p. (data not shown).
Fig. 2.

Determination of ideal i.p. dosage of FK506 to produce human-therapeutic trough concentrations in blood. Whole blood levels of FK506 were measured by LC-MS at 12 hours after the third injection of FK506 i.p. daily. All FK506 dosages were given according to body weight. Mice received 1, 2.5, 5 or 7.5 mg FK506/kg/day. Data are shown as mean ± s.e.m. of triplicate measurements. Dotted lines indicate the upper and lower limits for the trough therapeutic ranges for the drugs in transplant recipients.
We then compared the survival of BALB/c mice infected with an intranasal inoculum of 5×106A. fumigatus CEA10 conidia after immunosuppression from day 3 prior to infection (day −3) with either hydrocortisone (125 or 250 mg/kg subcutaneously, s.c., every 3 days) or FK506 (1 mg/kg i.p. daily). These studies showed that although 80% mortality was seen by day 9 using 250 mg hydrocortisone/kg s.c. every 3 days, and 60% mortality using 125 mg hydrocortisone/kg i.p. every 3 days, there was no mortality for FK506 (used alone) at 1, 5, 10 or 15 mg/kg up to 10 days post-infection (data not shown). RT-PCR for fungal burden and histopathological analysis by periodic acid-Schiff (PAS) stain showed no evidence of infection in any of these animals at day 10 post-infection.
FK506 causes incremental mortality from pulmonary aspergillosis when combined with steroids
We then compared the effect of immunosuppression by a combination of FK506 and hydrocortisone on the outcome for pulmonary aspergillosis with the effect of hydrocortisone alone. In two independent experiments, groups of eight BALB/c mice were immunosuppressed with 125 mg/kg hydrocortisone every 3 days from day −3 with or without the addition of 1 mg/kg FK506 daily i.p. These studies showed incremental mortality from invasive pulmonary aspergillosis in the FK506-hydrocortisone immunosuppression model (100% by day 5) compared with immunosuppression using hydrocortisone only (75% mortality by day 5, P<0.0041; Fig. 3A). We observed that all mice initially had dyspnoea, including those that subsequently survived in the steroid group, which was consistent with recovery from significant aspergillus infection. FK506 did not cause increased weight loss in sham PBS-infected animals immunosuppressed with hydrocortisone (supplementary material Fig. S1A). Weight loss data for A. fumigatus-infected mice indicated that weight loss was greatest in the combined immunosuppression group (supplementary material Fig. S1B). Analysis of the fungal burden at 6, 24 and 48 hours post-infection showed an increase in the fungal burden in the animals treated with FK506 and hydrocortisone (P<0.021; Fig. 3B). Furthermore, analysis of fungal killing in murine alveolar macrophages indicated a clear killing defect that was dependent upon FK506 (Fig. 3C).
Fig. 3.
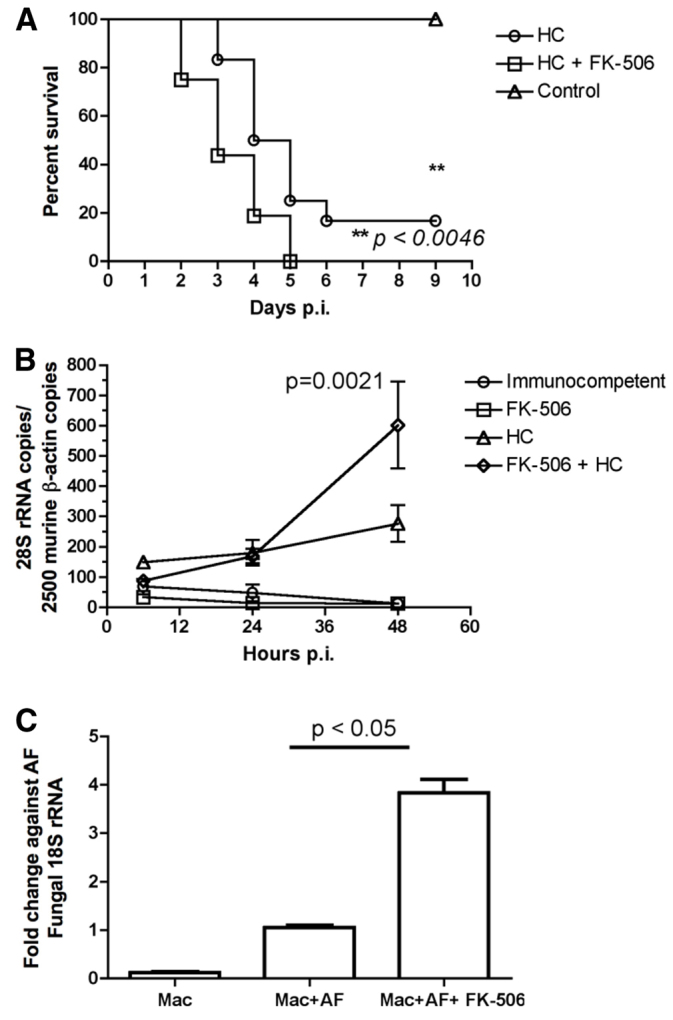
Survival from pulmonary aspergillosis and fungal burden in BALB/c mice immunosuppressed with FK506 and hydrocortisone. (A) Survival from pulmonary aspergillosis. Two groups of eight BALB/c mice were immunosuppressed with either hydrocortisone (125 mg/kg s.c. every 3 days) or with hydrocortisone (125 mg/kg s.c. every 3 days) in combination with FK506 (1 mg/kg i.p. daily) from day −3. They were then intranasally inoculated with 5×106A. fumigatus CEA10 conidia. Data are shown as mean from the two independent experiments. Survival differences were determined by Kaplan-Meier analysis. (B) Time course of pulmonary fungal burden. Groups of three mice were either untreated or immunosuppressed with FK506, hydrocortisone or both and sacrificed at 6, 24 and 48 hours post-infection and lungs harvested. Total DNA was isolated and A. fumigatus fungal burden estimated by semi-quantitative RT-PCR in comparison with murine β-actin. Statistical significance was determined by linear regression analysis. Data are shown as mean ± s.e.m. (C) Killing of A. fumigatus by murine alveolar macrophages. The alveolar macrophage cell line MH-S was pre-treated either with 10 ng/ml FK506 or with carrier. The ability to kill A. fumigatus CEA10 was assayed at 6 hours post-infection at an MOI of 1 by RT-PCR for fungal RNA after normalisation to control. p.i., post-infection; HC, hydrocortisone; Mac, alveolar macrophage; AF, A. fumigatus.
In order to determine whether the increased mortality in the FK506-hydrocortisone model was independent of fungal strain or mouse strain, we repeated these experiments in groups of eight mice using either a C57BL/6 background or with the A. fumigatus isolates AF293 or ATCC46645. These experiments confirmed the incremental mortality for the FK506-hydrocortisone model with both the sequenced strain AF293 and the clinical isolate ATCC46645 (Fig. 4). Survival studies in the C57BL/6 background mouse showed that they were also susceptible to the effects of FK506, with an incremental increase in mortality in the mice treated with FK506 and hydrocortisone (100% at day 5) as compared with the hydrocortisone-only group (50% at day 5, P<0.0016) (Fig. 4C).
Fig. 4.
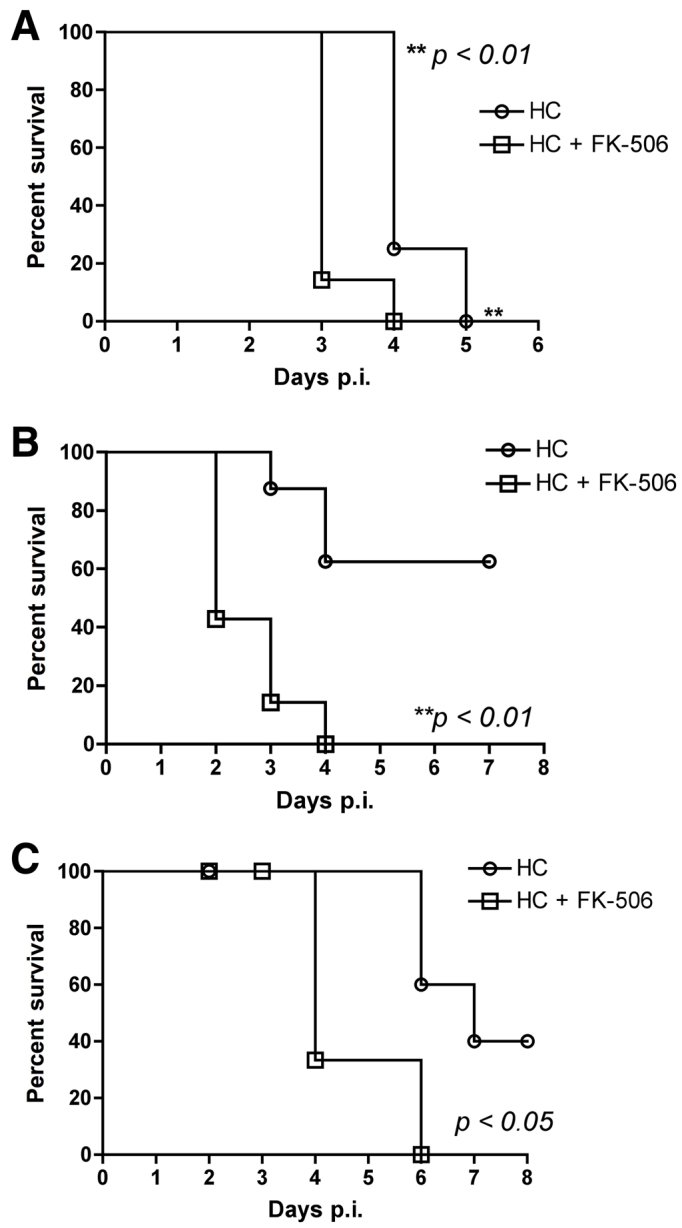
Incremental mortality in the FK506-hydrocortisone immunosuppression model is independent of murine or fungal strain. Groups of eight (A,B) male BALB/c and (C) male C57BL/6 mice were immunosuppressed with hydrocortisone (125 mg/kg s.c. every 3 days) alone or in combination with FK506 (1 mg/kg i.p. daily) from day −3, and then intranasally inoculated with 5×106A. fumigatus AF293 (A), ATCC46645 (B) or CEA10 (C) conidia. Survival differences were determined by Kaplan-Meier analysis. p.i., post-infection; HC, hydrocortisone; FK, FK506.
In invasive aspergillosis, FK506-mediated immunosuppression results in increased pulmonary inflammation and an increase in JE, KC and TNFα
We then undertook a histopathological analysis of the lungs from mice with pulmonary aspergillosis. Groups of three mice were immunosuppressed with hydrocortisone, FK506 plus hydrocortisone, or FK506 alone and the lungs harvested at 48 hours post-infection. Sections were stained by PAS and scored. Inflammation was quantified by threshold particle analysis using ImageJ software. There was incremental parenchymal inflammation in the mice immunosuppressed with both FK506 (1 mg/kg) and hydrocortisone (125 mg/kg) compared with the hydrocortisone-only (125 mg/kg) group (Fig. 5A). Hematoxylin and eosin (H&E) staining and Grocott sections from the lung tissue showed acute inflammation that surrounded and involved the airways. Some of the airways were occluded by an infiltrate (Fig. 5B). In others, the bronchial epithelium was disrupted and destroyed by the inflammatory response (Fig. 5B). Also noted were multiple areas of patchy consolidation with destruction of the lung parenchyma; there was often near-complete obliteration of the lung architecture by the acute inflammatory response. The cellular infiltrate was composed of neutrophils and histiocytes. Grocott silver staining showed a large number of septate fungal hyphae branching at acute angles and with the presence of bulbous heads.
Fig. 5.
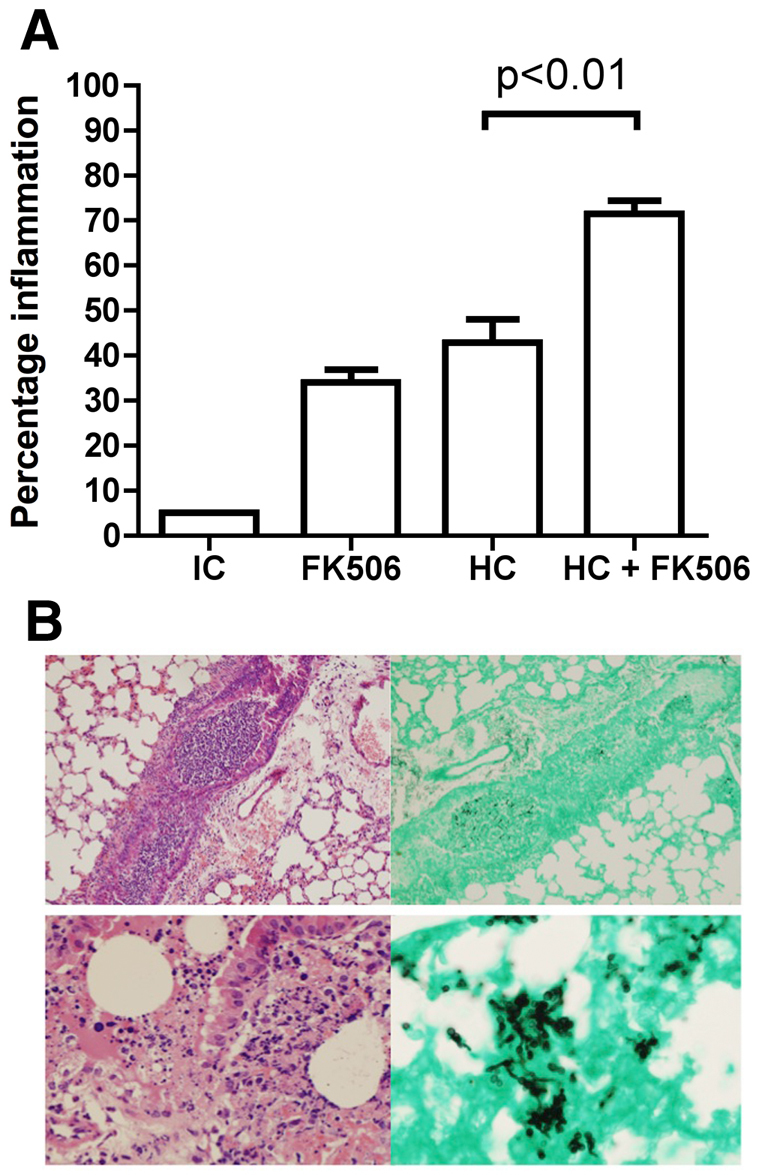
Immunosuppression with FK506 and hydrocortisone leads to increased inflammation in invasive pulmonary aspergillosis. Groups of three mice were either untreated or immunosuppressed with FK506, hydrocortisone or both and culled at 48 hours after infection. Lungs were fixed and three PAS stained sections from each mouse scored with ImageJ software by threshold analysis for the amount of inflammation. (A) Percentage of lung area inflamed (histopathology). (B) Representative Grocott-stained (right) and H&E-stained (left) lung sections for the combined FK506-hydrocortisone model, demonstrating airways occluded by inflammatory cells and fungal germlings, together with inflammation in and around the airway and damage to the bronchial epithelium. IC, immunocompetent; HC, hydrocortisone.
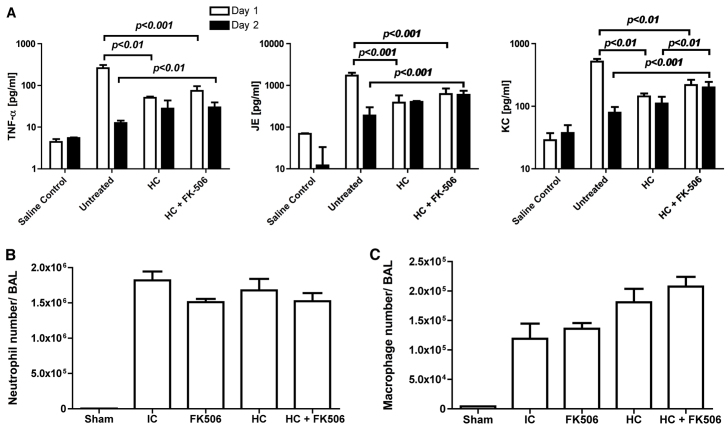
In order to determine the effects of FK506 immunosuppression on pulmonary cytokine and chemokine production, we then performed Luminex multianalyte profiling (MAP) cytokine analysis on whole lung homogenates and BAL samples in mice immunosuppressed with FK506 and hydrocortisone at 24 and 48 hours post-infection. These studies showed that immunocompetent mice (i.e. untreated) infected with A. fumigatus had significantly higher levels of JE (homologue of human MCP-1), KC (homologue of human IL-8) and tumour necrosis factor-α (TNFα) in their lungs at 24 hours compared with mice treated with either hydrocortisone or FK506 plus hydrocortisone (Fig. 6A). In immunocompetent mice, JE, KC and TNFα fell to near normal levels by 48 hours. This contrasted with hydrocortisone-treated animals, in which JE, KC and TNFα increased significantly. The highest levels of JE, KC and TNFα were seen at 48 hours post-infection in the lungs of the mice treated with FK506 and hydrocortisone (Fig. 6A). There were no significant increases in interferon-γ, IL-2, IL-4, IL-6 or IL-13. We further characterised neutrophil and macrophage numbers in the alveolar space by fluorescence activated cell sorting (FACS) analysis of broncho-alveolar lavage samples at 24 hours post-infection. These studies did not demonstrate any significant difference in inflammatory cell numbers in infected animals (Fig. 6B,C).
Fig. 6.
Immunosuppression with FK506 and hydrocortisone leads to increased levels of TNFα, JE and KC in the lung in invasive aspergillosis. (A) Groups of three mice were either untreated or immunosuppressed with hydrocortisone or FK506 plus hydrocortisone and culled at 24 and 48 hours after infection with 5×106A. fumigatus CEA10 conidia. Saline controls were uninfected. Lungs were homogenised and supernatant levels of TNFα, JE and KC measured by Luminex MAP analysis. Statistical significance was determined by one-way ANOVA and Tukey’s test. (B,C) Groups of three mice were either untreated or immunosuppressed with FK506, hydrocortisone or both and culled at 24 hours after infection with 5×106A. fumigatus CEA10 conidia. (B) FACS analysis for neutrophil influx into the bronchoalveolar space. There were no significant differences between infected groups. (C) FACS analysis for macrophages in the bronchoalveolar space. There were no significant differences between infected groups. Sham, uninfected group; HC, hydrocortisone; IC, immunocompetent; BAL, bronchoalveolar lavage. Data are shown as mean and s.e.m.
DISCUSSION
IFIs have emerged as a major cause of morbidity and mortality in organ transplant patients (Pappas et al., 2010) who are immunosuppressed with steroids and calcineurin inhibitors. However, this immunosuppressive drug regimen is not represented in current standard animal models of invasive fungal infections, which are typically based upon hydrocortisone alone (Sheppard et al., 2006). We have therefore developed a new model of murine immunosuppression using both FK506 and hydrocortisone. This model will enable detailed and clinically more representative mechanistic studies of disease pathogenesis and of antifungal drug evaluation. Importantly, this model has evolved from our existing standard hydrocortisone model of invasive aspergillosis and should therefore ensure that the outcomes can be compared with those relating to virulence, immunity and therapy from previous mouse studies (Tang et al., 1993).
Despite the in vitro antifungal activity of FK506 at transplant-therapeutic concentrations, due to the presence of the calcineurin pathway in fungi (Heitman et al., 1994), we found that mice immunosuppressed with hydrocortisone plus FK506 had an incremental mortality from pulmonary aspergillosis compared with the established hydrocortisone murine model. Paradoxically, this was associated with a significant increase in lung fungal burden, with the results reproducible in BALB/c mice, C57BL/6 mice, the genome-sequenced strains AF293 and CEA10, as well as the clinical isolate ATCC46645. There was histopathological evidence of increased parenchymal and trancheobronchial inflammation as assessed through blind scoring by a consultant histopathologist.
It was not possible to establish an FK506 monotherapy immunosuppression model of pulmonary aspergillosis without the use of a large fungal inoculum (5×107 conidia). Under these conditions, a hyperacute disease developed that was characterised by acute respiratory failure, dense peribronchial hyphal infiltrates and death within 24 hours. This syndrome was not consistent with the pattern of clinical disease in transplant patients, which is typically much less acute (Pappas et al., 2010). By contrast, we were able to demonstrate incremental susceptibility to infection in the more clinically representative FK506 plus steroid combination immunosuppression murine model. Dosing with 1 mg/kg FK506 i.p. daily enabled establishment of 12-hour trough levels of FK506 that were equivalent to those seen in human transplant patients (5–12 ng/ml). This resulted in an increase in mortality from 80% in the baseline hydrocortisone model to 100% in the FK506-hydrocortisone model. These observations suggest that the combination of steroids with calcineurin inhibitors is a key driver for susceptibility to fungal infection in organ transplant recipients. This is consistent with our observations in man where the ‘at risk’ population is typically immunosuppressed with a combination of steroids and calcineurin inhibitors.
The finding of incremental mortality with therapeutic levels of FK506 strongly suggests that calcineurin inhibitors contribute to increased susceptibility to IFIs in transplantation. Notably, our in vitro studies demonstrate that therapeutic concentrations of FK506 (e.g. 5–12 ng/ml) significantly impair radial growth of A. fumigatus in the EUCAST standard RPMI-based medium. However, and paradoxically, our in vivo studies demonstrated that similar dosages lead to higher fungal burden in the lung and increased mortality. This suggests that the immunosuppressive effects of FK506 outweigh its antifungal potential in this murine model, a finding that was reproducible across three different fungal strains (CEA10, AF293 and ATCC46645) and two mouse strains (BALB/c and C57BL/6). These findings were also consistent with those demonstrated in murine alveolar macrophages that exhibited impaired fungal killing in the presence of FK506. Taken together, the reults suggest that the calcineurin pathway contributes to innate immune responses to A. fumigatus and, as such, are consistent with prior observations in Candida albicans (Greenblatt et al., 2010). Further studies are required to define the molecular mechanisms underlying this phenotype. We also noted that a significant number of lung transplant patients with pulmonary aspergillosis also receive mycophenolate mofetil; however, our studies to date indicate that its addition to the immunosuppressive drug regimen does not increase susceptibility to IFIs in this murine model (unpublished observations).
The calcineurin pathway is highly conserved in eukaryotic organisms (Aramburu et al., 2004). In Saccharomyces cerevisiae, the primary targets of cyclosporin and FK506 have been shown to be cyclophillin and FKBP12, respectively. Both drugs have also been shown to have potent antifungal activity in C. neoformans, and A. fumigatus (Foor et al., 1992; Odom et al., 1997; Steinbach et al., 2007a). Previous microbroth dilution studies defined a geometric MIC of 1.25 μg/ml for FK506 against a range A. fumigatus strains; however, these studies employed the NCLS method (National Committee for Clinical Laboratory Standards, 2002) rather than the EUCAST method, and the strains tested differed (Steinbach et al., 2004). In comparison, although we found that the MIC for the highly-virulent genome-sequenced strain CEA10 was 16 μg/ml, we went on to assess the radial growth of three A. fumigatus strains on EUCAST agar. These studies indicated that the impaired radial growth still occurred at the much lower patient blood trough therapeutic concentration of 5 ng/ml of FK506 for all of the strains tested (CEA10, Af293 and ATCC46645).
These observations raise the possibility that FK506 has significant effects on fungal growth in vivo in transplant patients. Interestingly, in C. albicans, calcineurin inhibitors do not inhibit growth unless they are used in combination with azole antifungals such as fluconazole (Marchetti et al., 2000). Further studies have shown synergism of calcineurin inhibitors with the echinocandin class of antifungals that inhibit cell wall 1,3-β-glucan synthesis (Steinbach et al., 2007b). This suggests that significant interactions could exist between the calcineurin inhibitor drugs used as immunosuppressants in transplantation and the antifungal drugs used to treat IFIs. Thus, the development of a new murine transplant immunosuppression model of pulmonary aspergillosis that is based on a combination of steroid and FK506 will enable the further and detailed study of calcineurin inhibitor interactions with multiple drugs in a clinically relevant model. Its direct comparability to our current hydrocortisone immunosuppression model or the cyclophosphamide-induced neutropenia model is important because the same mouse strain, fungal strain, inoculum and steroid immunosuppression regimen were used.
Histopathological analysis of the lungs from FK506-hydrocortisone immunosuppressed mice with pulmonary aspergillosis demonstrated incremental inflammation defined by increased polymorphonuclear and histiocytic cell infiltrates and tissue necrosis in the lung compared with the baseline hydrocortisone immunosuppression model. Notably, there was an increase in tracheobronchial inflammation associated with numerous visible hyphae within the airway. Interestingly, Aspergillus tracheobronchitis is a disease syndrome that is particularly associated with lung transplant patients, suggesting that the combination of FK506 and hydrocortisone might, in some way, contribute to this disease (Kramer et al., 1991). It is further conceivable that the antifungal properties of calcineurin inhibitors alter the pathology of the disease and lead to a mucosally restricted phenotype.
Our characterisation of pulmonary Aspergillus infections in 406 lung transplant patients over a 6-month period found an incidence of 39 cases of proven or probable IFI, of which 19 were due to pulmonary aspergillosis. Of these, 58% had tracheobronchitis as their clinical diagnosis, with all except one patient receiving steroids and a calcineurin inhibitor at the time of diagnosis. This was a clinical demonstration of the association of the steroid and calcineurin drug regimen with an increased susceptibility to IFIs.
Aspergillus tracheobronchitis is common in other groups of organ transplant patients and this also suggests a relationship with calcinerin inhibitor use. In order to further determine the mechanism that leads to increased inflammation and a cellular infiltrate in the lung in our new mouse model, we undertook Luminex® Fluorokine® MAP cytokine analysis of whole mouse lung homogenates. Immunocompetent mice showed an early (24 hours) pro-inflammatory response to pulmonary aspergillosis with high levels of lung TNFα, JE and KC compared with hydrocortisone-only immunosuppressed mice. However, by 48 hours, there was a dramatic reduction in lung cytokine production in immunocompetent mice that was associated with rapid fungal eradication, whereas mice immunosuppressed with hydrocortisone or FK506 plus hydrocortisone continued to increase their lung TNFα, JE and KC. These changes were greatest in the combination FK506-hydrocortisone murine model where the fungal burden was also highest. This suggests an initial failure to generate an adequate and appropriate innate cytokine response in the transplant model at 24 hours, followed by a late sustained cytokine response at 48 hours that is associated with the presence of the fungal pathogen in the lung. Therefore, the inflammatory cytokine TNFα, the macrophage chemokine JE and the neutrophil chemokine KC might drive the continuing cell-mediated inflammatory pathology in this model. These observations lead us to hypothesise that calcineurin inhibition might directly impair innate fungal killing. Our studies in murine alveolar macrophages further support these findings, where exposure to FK506 significantly impaired killing of A. fumigatus in vitro. Taken together, these in vitro and in vivo observations strongly suggest that FK506 impairs fungal killing in alveolar macrophages, leading to increased fungal burden in the lung. This increased fungal burden appears to drive a sustained hyperinflammatory response in the lung, leading to tissue destruction and increased mortality. Further studies are required to confirm these preliminary observations and to further define the relationship between tracheobronchitis, chemokine expression and calcineurin inhibition.
In summary, we have developed a new mouse model of pulmonary aspergillosis that is based upon immunosuppression with FK506 and hydrocortisone and is relevant to organ transplantation as it is practised today. Because IFIs have emerged as a major cause of morbidity and mortality in this rapidly expanding group of patients, the hydrocortisone-only mouse model for studies of host immunity, immunotherapy and antifungal therapy is no longer suitable (Pappas et al., 2010). It should now be possible to obtain a better understanding of the complex drug interactions that occur and to enable the development of rational strategies to improve the outcome for those patients with life-threatening IFIs.
MATERIALS AND METHODS
Estimation of prevalence of pulmonary aspergillosis in lung transplant patients
We prospectively identified lung transplant patients at the Royal Brompton and Harefield NHS Trust (London, UK) who started antifungal drug therapy over a 6-month period. This study was approved by the ethics committee of the Royal Brompton and Harefield NHS Trust Biomedical Research Unit. Case notes were reviewed and patients categorised into proven, probable and possible invasive aspergillosis according to the revised EORTC criteria (De Pauw et al., 2008). Probable aspergillus tracheobronchitis was defined as typical bronchoscopic appearances (tracheobronchitis with or without ulceration) or radiographic appearances (tree-in-bud) in the presence of positive bronchoscopic culture for A. fumigatus in patients with otherwise unexplained deterioration in lung function and no other identified cause. Immunosuppressive regimens at the initiation of antifungal drug therapy were identified from electronic pharmacy records.
A. fumigatus strains and growth conditions
A. fumigatus CEA10 was the primary isolate used. A. fumigatus 293 and ATCC46645 were used for confirmatory studies. For in vivo studies, strains were cultured on solid Aspergillus complete medium (ACM) for 3–5 days (Barratt et al., 1965) prior to harvest in 0.1% Tween and then filtered through MIRACLOTH (Calbiochem, UK). Conidial suspensions were washed twice in sterile normal saline (Baxter Healthcare, UK), enumerated by haemocytometry and re-suspended at the concentrations indicated. For in vitro studies, all radial growth and minimum inhibitory concentration assays were performed according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) procedures (Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing, 2008). Stock FK506 (Cambridge Bioscience, UK) was re-suspended in 100% ethanol at 5 mg/ml, and diluted in RPMI medium (Sigma, UK). The drug was added to EUCAST medium at the desired concentration as indicated.
Murine infections
All mouse experiments were approved by the United Kingdom Home Office and performed in accordance with project license PPL 70/7324. Groups of five BALB/c or C57BL/6 male mice (18–22 g; Harlan Ortech, UK) were housed in individually vented cages with free access to autoclaved food and water. Mice received hydrocortisone (Hydrocortistab, Sovereign Medical) by s.c. injection and FK506 (Prograf, Astellas) by i.p. injection at the concentrations and time-points shown. All mice received 1 mg/l tetracycline hydrochloride (Sigma) and 64 mg/l ciprofloxacin (Sigma) in drinking water as prophylaxis against bacterial infections. Mice were anaesthetised by isoflourane inhalation and intranasally inoculated with the indicated number of A. fumigatus conidia in 40 μl of normal saline. For mortality studies, mice were culled at 20% weight loss as a UK Home Office established end-point and organs harvested.
For histopathological studies, lungs were fixed in 10% buffered formalin for 24 hours and embedded in paraffin. Sections (4 μm) were stained with PAS, H&E or Grocott stains. Analysis of lung histopathological inflammation was performed using ImageJ software (rsb.info.nih.gov/ij/). Quantitative analysis of total lung area was performed by threshold particle analysis in ImageJ (http://rsbweb.nih.gov/ij/). Further thresholding procedures were performed within selective areas of inflammation that were visually identified and a subsequent percentage affected proportion calculated.
FK506 concentration in blood
Blood was taken by terminal cardiac puncture and stored in Microcontainer K2E tubes (Becton Dickinson). Whole blood FK506 concentrations were determined by liquid chromatography-mass spectrometry (LC-MS) according to validated clinical methods.
Luminex cytokine analysis
Murine lungs were harvested and immediately homogenised in 1 ml phosphate-buffered saline (PBS) per 100 mg tissue. Lung homogenates were centrifuged at 3000 g for 10 minutes and supernatants snap-frozen in liquid nitrogen. Cytokine quantification was performed with a Luminex 200 analyser by Fluorokine® multianalyte profiling (MAP) and according to the manufacturer’s instructions (Luminex, USA).
Estimation of fungal burden
For in vivo studies, whole lungs were homogenised in 1 ml PBS per 100 mg lung tissue and DNA extracted with the DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer’s instructions. Fungal burden was estimated by semi-quantitative real-time PCR as described, after normalisation to murine β-actin (White et al., 2006). For in vitro studies, fungal burden was analysed by measuring A. fumigatus 18S rRNA (GenBank accession number AB008401) levels by real time PCR as previously described (Werner et al., 2009). The murine BALB/c-derived MH-S alveolar macrophage cell line was pre-treated either with 10 ng/ml FK506 for 1 hour or with vehicle, and infected with resting conidia (A. fumigatus CEA10; multiplicity of infection, MOI, 1:1) for 6 hours. Results were calculated as fold change against non-FK506 treated cells by the ΔCt method, whereby the input was adjusted to murine HPRT levels (forward 5′-GTAATGATCAGTCAACGGGGGAC-3′; reverse 5′-GCAAGCTTGCAACCTTAACCA-3′).
Flow cytometry
Mice were sacrificed and bronchoalveolar lavage performed by tracheal cut-down followed by intubation with a 20 Venflon intravenous catheter (Becton Dickinson). Bronchoalveolar lavage was performed by instillation of 1 ml of PBS twice. Cell suspensions were labelled with the following antibodies: anti-CD16/32 (blocking; eBioscience), anti-Ly-6G-BV421 (BioLegend), anti-F4/80-APC/Cy7 (BioLegend), anti-CD11c-FITC (eBioscience) and anti-CD45-PE/Cy7 (eBioscience). Cells were fixed with 4% paraformaldehyde and acquired on a Fortessa flow cytometer.
Supplementary Material
Acknowledgments
We are grateful to Paul Nya for histopathological sections. We also thank P. Lewis White for plasmids.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
D.A.-J., S.S., A.D. and E.B. conceived, designed and oversaw the experiments. S.H., D.A.-J. and G.C. performed the experiments. N.K. reported the histopathological sections. A.S. and M.C. undertook the clinical study. A.S. performed the histopathological analysis. D.A.-J., S.H., A.S. and S.S. analysed the data and wrote the paper. All authors revised and approved the manuscript.
FUNDING
This work was supported by a Medical Research Council (UK) Clinician Scientist Fellowship to D.A.-J. [grant number G0902260/1].
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.010330/-/DC1
REFERENCES
- Aramburu J., Heitman J., Crabtree G. R. (2004). Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep. 5, 343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddley J. W., Andes D. R., Marr K. A., Kontoyiannis D. P., Alexander B. D., Kauffman C. A., Oster R. A., Anaissie E. J., Walsh T. J., Schuster M. G., et al. (2010). Factors associated with mortality in transplant patients with invasive aspergillosis. Clin. Infect. Dis. 50, 1559–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt R. W., Johnson G. B., Ogata W. N. (1965). Wild-type and mutant stocks of Aspergillus nidulans. Genetics 52, 233–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pauw B., Walsh T. J., Donnelly J. P., Stevens D. A., Edwards J. E., Calandra T., Pappas P. G., Maertens J., Lortholary O., Kauffman C. A., et al. (2008). Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46, 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J. A. (2007). Infection in solid-organ transplant recipients. N. Engl. J. Med. 357, 2601–2614 [DOI] [PubMed] [Google Scholar]
- Fishman J. A. (2011). Infections in immunocompromised hosts and organ transplant recipients: essentials. Liver Transpl. 17 Suppl. 3, S34–S37 [DOI] [PubMed] [Google Scholar]
- Foor F., Parent S. A., Morin N., Dahl A. M., Ramadan N., Chrebet G., Bostian K. A., Nielsen J. B. (1992). Calcineurin mediates inhibition by FK506 and cyclosporin of recovery from alpha-factor arrest in yeast. Nature 360, 682–684 [DOI] [PubMed] [Google Scholar]
- Fox D. S., Heitman J. (2002). Good fungi gone bad: the corruption of calcineurin. Bioessays 24, 894–903 [DOI] [PubMed] [Google Scholar]
- Green C. J., Allison A. C. (1978). Extensive prolongation of rabbit kidney allograft survival after short-term cyclosporin-A treatment. Lancet 311, 1182–1183 [DOI] [PubMed] [Google Scholar]
- Greenblatt M. B., Aliprantis A., Hu B., Glimcher L. H. (2010). Calcineurin regulates innate antifungal immunity in neutrophils. J. Exp. Med. 207, 923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J., Cardenas M. E., Breuder T., Hemenway C., Muir R. S., Lim E., Goetz L., Zhu D., Lorenz M., Dolinski K. (1994). Antifungal effects of cyclosporine and FK 506 are mediated via immunophilin-dependent calcineurin inhibition. Transplant. Proc. 26, 2833–2834 [PubMed] [Google Scholar]
- High K. P., Washburn R. G. (1997). Invasive aspergillosis in mice immunosuppressed with cyclosporin A, tacrolimus (FK506), or sirolimus (rapamycin). J. Infect. Dis. 175, 222–225 [DOI] [PubMed] [Google Scholar]
- Kang Y. J., Kusler B., Otsuka M., Hughes M., Suzuki N., Suzuki S., Yeh W. C., Akira S., Han J., Jones P. P. (2007). Calcineurin negatively regulates TLR-mediated activation pathways. J. Immunol. 179, 4598–4607 [DOI] [PubMed] [Google Scholar]
- Kotton C. N., Fishman J. A. (2005). Viral infection in the renal transplant recipient. J. Am. Soc. Nephrol. 16, 1758–1774 [DOI] [PubMed] [Google Scholar]
- Kramer M. R., Denning D. W., Marshall S. E., Ross D. J., Berry G., Lewiston N. J., Stevens D. A., Theodore J. (1991). Ulcerative tracheobronchitis after lung transplantation. A new form of invasive aspergillosis. Am. Rev. Respir. Dis. 144, 552–556 [DOI] [PubMed] [Google Scholar]
- Marchetti O., Moreillon P., Glauser M. P., Bille J., Sanglard D. (2000). Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44, 2373–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar P. B. (1958). The immunology of transplantation. In The Harvey Lectures (1956–1957), series 52, pp.144–176 New York: Academic Press; [PubMed] [Google Scholar]
- Moore R., Lord R. (1994). Tacrolimus (FK506) versus cyclosporin in prevention of liver allograft rejection. Lancet 344, 948. [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards (2002). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard. NCCLS document M38-A. Clinical and Laboratory Standards Institute, Villanova, PA [Google Scholar]
- Ochiai T., Nakajima K., Nagata M., Suzuki T., Asano T., Uematsu T., Goto T., Hori S., Kenmochi T., Nakagoori T., et al. (1987). Effect of a new immunosuppressive agent, FK 506, on heterotopic cardiac allotransplantation in the rat. Transplant. Proc. 19, 1284–1286 [PubMed] [Google Scholar]
- Odom A., Muir S., Lim E., Toffaletti D. L., Perfect J., Heitman J. (1997). Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16, 2576–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas P. G., Alexander B. D., Andes D. R., Hadley S., Kauffman C. A., Freifeld A., Anaissie E. J., Brumble L. M., Herwaldt L., Ito J., et al. (2010). Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 50, 1101–1111 [DOI] [PubMed] [Google Scholar]
- Rüegger A., Kuhn M., Lichti H., Loosli H. R., Huguenin R., Quiquerez C., von Wartburg A. (1976). [Cyclosporin A, a peptide metabolite from Trichoderma polysporum (Link ex Pers.) Rifai, with a remarkable immunosuppressive activity]. Helv. Chim. Acta 59, 1075–1092 [DOI] [PubMed] [Google Scholar]
- Sheppard D. C., Graybill J. R., Najvar L. K., Chiang L. Y., Doedt T., Kirkpatrick W. R., Bocanegra R., Vallor A. C., Patterson T. F., Filler S. G. (2006). Standardization of an experimental murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50, 3501–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach W. J., Singh N., Miller J. L., Benjamin D. K., Jr, Schell W. A., Heitman J., Perfect J. R. (2004). In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus isolates from transplant and nontransplant patients. Antimicrob. Agents Chemother. 48, 4922–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach W. J., Reedy J. L., Cramer R. A., Jr, Perfect J. R., Heitman J. (2007a). Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5, 418–430 [DOI] [PubMed] [Google Scholar]
- Steinbach W. J., Cramer R. A., Jr, Perfect B. Z., Henn C., Nielsen K., Heitman J., Perfect J. R. (2007b). Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob. Agents Chemother. 51, 2979–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing (2008). EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14, 982–984 [DOI] [PubMed] [Google Scholar]
- Tamura K., Fujimura T., Iwasaki K., Sakuma S., Fujitsu T., Nakamura K., Shimomura K., Kuno T., Tanaka C., Kobayashi M. (1994). Interaction of tacrolimus(FK506) and its metabolites with FKBP and calcineurin. Biochem. Biophys. Res. Commun. 202, 437–443 [DOI] [PubMed] [Google Scholar]
- Tang C. M., Cohen J., Krausz T., Van Noorden S., Holden D. W. (1993). The alkaline protease of Aspergillus fumigatus is not a virulence determinant in two murine models of invasive pulmonary aspergillosis. Infect. Immun. 61, 1650–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C. J., Dark J. H. (2012). Organ transplantation: historical perspective and current practice. Br. J. Anaesth. 108 Suppl. 1, i29–i42 [DOI] [PubMed] [Google Scholar]
- Werner J. L., Metz A. E., Horn D., Schoeb T. R., Hewitt M. M., Schwiebert L. M., Faro-Trindade I., Brown G. D., Steele C. (2009). Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182, 4938–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. L., Linton C. J., Perry M. D., Johnson E. M., Barnes R. A. (2006). The evolution and evaluation of a whole blood polymerase chain reaction assay for the detection of invasive aspergillosis in hematology patients in a routine clinical setting. Clin. Infect. Dis. 42, 479–486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.